Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Contact lens–induced corneal oedema was recognised in the first two written accounts of the clinical application of contact lenses published over a century ago. In his original treatise on contact lenses, published in 1888, Adolf Fick noted that the cornea became cloudy within hours of insertion of a glass haptic shell. Although Fick would not have been aware of the exact cause of this disturbing pathological change, it is clear that he was observing contact lens–induced corneal oedema. Even more remarkably, Fick observed that the onset of corneal clouding could be delayed by trapping an air bubble between the lens and cornea.
In his inaugural dissertation to the University of Kiel in Germany, August Müller provided a graphic subjective description of what was undoubtedly a marked contact lens–induced corneal oedema. Müller correctly identified inadequate tear exchange beneath the lens as the cause of this problem but was unable to find a solution at the time.
These pioneering works signalled the beginning of the battle against corneal oedema – yet, despite numerous significant advances in lens materials, designs, fitting techniques and possible modalities of wear, we are still unable to claim an absolute victory over lens-induced oedema.
Oedema refers to an increase in the fluid content of tissue. Since the cornea is only able to swell in the anteroposterior direction as a result of the collagen fibre network in the stroma, the physical dimensions of the cornea can only increase in that dimension – that is, in thickness. Corneal oedema is usually expressed as the percentage increase in corneal thickness. Thus, for example, a cornea that swells from a thickness of 550 μm before lens wear to become 605 μm thick after lens wear has swollen 55 μm, or has experienced 10% oedema. As will be discussed in this chapter, it is typically the corneal stroma that swells. The corneal epithelium can also become oedematous, but it usually swells in response to osmotic stress (see Chapter 20 ), whereas the stroma swells in response to hypoxic stress. As a result of this analysis, it can be seen that in a cornea suffering from only stromal oedema, the percentage increase in total corneal thickness will slightly underestimate the percentage increase in stromal thickness.
Laboratory scientists have developed techniques for the precise measurement of corneal thickness and have noted that oedema is a reliable and repeatable index of the physiological integrity of the cornea.
Early textbook accounts describing the detection of corneal oedema associated with the wearing of contact lenses made from polymethyl methacrylate (PMMA) cited ‘central corneal clouding’ as the key diagnostic criterion indicating corneal compromise. This condition occurred in patients wearing tightly fitted PMMA lenses, which restricted tear exchange beneath the lens and hence created hypoxic oedema. A discrete, round area of clouding could be clearly observed in the central cornea ( Fig. 22.1 ).

Central corneal clouding indicates a gross level of oedema that is rarely observed in modern contact lens practice. Fernandez-Velazquez reported three cases of severe bilateral central corneal clouding in association with ClearKone SynergEyes hybrid contact lenses for vision rehabilitation in patients with keratoconus. Despite attempts to modify the parameters of the lenses to reduce the oedema, two patients had to discontinue lens wear, and one patient was able to wear lenses only on a limited basis.
Central corneal clouding is an obvious sign of gross oedema; however, careful observation of the cornea using a slit lamp biomicroscope reveals the existence of more subtle signs that can be used to predict the level of oedema in response to lens wear. In this chapter, the oedema response to contact lens wear will be reviewed, techniques available to the clinician for evaluating the extent of oedema will be described and treatment alternatives for minimising the oedema response will be provided.
Every human experiences corneal oedema during sleep. Upon awakening in the morning, the cornea immediately begins to reduce in thickness (‘de-swell’). A new steady-state thickness – representing a thinning of about 3% – is reached after about 4 hours, indicating that the cornea had swollen about 3% overnight.
The prevalence of contact lens–induced oedema is essentially 100%, since all contact lenses induce some level of oedema, including extremely high oxygen permeable silicone elastomer lenses. The amount of oedema is related primarily to the extent of corneal hypoxia that is induced by the lens (see later).
In 1992, Stapleton et al. reported that of 1104 patients with contact lens–related disorders presenting to an eye clinic, 32(2.9%) displayed stromal oedema. Table 22.1 displays the results of hospital surveys of contact lens complications, conducted between 2009 and 2018 – carried out in China, India, Nepal, Singapore, the USA and the UK. Besides the relatively high figure of 8.3% reported by Lee et al. (8.3%), all other surveys noted a prevalence of stromal oedema of 2.1% or less.
| Author | Country | Year | Population size studied | Retrospective or prospective | Prevalence |
|---|---|---|---|---|---|
| Li et al. | China | 2018 | 141 | Retrospective | 0.7% |
| Sapkota et al. | Nepal | 2013 | 4064 | Retrospective | 0.0% |
| Lee et al. | USA | 2012 | 1369 | Prospective | 8.3% |
| Teo et al. | Singapore | 2011 | 953 | Prospective | 1.1% |
| Nagachandrika et al. | India | 2011 | 1255 | Retrospective | 0.1% |
| Radford et al. | UK | 2009 | 877 | Prospective | 2.1% |
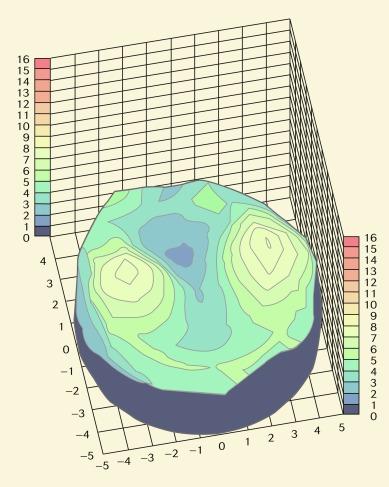
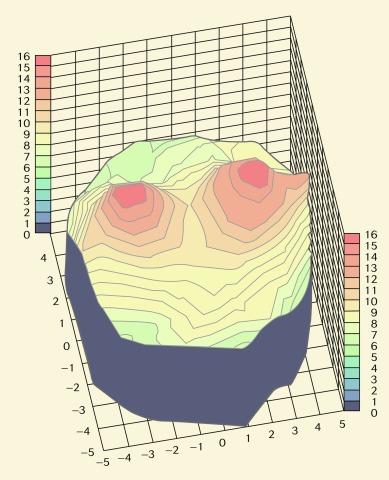
Although central corneal swelling data are usually quoted in the literature, it should be noted that the oedema response across the cornea is not uniform. This results from (a) the variation in thickness across powered contact lenses whereby blink-induced tear mixing does not act to equalise the distribution of oxygenated tears beneath the lens and (b) the resistance of the peripheral cornea to swell as a result of ‘limbal clamping’. Topographical corneal swelling plots for a silicone hydrogel lens and a conventional mid-water-content hydrogel lens are shown in Figures 22.2 and 22.3 , respectively. The apparently greater swelling of the temporal and nasal mid-peripheral cornea compared with the central cornea may be an artefact of the experimental methodology; nevertheless, the difference in overall swelling induced by these lenses is substantial.
A variety of sophisticated clinical and laboratory techniques can be employed to measure precisely the extent of lens-induced corneal oedema, the most popular being optical pachometry and ultrasonic recording. Orbscan topographical thickness mapping, specular microscopy, confocal microscopy, optical coherence tomography, femtosecond laser ranging and interferometry can also be used to measure corneal thickness. It is often necessary to use these more sophisticated techniques in clinical trials and laboratory research on contact lenses or for the provision of baseline information before corneal surgery. The clinical utilisation of these techniques has increased substantially in recent years as these instruments have become more affordable.
Clinicians can estimate the magnitude of corneal oedema via careful observation with the slit lamp biomicroscope, as a number of structural changes can be identified that correlate with various levels of oedema. These structural changes – striae, folds and haze – act as useful reference points or ‘yardsticks’ to form the basis of clinical decision-making.
When viewed under direct focal illumination, striae appear as fine, wispy, white, vertically oriented lines ( Fig. 22.4 ) and are always located in the posterior stroma. They can also appear as dark lines against the orange fundus pupillary reflex when observed by using retro-illumination. Striae are only observed when the level of oedema reaches about 5%. As the level of oedema increases, striae become greyer and thicker, and they increase in number. Striae do not cause vision loss.
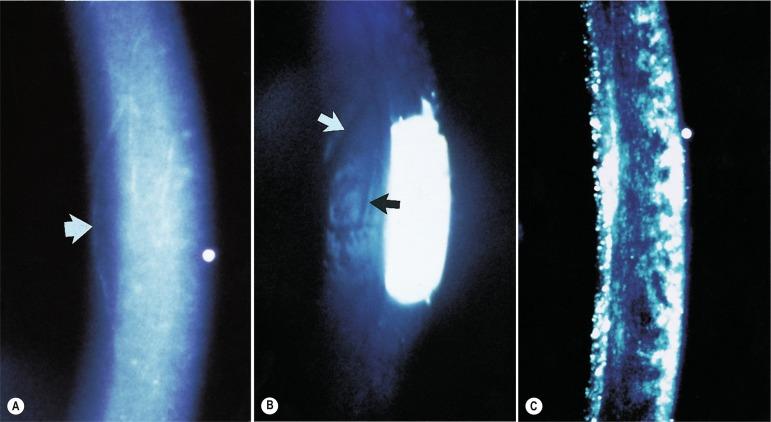
Folds can be observed in the endothelial mosaic as a combination of depressed grooves or raised ridges or as a general area of apparent buckling, when the level of oedema reaches about 8% ( Fig. 22.4 ). They also increase in number as the level of oedema increases. Folds are best observed by using specular reflection. Vision is thought to be unaffected by folds.
The stroma takes on a hazy, milky or granular appearance when the level of oedema reaches about 15% ( Fig. 22.4 ). In essence, the stroma has suffered a loss of transparency. This can be viewed using a variety of observation techniques. The milky appearance is evident when the cornea is viewed against the pupil under indirect illumination. Instead of the normal dark appearance, a fine grey haze is detected and fine iris detail is partially obscured. Sclerotic scatter technique will enhance this clinical picture. Contact lens–induced stromal haze can cause a slight degradation of vision when the level of oedema exceeds 20%.
In a clinical setting, stromal haze indicates gross oedema and will often be associated with other signs and symptoms of ocular distress. It would perhaps be more appropriate to consider oedema of this level as a frank bullous keratopathy. Contact lens wear is more likely to be an exacerbating factor rather than the primary cause of the development of stromal haze, so other possible causes of this complication ought to be investigated.
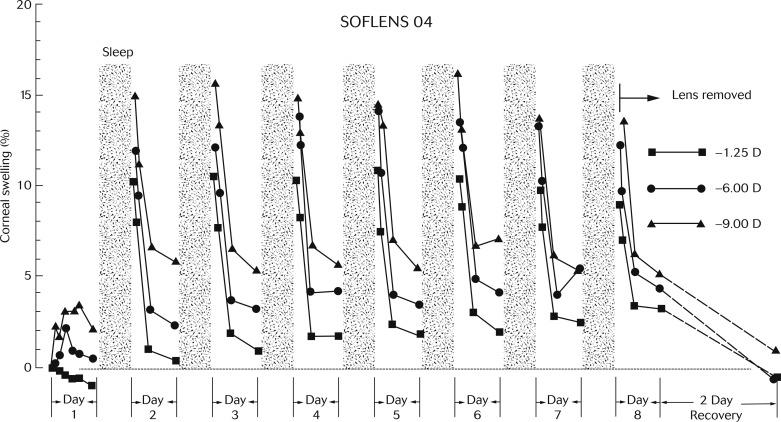
A large number of papers were published towards the end of the 20th century documenting the magnitude and time course of stromal oedema during lens wear. It is impractical to review all of these reports; however, one classic paper ‘pushed the boundaries’ and perhaps defined the maximum extent of stromal oedema during lens wear. This was the report of Holden et al., who demonstrated the large changes in stromal oedema that can occur during extended contact lens wear.
Figure 22.5 shows changes in stromal thickness recorded by Holden et al., who used optical pachometry in subjects wearing low-water-content (38% hydroxyethyl methacrylate) hydrogel contact lenses continuously over 8 days. Lenses of three different powers were worn: − 1.25 diopters (D), − 6.00 D and − 9.00 D. Over the 8-day period, stromal thickness was measured immediately upon awaking, during the day and immediately before going to sleep. As can be seen from Figure 22.5 , levels of oedema exceeding 15% were observed on some mornings, with the level of oedema dropping to about 1% to 3% during the day.
The findings from the study of Holden et al. can be contrasted with the corneal swelling response to 21st century lenses. Del Aguila-Carrasco et al. assessed the effect of seven representative daily disposable contact lenses on corneal thickness by using optical coherence tomography. Responses to seven different hydrogel and silicone hydrogel daily disposable soft contact lenses – Delefilcon A, Nelfilcon A, Omafilcon A, Filcon II3, Narafilcon A, Etafilcon A and Hilafilcon B – were assessed, each for a period of 12 hours of open-eye wear. Central corneal thickness was measured while patients were wearing these lenses at baseline and after 4, 8 and 12 hours. Measurements were also recorded without any contact lenses being worn during the day.
The results of this experiment are shown in Figure 22.6 . In the no-lens scenario, a small but significant (p < 0.05) thinning in the cornea was observed after the 12-hour period. Highly oxygen transmissible silicone hydrogel lenses caused a similar stromal de-swelling response to the no-lens condition. Unsurprisingly, a small amount of stromal oedema (about 1% oedema averaged across all lens types) was evident in response to daily hydrogel lens wear.
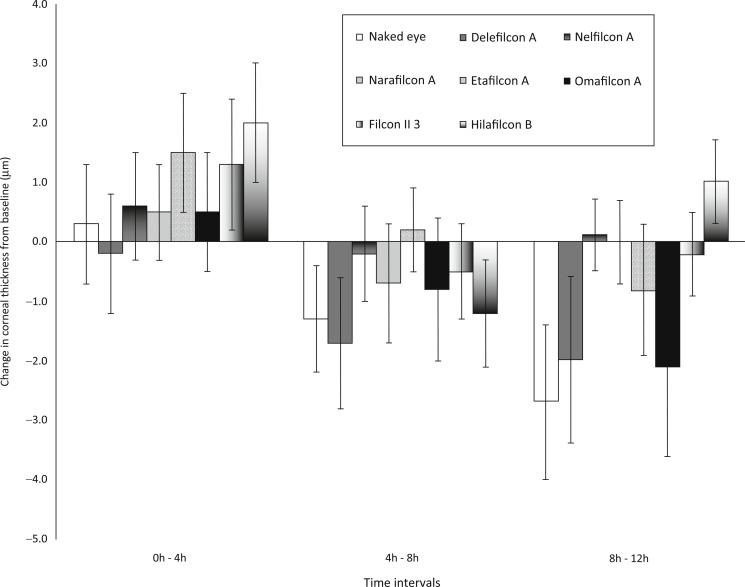
The lesson learned from these two studies is clear. In the latter part of the 20th century, low-oxygen-transmissibility lenses worn for extended periods caused high levels of stromal oedema. In stark contrast, in the 21st century, highly oxygen transmissible silicone hydrogel lenses worn on a daily-wear basis have a clinically insignificant effect on stromal thickness. When used on an extended-wear basis, silicone hydrogel lenses of a range of lens powers (− 3.00 D, − 10.00 D and + 6.00 D) induce 6% to 9% overnight oedema.
The cornea is a sophisticated five-layered tissue and is 78% water. The stroma, which constitutes 90% of the thickness of the cornea, has a constant tendency to imbibe water and swell. This tendency is counteracted by a fluid control mechanism (described as the ‘pump leak’ model ), which is located in the endothelium and acts to move water out of the stroma and back into the aqueous via a bicarbonate ion pump. If this mechanism is disrupted, or other physiological challenge is posed to the cornea, the demand on the endothelial pump to maintain deturgescence may become too great. Water will then enter the stroma, and the cornea will increase in thickness.
Striae are thought to represent fluid separation of the predominantly vertically arranged collagen fibrils in the posterior stroma ( Fig. 22.7 ). This creates a local refractile optical effect whereby stromal transparency is reduced in the immediate vicinity of the separated fibrils. It was originally postulated that the vertical orientation of striae is an artefact of the vertical orientation of the slit beam of the biomicroscope and/or the horizontal binocular displacement of the eyepieces of the objective; however, rotating the beam to a horizontal orientation does not alter this appearance (i.e. horizontally oriented striae do not appear suddenly).
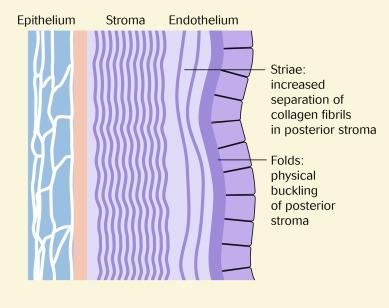
It is thought that folds indicate a physical buckling of the posterior stromal layers in response to high levels of oedema ( Fig. 22.7 ). Because of the inherent transparency of the stroma, it is not possible to directly observe folding of stromal tissue. Instead, folding can be seen as an alteration to the topography of the endothelial layer observed in specular reflection ( Fig. 22.8 ). Efron et al. confirmed the appearance of oedematous folds by using the confocal microscope ( Fig. 22.9 ). These folds appeared as long, straight, dark, orthogonal lines in the posterior stroma of the eyes of patients who had worn hydrogel lenses overnight. The fact that both vertical and horizontal folds were observed supports the notion that the appearance of only vertically oriented striae and folds with the slit lamp biomicroscope is an artefact of the configuration of the slit lamp biomicroscope earlier.
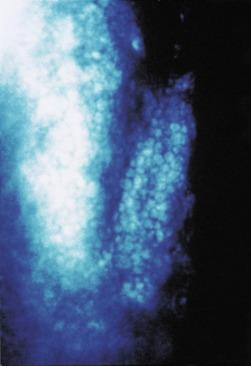
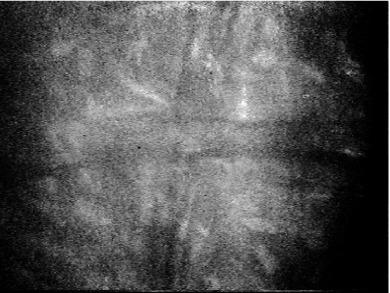
Haze is essentially a more advanced form of striae whereby there is a gross separation of collagen fibres throughout the full thickness of the stroma, disrupting the regular geometry and orderly arrangement of the stromal lamellae. This causes a failure of the optical coherence of the stromal collagen layers, and transparency is reduced. The greater the oedema, the greater is this disruption, and the greater is the extent of haze.
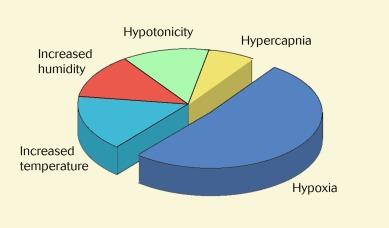
A number of possible mechanisms have been suggested as playing a role in lens-induced corneal oedema. These include hypoxia, retardation of carbon dioxide efflux (leading to tissue acidosis), mechanical effects, temperature changes, hypotonicity, inflammation and increased humidity. The extent to which these factors contribute to overnight corneal oedema is indicated in Figure 22.10 . Although the factors contributing to contact lens–induced corneal oedema have not been investigated in this manner, it would seem reasonable to explain lens-induced oedema primarily in terms of the effects of anterior surface hypoxia.
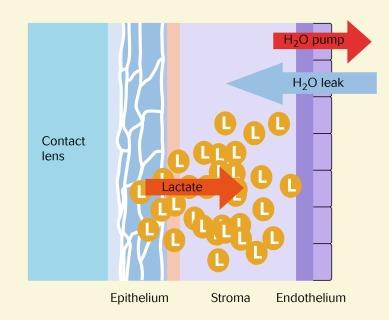
Contact lenses restrict corneal oxygen availability, creating a hypoxic environment at the anterior corneal surface. To conserve energy, the corneal epithelium begins to respire anaerobically. Lactate, a by-product of anaerobic metabolism, increases in concentration and moves posteriorly into the corneal stroma. This creates an osmotic load that is balanced by an increased movement of water into the stroma. The sudden influx of water cannot be matched by the removal of water from the stroma by the endothelial pump, resulting in corneal oedema ( Fig. 22.11 ).
Nguyen et al. showed that the variability in contact lens-induced corneal swelling is associated with both corneal metabolic activity and endothelial function (percentage of recovery per hour). This suggests that individuals with larger levels of corneal metabolic activity produce more lactic acid (i.e. more swelling), whereas stronger endothelial function resists swelling.
O’Donnell and Efron assessed the acute swelling response to contact lens–induced corneal hypoxia in patients with diabetes who wore contact lenses. A thick, low-water-content soft contact lens was fitted under a light patch to one eye of 23 patients with diabetes and one eye of 23 control subjects without diabetes in a single-masked, controlled clinical study. After 2.5 hours, an ultrasonic pachometer was used to measure the induced corneal oedema. The induced corneal oedema was significantly less in the patients with diabetes compared with the control subjects without diabetes (p = 0.004), suggesting that metabolic alterations to the cornea in diseases, such as diabetes mellitus, can alter the corneal swelling response to contact lens wear.
The level of corneal oedema in a contact lens wearer can be assessed using the grading scale for corneal oedema shown in Appendix A . An analysis of the clinical changes depicted in each level of grading is given in the following.
This is the normal non-lens-wearing situation of 0% oedema during the day.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here