Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The fallopian tubes, endometrium, myometrium, and cervix function in concert to receive gametes, facilitate fertilization, support embryo growth, and ultimately orchestrate the timely expulsion of a mature fetus. The preparation of a reproductive tract conducive to pregnancy is governed by ovarian steroid hormones acting directly on their cognate receptors and indirectly through multiple steroid-regulated growth factors, cytokines, and other extracellular and intracellular signaling molecules. This complex and redundant interaction between cells is made further complicated by bidirectional communications between the placenta trophoblast/trophectoderm and the endometrial cells. This chapter describes the structural and biochemical changes in the endometrium during the normal menstrual cycle and pregnancy, its clinical evaluation, and the pathophysiology of some relevant disorders related to endometrial function (see also Chapter 14, Chapter 25, Chapter 26 ).
The components of a receptive endometrium include the luminal epithelium, whose apical surface expresses cell adhesion molecules permitting adherence of the blastocyst; glandular epithelium, whose cells secrete substances that support the development of the blastocyst and placenta; and decidualized stromal cells and large granular lymphocytes that modulate trophoblast function and functional and structural modification of blood vessels. The interregulation of these components is orchestrated by the secretion and action of growth factors, growth factor-binding proteins, angiogenic factors, cytokines, and an extracellular matrix that facilitates trophoblast invasion. The combinatorial actions of paracrine and endocrine factors, together with the extracellular matrix, promote trophoblast proliferation and development into the endometrium while controlling excessive invasion.
Innate and adaptive immune functions, under the regulation of steroid hormones, collectively defend the reproductive tract environment against microbial invasion, but also must be modulated during pregnancy to allow the semiallogenic embryo and fetus to avoid rejection by the maternal host. The vascular system nourishes the endometrium in the initial receptive phase and is subsequently remodeled by invading trophoblasts to establish the placental blood supply. The coordinated contractile activity of the myometrium promotes sperm migration in a cycle of conception while also facilitating embryo transport prior to attachment.
In the absence of conception, the functionalis portion of the endometrium is shed through a well-controlled inflammatory-like reaction involving enzymes such as matrix metalloproteinases (MMPs), inflammatory cytokines, production of vasoactive substances, and uterine contractions, leaving behind the endometrial basalis, with its stem cells to allow regeneration. Specialized mechanisms ensure hemostasis and prevent scar formation. Through these mechanisms, a new and intact luminal endometrial surface is regenerated and prepared for the next round of oocyte release and potential fertilization.
The primary function of the endometrium is to provide a privileged site for blastocyst implantation and to provide an optimal environment for growth and development of the embryo/fetus and its associated placenta.
The cyclic differentiation of the endometrium depends on the actions of steroid hormones from the ovary including estrogen and progesterone.
The endometrium undergoes repetitive cycles of proliferation, differentiation, and menstruation, hundreds of times in a woman’s life, without apparent signs of aging.
Embryo implantation requires a complex series of endometrial changes to allow optimal receptivity to the embryo and to govern placental development.
Inflammatory signaling plays an important role in normal uterine function (menstruation and embryo implantation), but inappropriate inflammation in the endometrium may result in a phenomenon known as progesterone resistance, which appears to be involved in infertility and pregnancy loss.
The female reproductive tract is derived from the urogenital ridge, which, during week 6 of gestation, gives rise to paired mesodermal, paramesonephric tubes (the müllerian or paramesonephric ducts). Differentiation of the intermediate mesoderm precedes development of the gonads (ovaries/testicles), kidneys, urinary tract, and male/female reproductive tracts. , Mesenchymal to epithelial transformation within the intermediate mesoderm gives rise to tubules that eventually form the male and female reproductive tracts (see Chapter 16 ). , , Longitudinal invaginations of the coelomic epithelium form the Fallopian tubes or oviducts, with the caudal ends fusing by week 10 of gestation to produce the primordial uterus and upper portion of the vagina. A thin septum remaining after fusion eventually resorbs, yielding a single uterine cavity.
The primordial uterus is initially lined by a simple cuboidal epithelium that subsequently is specialized during postnatal development in domestic animals, laboratory rodents, and humans. Postnatal radial patterning establishes the major histological elements of the developed uterus, including stratification of the endometrial stroma, differentiation of the myometrium, and development and differentiation of the luminal (LE) and glandular epithelium (GE). Adenogenesis is initiated by differentiation of the GE from precursor LE. Nascent glands elongate into the stroma and then coil and slightly branch as they develop through the stroma toward the inner circular layer of the myometrium. By week 22 of gestation, the uterus has the primary structure of the adult organ. Glandular secretory activity, glycogen accumulation, and stromal edema are evident by week 32 under the influence of placentally derived steroid hormones. After delivery and the consequent precipitous fall in placental estrogens and progesterone, the endometrium regresses to an atrophic state, containing a few small glands and a poorly vascularized stroma.
The embryonic events described previously are driven, in large measure, by the secreted ligands of the wingless-type MMTV integration site (WNT) family (WNT4, WNT5A, WNT7A) and transcriptional regulators of the homeobox (HOX) gene family based predominantly on studies in mice ( Fig. 10.1 ). , This morphogenetic program can only be played out in the absence of antimüllerian hormone (AMH also known as müllerian Inhibiting Substance or MIS) is a member of the transforming growth factor beta (TGFB) family made by the Sertoli cells of the fetal testes. In the absence of testosterone and AMH, the müllerian ducts elongate and develop into the fallopian tubes, uterus, cervix, and upper part of the vagina. The elongation phase of the müllerian ducts requires a number of factors. Given their common embryonic origin, early development in the mouse of the kidneys, ureters, and reproductive tract are tightly linked and involve other specific genes, including Pax2, Lim1, Emx2 , as well as the members of the WNT and abdominal-B HOXA families of genes. Lim1 encodes a transcription factor that along with PAX2 is essential for urogenital tract development. Lim1 null mice lack uteri and oviducts. Pax2 null mice lack kidneys, ureters, and genital tracts. Caudal elongation of the paramesonephric duct is absent. EMX2 is another transcription factor of the homeodomain gene family that appears to be essential for urogenital tract development. , EMX2 is highly expressed in the adult uterus, and its expression is correlated with cell proliferation and appears to be inhibited by the HOX gene, HOXA10 . There is decreased expression of PAX2 and LIM1, and mesenchymal segmental polarity gene product, WNT4, is also absent in mice lacking EMX2, suggesting the essential role of this transcription factor.
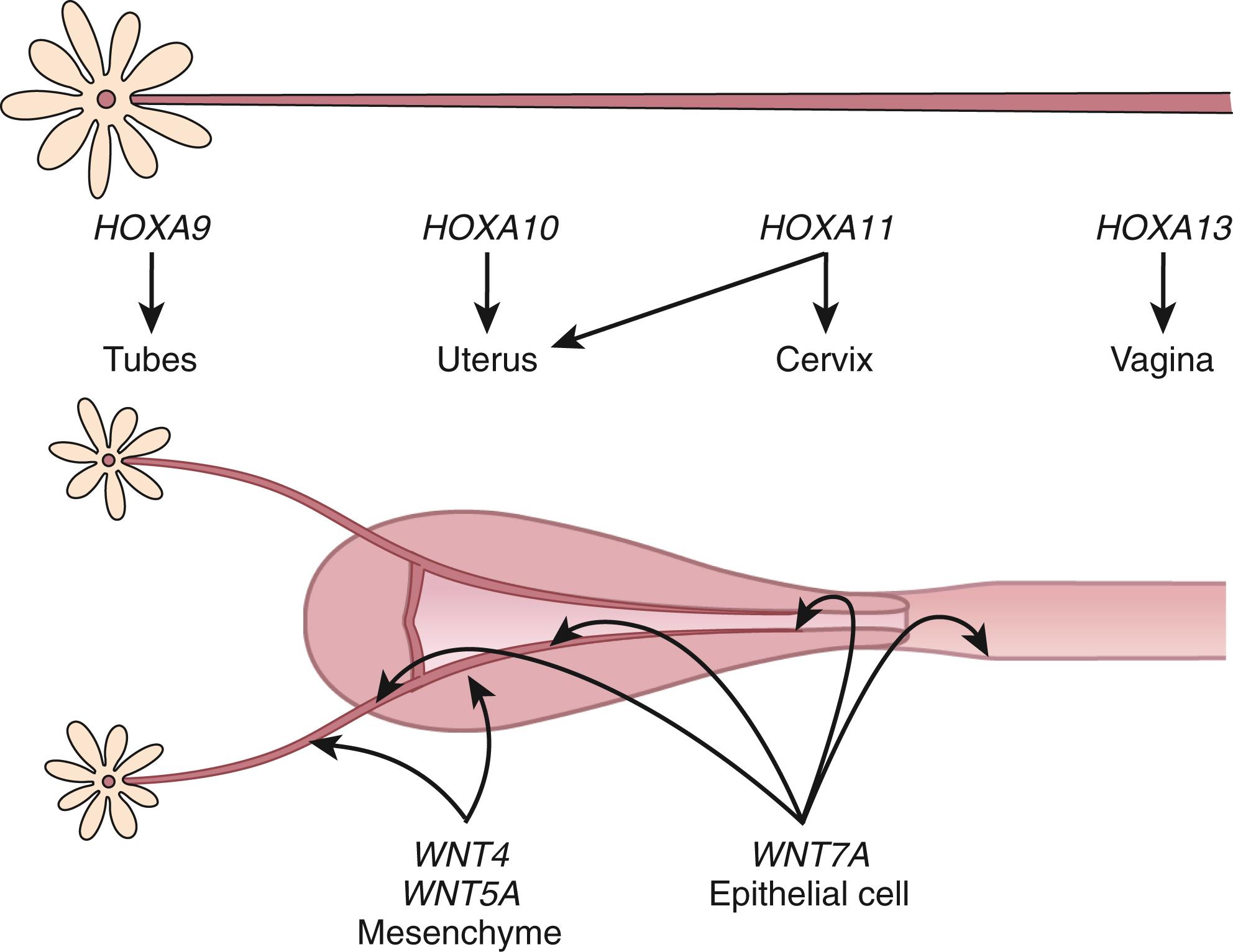
The function of individual WNTs in uterine development has been addressed by targeted deletion of specific WNT genes. Wnt4 and Wnt5a are expressed throughout the mesenchyme of the FRT, whereas Wnt7a is expressed uniquely in LE, and crosstalk between these compartments is essential for uterine development. , The müllerian ducts are absent in female mice lacking Wnt4 , a gene expressed in the mesenchyme. Moreover, female mice lacking WNT4 are partially sex-reversed due to the retention of the Wolffian ducts. Cases of Wnt4 null mutations associated with müllerian duct regression and a phenotype, including hyperandrogenemia, resembling that of the Wnt4 knockout mouse, have been reported in women. There is also a proposed role for WNT4 in postnatal uterine function, including progesterone signaling.
WNT9B is expressed in the Wolffian duct epithelium and is necessary for müllerian duct extension. Mutations in the WNT9B gene have been found in women with Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome. These müllerian defects have been reported to be associated with other gene defects, including PBX1, RBM8A, and TBX6 mutations. Other genes have been implicated as well, based on balanced translocation studies and breakpoint mapping.
Deficiency of Wnt5a , a gene expressed in the genital tubercle and genital tract mesenchyme, results in mice with stunted genital tubercles and the absence of external genitalia. WNT7A expression gives rise to the luminal and glandular epithelium of the fallopian tubes and uterus, while WNT5 is associated with stromal cells. , WNT7A is involved in paracrine signaling to the endometrial mesenchyme. Mesenchymal beta-catenin (CTNNB1) appears to be the essential downstream effector of the WNT7A pathway and mediates its effects on the oviduct and proper development of the uterus. Although mutations in Wnt7a have not been found in women with müllerian anomalies, , mice lacking Wnt7a develop an oviduct that is not clearly demarcated from the upper uterine horn, and the uterus develops cellular characteristics that are similar to the vagina (including a stratified epithelium without uterine glands), and the uterine smooth muscle is disorganized. Postnatal expression of HOXA10 and HOXA11 in the uterus is also lost. Mesenchymal CTNNB1 appears to be the essential downstream effector of the Wnt7a pathway and mediates its effects on the oviduct and the proper development of the uterus. The WNT family of genes, including receptors and downstream signaling molecules, are also expressed in a regulated fashion in the adult reproductive tract, indicating that they have additional roles beyond those involved in early morphogenetic events, including the regulation of steroid hormone action in adult tissues (discussed in subsequent chapter text). ,
Formation and anterior-posterior patterning of the müllerian duct is regulated primarily by homeodomain-containing transcription factors. The HOX genes encode an evolutionarily conserved family of transcription factors that contain a signature 60 amino acid DNA-binding homeodomain. They play critical roles in organizing cells along the anterior-posterior axis and directing them to select a particular pathway of development. Mammalian HOX genes are arranged in four different clusters, designated A through D, with each cluster organized in a linear arrangement that parallels the order of expression along the anterior-posterior body axis. Expression of HOXA genes in the human and mouse reproductive tract is conserved, with HOXA9 being expressed in the fallopian tubes, HOXA10 and HOXA11 in the uterus, HOXA11 in the cervix, and HOXA13 in the upper vagina. Although there is a consistent regional distribution of HOX gene expression along the reproductive tract, there is evidence for some functional redundancy among the adjacent genes. Like the WNT genes, the HOXA genes are also expressed in the adult uterus, and their expression is under estrogen and progesterone regulation.
The importance of the HOX gene family in reproductive tract function was demonstrated through targeted deletions in specific HOXA genes. Another significant discovery was that Hand–Foot–Genital syndrome and Guttmacher syndrome, autosomal dominant conditions that affect bones in the hands and feet and cause reproductive tract abnormalities (including the bicornuate uterus), are caused by mutations in the HOXA13 gene. , However, to date, mutations in HOXA7 to HOXA13 and HOX gene cofactor pre-B-cell leukemia homeobox1 (PBX1) have yet to be found in subjects with congenital absence of the uterus and vagina. HOXA10 and HOXA11 have both been implicated in the process of implantation. , Mice with targeted deletions in the HOXA10 and HOXA11 genes have subtle abnormalities in uterine morphology, including transformation of the upper uterine segment into oviduct-like histology ( HOXA10 mutants), and reduced endometrial stromal development and expression of leukemia inhibitory factor (LIF) are seen in HOXA11 mutants. Notably, both HOXA10 and HOXA11 nullizygous females are infertile due to a uterine factor, implicating these genes in the implantation process in the adult. Mice lacking H6 homeobox 3 (Emx3) , another HOX domain gene product, are also infertile due to an implantation defect associated with perturbations in WNT and LIF gene expression.
Müllerian anomalies represent a complex collection of developmental defects occurring in up to 5% of the general population. Depending on the stage of development at which they occur, physical differences in the reproductive tract can be mild (e.g., a partial uterine septum) or severe, with complete absence of the cervix, uterus, and Fallopian tubes. These can be associated with infertility, endometriosis, and miscarriage. Some of the abnormalities require surgical correction and are often discovered at the time of puberty, if not before. Given the close developmental interaction between the müllerian and urinary system, it is not surprising that combined renal and müllerian duct anomalies occur. The recent clinical success of uterine transplantation provides a promising surgical treatment option for uterine factor infertility in extreme cases (see Chapter 40 ; Uterine Transplantation). Continued studies on the genetics of müllerian development will provide critical insights into the origins of reproductive tract anomalies.
Many, but not all, of the uterine responses to steroid hormones are mediated by specific intracellular cognate receptors (see Chapter 5 ). These nuclear receptors serve as transcription factors undergoing striking spatial and temporal changes in expression during the menstrual cycle. The endometrial response to steroid hormones is determined by the number of bioavailable hormones, which is influenced by hormone production rates as well as local steroid metabolism; the repertoire of steroid receptors, coactivators, and corepressors expressed; and the action of growth factors and cytokines that modulate the action of steroid hormone receptors.
Postnatal patterning of the uterus is an ovarian steroid-independent event. , , The oviducts, uterus, cervix, and vagina form in mice with inactivating mutations of both nuclear estrogen receptors (ESR1 and ESR2). Despite this independence from maternal or fetal estrogens, normal differentiation of the female reproductive tract can, paradoxically, be disrupted by exogenous estrogens. Diethylstilbestrol (DES), a synthetic estrogen that causes uterine and cervical anomalies in exposed females (discussed later in this chapter), and polychlorinated biphenyls suppress expression of Wnt7a and alter the pattern of expression of HOXA9 and HOXA10 in the murine reproductive tract through ESR1. This suggests that alterations in HOXA and WNT gene expression are the likely molecular mechanism underlying the anatomical defects observed in human females exposed to DES in utero . Postnatal progesterone may also inhibit normal endometrial gland development, as shown in the neonatal ewe and mouse. , Using this model, it appears that progesterone inhibition of gland development involves disruption of the WNT system, and illustrates that development may be independent of hormones but exposure at the wrong time may alter the normal developmental pathways.
Estradiol is the primary trophic hormone for the uterus, mediating uterine growth through estrogen receptor alpha or ESR1, whose amounts are highest during the proliferative phase of the cycle and decline after ovulation in response to rising progesterone ( Fig. 10.2 ). Immunohistochemical studies observed estrogen receptors in the nuclei of epithelial, stromal, and myometrial cells during the proliferative phase, with the epithelial cell staining being most prominent. , After progesterone levels rise in the luteal phase, estrogen receptor staining is restricted to the deep basal glands and vascular smooth muscle. In situ hybridization studies demonstrate that mRNA transcripts for both ESR1 and ESR2 (coding for estrogen receptor alpha and beta, respectively) are expressed in the epithelial, stromal, and smooth muscle cells at all stages of the cycle.
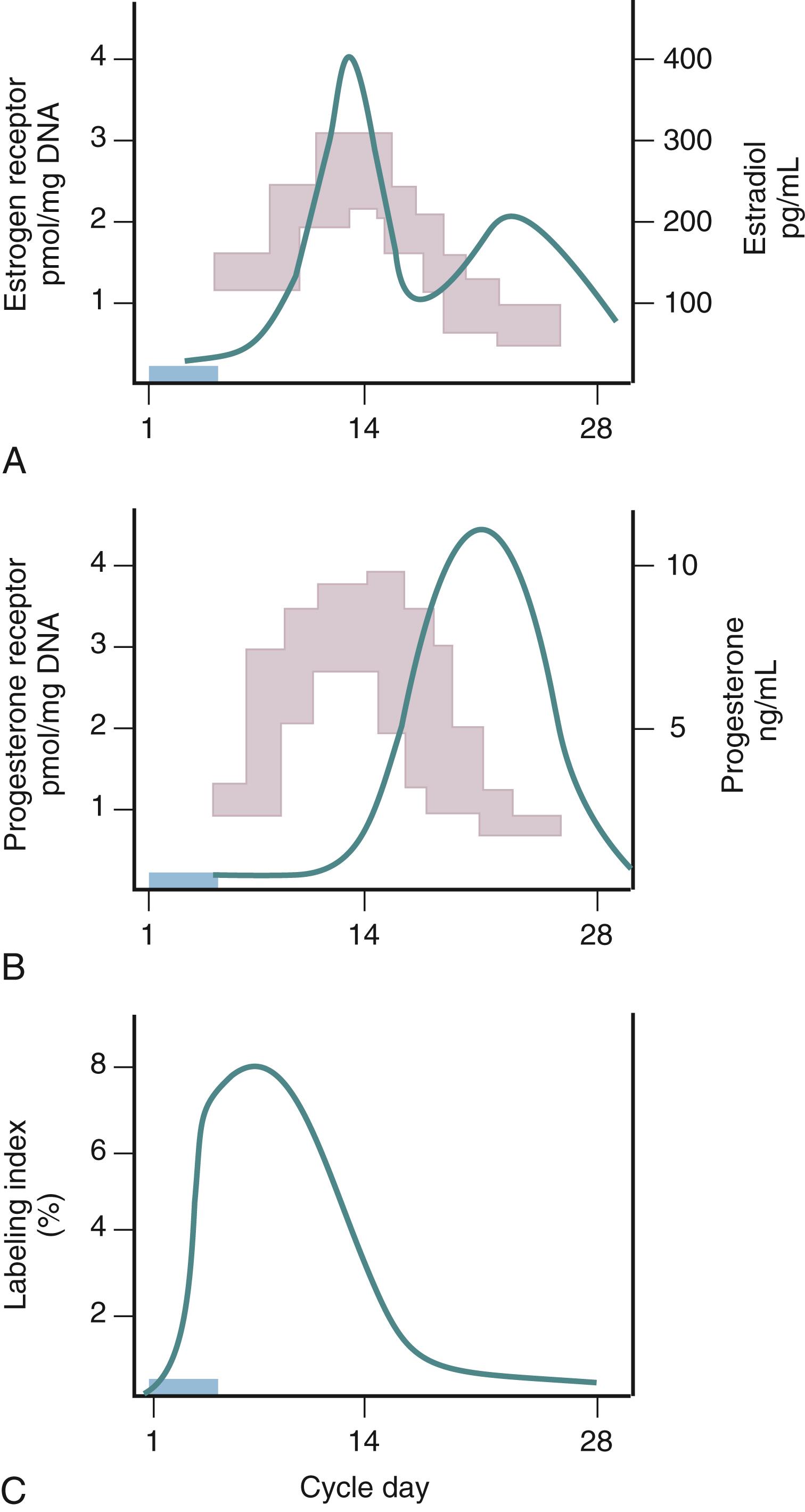
Is there a threshold dose of estrogen required to elicit a uterine growth response? Key and Pike proposed a threshold estrogen level of approximately 50 to 100 pg/mL, at which point endometrial proliferation is triggered and above which no further stimulation of endometrial proliferation occurs. This estimation is based on comparing endometrial proliferation assessed through ex vivo thymidine incorporation into endometrial explants from different stages of the menstrual cycle with corresponding estradiol levels at the different days of sampling. This hypothesis finds relevance in postmenopausal estrogen therapy where the effects of estrogen on bone, cardiovascular, and endometrial function may present differential risk/benefit profiles.
The decline in ESR1 at the time of implantation appears to be physiologically important and is a common finding across many species. , , Failure to decrease ESR1 indicates an imbalance in regulatory mechanisms of steroid hormone interactions and is associated with progesterone resistance, endometriosis, and infertility. , The mechanism of this downregulation is complex, involving progesterone signaling that is discussed in more detail later in this chapter.
ESR2 is expressed throughout the body expressed in almost all tissues. Despite a negligible effect on implantation in the knockout null mouse , ESR2 does appear to have importance in endometrial function. Like ESR1, ESR2 does appear to be upregulated by estrogen and downregulated by progesterone. Furthermore, in the ESR2 knockout mouse, progesterone receptor (PGR) levels rose, suggesting a suppressive action of ESR2 on this receptor, and may also modulate ESR1. In Esr1 depleted mice, treatment with estrogen blocked proliferation, suggesting an antiestrogenic effect and causing epithelial apoptosis through ESR2. In the human endometrium, ESR2 has been reported to be expressed in both glands and stroma, but some suggest it is present exclusively in the vascular compartment. Relative overexpression of ESR2 in ectopic endometrium of women with endometriosis has also been reported. Paradoxically, in humans, this increase in ESR2 noted in endometriosis has been shown to stimulate the progression of disease.
Other members of the estrogen receptor family include estrogen receptor-related alpha (ESRRA) and beta (ESRRB), orphan receptors, with homology to the classical ESR1. ERRA and its coactivator peroxisome proliferator-activated receptor gamma (PPARG) coactivator-1 alpha were found to show dramatically increased expression in the decidua and stimulate metabolic pathways for energy generation, perhaps in preparation for implantation. ERRB is observed throughout the endometrium during the menstrual cycle, including uterine natural killer (uNK) cells, but a precise role for this receptor has yet to be determined in the endometrium.
Membrane-bound receptors with specific recognition of steroid receptors also coordinate the paracrine, autocrine, and juxtacrine cellular mechanisms and provide an explanation for the rapid effects of steroid hormones. , , , Rapid effects of estradiol are mediated by at least two distinct receptors, a membrane-associated form of ESR1 and a recently described integral membrane receptor, G protein-coupled estrogen receptor (GPER), previously known as G protein-coupled receptor 30 (GPR30). , GPER has been characterized as an estrogen receptor and subsequent development of a GPER-specific agonist, G-1, and antagonist, G-15, have revealed important functions of this nonclassical estrogen receptor in multiple physiologic and pathophysiologic processes. , In the endometrium, this receptor is present in the endometrial epithelium during the late proliferative phase and transitions to the stroma and decidua in the latter stages of the menstrual cycle and pregnancy but may be dysregulated in endometriosis. , G-1 has been shown to induce cell cycle arrest and thereby has some potential value in proliferative diseases such as endometriosis. GPER may also function as an aldosterone receptor, and though a mechanism conferring steroid specificity remains unclear.
Progesterone antagonizes the actions of estrogen in the endometrium and promotes differentiation of the glands and stroma via PGR. The antagonism of the uterotropic actions of estradiol involves a complex series of events, including alterations in estrogen receptor expression, inhibition of estrogen-induced translocation of the cell-cycle regulators, and induction of enzymes that catabolize estradiol. , , All of these effects are mediated through specific cognate nuclear receptors that are induced by estrogen and modulate downstream events in a paracrine fashion between the epithelial and stromal compartments.
There are multiple isoforms of the PGR, but the majority of progesterone actions are served by the activities of either PGR-A or PGR-B. Both arise from a common PGR gene. Each isoform is expressed differentially in the endometrium, where the larger PGR-B is a strong transcriptional activator of endometrial genes, while PGR-A, 164 amino acid shorter, inhibits both PGR-B and other steroid receptors, including estrogen receptors. The A form predominates in the stroma, while B is more abundant in the epithelial phase of the early secretory phase of the cycle. Both PGR-A and PGR-B act as transcription factors, interacting with specific gene promoters and interacting with coregulators. PGR-B also has nongenomic mechanisms of action, able to interact with Src tyrosine kinases in the cytoplasm, which independently modify gene expression. While generally considered to have opposing functions, PGR-A is essential for stromal decidualization, and PGR-B is downregulated in the epithelial compartment at the time of pregnancy. Indeed, the persistence of epithelial PGR is thought to be a sign of defective endometrial receptivity and may signify progesterone resistance.
PGR isoforms A and B are both present in the epithelial and stromal compartments of the proliferative and early secretory endometrium. Progesterone through PGR serves to inhibit proliferation by estrogen, primarily by downregulation of the estrogen receptor, but also through induction of estradiol degrading enzymes. Unlike its antiproliferative effects in the glandular epithelium, stromal PGR stimulates proliferation through activation of the MAPK/AKT pathway.
Multiple other derived isoforms of PGR are thought to be present ( Fig. 10.3 ). In addition to PGR-A and PGR-B, there is a highly truncated isoform PGR-C, identified in the T47D breast cancer cell line. While still able to dimerize with PGR-A or PGR-B, PGR-C may be an inhibitory factor, unable to interact with gene promoters. A role in labor for PGR-C has been proposed.
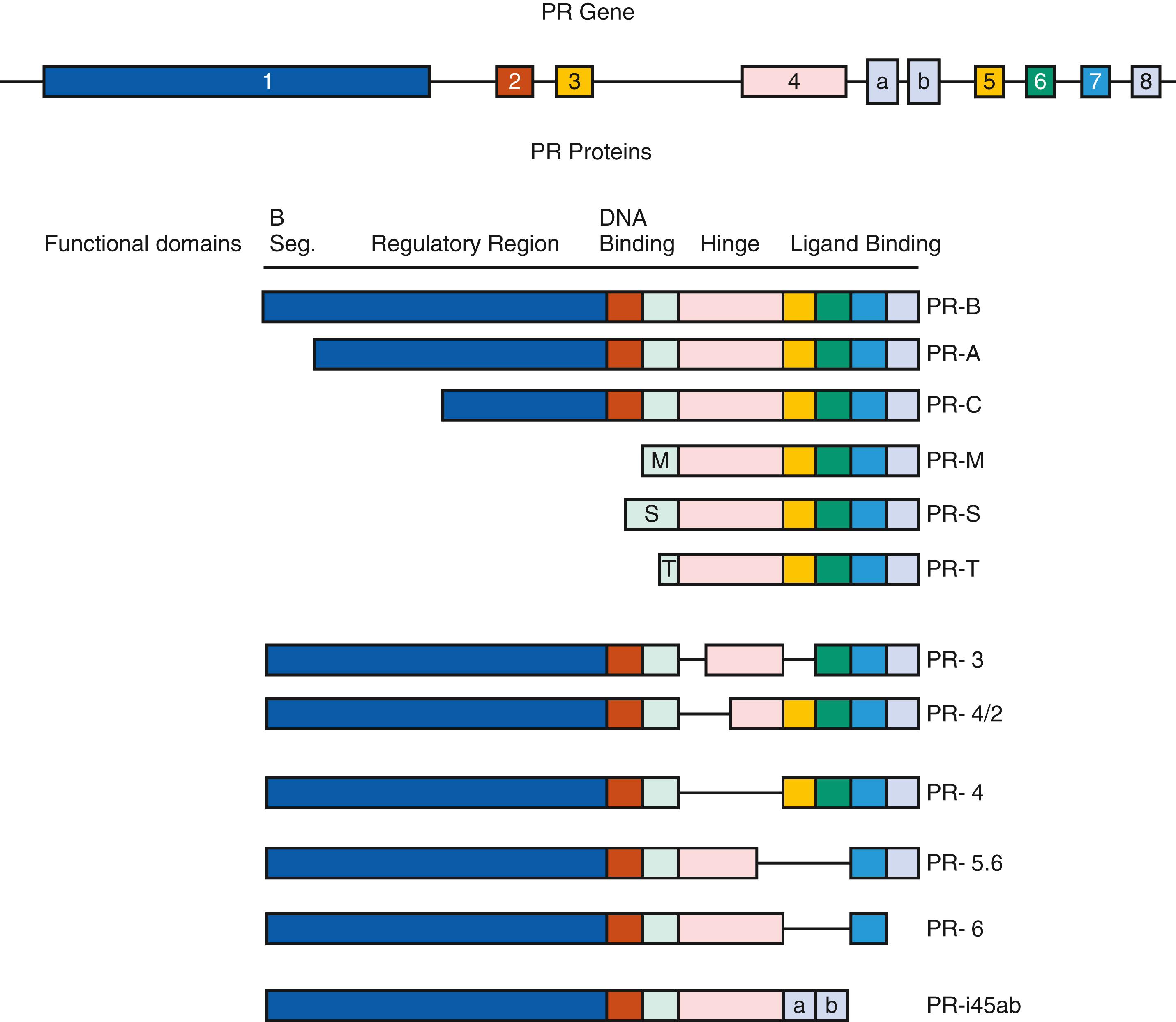
Classical endometrial PGR peak at the time of ovulation, localized to both epithelial and stromal cells, then declines. The rise in PGR is in response to estradiol, while the decline after ovulation is caused by elevated luteal phase progesterone that downregulates its own receptor. , , By 4 days after ovulation, PGR in the epithelial cells declines markedly in the epithelial compartment and remains weak or absent during the remainder of the secretory phase. In contrast, PGR expression in stromal cells remains strong throughout the menstrual cycle and into pregnancy, should it occur. In general, the A form predominates in the stroma, while the B is more abundant than the A form in the epithelial phase of the secretory phase of the cycle. PGR have not been detected in vascular endothelial cells or vascular smooth muscle, but they are abundant in the perivascular stroma. Consequently, the effects of progesterone or its withdrawal on the vasculature are likely indirect or perhaps via membrane PGR.
The significance of different ratios of these two major PGR forms, with respect to regulation of gene expression in the human uterus, is increasingly being studied. Clues are being provided from the study of mice with targeted deletion of the receptor isoforms. The uteri of mice lacking both the A and B forms of the PGR are hyperplastic and contain inflammatory infiltrates. , Hyperplasia reflects the lack of antagonism to the uterotropic actions of estradiol. Selective targeting of the A form of the PGR revealed that it is essential for progesterone-mediated actions on implantation and the decidual response. However, the examination of genes associated with the window of uterine receptivity known to be regulated by progesterone indicated that the A form controls expression of only a subset, while others appear to be under the control of PGR-B. Ablation of PGR-A in mice uncovered an unexpected role for the B form of the receptor in inducing epithelial proliferation. Administration of estradiol to PGR-A receptor-deficient mice resulted in uterine hyperplasia, but the combination of estradiol and progesterone resulted in even greater hyperplasia. It thus appears that PGR-A antagonizes the uterotropic responses mediated by both ESR1 and PGR-B. Regarding implantation and decidualization, however, when PGR-A null mice are examined, they are similar in their defects to mice that lack both PGR-A and PGR-B subunits, while PGR-B knockout mice were fertile. Clinically, reductions in PGR-B have been associated with proliferative states such as endometriosis, supporting this paradigm of counterregulatory mechanisms involving PGR isoforms. In addition, like estrogen, nongenomic actions of progesterone appear important in the reproductive tract as well.
Membrane forms of PGR have also been identified in the uterus. , [CR] This family of nonclassical, membrane progesterone receptors is structurally unrelated to PGR-A, B, or C, but each of the mammalian paralogues has been shown to specifically bind progesterone and can activate G-protein coupled signaling pathways. Indeed, membrane PGR (PGRMC) has been shown to rapidly activate MAP kinase and inhibit cyclic adenosine monophosphate (cAMP). Membrane PGR have been localized to the myometrium of humans and function to inhibit cAMP with a possible role of facilitating uterine contractions at term.
In addition to steroid hormone receptors, coactivators and chaperone proteins have an important impact on the uterine response to estrogens and progestogens. Uterine expression of p160 coactivators, steroid receptor coactivator-1 (SRC1), steroid receptor coactivator-2 (SRC2), and steroid receptor coactivator-3 (SRC3) has been examined. Decidualization of the endometrium does not occur without SRC2. SRC3 levels increased in the glandular epithelium in the late secretory phase, whereas SRC1 and SRC2 expression did not change. SRC3 has been linked to endometrial hyperplasia and in women with polycystic ovary syndrome (PCOS), SRC2 and 3, along with Erα, were elevated in the stroma and glandular epithelium, demonstrating that an abnormal endocrine milieu can alter coactivator levels, which could, in turn, result in endometrial dysfunction.
Based on the pioneering work of Cunha in mice, , the effects of estrogen and progesterone on epithelial and stromal proliferation and differentiation are, in large part, indirect, involving paracrine substances produced by the stroma that act on the epithelium. , , Estrogen acting through the stroma promotes DNA synthesis in the epithelium; under the influence of progesterone, the epithelium produces substances that affect the response of the underlying stroma and the epithelial cells to estrogen.
These indirect actions of estradiol on epithelial proliferation have been demonstrated in elegant reconstitution and grafting experiments employing stroma and epithelium from normal and Esr1 knockout (ERKO) mice. Epithelial cell proliferation does not occur when the stroma from an ERKO mouse uterus is paired with epithelium from a normal uterus. Conversely, epithelial cell DNA synthesis in response to estrogen occurs when normal stroma is paired with epithelium from ERKO mice. Studies on human endometrial cells in culture are consistent with the mouse studies. Estradiol increases epithelial cell proliferation when cocultured with stroma, but it does not increase proliferation in epithelial cells cultured in the absence of stromal cells.
What is the estrogen-stimulated signal from the stroma that promotes epithelial cell proliferation? Candidates include growth factors that are transcriptionally regulated by ESR1, including insulin-like growth factor-1 (IGF1), transforming growth factor alpha (TGFA), and epidermal growth factor (EGF). Alternatively, estradiol might suppress production of stromal factors that restrain epithelial cell proliferation. Among the growth factors, there has been particular interest in EGF and IGF1. Studies using mouse models, including transplantation of uteri and vagina from EGF receptor knockout mice, indicate that this receptor is required for the maximal fibromuscular stroma growth but not the epithelial cell proliferative response to estrogen.
IGF1 and IGF2 both stimulate the proliferation of human endometrial stromal cells via the type 1 IGF receptor. IGF1 expression is greatest in the late proliferative and early secretory phase, whereas IGF2 is most abundant in the midsecretory endometrium and decidua of the first trimester of pregnancy. The IGFs are bound by a family of binding proteins (IGFBPs) that modulate IGF activities in target tissues. One of the binding proteins, IGFBP1, is a major secretory product of decidualized stromal cells, and it has been hypothesized to play a role in controlling trophoblast invasion. Estrogen is the primary regulator of IGF1 expression in the uterus, which occurs predominantly in the stroma. , Estrogen also increases expression of IGF1 receptors, which are primarily found on epithelial cells. The mitogenic response of the mouse uterus to estrogen is absent in mice with targeted deletion of the IGF1 gene. Moreover, mice overexpressing IGFBP1, which results in reduced IGF1 bioavailability, have a blunted DNA synthesis response to estrogen treatment. Thus, IGF1 produced in the uterine stroma in response to estrogen acts on the epithelial cells to stimulate DNA synthesis; however, tissue grafting experiments showed that an IGF1 knockout mouse uterus responds to estrogen when placed into a normal mouse, whereas a wild-type uterus placed into an IGF1 knockout mouse showed minimal growth—indicating that systemic IGF1 is sufficient to support estrogen-driven uterine growth. These findings substantiate the importance of IGF1 in the uterine growth response to estrogen. Although uterine growth can occur in the absence of a paracrine IGF-1 system, these studies do not preclude a role for locally generated IGF1 as a redundant signaling mechanism or the role of other locally produced growth factors.
Progesterone is a vital steroid hormone that is involved in secretory preparation of the endometrium for implantation, decidualization, and suppression of myometrial contractility during pregnancy. Similar to estrogen, progesterone effects on the endometrium involve paracrine activities. , Progesterone possesses antiinflammatory characteristics and promotes immunotolerance during implantation and pregnancy. It is also associated directly, or indirectly, with most of the secretory proteins made by the endometrium that are present in the uterine lumen and glands to support embryo development and implantation.
Progesterone signaling in the endometrium affects each of the cellular compartments, including the epithelium, stroma, and resident immune cells. , It has become increasingly clear that progesterone has both direct and important indirect roles in both stroma and epithelial function. A primary role of progesterone after ovulation is to downregulate the actions of estrogen, which are unnecessary for normal secretory endometrial development , and may, in fact, inhibit implantation. During the early proliferative phase, when progesterone concentrations are low, cytoplasmic PGR is shielded from degradation by heat shock proteins and by interactions with coactivator proteins (SRC1 and SRC2) and chaperone proteins such as FKBP4 and FKBP5. In SRC2 conditional knockout mice ) , the females are infertile, similar to FKBP4 null mice, with each displaying implantation and decidualization failure. , In women, both FKBP4 and FKPB5 expression normally increases in the secretory phase, possibly regulated by HOXA10. Interestingly, blunted increases in FKBP4 are seen in endometrium from women with infertility and endometriosis, apparently regulated in part by changes in microRNA that regulate the degradation of mRNA coding for FKBP4 and other progesterone-regulated genes. This mechanism may be involved in the phenomenon of endometrial progesterone resistance. Much of our understanding of implantation and the paracrine actions of progesterone is based on studies in the mouse uterus. PGR is upregulated by estrogen but also requires the transcription factor GATA2. The action of progesterone begins with epithelial PGR binding to progesterone followed by the induction of a key epithelial target gene, Indian hedgehog (IHH) . IHH is secreted and communicates with the endometrial stroma in a paracrine fashion, binding to the stromal receptor Patched-1 (Ptch1) , resulting in increased stromal transcription factor, chicken ovalbumin upstream promoter transcription factor II (COUP-TFII or NR2F1), with downstream effectors including bone morphogenic protein-2 (BMP2) and WNT4. This pathway concludes with the induction of HAND2 in the stroma that is antiproliferative by inhibiting production of fibroblast growth factors (FGFs), which are required for the expression of epithelial estrogen receptors ( Fig. 10.4 ).
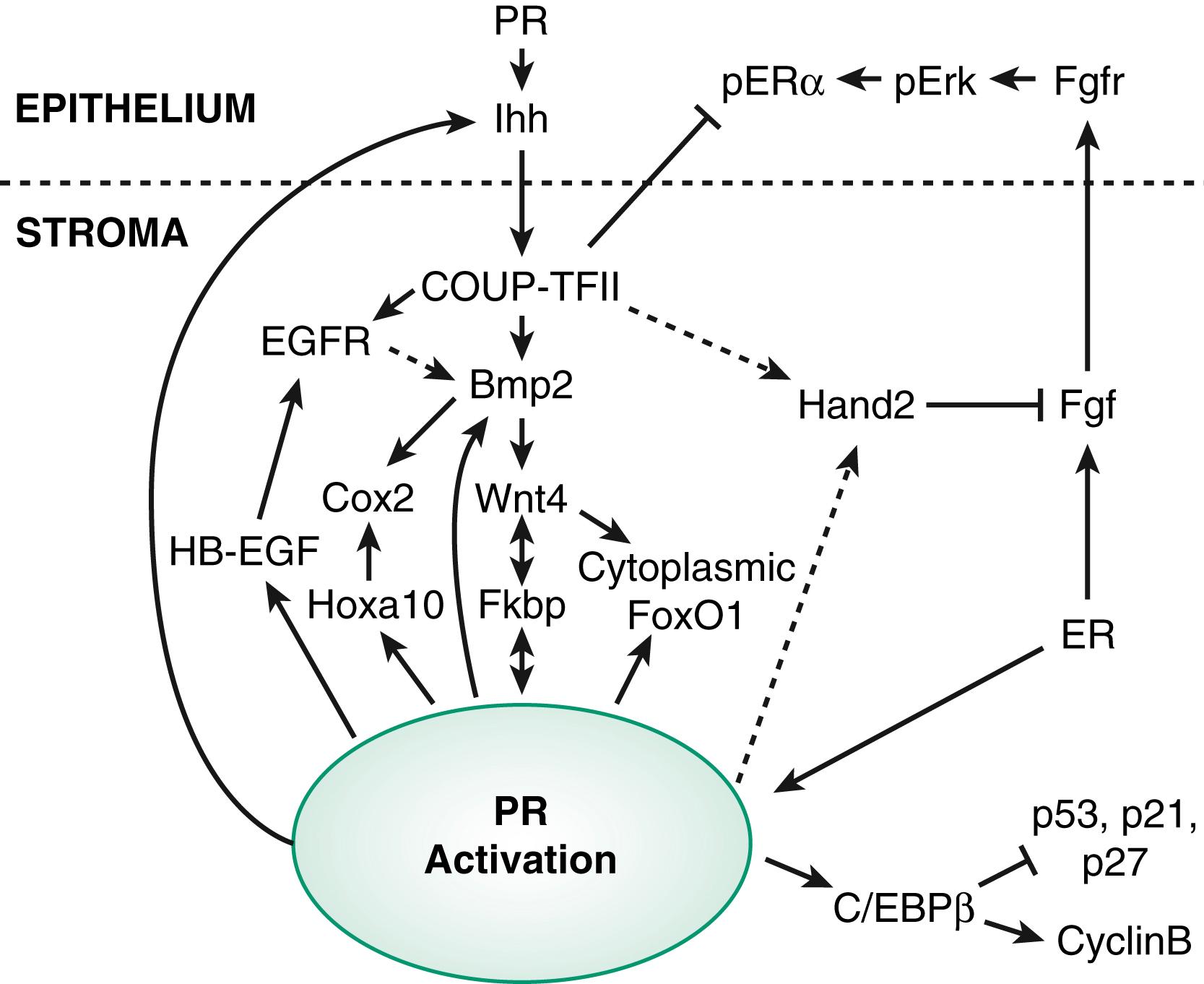
As mentioned earlier in this chapter, WNT4 is required for müllerian development and in adults is required for decidualization. WNT4 is also thought to be responsible for shuttling Forkhead box protein 1 (FOXO1), a transcription factor essential for decidualization, from the nucleus to the cytoplasm, preventing its apoptotic actions. Inadequate decidualization has been shown to be associated with implantation failure and associated with many critical genes.
Many of the stromal genes expressed in response to progesterone action include HOXA10 , heparin-binding EGF-like growth factor (Hbegf) , cyclooxygenase 2 (Cox-2, encoded by Ptgs2 ), and mitogen inducible gene 6 (Mig-6 or Errfi1) , are induced through the IHH pathway. Mig-6 is a negative regulator of EGF receptor, and transgenic knockout mice for this protein have endometrial hyperplasia, suggesting this progesterone-regulated gene is an important brake for cell proliferation in the endometrium. The HOX gene, HOXA10, is essential for uterine development, but in the adult has been shown to regulate FKPB4 and is therefore also essential for progesterone action and implantation. HOXA10 also directly upregulates epithelial receptivity genes such as the ανβ3 integrin, which was shown to be required for attachment of the embryo in animal models. , Mice lacking PGR, HOXA10, PTGS2, ARID1A, LIF, and other genes in transgenic mice have been found to exhibit decidualization defects and infertility. , ,
The uterus is also a target for androgens. Androgen receptors (AR) are expressed in the endometrium and myometrium, most prominently in the stromal cells during the proliferative phase and in the epithelial cells of the secretory phase. MAGE-11 was originally described as a primate-specific AR coactivator, reaches peak expression in the secretory phase, and colocalizes with AR. Interestingly, 5 alpha-reductase types 1 and 2, which convert testosterone to dihydrotestosterone, are expressed in epithelial cells throughout the cycle. Estradiol treatment increases endometrial stromal AR expression in the Rhesus monkey, and estradiol in combination with either testosterone or progesterone augments epithelial and myometrial AR levels. Consistent with these observations, AR expression is elevated in the endometrium of women with PCOS; this finding may explain, in part, the implantation defects and early pregnancy loss reported to be associated with this syndrome. Androgens and AR may also have essential roles in reproductive tract function in females, and this topic is becoming an active area of research.
The activity of steroid hormone in the endometrium is determined in part by the modulating effects of uterine enzymes that actively catabolize steroid hormones ( Fig. 10.5 ; see Chapter 4 ). These enzymes that carry out transformations of steroid hormones are subject to regulation during the menstrual cycle. Estradiol taken up from the plasma can be converted into estrone by the action of 17-beta hydroxysteroid dehydrogenases (HSD17B1) or converted to sulfated conjugates by estrogen sulfotransferase. Three different forms of HSD17B capable of oxidizing estradiol to estrone have been detected in primate endometrium: the type 2, type 4, and type 8 enzymes. Type 2 and type 8 enzymes are associated with microsomes; type 4 enzyme is in peroxisomes. Type 2 and type 4 enzymes use the oxidized form of nicotinamide adenine dinucleotide as a cofactor and are predominantly localized to the glandular epithelium in the secretory phase. The endometrial type 2 enzyme shows the greatest change in expression during the cycle, peaking in the secretory phase. The type 8 enzyme appears to be constitutively expressed. Progesterone enhances the conversion of estradiol to estrone in endometrial cells by inducing expression of the type 2 HSD17B and, to a much lesser degree, the type 4 enzyme. Progesterone also increases endometrial estradiol sulfation by increasing estrogen sulfotransferase activity. Thus progesterone activates enzymatic pathways that inactivate estradiol. However, there are regional differences in the balance of systems removing and restoring estradiol in the uterus. For example, estrogen sulfatase is detected only in the glandular epithelium of the basalis, where it may function to increase the level of estradiol from estradiol sulfate. The human uterus does not normally have the capacity to produce significant amounts of estrogen locally (either de novo or from circulating prohormones), but endometrial cancers, endometriotic lesions, and to a lesser extent, eutopic endometrium of women with endometriosis aberrantly express components of the steroidogenic machinery (P450 arom ) that endows the tissue with the capacity to synthesize estrogens from circulating adrenal or ovarian androgens or by de novo synthesis. Endometriosis lesions have been found to express StAR, the cholesterol side-chain cleavage enzyme, P450c17, aromatase, and type 1 HSD17B in stromal cells (see Chapter 25 ). Estradiol produced in these lesions can enhance the production of prostaglandin E2, which in turn stimulates transcription of the aromatase gene, resulting in a feedforward mechanism for increasing local estrogen levels. In addition, endometriotic lesions do not express the progesterone-regulated type 2 HSD17B that converts estradiol to estrone, which effectively increases the bioavailability of estradiol.
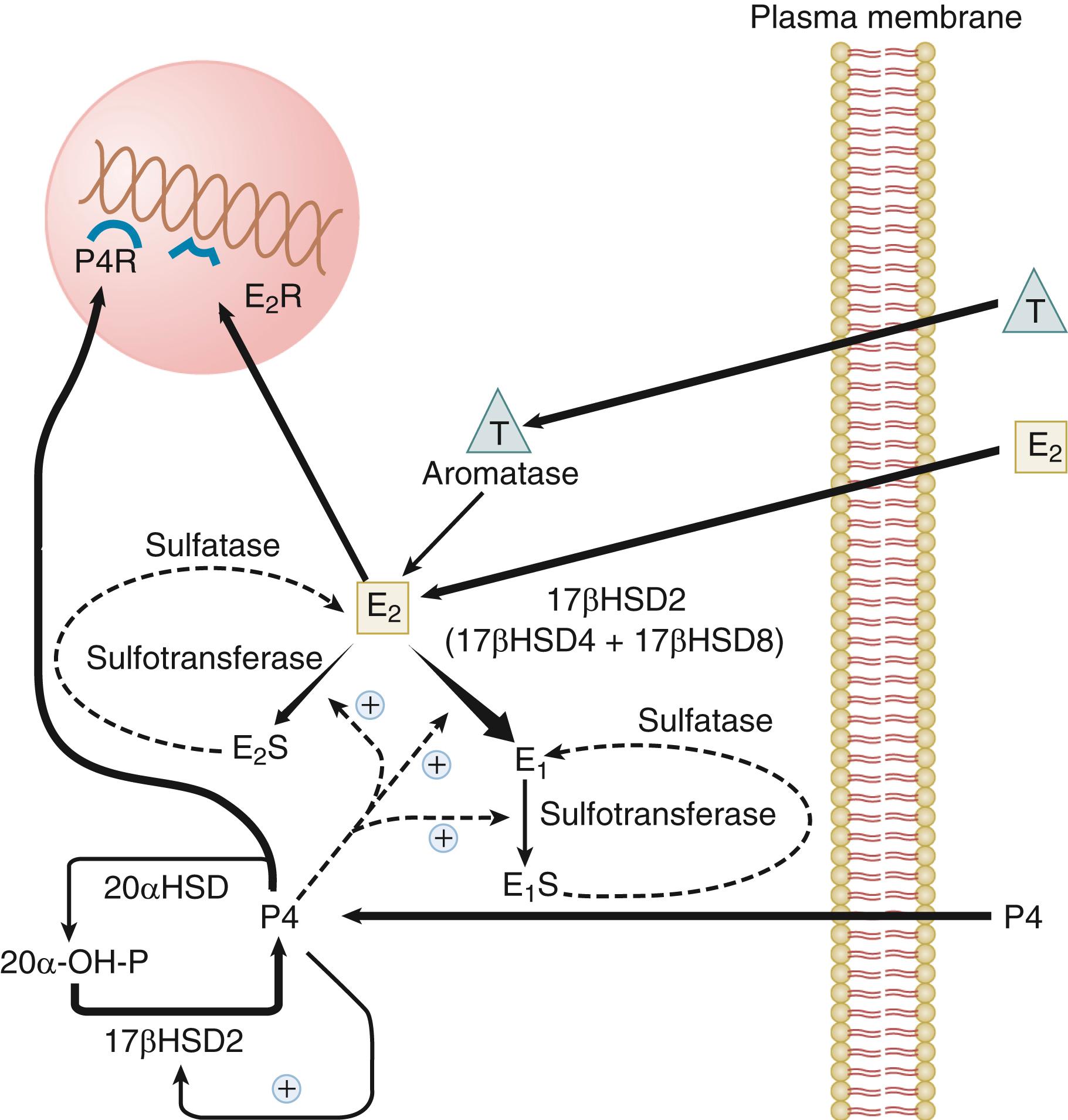
Progesterone is catabolized in the uterus into inactive 20α-hydroxyprogesterone by 20α-HSDs. The type 2 17β-HSD, which is increased in the secretory phase, is also a 20α-hydroxysteroid oxidase that converts 20α-hydroxyprogesterone back into progesterone. The induction of the type 2 HSD17B by progesterone in the secretory phase, therefore, contributes not only to the catabolism of estradiol but also to the maintenance of endometrial progesterone levels.
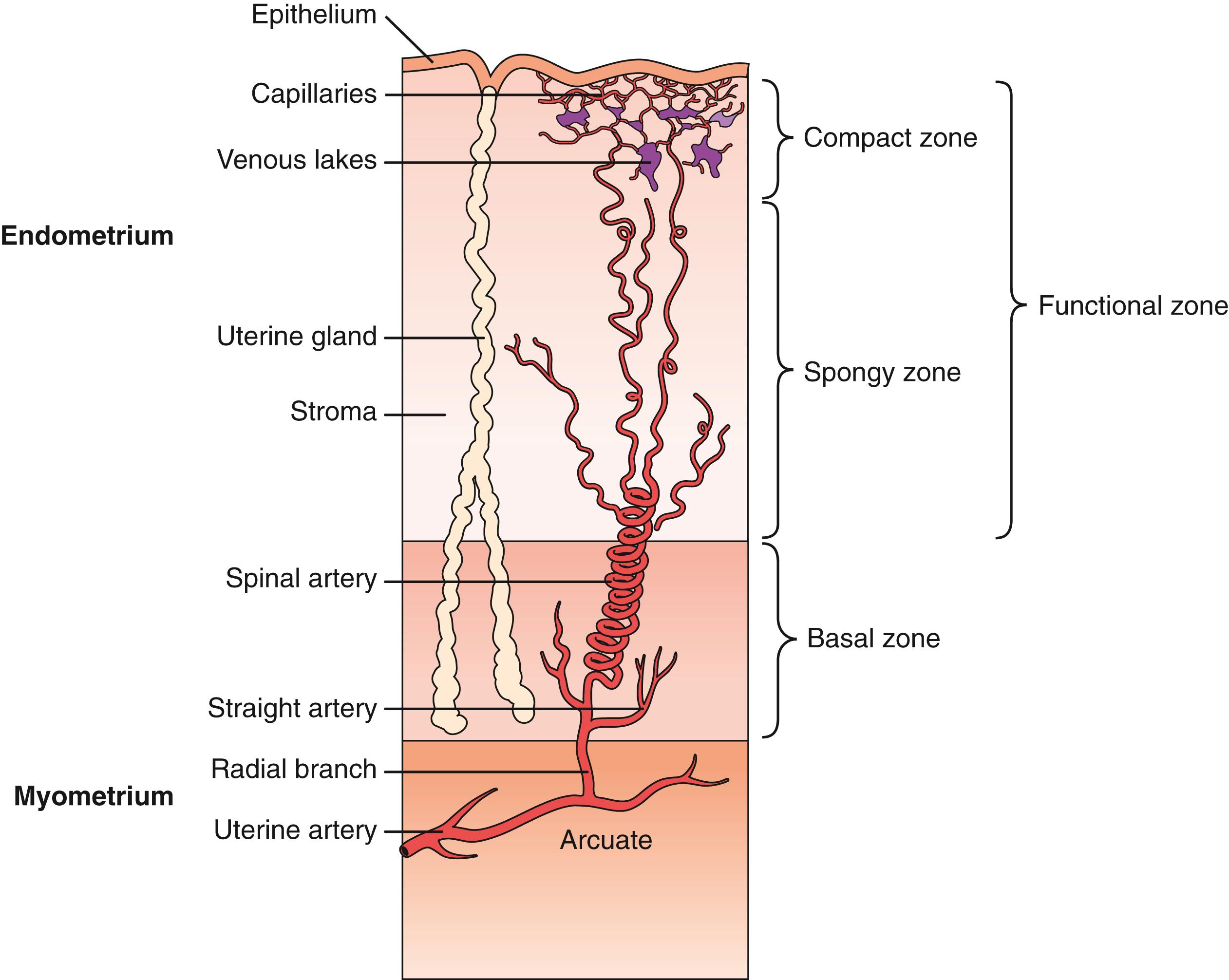
The endometrium undergoes cyclic changes in response to preovulatory and postovulatory steroid hormones, to prepare for blastocyst implantation and pregnancy. In discussions of structure and function, the primate endometrium is commonly described as consisting of two major layers, the functionalis, and basalis ( Fig. 10.6 ). The functionalis is a transient layer consisting of a compact zone that includes the stroma subjacent to the luminal epithelium and an intermediate spongy zone containing more densely packed glands. The basalis, or basal layer, lies beneath the spongy zone and adjacent to the myometrium. It contains the gland fundi and supporting vasculature and can regenerate the functionalis endometrium after it is shed at menstruation. These endometrial layers are histologically definable during the secretory phase. Through the menstrual cycle, the functionalis undergoes striking transformations, whereas the basalis remains relatively unchanged. Patterns of cell proliferation, programmed cell death, and gene expression also show gradients across the layers (described below). The majority of epithelial cell proliferation occurs in the upper regions of the functionalis, whereas proliferative activity in glands of the basalis is modest during the proliferative phase. Increased proliferative activity is observed in the midsecretory phase in the basalis layer. Of note, the epithelial cells of the basalis later maintain both ESR1 and PGR at a time when these receptors are normally depleted in the upper functionalis epithelium during the midsecretory phase.
During the early proliferative phase, the endometrium is usually less than 2 mm in thickness. Proliferation of cells in the basal zones and epithelial cells persisting in the lower uterine and cornual segments results in the restoration of the luminal epithelium by day 5 of the menstrual cycle. At that time, mitotic activity is evident in both the glandular epithelium and stroma. Remarkably, this recurrent “wound healing” process does not normally produce scarring. Endometrial stem cells capable of yielding progenitors of both the stromal and epithelial components of the endometrium presumably contribute to the regenerative process but are yet to be convincingly identified.
Rapid rejuvenation of the endometrium depends on many of the same factors that were involved in the ontogeny of the reproductive tract. WNT7A is expressed solely by the luminal epithelium and is a diffusible secreted factor that triggers cell proliferation through complex pathways. , Acting on the underlying stroma, soluble WNT7A binds to the receptor Frizzled (FZL) that phosphorylates the intracytoplasmic protein Disheveled (DSH; Fig. 10.7 A ). This protein inactivates glycogen synthase kinase beta (GSKB), turning off the breakdown of CTNNB1 by ubiquitination. Accumulation of CTNNB1 signals cell proliferation activities associated with endometrial growth acting as a transcription factor in the nucleus. The diffusion gradient of WNT7A downward into the growing endometrium is an attractive model for self-regulatory mechanisms to determine the growth of the endometrium.
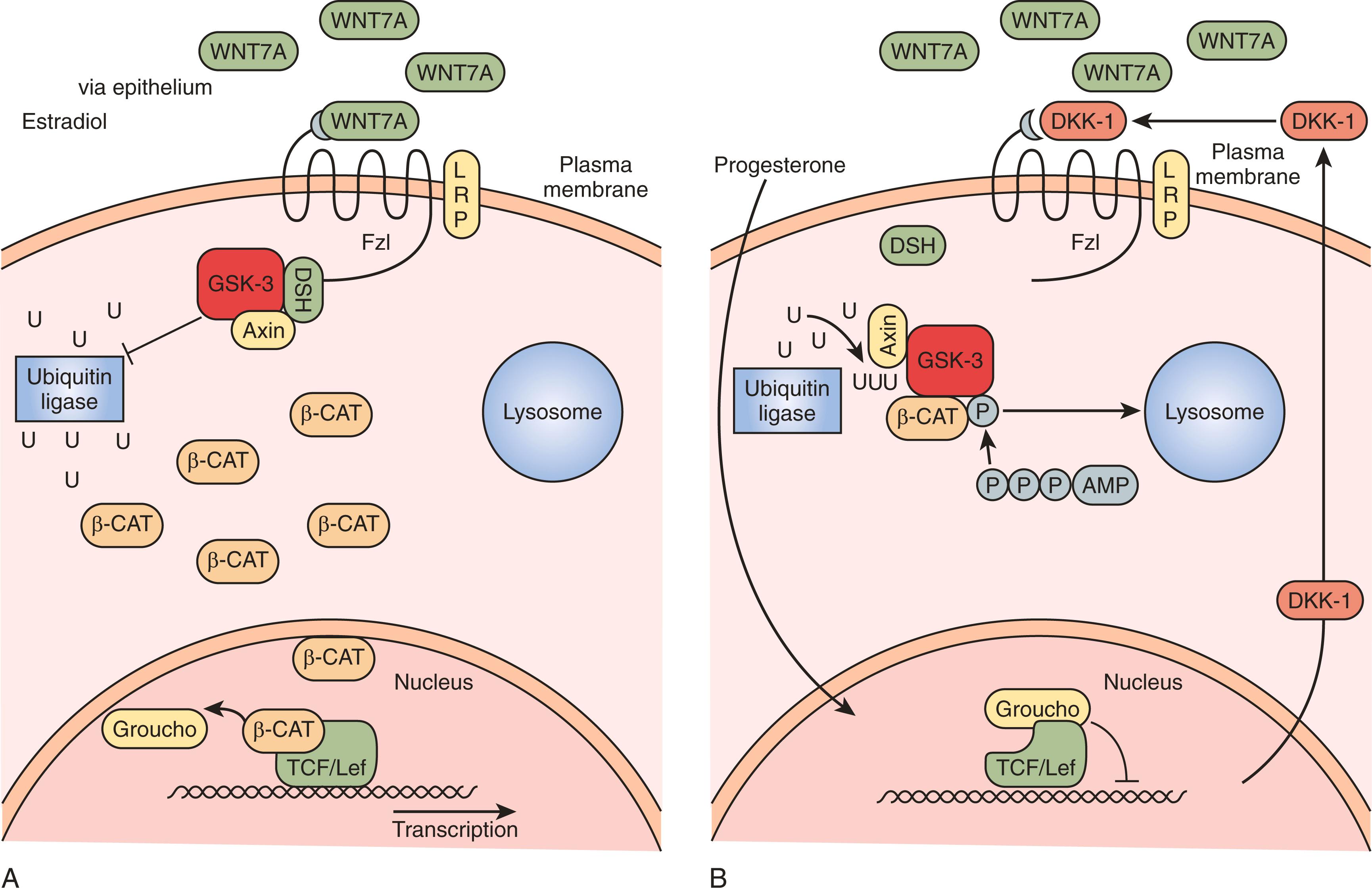
Counterregulatory mechanisms to disable WNT7A/FZL/DSH pathways include the action of progesterone, which stimulates the secretion of a protein called Dickkopf-1 (DKK1; see Fig. 10.7 B). , DKK1 binds to a coreceptor, LRP6, blocking the FZL receptor, turning off the action of WNT7A by preventing CTNNB1 accumulation. Defects in DKK1 production have been described in endometriosis and reflect progesterone resistance and might explain the proliferative phenotype of the endometrium in this condition. ,
The glands during the early proliferative phase are narrow, straight, and tubular, lined with low columnar cells that have round nuclei located near the cell base ( Fig. 10.8 ). At the ultrastructural level, the epithelial cell cytoplasm contains numerous polyribosomes, but the endoplasmic reticulum and Golgi complexes of these cells are not well developed.
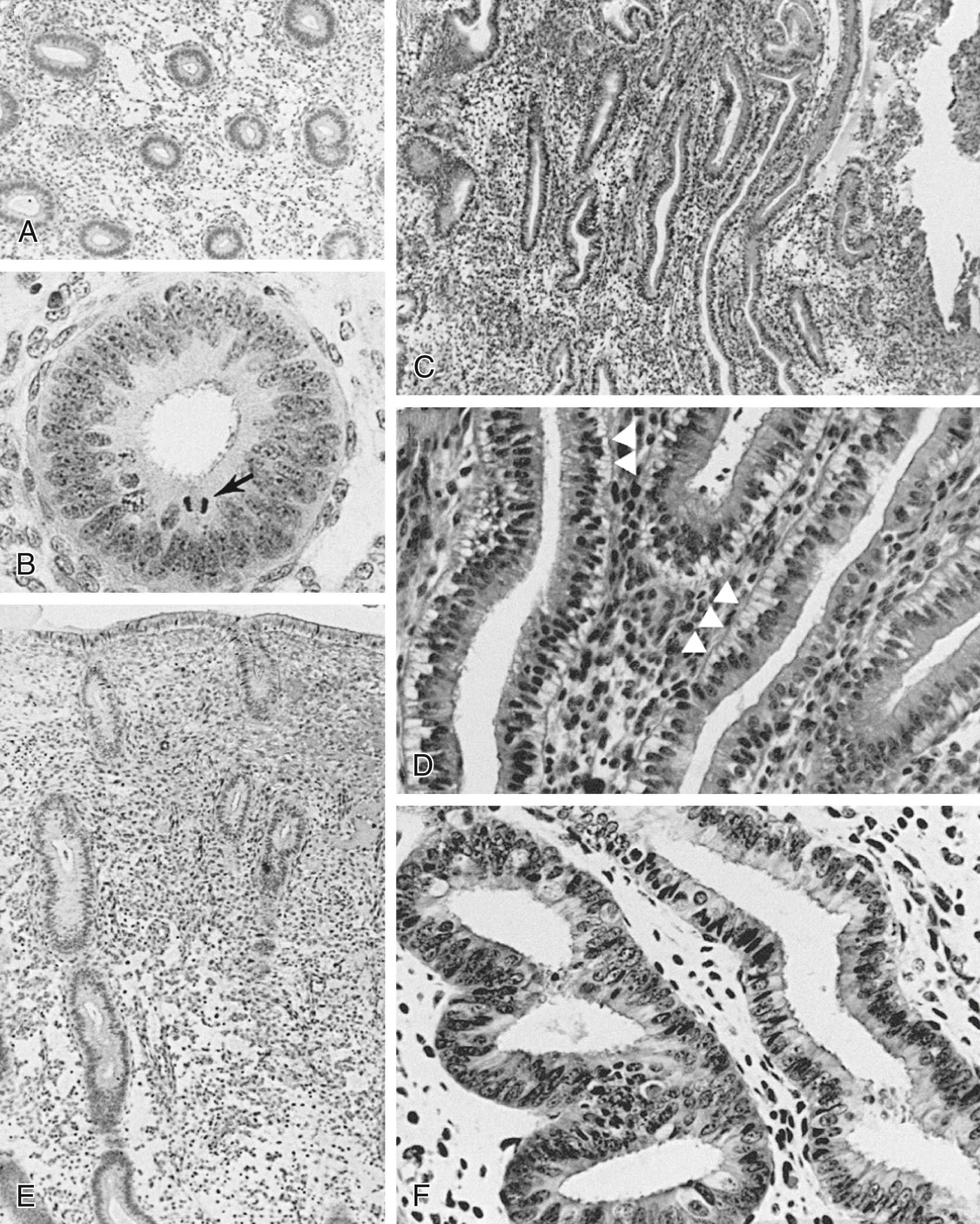
The endometrium thickens in the late proliferative phase due to glandular hyperplasia and an increase in the stromal extracellular matrix. The glands are widely separated near the endometrial surface and more crowded and tortuous deeper into the endometrium. The glandular epithelial cells increase in height and become pseudostratified as the time of ovulation approaches (see Fig. 10.8 D).
The effect of steroid hormones on the proliferation and secretion within the endometrium is highly dependent on the zones (basalis vs. functionalis layers). Studies from the rhesus macaque endometrium using specific labeling techniques show proliferation during the “proliferative” phase is confined to the functionalis layer.
Ovulation marks the beginning of the secretory phase of the endometrial cycle. However, it should be noted that the endometrial luminal and glandular epithelial cells also display secretory activity during the proliferative phase. Mitotic activity in epithelial and stromal cells is restricted to the first 3 days after ovulation and is rarely observed later in the cycle. The nuclei of glandular epithelial cells and stromal cells develop heterochromatin in the early secretory phase (see Fig. 10.8 ). The glandular epithelial cells begin to accumulate glycogen-rich vacuoles at their base, displacing the nuclei to the midregions of the columnar cells. Ultrastructural studies of endometrial epithelia reveal abundant endoplasmic reticulum and unusually large mitochondria with prominent cristae. A reticular network of argyrophilic fibers containing fibrillar collagens (collagen fiber type I and type III) is established in the stroma during this phase. Stromal edema contributes to the thickening of the endometrium.
A characteristic feature of this phase of the cycle is the development of the spiral arteries. These vessels become increasingly coiled as they lengthen more rapidly than the endometrium thickens. The endometrial glands are tortuous in the midsecretory and late secretory phases. Their secretory activity reaches a maximum about 6 days after ovulation, as reflected by the loss of vacuoles from the epithelial cell cytoplasm (see Fig. 10.8 E and F).
The nucleolar channel system, an ordered spherical stack of interdigitating tubules, appears transiently in the nucleoli of approximately 5% to 10% of the secretory phase epithelial cells between days 16 and 24. The nuclear channel system is thought to form from an invagination of the inner nuclear membrane, providing a direct connection to the perinuclear space for the transport of mRNA to the cytoplasm. Nucleolar and coiled-body phosphoprotein 1 (Nolc1 or Nopp140), a highly phosphorylated protein that associates with small nucleolar ribonucleoprotein particles required for RNA processing, appears to induce the formation of this intranuclear endoplasmic reticulum. The nucleolar channel system forms in response to progesterone and is an ultrastructural hallmark of the secretory phase during the expected time of implantation.
Stromal cells around blood vessels enlarge and acquire an eosinophilic cytoplasm and a pericellular extracellular matrix in the mid- to late secretory phase. These changes, referred to as predecidualization to distinguish them from the further transformation of the stroma that occurs in a fertile cycle, subsequently spreads, accentuating the demarcation between the subepithelial compact zone and the spongy zone. Unlike many laboratory animal species, an embryonic signal is not required for initiation of decidualization in the human uterus.
The fact that the predecidual changes occur first near blood vessels suggests that humoral or local factors provoke them. Among the local factors may be interactions with decidual granular lymphocytes, also referred to as uterine natural killer (uNK) cells. uNK cells encircle arterioles and closely associate with stromal cells through contacts that are remarkably similar to gap junctions. At the ultrastructural level, the predecidual stromal cells display well-developed Golgi complexes and parallel lamellae of the endoplasmic reticulum. Their surrounding matrix consists of laminin, fibronectin, heparan sulfate, and type IV collagen. , Substantial changes in gene expression also occur in stromal cells during decidualization.
The stromal cells of the midsecretory and late secretory phase also express a repertoire of proteins that promote hemostasis, including tissue factor (TF), a membrane-associated protein that initiates coagulation when it contacts blood, and plasminogen activator inhibitor type 1 (PAI1), also known as Serpin E1, which restrains fibrinolysis. , , PAI1 may prevent focal hemorrhage that might result from trophoblast invasion during implantation.
The main histologic features of the premenstrual phase are degradation of the stromal reticular network, which is catalyzed by MMPs; infiltration of the stroma by polymorphonuclear and mononuclear leukocytes; and “secretory exhaustion” of the endometrial glands, whose epithelial cells now display basal nuclei. Morphologic changes in the nuclei of granular lymphocytes, including pyknosis and karyorrhexis suggesting apoptosis, have been proposed to be some of the first events presaging menses; these changes occur prior to the breakdown of extracellular matrix and leukocyte infiltration. In the glandular epithelium, the nucleolar channel system and giant mitochondria characteristic of the early and midsecretory phases have vanished. The endometrium shrinks preceding menstruation, partly because of diminished secretory activity and the catabolism of the extracellular matrix. A broader discussion of menstruation in the context of abnormal uterine bleeding (AUB) is discussed later in this chapter.
Menstruation, caused primarily by progesterone withdrawal, marks a failure to achieve pregnancy and the need to shed the specialized uterine lining that results from spontaneous decidualization. The uniqueness of this process is highlighted by the fact that, although circulating progesterone and estrogen levels decline with corpus luteum regression in nonfertile cycles in all mammals, menstruation appears almost exclusively in humans and some Old World primates. In menstruating species, moreover, tissues that respond to estrogen and progesterone such as the fallopian tubes, vagina, and breast do not shed as ovarian steroid levels decline. The molecular mechanisms triggered by progesterone withdrawal include activation of the nuclear factor kappa beta (NFKB) transcriptional pathway (a major target of cytokines) and the resulting expression of genes like endometrial bleeding-associated factor (EBAF), an anti-TGFB cytokine that interferes with the action of other members of the TGFB family that promote endometrial integrity. This orchestrated blockade of the actions of TGFB appears to initiate many of the subsequent events of menstruation, including the elaboration of MMPs.
There have been two major models describing the mechanisms driving menstruation. One of these is vasoconstriction-induced hypoxia and reperfusion. The functional zone of the human endometrium is supplied by spiral arterioles that, in contradistinction to the radial and basal arteries that feed them, are highly sensitive to steroid hormones. The classic studies of Markee utilized autologous endometrial transplants into the anterior eye chamber of the rhesus macaque, allowing direct examination of the endometrium through the cycle. These studies demonstrated that vasoconstriction of the arterioles and spiral arteries precedes the onset of menstrual bleeding by 4 to 24 hours. It has been proposed that bleeding occurs after the arterioles and arteries relax, leading to hypoxia or reperfusion injury. However, later endometrial perfusion studies failed to reveal any significant reduction in endometrial blood flow in the perimenstrual phase or during menstruation. In addition, an analysis of expression and localization of hypoxia-inducible factor (HIF), a heterodimeric transcription factor induced by hypoxia and thus a biochemical marker of reduced oxygen availability, was unrevealing. No upregulation of the two HIF component subunits, HIF1A and HIF1B, was observed, and no nuclear localization of HIF subunits took place in the perimenstrual human endometrium. While these studies do not exclude the possibility of localized regions of vasoconstriction and hypoxia, they do bring into question the hypoxia/reperfusion model.
An alternative hypothesis to the vasoconstriction model is that menstruation is a controlled inflammatory response engendered by the withdrawal of progesterone. The inflammation hypothesis is supported by two features: the prominent accumulation of leukocytes in the endometrium in the premenstrual phase ; and the release of matrix-degrading enzymes characteristic of an inflammatory response. The hypothesis of progesterone withdrawal-induced inflammation is supported by the uterine inflammation observed in mice lacking PGR. Apoptotic cell death, which can be triggered by inflammatory mediators, occurs in the late secretory phase first in stromal cells and then gradually spreads throughout the functionalis. Rescue from apoptosis has been shown to occur in vivo with the administration of progesterone or exogenous human chorionic gonadotropin (hCG).
Changes in proteins involved in apoptosis appear to contribute to the regional programmed death in the endometrium. The antiapoptotic protein, BCL2, is prominently expressed in the glandular epithelium during the proliferative phase; expression declines in the secretory phase to reach low levels in the late secretory phase when apoptosis occurs. Studies report an inverse pattern of expression of survivin, a recently discovered inhibitor of apoptosis. Survivin binds to and blocks the effector cell death proteases, caspase-3 and -7. The activities of caspases-3, -8, and -9 are higher in the secretory phase. Low rates of survivin expression in the glandular epithelium are found in the proliferative phase, rising to peak expression in the late secretory phase. The protein is localized to the nuclei of cells in the functionalis and the cytoplasm in cells in the basalis. This differential distribution may indicate that survivin is not capable of suppressing apoptotic death in the late secretory phase functionalis but performs this role in the basalis, consistent with the observed patterns of apoptotic cell death. Elevated levels of survivin in endometriotic lesions correlate with reduced apoptotic death of cells in these lesions.
Although the vasoconstriction and inflammation hypotheses of menstruation might appear to be distinct, there are several overlapping biochemical features of hypoxia and inflammation, including the release of proinflammatory cytokines and apoptotic cell death that tend to blur the distinctions between these models. The vascular changes in the endometrium in the perimenstrual phase, resulting either from ischemia/hypoxia or from an inflammatory reaction, lead to extravasation of blood. Autophagy and heterophagy are evident, as is apoptotic cell death. The superficial endometrial layers become distended by the formation of hematomas; fissures subsequently develop, leading to the detachment of tissue fragments and the ultimate shedding of the functionalis. The resulting menstrual effluent contains fragments of tissue mixed with blood, liquefied by the fibrinolytic activity of the endometrium. Clots of varying sizes may be present if blood flow is excessive.
The inflammatory components of menstruation may be essential for the rapid restoration of tissue integrity that occurs following endometrial sloughing. The withdrawal of progesterone, as a well-known antiinflammatory mediator of the secretory phase, likely participates in the onset of inflammatory changes, including induction of MMPs, urokinase-type plasminogen activator and tissue-type plasminogen activator (uPA and tPA), and PAI1 expression. At menstruation and with progesterone withdrawal, prostaglandin-endoperoxide synthase 2 cyclooxygenase-2 (PTGS2 or COX-2) is dramatically elevated via NFKB, with induction of prostaglandins and lipoxygenases (LOX). One LOX, LOX15, is expressed with progesterone withdrawal. Since LOX15 is responsible for production of the antiinflammatory eicosanoid lipoxin A4 (LXPA4), expression may help curtail inflammatory responses. , Surprisingly, LXPA4 has also shown to be a potent estrogen receptor agonist and could function to facilitate endometrial repair, particularly during menstruation, when circulating estrogen levels are low.
The duration of menses in ovulatory cycles is variable, generally 4 to 6 days, but usually similar from cycle to cycle in any individual ovulatory woman. The duration of flow is abnormal if it is less than 2 days or more than 7 days. The amount of blood lost in a normal menses ranges from 25 to 60 mL, and loss of more than 60 mL per month is associated with iron deficiency anemia.
Menstruation and cyclic repair of the endometrial lining require both stem cells and an adaptation involving telomerase that imbues the endometrium with near immortality, compared with other tissues in the body. Disorders of endometrial regeneration can result in severe pathological conditions, including uterine cancer, endometriosis, and infertility. Over the last two decades, different approaches have been used to identify putative stem cells in the human endometrium. To date, no consensus concerning endometrial stem cell markers has been established. However, studies in mouse and human have suggested that the endometrium possess both endogenous and exogenous sources of endometrial stem cells that contribute to the endometrium’s regenerative capability. In 2004, endometrium in women who had undergone bone marrow (BM) transplantation was examined and found to contain endometrial epithelial and stroma cells that had clearly been derived from the donor BM. This observation supported the idea that BM-derived stem cells can traffic to and engraft in the endometrium, and secondly, that these undifferentiated BM stem cells undergo both mesenchymal and epithelial transformation adopting a phenotype indistinguishable from their host organ. This original observation was later confirmed in the mouse, using male BM donors and female recipients, proving that these were not endometrial stem cells that reside in the BM but rather BM-derived pluripotent stem cells.
Endometrial-derived pluripotent cells, whether native to the endometrium or derived from the BM, appear to have unusual properties that may revolutionize how we think about stem cell research. These cells have been manipulated in the mouse model to differentiate into neuronal cell types with the potential to produce dopamine, and pancreatic beta cells that produce insulin. , Mesenchymal stem-like cells that express CD140b and CD146 have also been identified that can be differentiated into adipocytes, osteocytes, chondrocytes, myocytes, and endothelium. Based on these early reports, the ready access to stem cells of endometrial origin could supplant the necessity to perform BM biopsy and offers the promise of a self-renewing source of autologous stem cells for grafting of a wide variety of cell types that might cure such human diseases such as Parkinson or type I diabetes, as well as treatment for Asherman syndrome with the possibility of endometrial renewal. Similarly, several studies have suggested the regenerative potential of platelet-rich plasma (PRP). Treatment of human endometrial primary cells and in vivo animal models of Asherman syndrome with PRP leads to increased proliferation and regeneration of endometrial tissue. Although initial results are promising, further mechanistic and clinical studies are necessary.
Early methods for identifying endometrial stem cells began with the study of retention of cells labeled with 5-bromo-2-deoxyuridine (BrdU). , These rare, highly proliferative and undifferentiated quiescent populations of cells maintain properties of clonicity, colony-forming unit activity, and the ability to reconstitute their tissue of origin. Hoechst 33342 exclusion to identify the side population (SP) cells have been used to show that in the endometrium, these SP cells are maintained at a constant level (∼1%) throughout a woman’s reproductive life. , Interestingly, it has been suggested that BM-derived cells do not contribute to this population of SP cells in the endometrium, suggesting they are a resident to the endometrium and therefore provide an endogenous source of self-renewing stem cells to the endometrium. Specific cell surface markers have been identified in endometrial mesenchymal stem cells (MSGs). These CD146 + PDGFRB − cells only represent less than 2% of endometrial stromal cells and are found in a perivascular location, similar to other BM-derived cells. These cells have increased proliferation capacity and may be important in the reconstitution of the endometrium following menstruation.
The endometrium, unlike other tissues in the body, does not appear to age. Despite up to 450 cycles of menstruation and regeneration, the endometrium of the typical woman continues to renew itself with remarkable reliability. Age is related to telomere length, which in other tissues determines the lifespan of a cell. Endometrium expresses the enzyme telomerase, at levels similar to certain cancers. Levels are cycle dependent, with higher levels in the proliferative phase reaching their nadir during the midsecretory phase. , Interestingly, telomerase is found in the epithelial but not the stromal compartment. Epithelial stem cells are thought to participate in endometrial renewal following menses, arising from the basalis layer. These cells appear to express stage-specific embryonic antigen 1 (SSEA-1 or CD15), a Lewis X epitope found on embryonic stem cells. The endometrial cells expressing this epitope had greater telomerase activity than cells without the epitope. Interestingly, telomerase may be indirectly regulated by estrogen, through WNT pathways involving CTNNB1 expression. In women and baboons with endometriosis and progesterone resistance, telomerase levels appear to be increased, suggesting a possible link to this disease and the pathophysiology of its chronicity and tendency to recur.
Angiogenesis , the formation of new blood vessels from preexisting vessels, rarely occurs without injury or disease in the normal adult, except in the female reproductive tract and ovary. Here the cyclic processes of endometrial shedding and regeneration and corpus luteum formation entail remarkable changes in vessel growth and remodeling. The angiogenic process involves multiple steps and is tightly regulated by activators and inhibitors. There are four phases of the endometrial cycle when important events relating to angiogenesis occur: (1) at menstruation, when there is a repair of ruptured blood vessels; (2) during the proliferative phase, when there is a rapid growth of endometrial tissue; (3) during the secretory phase, with the development of the spiral arterioles that feed a subepithelial capillary plexus; and (4) in the premenstrual phase, when there is evidence for vascular regression. If this angiogenic remodeling program is not properly executed, abnormalities in endometrial function can result, including menorrhagia.
Angiogenesis during the proliferative phase is by vessel elongation. In the secretory phase, intussusception appears to account for the increase in vessel branching; this proliferation of endothelial cells inside vessels ultimately produces a wide lumen that can be divided by transcapillary pillars or alternatively lead to capillary fusion or splitting. Although most prominent in the late menstrual and early and late proliferative phase, endothelial cell proliferation is continuous during the menstrual cycle. Thus vessel growth continues during the secretory phase, despite the fact that the surrounding endometrial tissue has ceased to grow, resulting in the coiling of spiral arterioles.
Endometrial angiogenesis and vessel remodeling are directed by a network of signaling molecules and receptors that include members of the vascular endothelial growth factor family, FGFs, angiopoietins, angiogenin, and the ephrins and their cognate receptors—which can also exist as secreted ligand-binding domains that function as inhibitors as a result of alternative splicing. , Although the temporal and spatial patterns of expression of several other angiogenic factors and their receptors have been defined in the endometrium, the specific roles of each of these factors in the endometrial angiogenesis–vessel remodeling cycle remain to be elucidated.
Of the members of the vascular endothelial growth factor family that includes VEGF-A, VEGF-B, VEGF-C, and VEGF-D, VEGF-A is most important for endometrial angiogenesis. VEGF-A acts on two different receptors: VEGF receptor-2 (VEGFR2), which may play the dominant role in signaling endothelial cell proliferation; and another tyrosine kinase receptor, VEGFR1 (also known as FLT-1), which may play the dominant role in mediating VEGF effects on vascular permeability. Both receptors are present on endothelial cells. VEGFR2, also known as kinase domain receptor (KDR), has been detected in stromal and epithelial cells of the premenstrual endometrium; this presence suggests actions on nonvascular compartments.
The premenstrual phase is characterized by a dramatic upregulation of the VEGFR2 receptor in stromal cells of the superficial layers of the endometrium in response to progesterone withdrawal. The action of VEGF on VEGFR2 may participate in the increased expression of MMP-1 in the stromal in the premenstrual phase.
VEGF-A expression is detectable in glandular epithelial and stromal cells in the proliferative phase, stimulated by estrogen through the actions of hypoxia-induced factor-1 alpha (HIF1A) . , HIF1A is also induced by prostaglandins E2, which is maximally produced at the time of menstruation. VEGF released from neutrophils in intimate contact with the endothelial cells is thought to stimulate endometrial vessel growth. It is also present in uterine NK cells. During the secretory phase, VEGF-A can be identified in surface epithelial cells, which presumably secrete into the uterine cavity. The release of VEGF from subepithelial NK cells has been suggested to play a role in directing the development of the subepithelial capillary network in the secretory phase.
VEGF-A has four common isoforms. It has four common splice variants (VEGF121, VEGF165, VEGF189, and VEGF 206). After ovulation, there is a remarkable shift in VEGF-A isoforms expressed in the uterus with the appearance of VEGF-A189 in the perivascular stromal cells; the VEGF-A189 isoform can be processed by proteolytic cleavage by plasminogen activator. VEGF-A189 increases vascular permeability acting on VEGFR1, while its processed form binds to the VEGFR2 receptor, which mediates the mitogenic action on endothelial cells.
The highest VEGF-A levels are found in the menstrual phase, probably a response to proinflammatory cytokines. The surge might also be attributed to focal hypoxia, which is a potent stimulus to VEGF-A gene transcription. Expression of VEGFR1 and VEGFR2 is also greatest in the menstrual phase. The elevated levels of VEGF and cognate receptor expression at this time are presumed to be important for vessel repair and the preparation for angiogenesis in the proliferative phase. Notably, the functional activity of VEGFR2 (as assessed by receptor phosphorylation), a signature indicating ligand activation, is relatively low in the early menstrual phase when levels of soluble VEGFR1 (sFLT-1), which sequesters VEGF, are highest. VEGFR2 receptor phosphorylation increases substantially in the late menstrual phase, remaining modestly elevated in the early and late proliferative phase when sFLT-1 levels decline. The FGF family of proteins may also participate in endometrial angiogenesis through interactions with the VEGF system. FGFs upregulate VEGFR2 and VEGF-A expression, and in a feedforward loop, VEGF-A promotes the release of FGFs from the extracellular matrix. Basic FGF is a potent stimulus for ανβ3 integrin expression. This integrin is present at the site of active angiogenesis and fundamental to endothelial invasion and vessel elongation during angiogenesis.
The angiopoietins (Ang) regulate vessel stability. Ang-1, expressed in vascular smooth muscle cells, binds to a cognate receptor, Tie-2, on endothelial cells, resulting in vessel stabilization. Ang-2 is a physiologic antagonist of Ang-1. It also binds to the same Tie-2 receptor. Vessels atrophy when Ang-2 acts in the absence of VEGF-A, whereas angiogenesis is promoted in the presence of VEGF-A. In situ hybridization studies indicate that Ang-1 expression is most abundant in the glands and stroma of the early and midproliferative phase and reduced in the late proliferative phase. Ang-2 expression is detected in the glands and stroma throughout the cycle, with the highest expression occurring in the early proliferative and mid- to late secretory phases. In endometrium from women with menorrhagia, Ang-1 expression is consistently downregulated; as a result, the ratio of Ang-1 to Ang-2 is reduced, which contributes to vessel instability.
Angiogenin is a heparin-binding molecule that is expressed by endometrial epithelial and stromal cells at the greatest levels in the mid- to late secretory phases and the decidua of early pregnancy. Angiogenin is thought to contribute to the proliferation of vascular smooth muscle cells around the spiral arterioles. Like VEGF-A, expression of angiogenin is stimulated by hypoxia. It is also increased by progesterone.
Ephrins , a family of molecules and their cognate tyrosine kinase receptors, are believed to guide endothelial cells to specific targets. Ephrins have been detected in endometrial endothelial and stromal cells, but the functional roles of these molecules and their receptors in the uterus remain to be clarified.
The physiologic consequences of angiogenesis are reflected in changes in endometrial blood flow. By measuring the clearance of radioactive xenon gas, the highest endometrial perfusion was reported between days 10 and 12 and days 21 and 26 of the cycle. Microvascular perfusion has been assessed by laser Doppler fluximetry with transvaginal placement of a fiberoptic probe into the uterine cavity. With the use of this technique, endometrial perfusion was found to be highest during the proliferative phase and the early secretory phase, not too dissimilar from the finding based on xenon clearance. Uterine blood flow is greatest in the fundus, and higher flow rates are associated with better outcomes in assisted reproduction. Notably, diminished uterine blood flow has not been found in the perimenstrual period, but these methods cannot easily identify localized areas of vasoconstriction.
The biochemical basis for the dramatic structural changes in the endometrium in the perimenstrual period includes the action of specific matrix-degrading proteases, the MMPs. , Studies on human endometrial explants in culture demonstrated that degradation of the extracellular matrix occurs in the absence of progesterone and estrogen, which suppress the expression MMPs. Moreover, this degradative process can be blocked by MMP inhibitors but not by inhibitors of lysosomal cysteine proteinases—directly implicating MMPs in the catabolism of the endometrial extracellular matrix.
Enzymes of the fibrinolytic system (urokinase and tissue plasminogen activators) are increased in the endometrium as progesterone is withdrawn in the perimenstrual period. Moreover, PAIl expression is reduced, allowing the plasminogen activators to activate plasmin and proteolytically cleave and activate the latent MMP proenzymes. MMPs represent a large family of proteinases that play a major role in the remodeling of the extracellular matrix ( Fig. 10.9 ). In situ hybridization and immunocytochemistry have been used to map the expression of MMPs and the endogenous inhibitors, tissue inhibitors of the matrix metalloproteinases (TIMPs), in the primate endometrium. Cell-specific and menstrual cycle-specific patterns were revealed, with the most profound changes occurring during the perimenstrual period. , After ovulation, the expression of interstitial collagenase (MMP-1), stromelysin-1 (MMP-3), and stromelysin-2 (MMP-10) in the endometrial stroma is essentially restricted to the perimenstrual and menstrual phase.
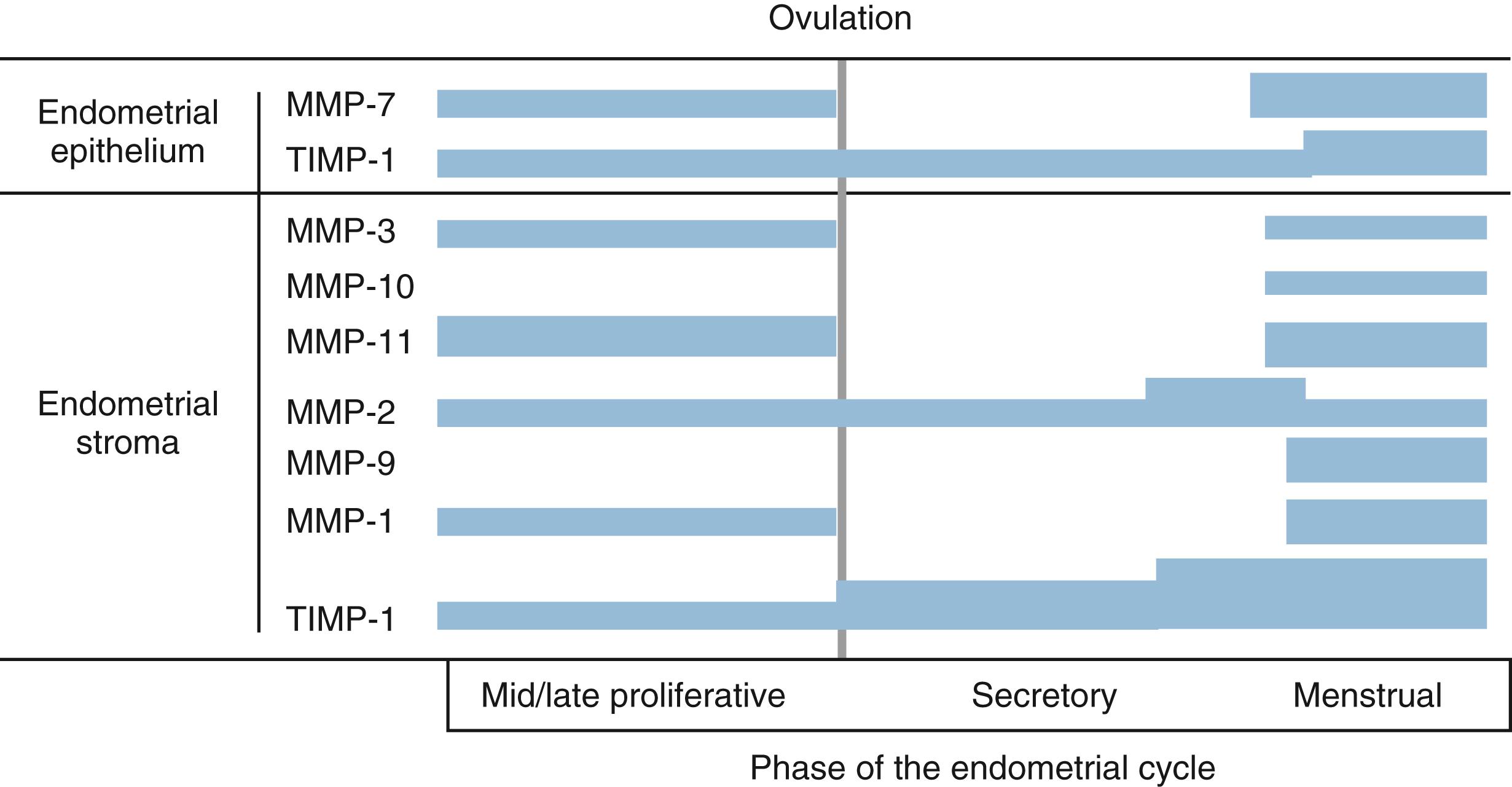
Other MMPs are detected during the proliferative and secretory phases but are significantly increased in expression perimenstrually. These include the type IV collagen-degrading enzymes, MMP-2 and MMP-9. The membrane-bound MMP, MMP-14 (which activates MMP-2), is detected during menstruation in stromal inflammatory cells and epithelial cells. TIMP-1, which is detectable in the endometrium throughout the cycle, is increased in the stroma, epithelium, and arterioles at menstruation.
The importance of progesterone withdrawal in regulating endometrial MMPs and the different temporal patterns of expression have been well-documented in in vivo and in vitro systems. In a primate model in which hormone levels were manipulated by steroid implants, progesterone withdrawal resulted in upregulation of MMP-1, -2, -3, -7, -10, -11, and -14. It is important to note that the expression of MMPs in the endometrium is heterogeneous. ,
At the start of menstruation, MMP-1 is found in patches of stromal cells in the superficial zone; these patches are colocalized with areas of reduced stromal and epithelial expression of estrogen and PR and focal disruption of the extracellular argyrophilic fibrillar network—reflecting the degradative activity of MMP-1. As the process of menstruation proceeds, MMP-1 expression spreads to include the entire functionalis. MMP-2 and MMP-3 expression is also limited to the stromal cells in the functionalis. During menses, MMP-1, -2, -3, and -9 localize primarily in and around arteriolar walls. The heterogeneity of MMP expression suggests that MMP gene transcription is under the control of local rather than systemic (steroidal) factors. In other words, steroids are indirectly influencing MMP expression.
Progesterone, particularly in the presence of estradiol, can suppress the expression of certain MMPs (i.e., MMP-1, -2, -7, -9, and -11) in endometrial explant culture. This action is most likely explained by changes in autocrine/paracrine signals—particularly proinflammatory cytokines or members of the transforming growth factor family, which respectively are potent inducers and suppressors of MMP gene transcription. In culture systems, IL-lα has been implicated as the mediator of MMP-1, MMP-3, and MMP-7 expression in response to withdrawal of progesterone. Neutralizing antibodies to TGF-β prevents the action of progesterone in blocking MMP-3 and MMP-7 expression.
EBAF is the orthologue of the murine gene named Lefty and another likely candidate for a progesterone-regulated cytokine controlling MMP expression. LEFTY was originally identified in human endometrium as a gene upregulated in the late secretory and menstrual phases of the normal cycle, being absent in the proliferative, early, and midsecretory endometrium. EBAF expression, which is predominantly found in the endometrial stroma and to a much lesser extent in the glandular epithelium, is suppressed by progesterone. Interestingly, endometrium from women with a history of abnormal bleeding and endometriosis expressed Lefty at unusual times including the proliferative, early, and midsecretory phases.
Unlike other members of the TGF-β family that promote the formation and stability of the extracellular matrix, EBAF downregulates the elaboration of collagen in association with reduced expression of connective tissue growth factors while upregulating expression of collagenolytic and elastinolytic enzymes. These actions of EBAF are the result of antagonism of the SMAD signaling pathway that is activated by the other TGF-β growth factors. Thus the decline in progesterone and estradiol in the late luteal phase initiates alterations in the endometrium that include upregulation of proinflammatory cytokines (some of which may be contributed by immune cells that accumulate in the endometrium) and a natural TGF-β antagonist. The collective result is focal and widespread expression of matrix-degrading enzymes that result in the remodeling of stroma and blood vessels in the functionalis.
Lysosomal involvement in the process of menstruation has been proposed because of three observations: an increase in the abundance of lysosomes in the endometrium during the late secretory phase, the cytochemical demonstration of acid phosphatase in the perimenstrual endometrium, and the high specific activity of certain lysosomal hydrolases in endometrial tissue in the menstrual phase. However, inhibitors of these enzymes, leupeptin and E-64, do not prevent the progesterone withdrawal-induced breakdown of extracellular matrix in endometrial explants, as do the inhibitors of MMP activity. These observations suggest that lysosomal proteinases are not major contributors to the remodeling of the perimenstrual endometrium.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here