Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Rheumatic diseases involve abnormalities of multiple organs and systems, but it is predominantly the musculoskeletal system that is affected. The musculoskeletal system consists of bones, joints, cartilage, tendons, ligaments, muscles, and associated connective tissues. This chapter is an overview of normal musculoskeletal biology. In other sections, pathology of the musculoskeletal system and other systems pertinent to rheumatic diseases, including immune, vascular, and neurological systems, are discussed in relevant contexts.
The musculoskeletal system is derived from embryonic mesoderm, a layer that first appears at approximately the fourth week of gestation. As the embryonic neural crest closes to create the notochord, cells from the dorsal margins migrate to form the teeth and some facial bones, cartilage, and connective tissue of the anterior skull. Mesodermal cells arising from the prechordal region (located rostral to the notochord) form cartilage and bone for the posterior skull, paraxial mesodermal cells (somites) generate the axial skeleton, and lateral plate mesodermal cells form the appendicular skeleton.
As with all large organisms, the human endoskeleton provides a rigid framework, allowing movement to occur with power and precision. The axial skeleton comprises the skull, mandible, auditory ossicles, hyoid bone, vertebral column, sternum, and ribs, and the appendicular skeleton includes the limbs and limb girdles. Ossification of the fibrous membranes and hyaline cartilage that constitute the primordial skeletal scaffold begins at approximately the seventh gestational week, and by birth it is almost, but not entirely, complete.
The skeleton is the framework that maintains body shape; supports muscles and connective tissues, neurovascular networks, and skin; protects the body’s vital organs; and makes movement possible through articulations and muscle and tendon attachments. The skeleton is the repository for hematopoietic and mesenchymal progenitor cells, a source of energy derived from yellow marrow adipocytes, a mediator of the immune response, and functions as an endocrine organ contributing to calcium, phosphate, and glucose homeostasis. Further, the musculoskeletal system aids other bodily functions, including digestion by mastication, respiration by moistening inspired air passing through bone sinuses, and communication by way of auditory ossicles for hearing and the larynx for vocalization.
The skeleton is a dynamic organ both anatomically and physiologically. It is modified structurally during growth and maturation, in response to fluctuating mechanical loads, and as a normal physiological process throughout life. It is continuously generating new blood cells and contributing to the maintenance of the body’s biochemical and energy balance.
Bone is a composite of organic and inorganic materials. The hardness of bone is a consequence of the interaction of organic constituents such as collagen, proteoglycans, and matrix proteins, which confer tensile strength and permit flexibility to withstand stress, and inorganic materials such as hydroxylapatite and other calcium and phosphate salts, which account for bone’s compressive strength.
Bones are categorized based on either their respective shapes and attendant functions or the process by which they become ossified.
All bones share similar ultrastructural characteristics, but gross anatomical shapes differ, reflecting adaptations to their respective functions. Long bones, with a greater length than width, have a thick wall of cortical bone to withstand and adapt to fluctuating stresses of weight-bearing and to allow for rotation and leverage. Short, cuboid-shaped bones, such as carpal bones, support and facilitate movement. Broad flat bones, such as cranial bones, sternum, and ribs, are thin and curvilinear; they serve as attachment sites for musculature and they shield vital organs. Irregular-shaped bones protect the face and spinal cord. Sesamoid bones develop as smooth, round ossifications to counteract compressive forces within tendons; patellae are the only sesamoid bones consistently present in humans, although the hallux sesamoid bone of the foot and the pisiform of the wrist are usually present as well.
Bone is formed by either intramembranous or endochondral ossification. Intramembranous ossification occurs in facial, cranial, and clavicular bones as mesenchymal cells within the unossified skeleton coalesce and then differentiate into either capillaries or osteoblasts. The osteoblasts secrete unmineralized osteoid matrix into which mineral salts (notably calcium and phosphorus) are then deposited. When ossified, osteoid produced around capillaries becomes honeycomb-like trabecular bone (also referred to as cancellous or spongy bone ), which encroaches on the vasculature, compacting it into red marrow.
Endochondral ossification occurs in most appendicular and axial skeleton bones. Endochondral ossification involves the progressive replacement of the primordial cartilaginous scaffold with bone through a sequence in which chondrocytes proliferate, mature, and enlarge. By 8 weeks gestational age some mesenchymal cells have differentiated into cartilage-producing chondrocytes that create the cartilaginous skeletal framework. Building the skeletal scaffold begins as mesenchymal stem cells form condensations at the site of future bone. Cells within the condensations do not differentiate directly into bone-producing osteoblasts; instead, they become proliferating chondrocytes, which synthesize the extracellular matrix comprising predominantly type II collagen, some type IX and XI collagens, and proteoglycans. Then, as mature, hypertrophied chondrocytes, they secrete a matrix rich in type X collagen, which provides the supporting framework for endochondral ossification.
As the cartilage matrix ossifies, blood flow to the chondrocytes is impeded, the cells die, and adjacent cartilage breaks down, leaving a medullary cavity into which new vessels invade to deliver bone-producing osteoblasts. While ossification continues from the primary ossification center in the diaphysis, cartilage continues to be produced at the ends of bones as the epiphyses. At birth, cartilage remains at the articular surface and the growth plate (the physis) situated between the epiphysis and the diaphysis. A mature long bone includes the epiphysis at the end of the bone, the diaphysis (the shaft of the bone), and the interposing metaphysis, which represents the region of the previous cartilaginous growth plate. Apophyses, such as the tibial tuberosity, posterior calcaneus, greater and lesser trochanters, and iliac crests, like epiphyses, are the sites of new bone formation but do not contribute to bone length; instead, they generate new bone in response to tendinous or ligamentous traction.
Bone includes an outer layer of thick, compact, cortical bone and an inner layer of interlacing ossified struts (trabeculae). Alignment of the trabecular network with the long axis of the bone confers maximal tensile strength and compressive tolerance so that less bone mass is required. Bones subjected to predominantly tensile and compressive forces, such as vertebrae, have a thin cortex of compact bone; they exert their support by their shapes and trabecular architecture. Bones that are required to tolerate torsion, bending, and shearing forces, such as the bones of the leg, tend to be cylindrical with a thick collar of cortical bone and a marrow cavity.
Type I collagen constitutes 90% of bone matrix. The triple helical strands of collagen, when densely packed into parallel alignments (lamellae), confer optimal tensile strength to accommodate mechanical loading. Other organic constituents of bone contribute to regulating mineralization, growth, and energy metabolism.
The circular osteon is the fundamental physiological unit of most mammalian compact bone ( Fig. 1.1 ). The microstructure of the osteon is characterized by 5 to 20 concentric layers of compact (cortical) bone that encircle a central haversian canal (first identified by 17th-century English physician Clopton Havers), through which neurovascular bundles traverse to supply the adjacent osteocytes ( Fig. 1.1 ). Osteons, measuring a few millimeters in length and less than 0.25 mm in diameter, tend to orient to the long axis of the bone. Osteons are characteristically present in mature bone that has undergone new bone growth, remodeling, or repair. Osteons and their associated intra-haversian blood vessels communicate by interconnecting transverse Volkmann canals (named for 19th-century German physiologist Alfred Volkmann).
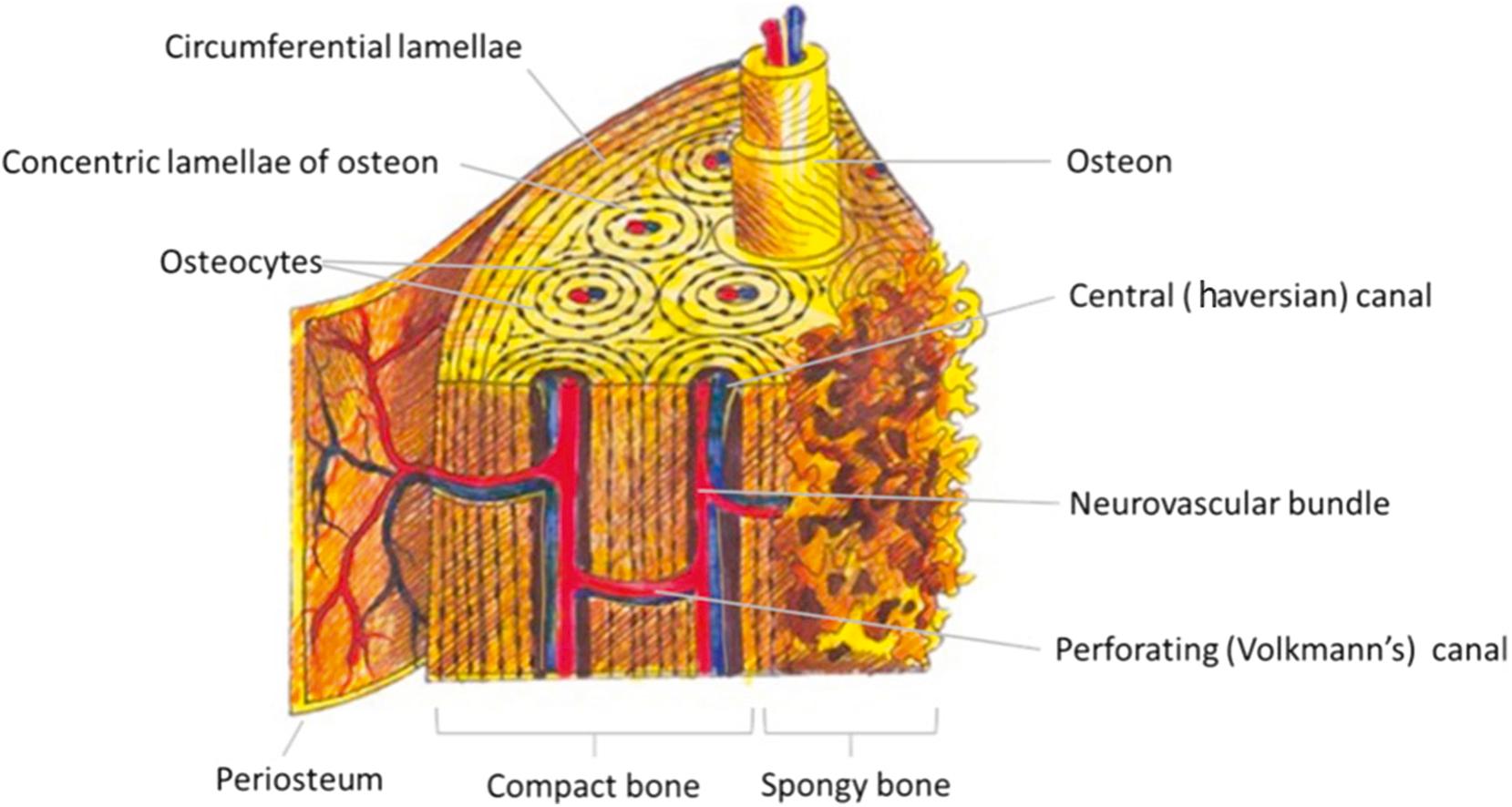
Deeper layers of bone are made of spongy bone, the normal physiological consequence of bone breakdown induced by osteoclasts.
Bone contains four cell types: mesenchymal stem cells, osteoblasts, osteocytes, and osteoclasts. Mesenchymal stem cells, located in the intertrabecular loose connective tissue adjacent to vascular channels and in the periosteum, have the potential to differentiate into cells of bone (osteoblasts, osteocytes, and osteoclasts), cartilage (chondrocytes), muscle (myocytes), or fat (adipocytes).
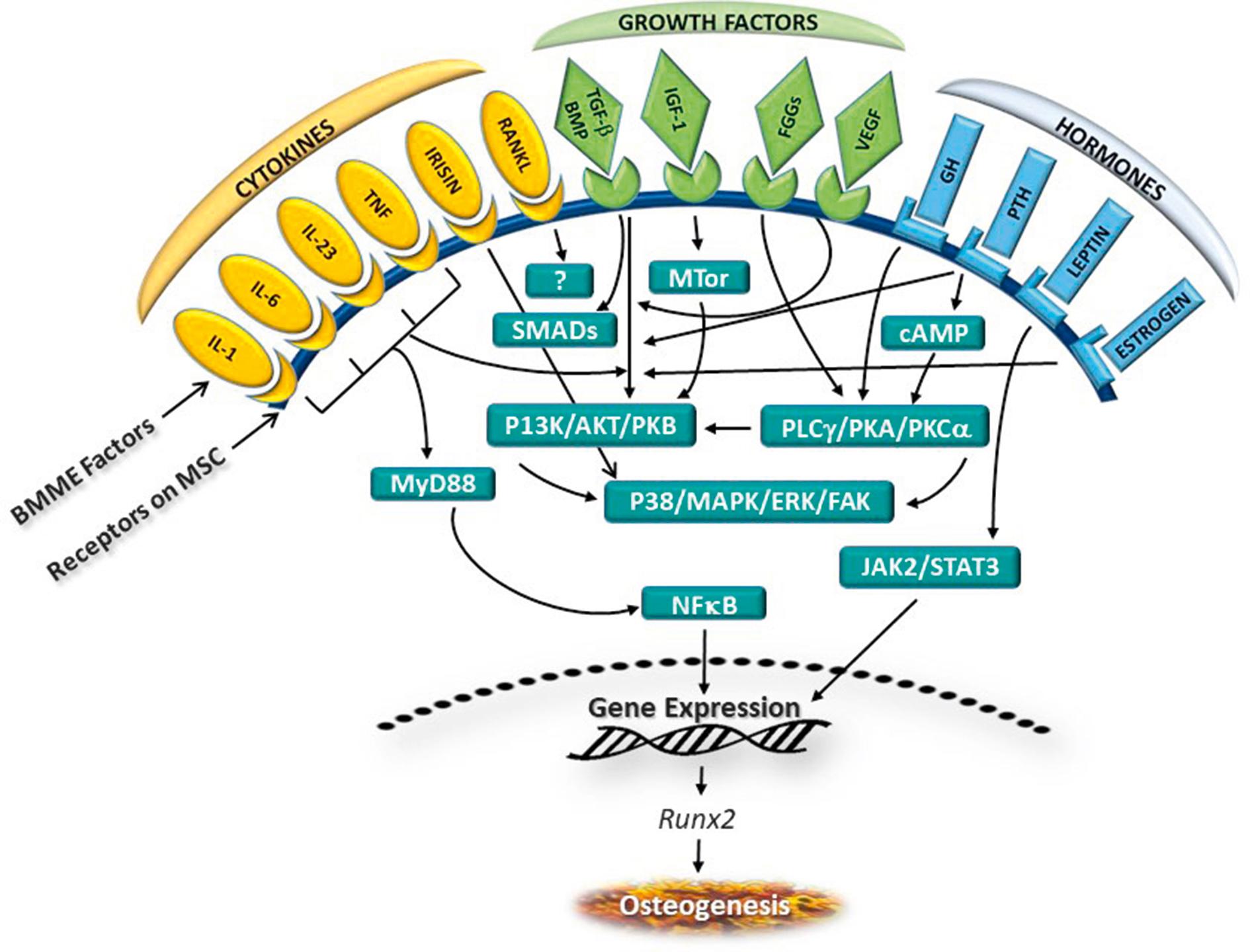
The differentiation of mesenchymal stem cells into osteoclasts is regulated by a wide array of cytokines, growth factors, and hormones that, respectively, promote or inhibit osteoclastogenesis ( Fig. 1.2 ). Groups of osteoblasts, which assemble into clusters to form the osteon, produce the organic constituents of bone, mainly collagen and to a lesser extent osteocalcin and osteopontin. Osteocalcin is a hormone that acts on pancreatic beta cells to promote insulin production in response to leptin and can activate bone-based adipocytes to regulate insulin responsiveness. Thus, there is an interaction between bone development, fat cell function, and insulin production. Osteopontin participates in bone remodeling by securing osteoclasts to the inorganic (mineral) matrix of bone.
After becoming entrapped in fully formed bone, osteoblasts become osteocytes within intramatrix cavities (lacunae). The mechanosensory osteocytes begin mineralizing hydroxylapatite, the bone’s organic matrix. Osteocytes are interconnected by cytoplasmic tendrils that traverse a network of canaliculi through which nutrients and metabolites are delivered to ensure osteocyte viability. Osteocytes, the most abundant bone cells, maintain the physiological balance between bone formation by osteoblasts and bone resorption by osteoclasts, thus preserving bone integrity and mediating bone remodeling in response to mechanical stresses.
Osteoclasts mediate resorption at the inner surfaces of bone, such as the marrow cavity and cancellous bone trabeculae, and the outer surfaces of bone, such as the epiphyses. Osteoclasts also participate in the conversion of cancellous bone into lamellar (compact) bone by creating spaces in which osteons can develop. Osteoclasts degrade bone by secreting lysosomal enzymes, resulting in calcium release to maintain mineral homeostasis.
After birth, longitudinal bone growth occurs from the epiphyseal plate, which has four zones: reserve (resting), young proliferating cartilage, maturing cartilage with hypertrophic chondrocytes, and calcifying matrix ( Fig. 1.3 ). The epiphyseal side of the plate is retained as articular cartilage, whereas on the diaphyseal side, the epiphysis ossifies to increase bone length. The dense cortex of long bones is enveloped by an outer layer of periosteum and an inner layer of endosteum.
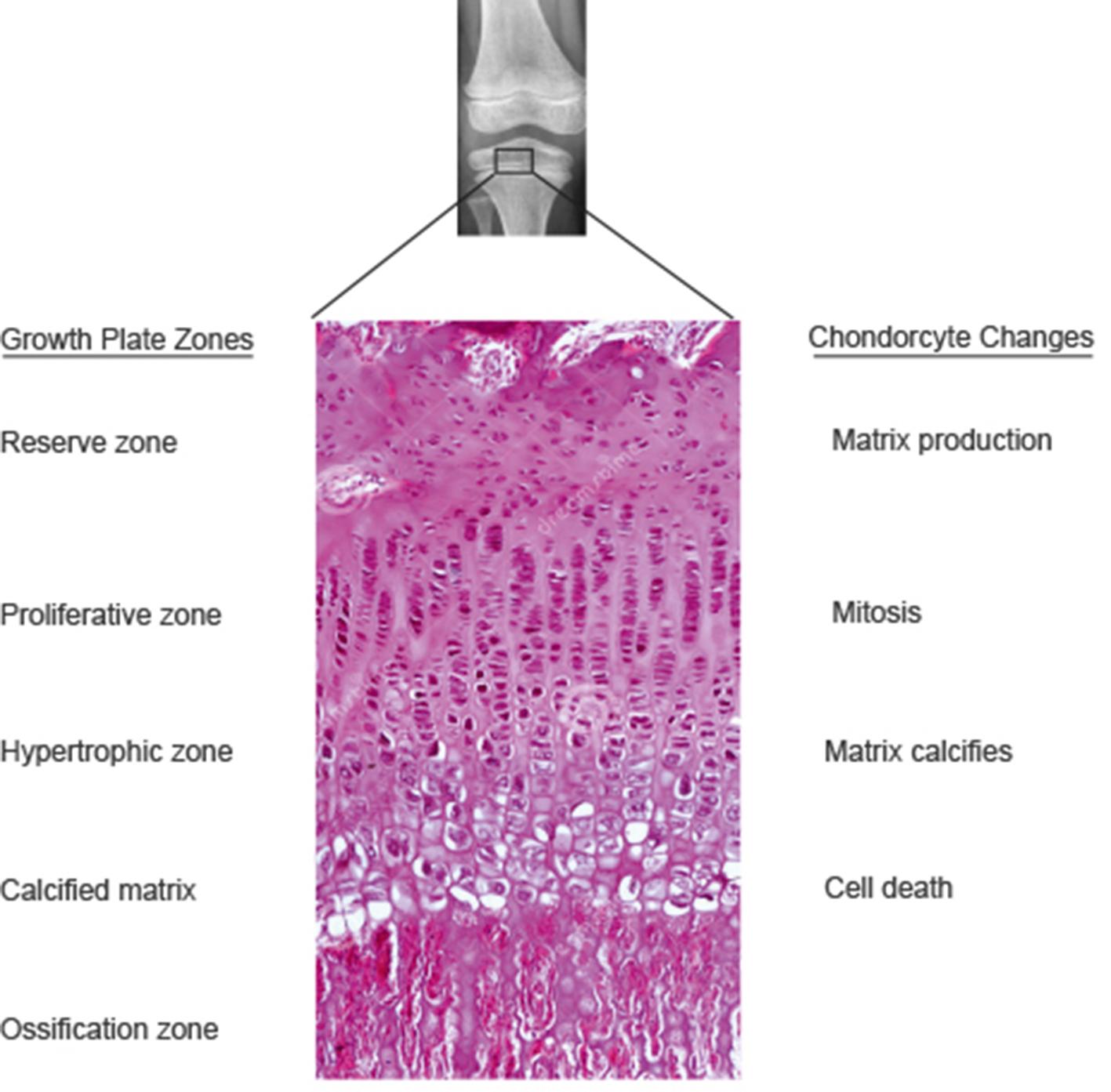
The stages of skeletal maturation are reflected by the radiographic appearance of secondary ossification centers in long bones and physeal closure. The Tanner-Whitehouse , and Greulich-Pyle protocols remain the conventional methods for assessing skeletal maturation (bone age) using left hand and wrist radiographs, although newer assessment methods have been considered. Bone growth at the physis is governed by thyroid, growth, and sex hormones. Peak bone mass development during adolescence is determined by a variety of factors, including diet, physical activity, and synergistic effects of growth hormone and insulin-like growth factor. During the most rapid phases of adolescent growth, testosterone promotes cell division resulting in widening of the physis, and estrogen promotes calcification and closure of the physis. , Completion of ossification of the iliac apophyses is temporally related to cessation of the appendicular skeleton growth, although vertebral height may continue to increase and contribute to increasing height until the third decade of life.
The endochondral ossification that leads to longitudinal bone growth during childhood and adolescence requires the continuous availability of chondrocytes at the growth plate. During fetal and early neonatal periods, chondroprogenitor cells are progressively depleted. In contrast, as secondary ossification centers develop later in the bone growth process, chondrocytes in the physis begin to acquire characteristics of stem cells and, under the influence of two signaling pathways (hedgehog and mammalian target of rapamycin complex 1), differentiate into chondroprogenitor cells. In this way, a continuous supply of chondrocytes is available during growth.
Bone growth is accompanied by coincident angiogenesis, a process regulated by bone cells. , Osteocytes, which have dendritic processes connected to blood vessels, induce angiogenesis by the vascular endothelial growth factor and mitogen-activated phosphorylated kinase signaling pathways.
Growth factors are delivered to the physis through three routes of arterial supply: nutrient arteries, a metaphyseal-epiphyseal network, and a periosteal system ( Fig. 1.4 ). In the resting state, bones receive approximately 10% to 15% of the cardiac output. Pathological states, such as joint inflammation, are associated with increased blood flow and acceleration of bone growth.
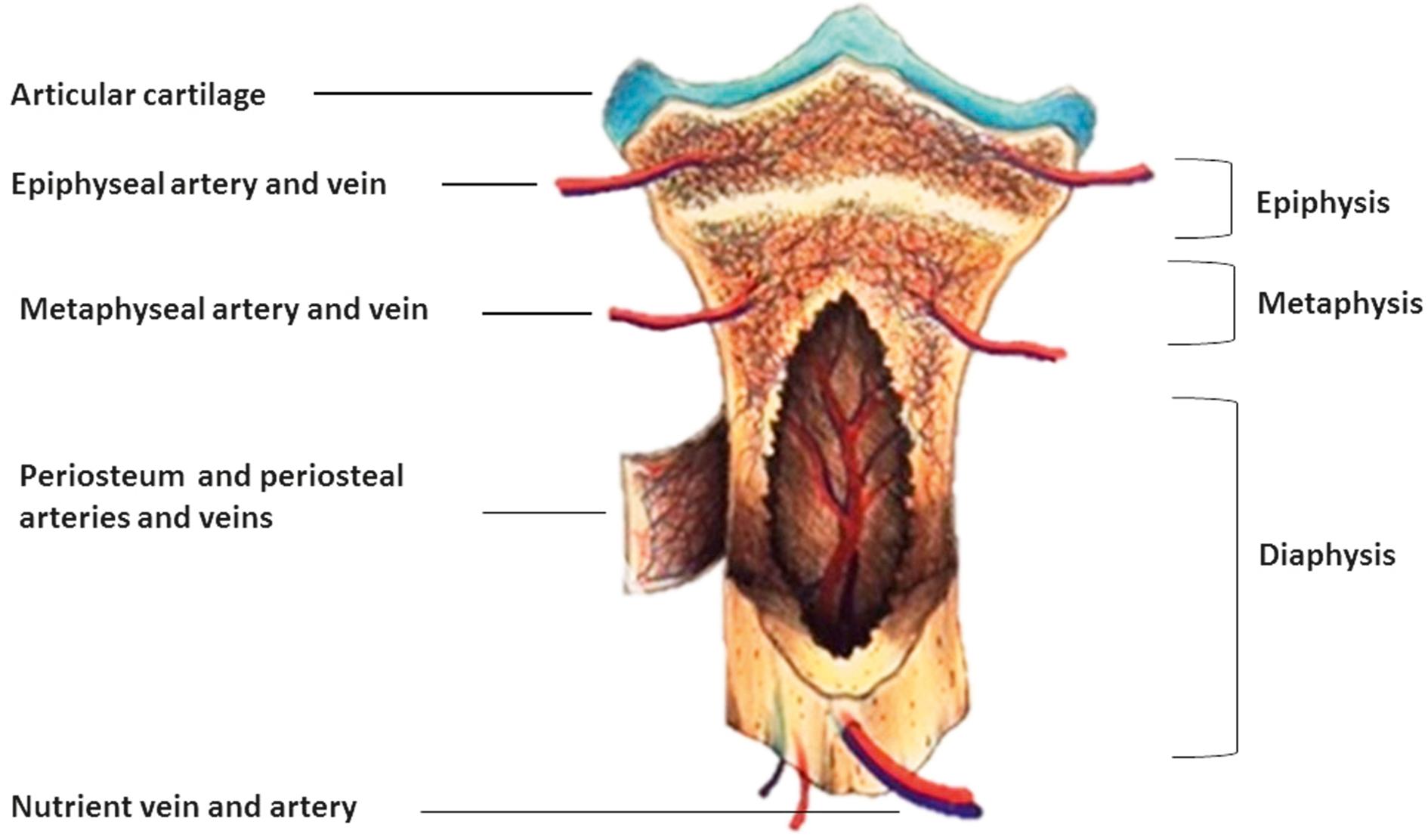
The nutrient blood vessels (together with nerves) penetrate the cortex through nutrient foramina, enter the medullary cavity, and then branch into ascending and descending arterioles that travel through the haversian system to supply blood at high-pressure flow to the inner two-thirds of mature bone ( Fig. 1.4 ). The metaphyseal-epiphyseal vascular supply is derived from arteries arising from the periarticular vascular plexus that include metaphyseal, perichondral, and epiphyseal arteries. The periosteal vascular flow is a low-pressure system supplying the outer one-third of bone through the haversian and Volkmann canals. Evidence indicates that an elaborate and dense transcortical capillary network is the terminal pathway for blood delivery to bone.
Circumferential growth, an increase in bone width, results from appositional subperiosteal membranous ossification on the bone surface.
Maintaining the integrity of mature bone, repairing damaged bone, and releasing calcium from bone are mediated by a shared process called bone remodeling . Bone remodeling, which occurs continuously throughout life, is more rapid during childhood and adolescence. The regulation of bone modeling during development and subsequent bone remodeling that occurs after birth requires direct interactions between osteoblasts and osteoclasts, endocrine and neurological controls, and innate immune and inflammatory regulators.
The metabolic events that initiate bone remodeling are unclear, but the death of osteocytes is likely to be an early event in the repair/remodeling cascade. After osteocytes die, osteoclasts degrade bone matrix, forming lacunae into which mesenchymal cells migrate. Intralacunae mesenchymal cells differentiate into osteoblasts resulting in new bone matrix formation and subsequent mineralization. Depending on bone size and patient age, this remodeling process might take up to 6 months. Bone remodeling occurs continually even in adults; it is estimated that the entire healthy adult skeleton is remodeled several-fold during a lifetime.
The controlled balance between bone-forming osteoblasts and bone-resorbing osteoclasts is fundamental to sustaining bone health. Intercellular signaling by the wingless-related integrated site (Wnt) family of glycoproteins regulates the transcription cofactor β-catenin, which is required for signaling the expression of genes involved in development. , The Wnt/β-catenin signaling pathway (referred to as the canonical Wnt signaling pathway ) is crucial for growth and development of a wide array of tissues, including bone. Wnt signaling is integral for skeletal formation and embryonic limb development, chondrogenesis, production of bone matrix by osteoblasts, and the differentiation and activity of osteoclasts. Wnt/β-catenin signaling prods mesenchymal stem cells to differentiate into osteoblasts, chondrocytes, or osteocytes. The Wnt/β-catenin pathway enhances bone mass by promoting stem cell renewal and osteoblastogenesis, suppressing osteoblast and osteocyte apoptosis, and sensing mechanical loading. Bone remodeling also occurs in response to endocrine influences such as thyroid, growth, and sex hormones.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here