Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Staphylococcus aureus is a highly successful opportunistic pathogen. It is a frequent colonizer of the skin and mucosa of humans and animals (it is present in the anterior nares of up to 30% of the healthy human population) and can produce a wide variety of diseases. These diseases encompass relatively benign skin infections, such as folliculitis and furunculosis, and life-threatening conditions, including erysipelas, deep-seated abscesses, osteomyelitis, pneumonia, sepsis, and endocarditis. In addition to infections in which the organism is physically present at the infected site, S. aureus is also capable of producing “distant” diseases, which are mediated by the secretion of toxins. The toxins can be produced directly by bacteria that colonize the skin or mucosa or indirectly by microorganisms that colonize food or beverages. The former is exemplified by staphylococcal scalded skin syndrome (SSSS), which is the result of skin, mucosal, or wound colonization by S. aureus –producing exfoliative toxin A or B (ETA or ETB) and by staphylococcal toxic shock syndrome (TSS), which is the result of the production of toxic shock syndrome toxin 1 (TSST-1) or exotoxins B or C. The latter is exemplified by S. aureus food intoxication, in which the toxin is ingested with the contaminated dish, and disease follows shortly thereafter in the form of vomiting and diarrhea. Food intoxication is the result of staphylococcal toxins called enterotoxins. These toxins are heat stable. Cooking may kill the contaminants but does not denature the toxins. Hence, subsequent culture of the dish may fail to grow the culprit bacterium.
S. aureus has an extraordinary capacity to adapt and survive in a great variety of environments. During the past decades, molecular and genetic dissection of S. aureus has revealed a great number of surface adhesins, which mediate adherence to and colonization of target tissues, and secreted enzymes, toxins, superantigens (SAgs) and immune evasion determinants that are responsible for invasion and distant disease ( Table 194.1 ). The availability of now several thousands of S. aureus genome assemblies and annotation reports ( www.ncbi.nlm.nih.gov/genome/genomes/154 ) has helped complete this portrait. S. aureus is part of the Firmicutes phylum and shares approximately 50% of orthologue genes with notoriously nonpathogenic Bacillus subtilis, which indicates that the two organisms have evolved from a common ancestor. Homology searches on the chromosome revealed numerous new surface-attached and secreted factors that represent additional pathogenic factors. S. aureus harbors a large number of mobile genetic elements (MGEs) from exogenous origin, including insertion sequences, transposons, bacteriophages, pathogenicity islands, and genomic islands, which contain specific determinants responsible for disease and antibiotic resistance. The presence of these exogenous elements attests to the high capacity of S. aureus to undergo horizontal gene transfer and exchange genetic elements with other organisms, including staphylococcal and nonstaphylococcal genera. Because gene exchange is a key player of evolution, this peculiar genetic plasticity is a likely explanation for the success of S. aureus, both as a colonizer and a disease-producing microbe. In the case of SAgs (see later discussion), one of the trading partner is suspected to be Streptococcus pyogenes .
| GENE | LOCATION | PRODUCT | ACTIVITY OR FUNCTION | TIMING a | ACTION OF REGULATORY GENES b | |||
|---|---|---|---|---|---|---|---|---|
| agr | saeRS | rot | sarA | |||||
| Surface Proteins | ||||||||
| spa | Chromosome | Protein A | Blocks IgGs, binds von Willebrand factor | Exp | − | See footnote c | + | |
| cna | Chromosome | Collagen BP | Collagen binding | Exp | − | |||
| fnbA | Chromosome | Fibronectin BPA | Fibronectin binding | Exp | − | - | ||
| fnbB | Chromosome | Fibronectin BPB | Fibronectin binding | Exp | − | + | ||
| clfA | Chromosome | Clumping factor A | Fibrinogen binding | Exp | 0 | |||
| clfB | Chromosome | Clumping factor B | Fibrinogen binding | Exp | 0 | + | 0 | |
| sdrC | Chromosome | Serine-aspartate repeat protein | Fibrinogen binding Cytokeratin binding |
Exp | + | |||
| Capsular Polysaccharides | ||||||||
| cap5 | Chromosome | Polysaccharide capsule type 5 | Antiphagocytosis | Pxp | + | + | ||
| cap8 | Chromosome | Polysaccharide capsule type 8 | Antiphagocytosis | Pxp | + | |||
| Cytotoxins | ||||||||
| hla | Chromosome | α-Hemolysin | Hemolysin, cytotoxin | Pxp | + | + | − | See footnote c |
| hlb | Chromosome | β-Hemolysin | Hemolysin, cytotoxin | Pxp | + | + | − | See footnote c |
| hld | Chromosome | δ-Hemolysin | Hemolysin, cytotoxin | Pxp | + | 0 | + | |
| hlg | Chromosome | γ-Hemolysin | Hemolysin, cytotoxin | Pxp | + | − | See footnote c | |
| lukS/F | PVL phage | PVL | Leucocidin | Pxp | + | − | ||
| Superantigens | ||||||||
| sea | Bacteriophage | Enterotoxin A | Food poisoning, TSS | Xp | 0 | |||
| seb | SaPI3 d | Enterotoxin B | Food poisoning, TSS | Pxp | + | See footnote c | ||
| sec | SaPI4 d | Enterotoxin C | Food poisoning, TSS | Pxp | + | |||
| sed | Plasmid | Enterotoxin D | Food poisoning, TSS | Pxp | + | |||
| eta | ETA phage | Exfoliatin A | Scalded skin syndrome | Pxp | + | |||
| etb | Plasmid | Exfoliatin B | Scalded skin syndrome | Pxp | + | |||
| tst | SaPI1,2, bov1 d | Toxic shock toxin 1 | TSS | Pxp | + | See footnote c | ||
| Enzymes | ||||||||
| SplA-F | Chromosome | Serine protease-like | Putative protease | + | − | |||
| ssp | Chromosome | V8 protease | Spreading factor | Pxp | + | 0 | − | |
| aur | Chromosome | Metalloprotease (aureolysin) | Processing enzyme? | Pxp | + | − | ||
| sspB | Chromosome | Cysteine protease | Processing enzyme? | ? | − | |||
| scp | Chromosome | Staphopain (protease II) | Spreading, nutrition | Pxp | + | − | ||
| geh | Chromosome | Glycerol ester hydrolase | Spreading, nutrition | Pxp | + | 0 | − | See footnote c |
| lip | Chromosome | Lipase (butyryl esterase) | Spreading, nutrition | Pxp | + | 0 | See footnote c | |
| fme | Chromosome | FAME | Fatty acid esterification | Pxp | + | See footnote c | ||
| plc | Chromosome | PI-phospholipase C | Pxp | + | ||||
| nuc | Chromosome | Nuclease | Nutrition | Pxp | + | + | ||
| has | Chromosome | Hyaluronidase | Spreading factor | Xp | See footnote c | |||
| coa | Chromosome | Coagulase | Clotting, clot digestion | Exp | + | + | + | |
| sak | Bacteriophage | Staphylokinase | Plasminogen activator | Pxp | + | 0 | ||
a Timing: Xp, throughout exponential phase; Exp, early exponential phase only; Pxp, postexponential phase; 0, no effect of gene on. Expression: +, upregulated; −, downregulated.
b agr, Accessory gene regulator; PVL, Panton-Valentine leukocidin; saeRS, S. aureus exoproteins; rot, repressor of toxins; sarA, Staphylococcus accessory regulator.
Members of the Staphylococcus genus are gram-positive cocci (0.5–1.5 µm in diameter) that occur singly and in pairs, tetrads, short chains, and irregular grapelike clusters. Ogston introduced the name Staphylococcus (Greek staphylé, “a bunch of grapes”) to describe micrococci responsible for inflammation and suppuration. Staphylococci are nonmotile, non–spore forming, and usually catalase positive, and they are often unencapsulated or have a limited capsule ( Fig. 194.1 ). Most species are facultative anaerobes.
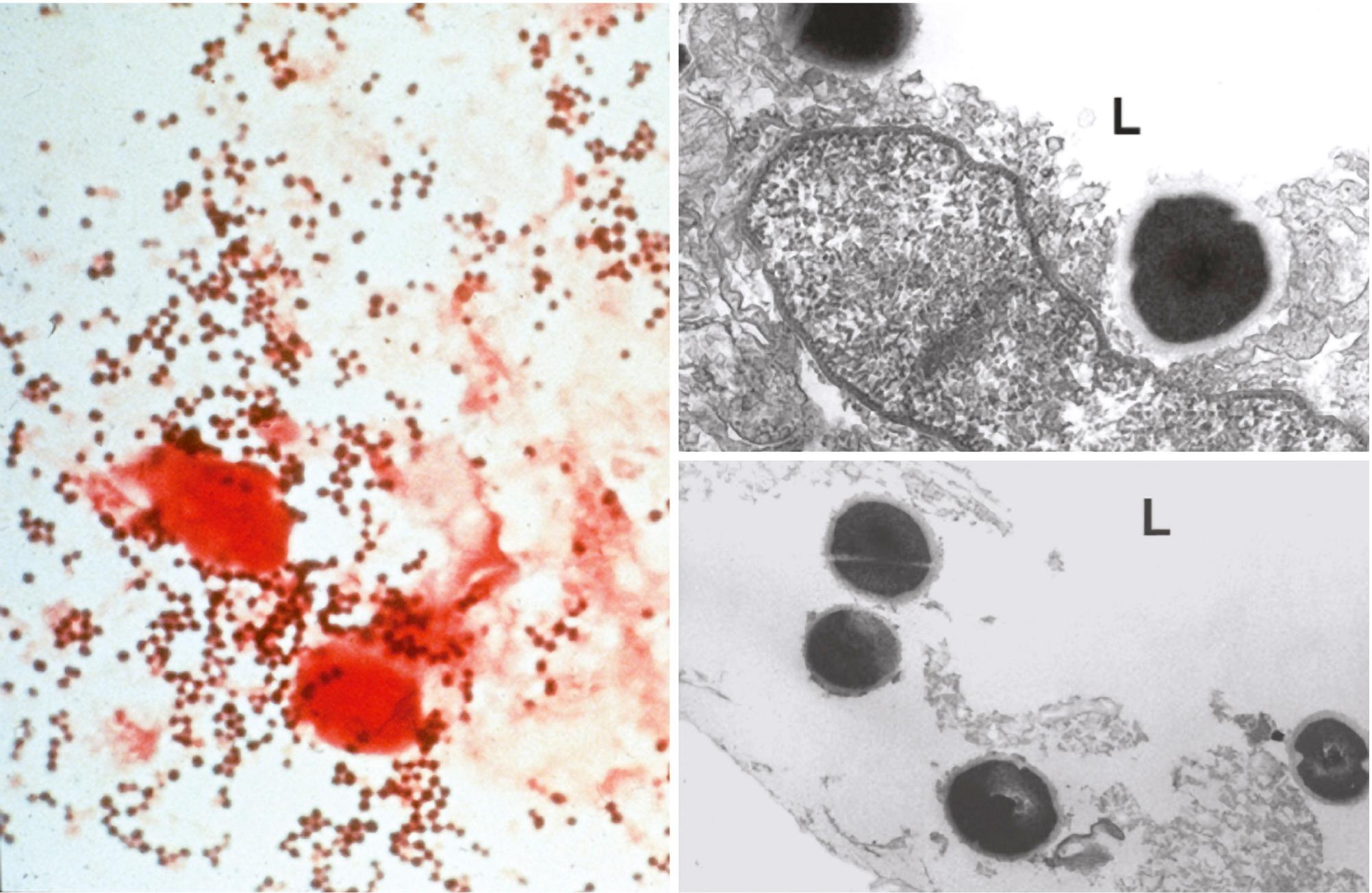
The genus Staphylococcus contains up to 40 taxa, 16 of which are commonly found in humans ( Table 194.2 ). Only a few are pathogenic in the absence of predisposing immunosuppression or implanted foreign material. The most virulent ones include S. aureus, Staphylococcus lugdunensis, and Staphylococcus schleiferi in humans, and S. aureus and Staphylococcus intermedius in animals. Although Staphylococcus epidermidis, Staphylococcus haemolyticus, and Staphylococcus saprophyticus are commonly responsible for device-related and urinary tract infections, they produce substantially less devastating disease syndromes than S. aureus.
| HOST | SPECIES | COAGULASE a | CLUMPING FACTOR a | VIRULENCE a |
|---|---|---|---|---|
| Human and other primates | S. aureus | ++ | ++ | +++ |
| S. epidermidis | − | − | + | |
| S. capitis | − | − | ± | |
| S. caprae | − | − | ± | |
| S. saccharolyticus | ± | − | − | |
| S. warneri | − | − | − | |
| S. pasteuri | − | − | − | |
| S. haemolyticus | − | − | + | |
| S. hominis | − | − | ± | |
| S. lugdunensis | − | ± | + | |
| S. auricularis | − | − | ± | |
| S. saprophyticus | − | − | + | |
| S. cohnii | − | − | − | |
| S. xilosus | − | − | − | |
| S. simulans | − | − | − | |
| S. schleiferi | ± | + | + | |
| Carnivores | S. intermedius | + | − | ++ |
| S. felis | − | − | ++ |
a Semiquantitative estimate of production of coagulase and clumping factor and relation to virulence.
S. aureus harbors some unique features when compared with its less–disease-producing congeners. These include coagulase and clumping factors (or fibrinogen-binding proteins), which have laboratory diagnostic value because they help rapidly discriminate between coagulase-positive (i.e., S. aureus ) and coagulase-negative staphylococci (CoNS; see Table 194.2 ). Moreover, S. aureus carries between more than 20 and more than 30 adhesin and toxin genes, respectively, as compared with 10 or fewer adhesin genes and virtually no toxin genes for the CoNS mentioned previously. Thus, S. aureus is a distinct pathogen within the Staphylococcus genus.
Staphylococci are ubiquitous colonizers of the skin and mucosa of virtually all animals, including mammals and birds. Some species have preferential niches as indicated by their names (see Table 194.2 ). S. epidermidis and Staphylococcus capitis are constant colonizers of the skin and scalp, respectively. Staphylococcus pseudintermedius is a colonizer of cats and dogs and may be misidentified as S. aureus (tube coagulase positive, slide coagulase negative) when transmitted by animal bites.
In animals, S. aureus is a major cause of livestock infection, including mastitis in bovine and ovine herds. In humans, S. aureus has a niche preference for the anterior nares, especially in adults, and is shed onto healthy skin, including axilla and perineum. However, certain clones may have preferences for more hidden niches, as was shown in the case of a peculiar epidemic hospital methicillin-resistant S. aureus (MRSA) clone that colonized the groin and rectum. S. aureus can exist as a resident or a transient member of the normal flora. Nasal carrier rate varies from 10% to 40% in both the community and the hospital environment. S. aureus carriage in various anatomic sites may put certain populations at an increased risk for infection, such as patients with recurring furunculosis and patients who are subject to medical procedures, including hemodialysis, peritoneal dialysis, and surgery (see later section “ Carriage of Staphylococcus aureus ”).
S. aureus carriage has also become a way of persistence and spread of multiresistant staphylococci, especially MRSA. Because MRSA can resist many of the antibiotics in common use, it has risen to the level of a public health threat in the hospital for 3 decades and in the community since the beginning of this century.
Live organisms obtained by means of culture are critical for phenotypic diagnosis and revealing emerging antibiotic resistant phenotypes from as yet unknown mechanisms. In addition, molecular diagnosis helps speed up the results, which take a few hours instead of 1 to 3 days with bacterial subculturing. Molecular methods also help detect the presence of nonculturable microbes, mostly when patients have taken antibiotics before sample collection.
Techniques for culture and identification of staphylococci have been described. Specimens should be inoculated both on blood agar and into rich liquid media such as Mueller-Hinton broth. With S. aureus, abundant growth occurs normally within 18 to 24 hours. However, morphologic variants (see subsequent discussion) may require prolonged growth periods, and plates should be kept 2 to 3 days in order to detect them. Colonies should be Gram stained, subcultured, and tested for genus, species, and antibiotic susceptibility when appropriate. Phenotypic tests for species identification include coagulase tests and agglutination tests, which detect the presence of surface determinants, including clumping factor, protein A, and polysaccharides. Phenotypic antibiotic susceptibility tests vary from agar-diffusion methods (e.g., Kirby-Bauer and Etests) to automated measurement of metabolic activity or growth rates. Macro broth or agar dilution methods are precise but are not routinely performed in the laboratory.
Molecular specification may be necessary in case of unclear phenotype, such as, for instance, in the case of morphologic variants (see next section).
Prolonged incubation is particularly important for the detection of morphology variants such as small colony variants (SCVs). SCVs grow into tiny colonies that are difficult to distinguish and may be mistakenly disregarded as contaminants. They are usually recovered from protracted, difficult-to-treat infections such as chronic osteomyelitis and infected osteosynthetic prostheses, and have also been described in patients with cystic fibrosis.
The most classic types of SCVs are selected during aminoglycoside therapy and result from alterations in the respiratory chain. Such SCVs have a lower transmembrane potential, which impedes the intake of the drug. Interesting to note, switching from normal colonies to SCVs occurs naturally in the absence of antibiotic at a high rate (about 10 −6 ), and switching back from SCVs to normal colonies also occurs. Hence, SCVs are proposed to result from an intrinsic capacity of the bacterium to survive in unfavorable conditions rather than fortuitous mutations.
SCVs are also selected by other antimicrobial agents, including triclosan. They were recovered from the sputa of up to 25% of children with cystic fibrosis and were statistically significantly associated with previous trimethoprim-sulfamethoxazole (TMP-SMX) therapy. Such SCVs carry mutations in the thymidylate synthase gene (thyA) and are dependent on exogenous thymidine to grow. S. aureus synthesizes thymidine by using thyA plus tetrahydrofolate to convert uridine monophosphate into thymidine monophosphate. TMP inhibits the synthesis of tetrahydrofolate, thus making thyA useless. By mutating the thyA gene, S. aureus forces itself to rely on exogenous vital thymidine by importing it. This makes the bacterium resistant to TMP. Thymidine is available in DNA-rich lung secretions of patients with cystic fibrosis and in abscesses. However, the rate of thymidine import is limiting, which results in slow growth and SCV phenotype.
In spite of slow growth, SCVs are equally as or more infective than their fast-growing parents in experimental infections such as osteoarthritis and endocarditis. Moreover, SCVs are particularly prone to invade eukaryotic cells and persist in them, which may explain their occurrence in latent infections. SCVs are cross-resistant to drug-induced killing by most antibiotics, and their eradication necessitates prolonged antibiotic therapy including drug combinations with rifampin.
Molecular diagnosis plays an increasing role in rapid detection of microbial pathogens and identification of drug-resistance determinants. Techniques based on molecular probing have been reviewed. One of these techniques relies on fluorescent detection of 16S rRNA with a peptide nucleic acid probe (peptide nucleic acid fluorescence in situ hybridization [PNA-FISH]). Such a technique has been shown to be highly specific and to help in discriminating S. aureus from CoNS in blood culture within 4 hours. In addition, its usefulness in clinical therapeutic decision making has also been demonstrated.
Multiplex real-time polymerase chain reaction (PCR) assays are being developed to quantify organisms directly in clinical samples. Genes representative of both species and resistance mechanisms are amplified simultaneously. For MRSA, the resistance gene sought is mecA, which encodes low-affinity penicillin-binding protein A (PBP2A).
However, mecA is also present in methicillin-resistant CoNS and thus detects simultaneously both MRSA and commensal methicillin-resistant CoNS, which may result in false diagnosis. One way to bypass this limit is to extend the mecA amplification product to orfX . orfX is an open reading frame that is specific for S. aureus, and its amplification ensures the correct diagnosis. Another way is to choose additional S. aureus or CoNS specific genes. These include typical S. aureus gene versions such as femA, protein A (spa) , coagulase (coa) , and nuclease (nuc) . Other genes or gene combinations were also successfully used to discriminate between S. aureus and CoNS in clinical samples.
Limits may occur with PCR amplification techniques. In some cases, proprietary DNA targets, known only to the manufacturer, can make it difficult to assess the vulnerability of commercial molecular assays to changes in the DNA sequence of isolates being tested. In a large multicenter US study of one molecular platform for identifying MRSA, 3 of 93 MRSA isolates were called methicillin-susceptible S. aureus (MSSA), and 8 of 102 MSSA isolates were called MRSA, indicating that although molecular typing may be useful for rapid screening of carriers, it may carry the risk of misdiagnosis in clinical care. Likewise, PCR amplification using standard primers failed to amplify a new version of the mecA gene, renamed mecC, that emerged in livestock MRSA. In this very case, methicillin resistance was detected with phenotypic tests. Moreover, MSSA isolates have been found that had PCR-detected mecA elements but later reverted to methicillin resistance under therapy. The loss of a transposon that interrupted mecA and replication errors accounted for this conversion.
Currently, more rapid identification by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry is being developed, allowing MRSA identification from a colony within 5 minutes as compared with several hours with PCR.
However, although more rapid than phenotypic tests, these molecular techniques still require prior growth of the organisms, which may take 12 to 24 hours, and do not test all possible antibiotic resistance genes. Whole-genome sequencing may become an option to screen for species and resistance genes, but the bioinformatic workload remains a limiting factor. One emerging technique relies on metagenomic analysis of DNA amplified directly from clinical samples, without prior culturing, through use of next-generation sequencing (NGS). Here, identification of genus, species, and known resistance genes will rely on comparison with large databases, but unknown resistance genes will be missed. Thus, phenotypic testing must always be kept in mind.
There is a dual interest in studying the genealogy of life. One is academic and aims at solving the evolutionary journey of peculiar organisms. The other is epidemiologic and aims at tracing a peculiar pathogen responsible for clinical problems. S. aureus is a common pathogen both in the hospital and in the community. Although the prevalence of MRSA has been slowly decreasing over the last decade in the United States and Europe, this trend is not global and the proportion of MRSA in health care–related infections remains over 50% in other geographic locations.
MRSA is highly clonal, and a few highly successful clones, named according to the place where they were described, can be recovered at multiple locations both nationwide and worldwide (i.e., the Iberian, Brazilian, Hungarian, New York/Japan, Pediatric, and EMRSA-16 pandemic clones). The main molecular typing methods underlying this comprehension are briefly presented later. More complete total genome sequencing may not be required for routine tracing of epidemic strains.
The seminal method is a restriction-fragment length technique based on large chromosomal fragments generated by digestion with the low-frequency cutting enzyme Sma I. The fragments are separated with pulsed-field gel electrophoresis (PFGE) and yield banding patterns specific for particular clones. Banding comparison allowed identification of the major epidemic clones listed earlier, which represented 70% of more than 3000 MRSA isolates recovered worldwide.
One limitation of PFGE is that it does not provide accurate information on the genealogy of the organism. Indeed, the length of chromosomal fragments, and thus the clone-specific banding, may be modified with acquisition or loss of mobile DNA (MGEs) such as transposons, prophages, or pathogenicity islands. The new banding pattern may identify a different clone, which is in fact the same bacterium that has gained or lost MGEs. This is exemplified by the fact that several PFGE major MRSA epidemic clones belonged to the same multilocus sequence typing (MLST; see later) group. Thus, PFGE is useful to follow epidemic clones, but not to build the parental staphylococcal genealogy.
In contrast to PFGE, MLST is a sequence-based method that allows the unambiguous assignment of the ancestral phylogeny of the staphylococcal population. It consists of sequencing seven housekeeping genes (i.e., arcC, aroE, glpF, gmk, pta, tpi, and yqiL ) and comparing them with the sequences of other isolates collected in a central database ( www.mlst.net ). It compares allelic diversity based on approximately 500-bp internal gene fragments. Thousands of sequences have been submitted, generating numerous sequence types (STs). Organisms that share all seven alleles are defined as clones, those that share five of seven identical alleles are defined as clonal complexes (CC), and those that share less than five alleles are defined as unrelated . Within such arborescence, STs can be considered as founders of further evolutionary groups such as CCs.
Because housekeeping genes are independent of acquired MGEs, MLST traces staphylococci back to their latest common ancestor. Of the seven pandemic clones mentioned previously, six could be traced back to three ancestral MSSA types (i.e., CC5, CC8, and CC30; Fig. 194.2 ). Thus, a few ancestral clones of MSSA took the lead and successfully colonized humans and animals before antibiotic resistance developed. Later acquisition of MGEs carrying drug-resistance or virulence genes helped further adaptation to new conditions (e.g., antibiotic use in hospitals), generating a new PFGE makeup on similar ancestral parents (see “ Comparative Genomics and Evolution ”). Moreover, genomics now shows that acquisition of antibiotic resistance genes is reversible and that the contemporary decrease in MRSA prevalence is associated with the loss of the methicillin-resistance determinants.
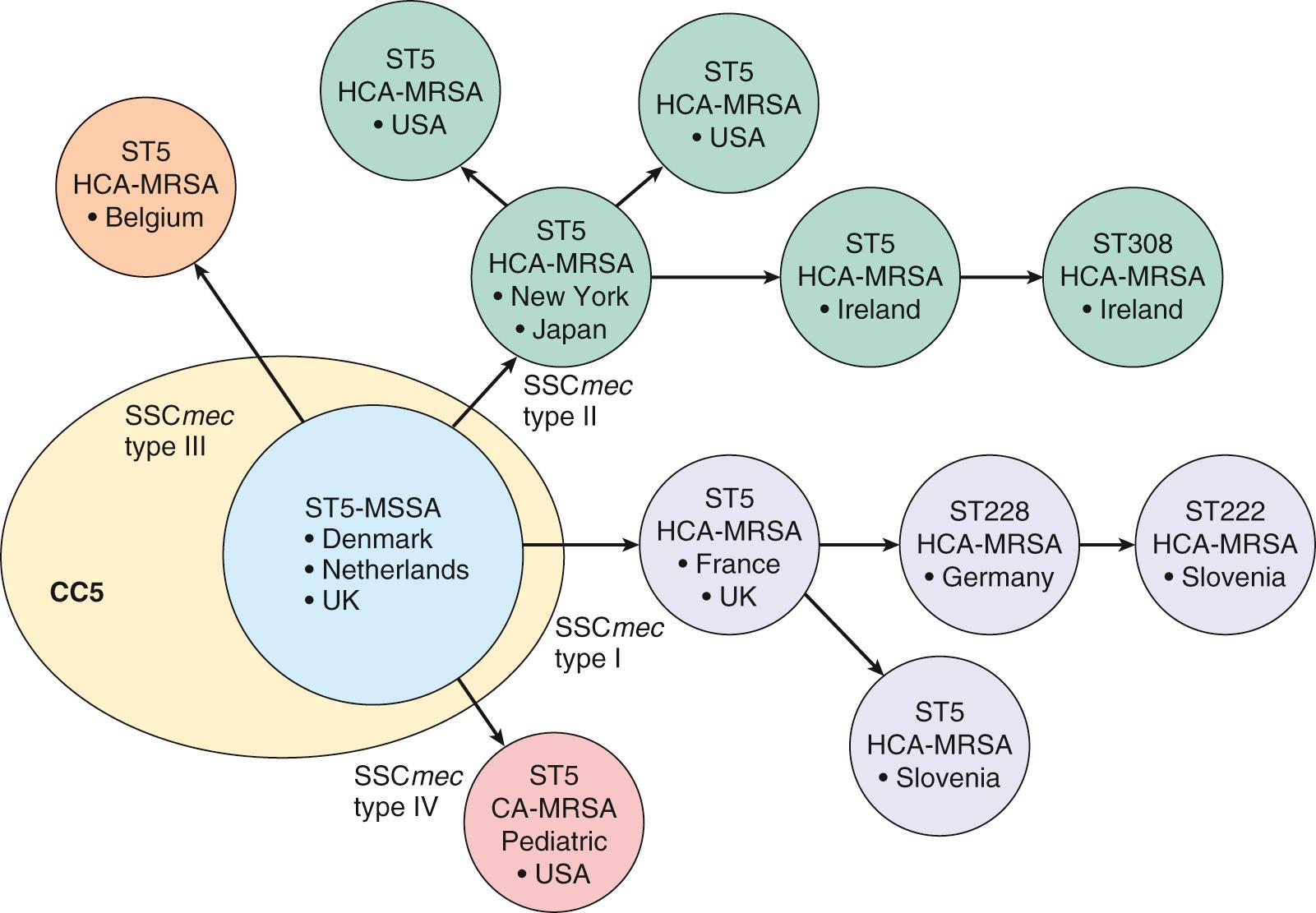
Spa typing and double-locus spa-clfB typing rely on PCR amplification of strain-specific regions within hypervariable segments of the spa (protein A) or clfB (clumping factor B) genes. The variable regions are made of 24 nucleotide repeats in spa and serine-aspartate repeats in clfB, the length of which may vary from duplication or accidental loss of DNA material. Single PFGE or MLST types can evolve into different spa or clfB sublineages. Hence, combining these techniques generates unambiguous data sets that can be compared in multicenter studies.
Typing is critical in order to understand the S. aureus epidemiology. On the other hand, although a handful of founding ST types appear to be prevalent in MRSA and PVL-positive strains, no specific types could be attributed to disease-producing versus mere colonizing strains.
S. aureus is extremely well equipped in surface factors and secreted proteins that mediate host colonization and disease (see Table 194.1 ). In addition to these features, S. aureus is equipped with regulatory systems that sense environmental conditions and respond by fine-tuning the expression of given metabolic and virulence determinants (for review, see Novick and Geisinger, Pragman and Schlievert, and Balasubramanian and colleagues ). Some aspects of this subtle adaptation machinery are described subsequently.
At least three families of regulatory elements intertwine to adjust gene expression to specific environmental conditions: first, two-component regulatory systems, of which agr (for accessory gene regulator) is a paradigm; second, DNA-binding proteins, largely represented by the Sar (for staphylococcal accessory regulator) family of proteins; and third, small regulatory RNAs.
The paradigm of two-component regulatory systems virulence gene regulation is agr, which is schematized in Fig. 194.3 . agr functions as a quorum sensing control that reacts to bacterial density, allowing the preferential expression of surface adhesins during the exponential phase of growth (low cell density) and switching to the expression of exoproteins during the postexponential and stationary growth phases (high cell density). The switch is composed of two divergent operons (see Fig. 194.3 ). On the left hand, promoter P2 drives the transcription of a series of components that comprises (1) a transmembrane protein (AgrB); (2) an autoinducing peptide precursor (AgrD), which is processed and exported by membrane-spanning AgrB; (3) a transmembrane sensor (AgrC), which is the cognate receptor of the AgrD-derived autoinducing peptide; and (4) a transcription regulator (AgrA) that can be activated by AgrC. At low cell density (exponential growth phase), the P2 promoter is off and the operon is transcribed at a low level. As cell growth proceeds, the concentrations of both bacteria and extracellular autoinducing peptide increase in the milieu, thereby augmenting the chance of the autoinducing peptide to make contact with its cognate AgrC receptor. On contact, AgrC activates the response regulator AgrA, a process that may involve AgrA dephosphorylation.
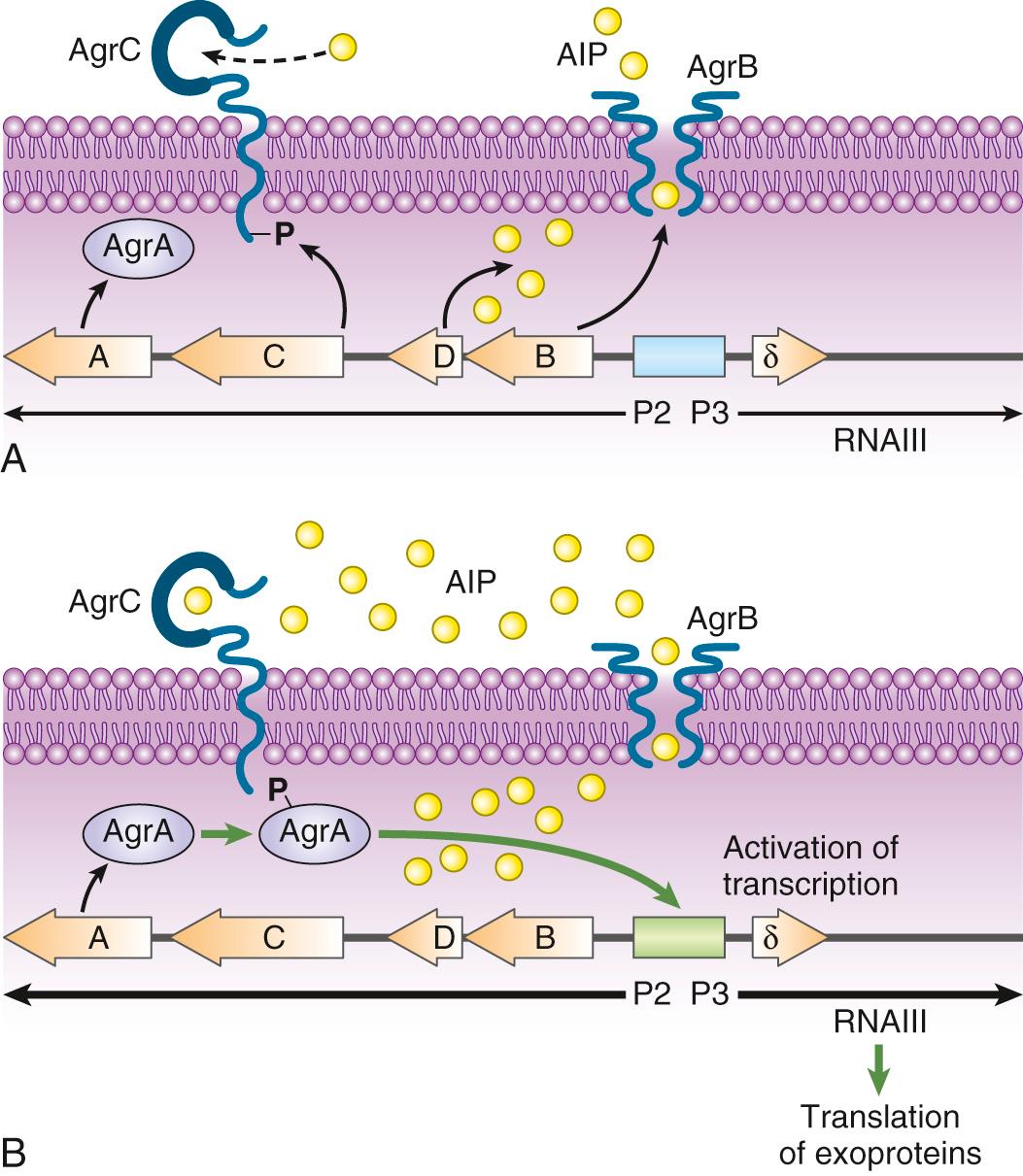
Activated AgrA is a DNA-binding protein that turns on the transcription from both promoter P2, generating a positive feedback on the system, and promoter P3, which drives the transcription of δ-hemolysin and of a peculiar effector called RNAIII. RNAIII has a reciprocal effect and activates the expression of several secreted proteins while downregulating the expression of surface-bound factors (see Table 194.1 ). RNAIII has a complex three-dimensional structure and a long half-life (up to 15 minutes). It regulates gene expression in several ways, including at the translational level by blocking the messenger RNA (mRNA) ribosome-binding site (RBS) of the target genes, or by prolonging the half-life of mRNA of downstream pleiotropic transcriptional regulators such as MgrA.
The S. aureus chromosome encodes for up to 16 two-component regulatory systems involved in both metabolic environmental control and virulence gene regulation. Important two-component regulatory systems regarding virulence genes include saeR/S (for S. aureus exoproteins), srrAB (for staphylococcal respiratory response), and arlS (for autolysis-related locus sensor). saeR/S was identified with transposon mutation in a pleiotropic mutant defective in exoprotein synthesis other than that regulated by agr (e.g., coagulase and nuclease; see Table 194.1 ). saeR/S acts independently of agr and responds to environmental stimuli such as high salt, low pH, glucose, and subinhibitory antibiotic concentrations. srrAB and arlS interfere with growth in microaerobic conditions and autolysis, respectively. srrAB represses the expression of TSST-1 and protein A in microaerobic conditions, an observation that may be relevant for the pathogenesis of tampon-related TSS (see later discussion). Both srrAB and arlS interact reciprocally with agr.
sar is an important locus that encodes the DNA-binding protein SarA, which positively controls agr ( Fig. 194.4 ), and maybe also sae and arlS. In addition, sar directly regulates adhesin genes (see Table 194.1 ). The sarA transcripts peak at the end of the logarithmic phase of growth, thus promoting agr expression. Moreover, sarA itself is transcribed downstream of three alternate promoters, which can themselves be regulated by as yet incompletely solved factors.
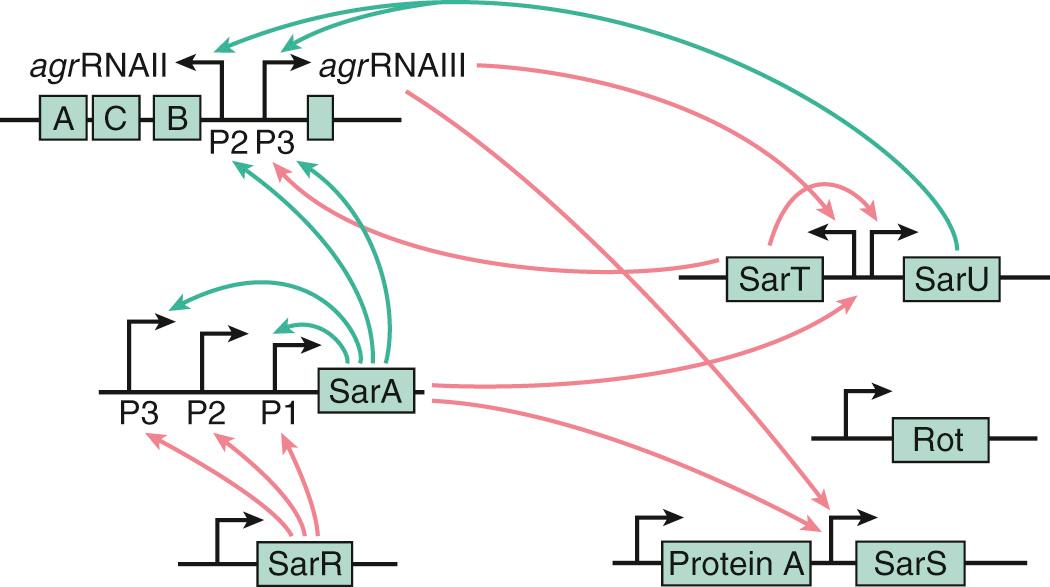
SarA is the prototype of a growing family of DNA-binding proteins that may drive a number of transcriptional activities, including the expression of housekeeping genes and phage-related genes. sarA homologues include sarR, sarS, sarT, sarV, sarU, sarY, rot, and mgrA. rot stands for “repressor of toxins” and counters toxin expression by repressing agr. Inactivation of rot partially restored the agr phenotype in agr -negative mutants, probably by alleviating a repressing effect on the downstream P3 cascade of the agr. This downstream cascade might be the target of several additional regulators that also affect the agr phenotype (see Fig. 194.4 ). mgrA stands for multiple gene regulator. It controls the transcription of up to 355 genes (175 upregulated and 180 downregulated), including capsule, and protein A and α-hemolysin genes in an agr -dependent way.
Sigma factors (σ) are another major mechanism of response to environmental stimuli. In bacteria, σ factors combine with and activate RNA polymerase to transcribe specific sets of genes. S. aureus contains one σ A and two alternative σ B and σ C . Alternative σ B is important for the microbial response to a variety of stresses, including temperature, energy depletion, and chemical stimuli. It acts mostly via the global regulatory network and affects the expression of up to 251 genes (198 positively, 53 negatively), but also has some direct effect by activating the expression of coagulase and fibronectin-binding proteins at the early growth phase, and downregulating certain secreted proteins in the stationary phase. Mutants overexpressing σ B were more virulent in experimental endocarditis, probably by increasing the expression of surface adhesins. Conversely σ B defective mutants were less infective in a model of catheter-related systemic infection.
Small RNAs (sRNAs) are increasingly recognized as major players in regulation of gene expression. They act mainly at the translational level via antisense hybridization with mRNA, where they can alter mRNA stability, hide RBSs from ribosome recognition, or conversely reveal RBSs that are hidden in secondary mRNA structures by unfolding these very structures. Alternatively, sRNA can also bind regulatory DNA-binding proteins, thus sequestering them from their original gene regulatory function. A genome-wide analysis generated a “Staphylococcal Regulatory RNA Database” (SRD; http://srd.genouest.org/ ) and identified at least 550 potential regulatory sRNAs. The best functionally characterized of them are RNAIII, which orchestrates the agr response, and RNAI, which regulates the replication of multiresistance plasmid pSK41.
In symmetry, posttranscriptional expression is also modulated by direct RNA alteration via endoribonuclease III (RNase III). This RNA double-stranded endonuclease plays a critical role in RNA processing and decay. It has been shown to modulate posttranscriptional expression through various mechanisms, including turnover of transcribed and nontranscribed RNAs, and by maturating the 5′ untranslated region (5′UTR) of the mRNAs of the cold-shock protein cpsA and maybe the protein A spa genes, to increase their stability and translation.
The regulatory network must be considered as a metabolic hub that integrates both external and internal information and responds in the most appropriate way. The observed phenotypes result from complex interplays among sometimes contradicting signals of sensors and transcriptional and posttranscriptional regulators, the understanding of which will require a systems biology approach. Moreover, experimentally interrupting one of these circuitries may cause compensation by others, thus introducing biases in the observed phenotype. In this complex system, agr appears to be a central switch toward which many other regulators converge (see Fig. 194.4 ).
The intuitive agr -based model suggests that scattered growing bacteria produce primarily adhesins, promoting tissue colonization, whereas installed organisms that form dense populations switch to the production of hydrolytic enzymes and toxins for the purpose of feeding and escaping host defenses. Accordingly, inactivation of the function of agr alone decreased pathogenicity in experimental models of tissue destruction (e.g., subcutaneous abscesses), where exoprotein production is likely to be important. On the other hand, agr inactivation did not much influence the course of experimental endocarditis, where bacterial surface adhesins are critical for valve colonization. Indeed, although agr -negative mutants are hampered in exoprotein production, they are still fully equipped with surface-bound colonizing determinants (see Table 194.1 ). In contrast, inactivation of sar decreased infectivity in experimental endocarditis because in addition to its effect on agr expression (see Table 194.1 and Fig. 194.4 ), sar also acts directly on expression of surface-bound fibronectin-binding protein A (FnBPA), which promotes experimental endocarditis.
In addition, in vivo gene expression revealed a further level of complexity. For instance, although sar transcripts were detected in infected vegetations during experimental endocarditis, they were expressed from both P1 and P2 promoters, rather than only from the P1 promoter as observed in vitro. Likewise, in vivo expression of several genes appeared dissociated from their control regulator as described in vitro. Although agr positively regulates TSST-1 in vitro (see Table 194.1 ), the toxin was still expressed by an agr -negative mutant in a rabbit model of TSS in vivo. This may result from alternative regulation by other regulators that act either downstream of the agr locus or directly on the tss gene promoter. Eventually, agr -negative mutants can be recovered from clinical samples as in cystic fibrosis and in carrier and bacteremic patients. Such agr -negative clinical isolates, and agr -negative laboratory mutants, have increased surface adhesins and an increased ability to form biofilms, and are found in chronic infections such as osteomyelitis and device infections.
Hence, the pathogenic implication of regulatory circuitries cannot be drawn merely from in vitro observations. In vivo experimentation reveals the plurality of S. aureus infection forms, which may be variously altered by novel antivirulence therapies. For instance, inhibition of the agr loop by action on the autoinducing peptide impedes acute tissue destruction but might promote biofilm formation and chronic infection.
Genetic and functional experiments revealed the existence of at least four agr groups in S. aureus, which were characterized by specific variations in all three AgrB, AgrD, and AgrC proteins (see Fig. 194.3 ). Whereas the autoinducing peptide of a given agr group stimulated signaling in other strains sharing the same agr group, it either cross-inhibited (e.g., group I and group IV) or cross-activated (e.g., group I and group II) members of other groups. This suggests that certain antagonistic agr groups could be mutually exclusive with attempts to simultaneously colonize the same niche. However, studies regarding this hypothesis gave conflicting results. In particular, patients with cystic fibrosis colonized with S. aureus can successfully harbor organisms from two antagonistic agr groups.
Although agr and other global regulators control the timely expression of pathogenic genes, they are not bona fide pathogenic factors themselves. The agr locus has homologues in numerous nonpathogenic staphylococci. A phylogenic study of nonpathogenic CoNS indicated that variations in agr genes followed parallel variations in species-specific rRNA genes. In fact, agr groups diverged very early during the evolution of staphylococci (see “ Comparative Genomics and Evolution ”) and represent a lineage marker of strains that evolve in distinct environments rather than a strategy to exclude potential competitors. Thus, global regulators were originally meant to control the expression of useful metabolic genes. How exogenous virulent genes, which were acquired later, succeeded in taking advantage of such systems remains a fascinating question of evolutionary genetics.
Although S. aureus is an innocuous resident of the skin and mucosal flora in up to 30% of the human population, healthy carriers are notoriously more prone than noncarriers to develop invasive S. aureus infections. This is exemplified by recurrent skin and wound or bloodstream infections (BSIs), which are due to the patient's own carriage strain in up to 80% of the cases.
Colonization of the anterior nares is ideal for microbial dissemination. Outward dissemination is illustrated in Fig. 194.5 . A few drops of fluorescein were instilled intranasally in a volunteer, followed by ultraviolet imaging. Two hours after instillation, fluorescein was all over the hands and clothes, ensuring both sneezing-induced and contact dissemination.

Inward dissemination by host invasion is an opportunity for ample bacterial proliferation. Host invasion is often considered a bacterial dead end, because invading microorganisms may be destroyed by the immune system. However, this only holds true if the immune system can eliminate the invading organisms, which is mostly not the case with S. aureus (see “ Immune Evasion ” later). Alternatively, invading microbes can kill the host, but then incur the risk of disappearing with the decaying corpse. Nevertheless, although this is expected in humans wherein dead bodies are eliminated through burial or cremation, it is different in the wild, where scavengers eat corpses, thus contributing to further dissemination. As a result, S. aureus has little evolutionary pressure to dampen its invasive lifestyle—which comes in addition to commensalism—whereas it has ample reasons to withstand host defenses, including resistance to antibiotics.
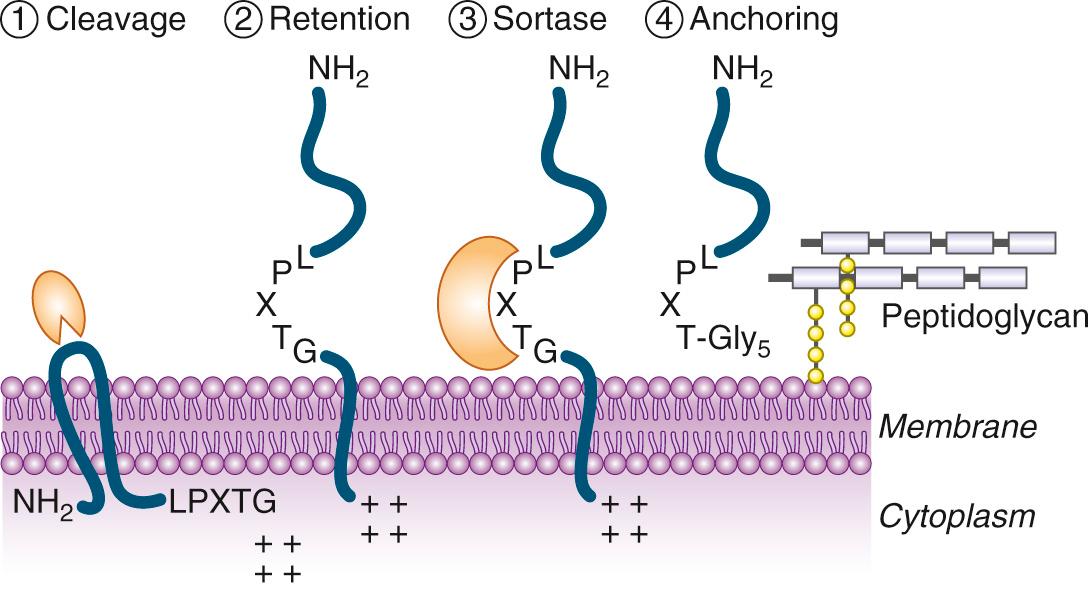
Persistent mucosal and skin colonization is critical. Factors involved in attachment to nasal epithelia involve teichoic acids, which may attach to lectin glycoproteins on the surface of mucosal cells, fibrinogen-binding protein B (clumping factor B or ClfB), serine-aspartate rich proteins C and D (SdrC and SdrD), SasG, and IsdA ( Table 194.3 ). ClfB, SdrC, SdrD, SasG, and IsdA are members of a family of S. aureus surface-bound proteins referred to as MSCRAMMs (for matrix surface components recognizing matrix molecules). MSCRAMMs are covalently attached to the S. aureus peptidoglycan via the membrane-bound transpeptidase sortase A (SortA) ( Fig. 194.6 ). SortA-attached proteins include at least 21 members, some of them having functions different than host-matrix adherence (see Table 194.3 ). However, all to them are recognized by SortA at an LPXTG signature motif, cleaved by SortA between LPXT and G and covalently attached to the last G (glycine) residue of the peptidoglycan pentaglycine side chain (see Fig. 194.6 ).
| GENE | PROTEIN | AA | SORTASE | MOTIF | LIGAND SPECIFICITY | POTENTIAL IMPLICATION IN DISEASE |
|---|---|---|---|---|---|---|
| Spa | Protein A | 508 | SrtA | LPETG | Antibody Fc fragment (IgG, IgM) von Willebrand factor, TNFR1, platelets | Experimental sepsis, experimental osteoarthritis |
| clfA | Clumping factor A | 933 | SrtA | LPDTG | Fibrinogen, platelets | Experimental endocarditis |
| clfB | Clumping factor B | 913 | SrtA | LPETG | Fibrinogen, cytokeratin 10, platelets | Colonization of nasal mucosa |
| cna | Collagen-binding protein | 1183 | SrtA | LPKTG | Collagen | Experimental osteomyelitis, septic arthritis |
| fnA | Fibronectin-binding protein A | 1018 | SrtA | LPETG | Fibronectin, fibrinogen, elastin | Experimental endocarditis |
| Platelets | Cell invasion, experimental mastitis | |||||
| fnB | Fibronectin-binding protein B | 914 | SrtA | LPETG | Fibronectin, fibrinogen, elastin, platelets | Experimental mastitis |
| sdrC | Serine-aspartate repeat protein | 947 | SrtA | LPETG | Fibrinogen, cytokeratin of nasal epithelia | Nasal colonization |
| sdrD | Serine-aspartate repeat protein | 1315 | SrtA | LPETG | Fibrinogen, desmosomal desmoglein | Nasal, deep skin colonization, biofilm |
| sdrE | Serine-aspartate repeat protein | 1166 | SrtA | LPETG | Bridges fibrinogen and complement factor H on the S. aureus surface | Immune evasion |
| pls | Plasmin-sensitive protein | 1637 | SrtA | LPDTG | Cellular lipids, ganglioside M3; nasal epithelial cells | Colonization of nasal mucosa |
| sraP (or sasA) |
Serine-rich adhesin for platelets | 2261 | SrtA | LPDTG | Platelets | Experimental endocarditis |
| IsdA | Iron-regulated surface determinant A | 354 | SrtA | LPKTG | Fibrinogen, fibronectin | Nasal colonization |
| (sasE) | Hemoglobin/transferrin | |||||
| IsdB | Iron-regulated surface determinant B | 645 | SrtA | LPQTG | Hemoglobin/hemin | Experimental bacteremia and renal abscesses |
| (sasJ) | ||||||
| isdC | Iron-regulated surface determinant C | 227 | SrtB | NPQTN | Hemin | Experimental bacteremia and renal abscesses |
| isdH (or haR) |
Iron-regulated surface determinant H | 895 | SrtA | LPKTG | Haptoglobin/hemoglobin complex | Nasal colonization |
| sasI | Putative S. aureus surface protein I | Undetermined | Associated with bovine gangrenous mastitis strains | |||
| sasB | S. aureus surface protein B | 937 | SrtA | LPDTG | Undetermined | — |
| sasC | S. aureus surface protein C | 2186 | SrtA | LPNTG | Intercellular adhesion | Involved in biofilm |
| sasD | S. aureus surface protein D | 241 | SrtA | LPAAG | Undetermined | Involved in biofilm |
| sasF | S. aureus surface protein F | 637 | SrtA | LPKAG | Undetermined | — |
| sasG | S. aureus surface protein G | 1117 | SrtA | LPKTG | Nasal epithelial cells | Associated to invasive disease |
| Sas (or adsA) |
S. aureus surface protein H | 308 | SrtA | LPKTG | Cell wall associated adenosine synthase | Escape phagocyte-induced killing |
| sasK | S. aureus surface protein K | 211 | SrtA | LPKTG | Undetermined | — |
| fmtB | Formyl transferase B | SrtA | LPXTG | Cell wall synthesis, β-lactam resistance | Antibiotic resistance |
ClfB, SdrC, and SdrD are able to bind fibrinogen and keratin in vitro. SdrD was also shown to bind desmoglein 1, a desmosomal transmembrane protein that binds epidermal cells to keratin, which is likely to facilitate S. aureus persistence in deeper layer of keratinized epithelia. The epithelial ligand of SasG is not known, but SasG is involved in biofilm formation, which is an ingredient of local persistence. Likewise, the exact role of IsdA in nasal colonization is unclear. However, because IsdA is a heme-binding protein, it might help acquire essential iron for the colonizing bacteria. Finally, S. aureus can also survive in a dormant state inside nasal epithelial cells. This is one reason, in addition to biofilm, that explains why it is difficult to eradicate chronic carriage, especially with antimicrobials that do not penetrate inside eukaryotes cells—for instance, β-lactams.
Whether the fibrinogen-binding capacity of ClfB, SdrC, and SdrD may facilitate further invasion in case of mucosal or skin breaches is not known. On the other hand, SasG (among few others) elicited an antibody response in patients with invasive S. aureus diseases, a finding that supports its involvement in deep-seated infections.
Most interestingly, there is an as yet unexplained privileged liaison between S. aureus and chronic nasal carriers. In one study, S. aureus carrier and noncarrier volunteers had the nose disinfected and reinoculated with a mixture of four S. aureus strains, including the carriage strain in case of chronic carriers. Over a few days, noncarriers tended to eliminate all inoculated strains, whereas chronic carriers eliminated all foreign strains and reselected their own.
In certain occurrences, mucosal or wound colonization with S. aureus may produce distant diseases such as SSSS or TSS. These issues are discussed in dedicated sections later.
Taken together, although the subtle relation between S. aureus and its host remains incompletely solved, it is critical to detect and eliminate S. aureus carriage in groups at risk of severe infection, such as patients undergoing operation, dialysis patients, and possibly patients with prosthetic heart valves, in whom the 1-year mortality of S. aureus valve infection is up to 50%.
Because S. aureus is nonmotile, invasion takes advantage of mucosal or skin breaches, where the microorganism engages with constituents of deeper tissue and blood compartments. The first encounters are constituents of microthrombi, which occur as a normal healing process of tissue breaches. S. aureus avidly binds to soluble fibrinogen and clotted fibrin via clumping factor A (ClfA) and the fibrinogen-binding domain of fibronectin-binding protein A (FnBPA). ClfA and FnBPA are SortA-LPXTG wall-associated MSCRAMMs (see Fig. 194.4 and Table 194.3 ), that serve at least two purposes. On one hand, they encourage S. aureus attachment at the place of preexisting lesions. On the other hand, they also act as immune camouflage factors against complement-induced phagocytosis by surrounding the bacterium with a shield of soluble fibrinogen and fibronectin (see Table 194.3 ).
Once attached to microthrombi, S. aureus may encourage further thrombus formation via the action of two secreted coagulases—coagulase (Coa) and von Willebrand–binding protein (vWbp). Coa and vWbp bind to prothrombin and induce a conformational change that converts it into active staphylothrombin. Staphylothrombin is unique in that it polymerizes fibrinogen into fibrin and activates platelets even in blood anticoagulated via coumarin therapy, or exposed to heparin or calcium chelators.
Then, in order to avoid local trapping and hamper further spread, S. aureus needs to control local coagulation. To this end, coagulase is only transiently produced in the early exponential phase of growth. Moreover, S. aureus can escape clots by secreting staphylokinase (Sak), a protease that activates host plasminogen into active plasmin, which in turn disintegrates fibrin clots and promotes extension of local infections. Sak also cleaves complement opsonin C3b and preformed antibodies, contributing to the antiphagocytic properties of S. aureus (see “ Immune Evasion ” later). Sak is produced in both the early exponential and the late stationary growth phases. Its gene (sak) is located, together with SCIN (for staphylococcal complement inhibiting protein) and CHIPS (for chemotaxis inhibitory protein of Staphylococcus ), on a so-called immune escape cluster (IEC) as part of a ϕSa3 β-hemolysin–converting prophage, which is present in >90% of human S. aureus isolates, but usually not in animal isolates. Thus, the ϕSa3 prophage and its IEC cargo are believed to participate to the S. aureus specificity for human hosts.
It was originally thought that S. aureus surface MSCRAMMs would mediate direct binding to ligands present in target organs, such as binding to collagen via collagen-binding protein (Cna) in osteoarthritis. However, in the case of hematogenous dissemination, reaching the target organ requires prior S. aureus extravasation from the microcirculation. It was shown that ClfA and FnBPA are critical to colonize and invade damaged or inflamed endothelia, and this presumably occurred by direct attachment to the injured tissues. However, in the bloodstream, ClfA and FnBPA become rapidly saturated with soluble fibrinogen and fibronectin, which interfere with direct binding to injured vessels. Thus there is a missing link, which S. aureus circumvent by hijacking the coagulation system.
While circulating S. aureus organisms become saturated with soluble fibrinogen and fibronectin, ClfA and FnBPA induce a fibrinogen conformational change that triggers its docking to the platelet GPIIb/IIIa receptor and activates platelets. In addition, preexisting anti-ClfA or anti-FnBPA antibodies, if present, activate platelets by docking to the immunoglobulin G (IgG) platelet receptor FcγRIIa. These microaggregates are then conveyed through the blood to inflamed endothelia or to nascent platelet-fibrin clots present on injured tissues, to which activated platelets attach.
The convergence of these S. aureus– platelet microaggregates to inflamed endothelia is further strengthened by the second staphylocoagulase vWbp. vWbp has the dual capacity to activate blood coagulation and to bind endothelial-attached von Willebrand factor (vWF). vWF is secreted as monomer by inflamed endothelia or activated platelets. vWF monomers attach to injured tissues and polymerize into discrete strings floating in the vascular flow, which bind activated platelets and microaggregates in a sheer-dependent manner. S. aureus– secreted vWbp binds on one hand to the floating WWF strings, and on the other hand to S. aureus– attached ClfA, thus also favoring the halt of circulating S. aureus onto inflamed endothelia.
This platelet-staphylothrombin scenario was validated in experimental models of endovascular colonization and endocarditis, in which prophylaxis with antiplatelet (acetylsalicylic acid and ticlopidine) or antithrombin (dabigatran) agents successfully prevented S. aureus endovascular infections. In contrast, coumarin anticoagulation, which relies on a different mechanism, did not prevent experimental endovascular infection.
Of note, platelet activation may be a double-edged sword in that platelet degranulation produces platelet-microbicidal peptides (PMP) that destabilize bacterial membranes and can kill bacteria. However, S. aureus strains that produce successful endovascular infection are known to resist PMP-induced killing via plasma membrane modification.
Eventually, inflamed endothelial cells also express α5β1 integrins, which bind soluble fibronectin and normally act as a landing runway for neutrophils. However, α5β1-bound fibronectin also promotes S. aureus attachment via FnBPA, which triggers active bacterial internalization by endothelial cells. Local tissue destruction ensues, and more specific molecules can enter into action, including specific MSCRAMMs (e.g., Cna), biofilm facilitating factors, hemolysins, and immune evasion molecules.
Along with invasion and tissue colonization, S. aureus has to confront several layers of host defenses against which it applies an extremely sophisticated immune evasion armamentarium, which is briefly described in the following sections ( Table 194.4 ) (for review, see Foster, Kim and colleagues, and Guerra and colleagues ).
| INTERFERENCE WITH | DETERMINANT | LOCATION | FREQUENCY IN STAPHYLOCOCCUS AUREUS ISOLATES | ACTION |
|---|---|---|---|---|
| Neutrophil chemotaxis, migration and phagocytosis | Polysaccharidic capsule (mainly types 5 and 8) | Core genome | 20%–60% | Not recognized as PAMPs Steric blockage of neutrophil access to deeper cells wall structures including wall attached C3 and C3b |
| CHIPS (chemotaxis inhibitory protein of Staphylococcus ) |
ϕSa3 IEC | >60% | Blocks neutrophil C5a receptor Blocks neutrophil formyl-peptide receptor |
|
| Eap (or MAP) (extracellular adherence protein) |
Core genome | >95% | Interferes with neutrophil migration and extravasation by blocking the docking of neutrophil LFA-1 to endothelial ICAM-1 (See anticomplement activity below) |
|
| AdsA (or SasH) (adenosine synthase A) |
Core genome | 70%–80% | Converts adenosine monophosphate to adenosine Interferes with inflammation and phagocytosis | |
| Complement | SdrE (serine-aspartate repeat protein) | Core genome | 40%–60% | Antiopsonic Binds factor H on the S. aureus surface |
| SCIN | ϕSa3 IEC | >60% | Antiopsonic Inhibits lectin and alternative complement pathways Binds to and inhibits the C3 convertases C4b2 and C3bBb, thus blocking the generation of the C3b opsonin |
|
| Sak (staphylokinase) | ϕSa3 IEC | >60% | Antiopsonic Converts plasminogen into plasmin which cleaves fibrin (solubilizing clots), IgGs, and bacterial attached C3b (See anti-AMP activity below) |
|
| Eap (or MAP) (extracellular adherence protein) |
Core genome | >95% | Antiopsonic Inhibits classical and lectin complement pathways Binds to and inhibits C3 convertase C4b2 and further C3b-mediated opsonization |
|
| Efb (extracellular fibrinogen-binding protein) | IEC2 | 60%–70% | Antiopsonic Binds C3 components and fibrinogen Binds to staphylococcal wall-attached C3b and attracts plasma fibrinogen over it, thus shielding it from recognition by neutrophils |
|
| Ecb (Extracellular complement binding protein) | IEC2 | >95% | Antiopsonic Blocks binding of the neutrophil complement receptor CR1 to bacterial-attached C3b, thus preventing phagocytosis |
|
| Oxidative burst | SodA and SodM (super oxide dismutases) |
Core genome | Convert superoxide radicals to H 2 O 2 and O 2 (using manganese as a co-factor) | |
| KatA (catalase) | Core genome | Converts H 2 O 2 into H 2 O and O 2 | ||
| AhpC and AhpF (alkyl hydroperoxide reductases) |
Core genome | Convert H 2 O 2 to H 2 O and O 2 Convert alkyl hydroperoxides to alcohol and water |
||
| bNOS (bacterial nitric oxide synthase) | Core genome | Scavenges HOCl (hypochlorous acid). | ||
| Bacillithiol Coenzyme A Staphyloxanthin |
Core genome | Protection by S -thiolation of oxidants Antioxidant mechanism unclear |
||
| Antimicrobial peptides (AMPs) | Dlt ( d -alanine (lipo)teichoic acid ligase) | Core genome | >95% | Neutralizes negatively charged wall surface by alanine substitution of ribitol teichoic and lipoteichoic acids Decreases surface affinity for positively charged AMPs |
| MprF (muropetide resistance factor) | Core genome | >95% | Idem by adding l -lysine residues to phosphatidylglycerol at the extracellular side of the plasma membrane | |
| Sak (staphylokinase) | ϕSa3 IEC | Proteolytic degradation of fibrin (via plasmin activation), C3 components, IgGs, and AMPs | ||
| Aur (aureolysin) | Core genome | Proteolytic cleavage of cathelicidin AMPs | ||
| Leukocyte lysis | Hla (α-hemolysin) | Core genome | Forms heptamer barrels in the plasma membrane of target cells | |
| Hlg (γ-hemolysin) | Core genome | >95% | Bicomponent leukocidin Lyses both erythrocytes and leukocytes |
|
| Luk E/D (leukocidin E/D) | Genomic island beta | 30%–40% | Synergohymenotropic bicomponent leukodicin | |
| Luk F/M (leukocidin F/M) | ϕSa1 | Synergohymenotropic bicomponent leukocidin | ||
| Panton-Valentine leukocidin | ϕSa2 | 2%–4% | Synergohymenotropic bicomponent leukocidin | |
| Hld (delta-hemolysin) | Core genome | >95% | Idem phenol-soluble modulins (PSMs) below | |
| PSM alpha 1–4 PSM beta-1 and 2 (phenol-soluble modulins) |
Core genome | 100% | Phagocyte lysis by membrane destabilization Mechanism analogous to the delta-hemolysin mechanism of membrane damage |
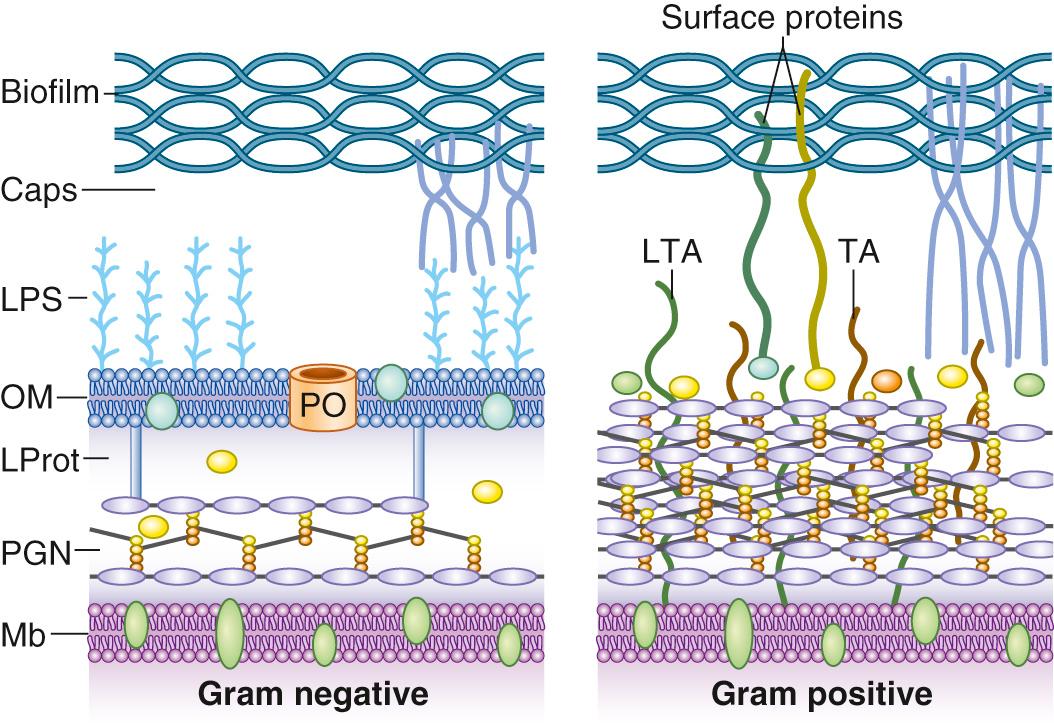
The first line of anti– S. aureus host defense is phagocyte engulfment, primarily by neutrophils, either by direct recognition of pathogen-associated molecular patterns (PAMPs), or via complement-mediated opsonization. Direct PAMP recognition is hampered by the production of polysaccharidic capsules (mostly type 5 or 8 in human S. aureus isolates), which are not recognized by professional phagocytes and physically block their access to underlying PAMPs, such as teichoic acids, lipoteichoic acids, peptidoglycan, and even C3b complement opsonins attached to these PAMP structures.
In addition, S. aureus uses several secreted and SortA-LPXTG anchored surface factors to counters phagocytosis. Secreted factors include the chemotaxis inhibitory protein CHIPS and the extracellular adherence protein Eap (or Map). CHIPS belongs to the ϕSa3 prophage IEC and blocks the neutrophil receptor for formyl-peptides, a universal signature of bacterial protein synthesis, and the C5a receptor for chemotaxis. Eap binds intercellular adhesion molecule 1 (ICAM-1) and fibrinogen and vitronectin, and blocks leukocyte adhesion and neutrophil recruitment mediated by β2-integrin and urokinase receptors in vitro and in vivo.
SortA-LPXTG anchored molecules include protein A (Spa), ClfA, and adenosine synthase A (AdsA, also called SasA) (see Tables 194.3 and 194.4 ). Spa blocks antibody-mediated phagocytosis by binding IgGs by their Fc fragments and exposing the Fab fragments instead, which are not recognized by complement. ClfA interferes with phagocytosis in a fibrinogen-dependent manner (probably by bacterial shielding) and an as yet unclear fibrinogen-independent manner. AdsA converts adenosine monophosphate into adenosine, a dual immuno-modulator compound that has proinflammatory antiinflammatory properties. S. aureus– generated adenosine was shown to impede neutrophil-mediated bacterial clearance and to promote abscess formation in a mouse model of sepsis and kidney abscess.
If not directly triggered by PAMPs, phagocytosis may be promoted by complement-mediated opsonization. The lectin and the alternative complement pathways are mainly involved against S. aureus . The classical pathway, which requires prior antibodies, is largely hampered protein A (Spa) and Sak, as mentioned earlier, and by secreted staphylococcal binder of immunoglobulin (Sbi). Sbi is both secreted and loosely attached to the bacterial envelope. Its envelope-attached form binds immunoglobulin Fc fragments similarly to Spa, and its secreted form binds complement factor H and C3d, which accelerate the decay of preopsonin C3. Soluble Sbi-C3d-factor H complexes also bind the complement receptor CR2 of B lymphocytes, promoting their apoptosis and impeding antibody production. In addition, direct complement-induced bacterial killing via the C8-C9 polymerization membrane attack complex (MAC) is not effective against gram-positive bacteria, because their plasma membrane is physically protected from MAC by the thick peptidoglycan cell wall (for review see Zipfel ).
The lectin and alternative complement pathways are triggered by PAMPs, which activate the lectin or alternative pathway-dependent convertases C4b2a and C3bBb. The convertases cleave C3 into C3a, which amplifies the chemoattractant loop, and C3b, which binds to staphylococcal teichoic acids and attracts phagocytes. S. aureus counteracts complement-mediated opsonization by means of several mechanisms. First, as mentioned earlier, it can produce polysaccharidic capsules, hindering phagocyte access to teichoic acid–attached C3b. Second, it secretes Sak, which cleaves C3 and C3b. Third, it produces staphylococcal complement inhibitory protein SCIN, which binds to and inhibits the C4b2a and C3bBb convertases, thus blocking the production of C3a and C3b. Like Sak, SCIN and CHIPS are located on the ϕSa3 prophage EIC. They are expressed in the exponential phase of growth, whereas Sak is also expressed later in the late stationary growth phase.
A fourth mechanism involves secreted extracellular fibrinogen binding protein (Efb), a dual adhesin capable of binding bacterial-attached C3b proximally, and soluble fibrinogen distally. As a result, Efb contributes an additional external fibrinogen shield, preventing the contact of neutrophils with C3b.
Finally, a most astounding host-hijacking mechanism is conferred by SortA-LPXTG anchored SdrE. In addition to binding fibrinogen in vitro, SdrE binds to and attracts complement factor H on the S. aureus surface. Factor H is a complement regulatory protein that normally binds to host cells and accelerates the decay of C3b in order to protect them from nonspecific assaults from self-host defenses. By attracting factor H on the S. aureus surface, SdrE usurps the complement host control system to its advantage.
Activated neutrophils trigger oxidative burst and bacterial killing via NADPH oxidase and myeloperoxidase (MPO), or via nitric inducible oxide synthase (iNOS). The cascade uses superoxide (O 2 − ) to produce highly oxidative molecules such as H 2 O 2 or hypochlorous acid (HOCl). Oxidation results in protein, lipid, and nucleic acid damage that can kill pathogens either extracellularly or inside phagolysosomes. Extracellular oxidative burst is exemplified by the neutrophil extracellular traps (NETs), which are constituted of neutrophil granules and chromatin proteins and contain up to 80% of neutrophil-released MPO. S. aureus can disable these mechanisms by reducing enzymes such as catalase, which converts H 2 O 2 to water and O 2 , or direct rescue of oxidized molecules by means of several reducing agents listed in Table 194.4 .
Insects and animals produce an array of antimicrobial peptides (AMPs) consisting most often of 20– to 100–amino acid pore-forming β-sheet structures. Human produces various AMPs in skin and mucosal tissues, and large quantities that are stored in granules of neutrophils and platelets (see “ Contribution of Coagulation ” earlier). A hallmark of these AMPs is that they are positively charged and are attracted by the negatively charged wall teichoic acids ( Fig. 194.7 ) and membrane phospholipids of the gram-positive bacterial envelope. S. aureus modulates its susceptibility to AMPs by modulating its surface charge, either by means of the d -alanine lipoteichoic acid ligase (dlt) operon, which decorates teichoic acids with alanine residues, or by means of a lysyltransferase that transfers lysine residues to membrane phospholipids. Both mechanisms result in a more positively charged bacterial envelope and thus in AMP repulsion. In particular, successful endocarditis S. aureus strains were shown to be consistently resistant to platelet-secreted PMPs, a property that may discourage the development of AMPs for therapeutic purposes.
S. aureus kills eukaryotic cells via secreted hemolysins, leukocidins, and phenol-soluble modulins (PSMs). There are four types of hemolysins, referred to as α-hemolysin (Hla), β-hemolysin (Hlb), δ-hemolysin (Hld), and γ-hemolysin (Hlg).
Hla and Hld are secreted in nontoxic soluble forms and multimerize on eukaryotic membranes to form lytic pores. α-Hemolysin (or α-toxin) is involved in a great variety disease. It multimerizes as heptamers on phosphocholine-containing membranes, a process which depends on the presence of host cell transmembrane protein ADAM10, which reunifies both metalloprotease and disintigrin (integrin-binding) properties. Moreover, α-hemolysin interferes with adherens junction proteins to induce cell killing, most notably plekstrin-homology domain-containing protein 7 (PLEKHA7). Indeed, PLEKHA7-deficient cells can readily recover from Hla cytotoxicity. Hence, polymorphism in this determinant could influence individual susceptibility to infection.
Hld acts in a similar way and belongs to the same family as PSMs, which were described more recently. There are four PSMα types (PSMα 1–4 ), consisting of approximately 20 amino acids, and two PSMβ types (PSMβ 1–2 ), consisting of approximately 40 amino acids, the genes for which are located on the staphylococcal chromosome. Hld is located upstream of agr RNAIII regulatory RNA (see “ Regulation ” section). The structure of Hld and PSMs consists of amphipathic α-helices, which confer several properties aside from membrane cell damage, including biofilm turnover and inflammatory responses.
Hlb is distinctive because it is a sphingomyelinase that damages membranes by means of enzymatic alteration of their lipid content.
Hlg is also peculiar in that it is composed of two types of proteins called S and F, for slow and fast elution at chromatography. It promptly lyses white blood cells in addition to other cells and is sometimes referred to as leukocidin. It is encoded by two distinct operons, one that encodes a unique HlgA (S protein) and another that encodes for one S protein (HlgC) and one F protein (HlgB). S and F proteins must assemble to form membrane-perforating complexes. Therefore, this class of hemolysins is also referred to as synergohymenotropic toxins. Active α-Hemolysin exists in two bioactive forms: HlgA-HlgB and HlgA-HlgC.
PVL is a peculiar homologue of Hlg, which was originally reported in 1932 by Panton and Valentine. PVL is encoded by two genes, lukS and lukF, which can assemble either between themselves or with the components of Hlg, thus producing chimera complexes. Like the other hemolysins, PVL is regulated by agr (see Table 194.1 ). Unlike the other hemolysins, PVL is encoded by mobile phages, including ϕSLT, ϕSa2958, ϕSa2MW, ϕPVL, ϕ108PVL, ϕ7247PVL, ϕSa119, ϕTCH60, and ϕSa2USA, which can transfer PVL to other strains. Also unlike the other hemolysins, the prevalence rate of PVL is usually low (≤2%) in MSSA and health care–associated MRSA (HCA-MRSA), whereas it is present in almost 100% of isolates of the community-acquired MRSA (CA-MRSA) USA300 cluster, which is peculiarly prevalent in North America.
PVL-producing S. aureus is associated with skin and soft tissue infection (SSTI) and severe hemorrhagic pneumonia in children and young adults. In contrast, it is rarely responsible for other infections, such as osteomyelitis, septicemia, and endocarditis. The reason for clustering in young patients is unclear. The clustering could be linked to an age-related permissive milieu or permissive immunologic window. Nevertheless, the connection is important; a young adult with recurrent boils and pneumonia should receive particular attention because the mortality rate of hemorrhagic lung disease is high ( Fig. 194.8 ).
Among the first lines of skin and mucosal innate defenses are γ/δ T cells and antigen-presenting Langerhans cells. γ/δ T cells are not major histocompatibility complex (MHC) restricted and respond to epithelial stress and injury. They promote healing via the production of growth factors and attract neutrophils and T cells via the production of IL-17A, which also upregulates the production of AMPs. Stimulation of neutrophils by IL-17A decreased the severity of experimental S. aureus SSTIs and facilitated S. aureus nasal eradication. Thus, γ/δ T cells and the production of IL-17A comprise an important nonspecific first-line defense against invading microbes. Besides, Langerhans cells should phagocytose invading organisms and present surface antigens to boost humoral immunity, thus completing the continuum from innate to acquired host immunity.
However, S. aureus is well equipped to counter recognition by phagocytes and migration of neutrophil and lymphocytes, and impede cytokine-mediated cell recruitment and antibody production, including Eap (or Map), which interferes with lymphocyte migration, Spa, Sbi, and Sak (see “ Escaping Phagocytosis ” earlier). In addition, the most impressive interference of S. aureus with cell immunity is the ubiquitous production of SAgs, which trigger massive and nonspecific activation of the T-lymphocyte compartment, resulting in TSS. One consequence of this T-cell distraction is immune paralysis and anergy. SAgs also aggravate atopic dermatitis and psoriasis by promoting local inflammation, serum suffusion, and access to nutrients. SAgs, of which TSST-1 is a paradigm, are discussed in the “ Superantigens ” section later.
Biofilm is an ultimate way to settle and escape host defenses. It consists in an extracellular polysaccharidic and proteinaceous meshwork that gathers bacterial communities within a mechanically cohesive scaffold. Biofilm-trapped bacteria cannot be physically phagocytized, a phenomenon referred to as frustrated phagocytosis, and are dormant. As result, they are phenotypically tolerant to antibiotic-induced killing.
Biofilm formation is a major therapeutic problem. It was widely described in CoNS but is also formed by S. aureus, especially in the settings of colonization of catheters and biomaterials. Biofilm-producing staphylococci were associated with persistence and virulence in various experimental models, including Caenorhabditis elegans and mice with foreign-body infection.
Biofilm formation evolves in three steps, starting with nonspecific adherence of individual cells to the materials, followed by growth and biofilm formation, and ending with detachment of surface bacteria. In CoNS, it is associated with the production of polysaccharide intercellular adhesion (PIA), which consists of β-1,6-glucosamine chains that are N -substituted with succinate residues. PIA is synthesized by an operon called ica composed of a regulator (icaR) and biosynthetic (icaADBC) genes.
An ica homologue has also been described in S. aureus. Its role in colonizing amorphous surfaces might be identical to that shown in CoNS. However, its role in disease initiation is debated. In S. aureus, biofilm production relates to a large network of genes including surface-attached and secreted proteins in addition to complex regulatory circuitries. For instance, although biofilm deep-seated bacteria must express adherence molecules, surface bacteria must be prone to detach in order to colonize additional organs. Detachment depends on, among other factors, agr expression, which represses expression of adhesins and promotes that of secreted factors including PSMs. In turn, PSMs are involved in remodeling biofilm surfaces and creating channels to feed inner parts of the structure. Thus, ica could be a relatively ancestral colonization mechanism that is still present in S. aureus but is surpassed by more effective means.
Taken together, the myriad immune evasion strategies collected by S. aureus highlight its remarkable adaptation to the animal world and make it a major challenge for host defense-mediated elimination. This explains the as yet unsuccessful attempts to develop an antibacterial vaccine against it, leaving only hope for antitoxin neutralizing vaccines, which will not eradicate the bacterium but might help reduce tissue destruction and disease symptoms.
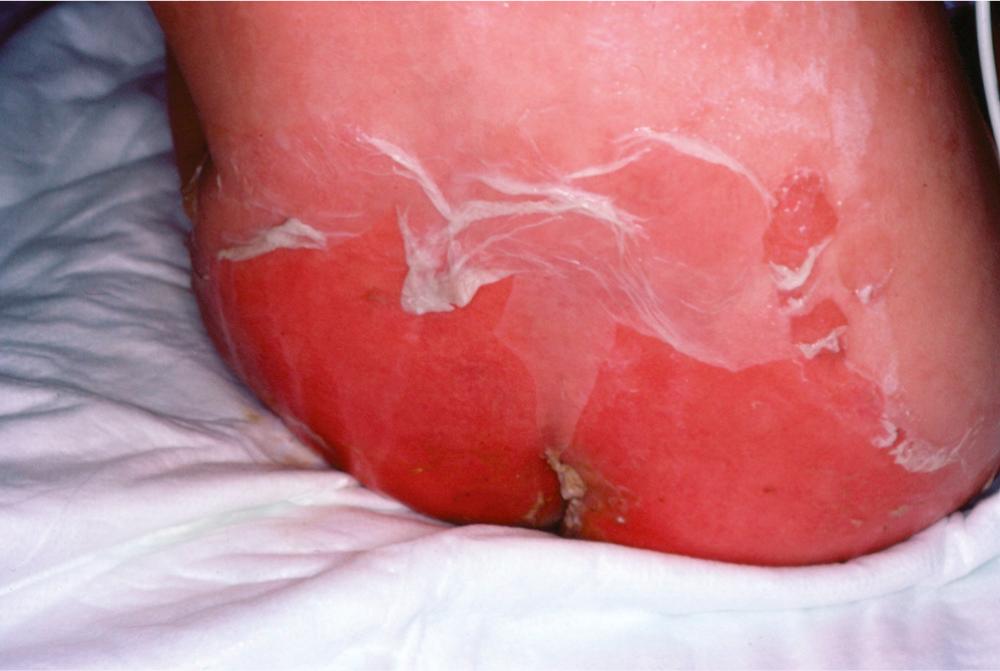
SSSS is a superficial skin disorder that varies from local blistering to impressive generalized scalding ( Fig. 194.9 ). It was originally described by the German physician Baron Gotfried Ritter von Rittershain, who published a series of 297 cases in young children in 1878. Hence, the syndrome is sometimes referred to as Ritter disease. SSSS clusters in neonates and infants younger than 1 year and rarely in adults. It is typically the result of mucosal or skin colonization (e.g., umbilical cord) with a toxigenic S. aureus strain that produces either ETA or ETB, encoded by the eta and etb genes, respectively. The toxin genes are located either on a phage (eta) or on a plasmid (etb). Two additional isoforms of SSSS toxins (exfoliative toxins C [ETC] and D [ETD]) were isolated through pathologic observations in animals and with genome screen, but seem not to be involved in humans.
A US study estimated the annual incidence of SSSS to be 8 cases per million US children, increasing to 45 cases per million in children younger than 2 years. The crude inpatient mortality was low (0.33%) and similar to that in children without SSSS sharing comparable clinical conditions. Similar figures were reported in France. SSSS is often related to S. aureus infections or carriage in close contacts, and may evolve as small epidemics that result from clonally related strains, usually in nurseries. Nasal carriage of the organism may be found among the medical staff, and all caretakers should be screened for this possibility. The proportion of S. aureus carrying eta or etb in overall staphylococcal nasal carriers or clinical isolates is low (≤2% of isolates), which explains the rarity of the disease and its clustering in favorable milieus.
The toxins act by a direct effect on the stratum granulosum of the epidermis. Mucosa are never involved. This consideration is important for differential diagnosis with more severe Lyell syndrome, which usually involves mucosa. Lyell syndrome, or toxic epidermal necrolysis, results from cleavage below the dermoepidermal junction. It is associated with a reaction to more than 100 drugs and sometimes vaccination and has a high fatality rate.
The toxin is released by staphylococci locally, passes through the body, and localizes at the level of the stratum granulosum. The toxin is a glutamate-specific serine protease whose molecular target is desmoglein-1 (Dsg1). Dsg1 is a transmembrane desmosomal glycoprotein that is important to maintenance of interkeratinocyte adhesion. The human skin harbors four Dsg isoforms (Dsg1 to Dsg4) that are localized in various layers of the epidermis, but only Dsg1 is present at the level of the stratum granulosum and is the target of SSSS toxins, which remove its amino-terminal extracellular domain.
An incompletely solved question is why the disease primarily affects children and adults with peculiar skin diseases. One hypothesis is that the toxin targets Dsg1 in the vicinity of the cell membrane ganglioside (GM4), which is present only in the skin of young children or in adults with peculiar skin diseases. This could explain the clustering of SSSS in these particular populations. GM4-like gangliosides are present in the skin of suckling mice and can inhibit the effect of the toxin when coincubated before injection to susceptible animals. The toxin has a serine protease activity, but only after it has reached the skin, which suggests that a locally induced conformational change is needed for activity.
The two forms of SSSS are a generalized form and a localized form. In the generalized form, the toxin spreads throughout the body and localizes at the level of the skin, where it produces generalized scalding (see Fig. 194.9 ). The skin easily detaches by mere rubbing (Nikolsky sign). The blister liquid is clear. Because scalding is the expression of a distantly secreted toxin, the responsible staphylococci are usually not found in the lesions. The disease is self-limited and wanes within 4 to 5 days, which probably parallels the appearance of specific antitoxin immunoglobulins. Indeed, in addition to age-related expression of GM4 or other specific factors in the skin, the presence of antitoxin antibodies in older children and adults also explains the restriction of SSSS to the younger age groups.
The localized form of SSSS is sometimes referred to as bullous impetigo ( Fig. 194.10 ). It results from the local spread of the toxin around a colonized wound in individuals who already bear some immunity against the toxin, as is the case in neonates still benefiting from passive maternal immunity (often around the umbilicus), or in older individuals who are already immunized. The presence of antibodies hinders distant dissemination of the toxin but not local spread around the colonized area. Unlike the generalized form, scalding is localized and the blister liquid often contains bacteria and sometimes white blood cells.
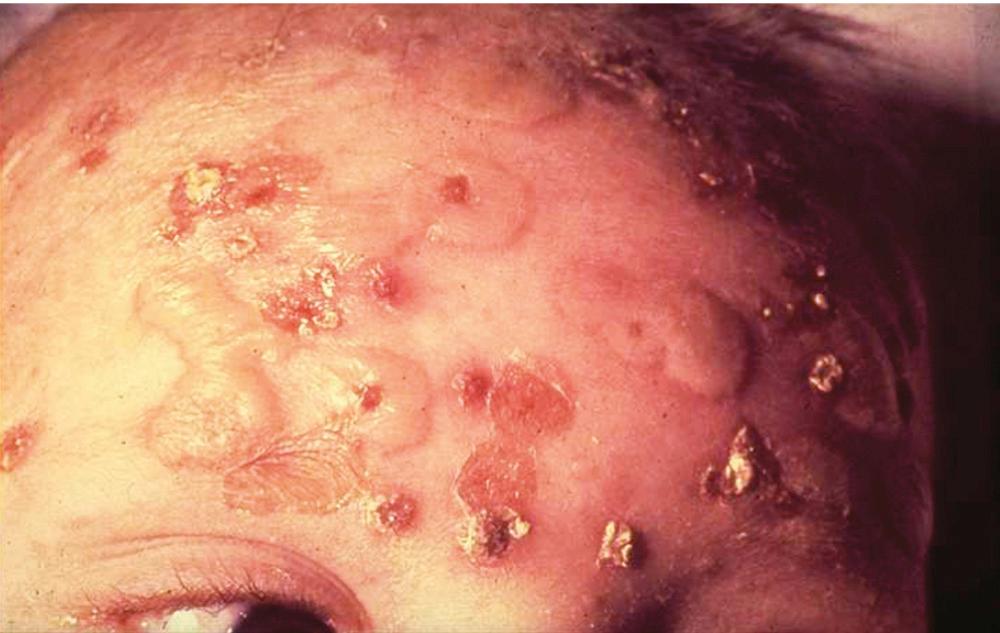
Patients may have general symptoms that include fever and lethargy, especially in the generalized form. Treatment includes general measures such as antiseptic wound dressing and fluid support, specific antibiotic therapy to eradicate the causative agent, and screening and decontamination of caretakers, especially in nurseries. If appropriately handled, the prognosis of SSSS in children is usually good and, as mentioned, the mortality rate far less than 5%. In contrast, the mortality rate can be very high in adults (>50%) and is usually associated with an underlying condition.
As mentioned, the differential diagnosis with Lyell syndrome (toxic epidermal necrolysis) is critical because the etiology, treatment, and prognosis of the diseases are different. In doubtful cases, skin biopsy is useful to provide the definitive answer.
TSST-1 and staphylococcal enterotoxins (SEs) are the paradigm of a large family of pyrogenic exotoxins called superantigens. SAgs are proteins that do not activate the immune system by means of normal contact between antigen-presenting cells and T lymphocytes. Normally, antigens are taken up by antigen-presenting cells, hydrolyzed, and presented as restricted peptides to cognate T lymphocytes. The peptides are expressed within a groove on the MHC class II receptor on the surface of the antigen-presenting cell. Cognate T cells recognize the peptide–MHC class II complex by specific contacts with the five variable domains of the α and β chain of the T-cell receptor (Vβ, Dβ, Jβ, Vα, Jα).
SAgs can bypass this highly specific interaction. They attach to an external portion of the Vβ domain from large quantities of lymphocytes and directly wedge them to the MHC class II receptors of antigen-presenting cells. This nonspecific contact activates up to 20% of the total pool of T cells, instead of approximately 1 per 10,000 during normal antigen presentation. The consequence is a massive burst in cytokine release, which drives an overwhelming inflammatory response that results in endotoxin-like shock, including endothelial leakage, hemodynamic shock, multiorgan failure, and possibly death.
S. aureus can produce a large number of SAgs. Aside from TSST-1, it can produce at least 15 different enterotoxins (SEs A, B, C n , D, E, G, H, I, J, K, L, M, N, O), which by definition are emetic when administered to rodents. However, the nomenclature has become more complicated with the discovery of enterotoxin homologues that did not have emetic properties, and which are now called SEl, for “staphylococcal enterotoxin-like,” followed by specific letterings. Moreover, additional screens have revealed a family of at least 14 proteins based on homologies in the conserved C- and N-terminal domains of SAgs. These proteins do not bind MHC class II molecules but can variously interact with immunoglobulins and complement. They are referred to as SSL for “staphylococcal superantigen-like” and tend to cluster together on staphylococcal pathogenicity islands (discussed in the “ Genomics and Mobile Genetic Elements ” section later).
Although quite some variation exists in the primary structure of many SAgs, they all share a common architecture, as shown with crystallography. They consist of A and B globular domains, which are made of β-sheet barrels and α-helices and rejoined by a discrete linking piece. In TSST-1, the region binding to the Vβ chain of the T-cell receptor has been mapped at the A-B hinge region.
A genealogy of SAgs was built on the base of their sequence homologies. The SAgs studied were segregated into five groups. Group I was represented only by TSST-1. Group III contained only staphylococcal SAgs (SEs H, I, K, L, and P), and group IV contained only streptococcal SAgs (SPs E, C, G, and SME Z). On the other hand, groups II and V contained both staphylococcal and streptococcal SAgs. Group II contained staphylococcal SEs B, C, and G and streptococcal SSA and SPE A, and group V contained staphylococcal SEs I, K, L, and P and streptococcal SPs E and H. This underlines the likelihood of horizontal gene transfer between these two genera, a fact that is becoming increasingly apparent with genome comparisons.
TSS has been sporadically reported as staphylococcal scarlet fever since 1927. Interest in TSS dramatically increased in the early 1980s, when a number of staphylococcal TSS cases occurred in young women who used high-absorbency tampons during menses. The disease was associated with a toxin called TSST-1 that was secreted locally by toxigenic strains. TSST-1 and other SAgs can cross the mucosal membrane by several means. At the level of mechanical barriers, tight junctions are not uniformly present on mucosal surfaces, and staphylococcal Hla may help further disrupt their surface. At the level of SAgs, a conserved dodecapeptide (YNKKKATVQELD) was shown to mediate transcytosis of the toxin to deeper mucosal layers, promoting contact with immune cells and triggering inflammation—a phenomenon referred to as outside-in signaling mechanism. There are two clinical forms of TSS: menstrual TSS and nonmenstrual TSS.
Menstrual TSS starts within 2 days of the beginning or the end of menses and is primarily associated with the use of high-absorbency tampons. Clinical signs include high fever, capillary leak syndrome with hypotension and hypoalbuminemia, generalized nonpitting edema, and a morbilliform rash, followed by desquamation after a few days. The toxin is produced locally, and blood culture results are typically negative. The organisms responsible were represented by a single clone in most reported cases.
The disease proceeds by SAg-induced hyperactivation of the immune system (see previous discussion). TSST-1 production is regulated by agr (see Table 194.1 ). However, its expression requires specific conditions that include (1) an elevated protein level; (2) a relatively neutral pH (6.5–8); (3) an elevated p co 2 ; and (4) an elevated p o 2 . All four conditions are met when menstruation is combined with the use of high-absorbency tampons. The high protein concentration and neutral pH are provided by blood proteins and their buffering capacity. The high P co 2 is ensured by the higher than atmospheric CO 2 content of venous blood. Eventually, the high concentration in O 2 is introduced into the vaginal anaerobic flora by the high-absorbency tampon. Thus, the O 2 brought in by the tampon might be the trigger that modifies an otherwise equilibrated ecosystem and stimulates the production of TSST-1 by colonizing staphylococci.
TSST-1–producing S. aureus may be found in up to 20% of isolates from both carrier and clinical specimens, and higher in MSSA than in MRSA. The fact that TSST-1 expression has special requirements may partially explain the comparatively low prevalence rate of the disease (approximately 1–3 cases per 100,000 patient-years).
Nonmenstrual TSS has attracted less attention than menstrual TSS, yet it can occur in any patient. In addition to TSST-1, nonmenstrual TSS can be the result of enterotoxins SEB and SEC, which are agr regulated (see Table 194.1 ). Responsible organisms may colonize virtually any site of the body, including surgical wounds (surgical TSS), lung (influenza-associated TSS), mucosa or skin (recalcitrant desquamative syndrome in patients with acquired immunodeficiency syndrome [AIDS]), contraceptive diaphragms, and dialysis catheters in patients undergoing chronic peritoneal dialysis. The development of general symptoms with high fever and cutaneous rash should suggest the possibility of nonmenstrual TSS in such patients.
A special feature of wound colonization is that the affected tissues often do not appear inflamed. This is believed to result from the toxin itself, which is able to prevent the influx of professional macrophages.
In addition to the use of high-absorbency tampons or colonization with a toxigenic strain, most patients who are TSS susceptible also lack specific antibodies that block the responsible SAg. In one study, antibody titers considered protective against TSST-1 (≥1 : 100) were detected in 30% of 2-year-old children and in more than 90% of women and men 25 years of age. However, low or negative titers of anti–TSST-1 antibodies (<5) were found in acute-phase sera from 90.5% of patients with menstrual TSS, and less than 50% had positive titers of anti–TSST-1 antibody that developed during convalescence. Hence, some patients remain susceptible to recurrent TSS.
An interesting feature of SAgs is that they primarily trigger a CD 4 + T-cell response, which privileges a helper T-cell type 1 (Th1) cytokine release response without a significant type 2 (Th2) response. A consequence of the dominant Th1 response is a decreased antibody expression, which could explain the relative lack of antibody response in patients with TSS. An additional explanation for the anergy could be SAg-induced apoptosis of responsive T cells, which could account for the prolonged anergy toward the deleterious toxin.
The diagnosis of TSS is based on a constellation of clinical and laboratory signs as proposed by the Centers for Disease Control and Prevention. Table 194.5 also proposes additional laboratory features, such as isolation of a toxin-producing organism to broaden the diagnostic tools. The criteria of streptococcal TSS, from toxigenic S. pyogenes isolates, are presented for comparison. Although both syndromes are the results of similar kinds of SAgs, they differ in two important aspects. First, in contrast to staphylococcal TSS, streptococcal TSS is almost always associated with the presence of streptococci in deep-seated infections, such as erysipelas or necrotizing fasciitis, which has been referred to as flesh-eating disease. Second, mortality rates are very different in staphylococcal and streptococcal TSS. Mortality rates of menstrual and nonmenstrual (in children) staphylococcal TSS were reportedly <1%. In contrast, mortality of streptococcal TSS in children was 28% and up to 45% in adults, especially in cases of necrotizing fasciitis, which necessitates aggressive treatment with generous surgical débridement of infected tissues, and sometimes amputation.
| STAPHYLOCOCCAL TOXIC SHOCK SYNDROME a | STREPTOCOCCAL TOXIC SHOCK SYNDROME |
|---|---|
| Fever Hypotension Diffuse macular rash with subsequent desquamation Three of following organ systems involved: Liver Blood Renal Mucous membranes Gastrointestinal Muscular Central nervous system Negative serologic studies for measles, leptospirosis, and Rocky Mountain spotted fever and negative blood or cerebrospinal fluid cultures for organisms other than Staphylococcus aureus |
Isolation of group A streptococci from: Sterile site for definite case Nonsterile site for probable case Hypotension Two of the following symptoms: Renal dysfunction Liver involvement Erythematous macular rash Coagulopathy Soft tissue necrosis Adult respiratory distress syndrome |
a Proposed revision of diagnostic criteria for staphylococcal toxic shock syndrome (TSS) includes (1) isolation of S. aureus from mucosal or normally sterile site; (2) production of TSS-associated superantigen by isolate; (3) lack of antibody to implicated toxin at time of acute illness; and (4) development of antibody to toxin during convalescence.
Treatment of staphylococcal TSS consists of elimination of the causative agent with antibiotic treatment and appropriate drainage of affected tissues if necessary. Antibiotic regimens should include active drugs such as β-lactams or vancomycin (in case of MRSA) plus protein inhibitors such as clindamycin or linezolid, which block the production of toxins. Supportive care that includes intravenous fluid and vasopressors might be necessary. The immunologic gap that allows the toxin to be active in susceptible patients suggests that passive immunotherapy such as intravenous immune globulin (IVIG) could be effective. However, the success of IVIG therapy has been disputed in several recent analyses. Because the mortality of menstrual staphylococcal TSS is low, immunotherapy should be considered only for life-threatening cases of streptococcal TSS.
Prevention is aimed at avoiding the use of hyperabsorbent tampons and preventing staphylococcal colonization of wounds and mucosa. In the case of nasal carriage, this is achieved with topical application of antibacterial agents such as mupirocin. In the case of extranasal colonization, additional complete body washing with antiseptics such as chlorhexidine is recommended for at least 1 week (see Table 194.8 later). Control cultures should be taken thereafter.
Active immunization with a TSST-1 vaccine could be a potential alternative. A phase I trial with recombinant TSST-1 demonstrated good tolerance and immunogenicity. Further evaluation is awaited.
S. aureus harbors up to 15 enterotoxins, which are defined as SAgs able to produce gastrointestinal symptoms that include vomiting and diarrhea in primate models. Although many of these toxins have potential SAg activity, not all of them have a clear role in human disease. As mentioned, SEB and SEC are associated with nonmenstrual TSS. Likewise, SEA is by far the most frequent culprit of food poisoning, whereas SED, SEB, and SEC are less frequently involved.
Foodborne disease is a major public health problem that may account for 6 to 8 million cases per year in the United States. S. aureus food poisoning follows ingestion of toxins that have been released into contaminated food stocks or beverages. The toxins are heat stable and thus are not denatured by cooking. The disease typically starts 2 to 6 hours after ingestion with general malaise, nausea, vomiting, abdominal pain, and diarrhea. No fever occurs, but the symptoms may be distressing enough to justify hospital consultation in approximately 10% of patients. The symptoms spontaneously resolve within 6 to 12 hours, and the prognosis is excellent, except in the case of severe dehydration in young children and elderly patients.
Although the mode of action of SAgs at the level of T lymphocytes is known, their mechanism at the surface of the intestinal mucosa is less clear. They might share transcytosis mechanisms with TSST-1.
Although SAgs can result in dramatic subversion of the host immune system, they are not ultimate bacterial weapons because they affect only a restricted subgroup of patients who do not mount an appropriate blocking antibody response. Many of these staphylococcal genes are physically contiguous, which suggests that they may have arisen by duplication, maybe for the purpose of diversity. The versatility of SAgs is further supported by the discovery that one of them (i.e., SHE) develops its SAg activity by binding to the Vα rather than the Vβ domains of the T-cell receptor, thus expanding different sets of T-cell lineages than classic SAgs.
The clinical relevance of this multiplicity of toxins is not entirely understood. Toxin genes are dispensable elements that are not needed for growth in rich media and in the absence of competition. Some SAgs (e.g., TSST-1 and SEA, SEB, and SEC) obviously provide a way for the bacterium to escape host immunity. For instance, SAgs have been involved in the etiology of psoriasis and atopic dermatitis, where SAg-induced skin modification could promote bacterial survival. On the other hand, the survival advantage of provoking allergic diseases including rhinitis and asthma is less intuitive, except maybe to promote airborne dispersal (see Fig. 194.5 ). Altogether, the multiplicity of SAgs could enable S. aureus to interfere with the immune response of various animal species, thus broadening its host spectrum.
At the time of writing, several thousands of S. aureus genome assemblies and annotation reports are available in public databases ( www.ncbi.nlm.nih.gov/genome/genomes/154 ). S. aureus genomes are circular and contain approximately 2.8 million base pairs that represent up to 2700 coding sequences, plus structural and regulatory RNAs. They are divided into (1) a core genome, which contains mostly housekeeping genes, is quite conserved along various staphylococcal species, and accounts for about 80% of the whole DNA, and (2) an accessory genome, which carries mobile DNA (MGEs), contains most S. aureus pathogenic and drug-resistance features, and may vary among different species and strains. In addition, certain elements of the core genome can vary according to lineages—for example, by the presence or absence of core genes that are specific of given clades. Therefore the core genome is sometimes subdivided into the core-stable and core-variable genome.
Genome evolution is driven by random point mutations that lead to single nucleotide polymorphism (SNP), larger variations in core genes (e.g., deletions or duplication of repeat regions) that may differ between lineages, and MGEs that include insertion sequences, transposons, viruses, and pathogenicity and genomic islands.
Beyond academic interest, understanding the evolution of S. aureus may help understand the fundamentals of successful clones and eventually help design strategies to block their spread. Based on 7- and 14-gene MLST analyses, an evolutionary scenario was proposed in which a common ancestor of Staphylococcus spp. first segregated into non– S. aureus and S. aureus species ( Fig. 194.11 ). The S. aureus branch acquired the genomic islands νSaα and νSaβ (see later), which are absent from other staphylococci and encode for type I restriction modification systems, and further evolved into two subbranches that gave rise to different STs and CCs. Although still discussed, it seems that the global regulator agr groups I to IV differentiated early in these two branches, which explains why the four agr groups may be found in different downstream CCs (see Fig. 194.11 ). Eventually, MGEs were acquired later and are dispersed in almost any of the STs or CCs. Yet, a few exceptions to this rule exist. Indeed, the TSST-1 gene and the PVL gene locus are classically associated with agr group III, the exfoliatin genes with agr group IV, and the vancomycin intermediate-resistance phenotypes with agr groups I or II. Whether this is due to peculiarly favorable agr -related genetic backgrounds or to favorable contemporary conditions for the extension of specific clones is unclear. Moreover, a functional expression of agr is dispensable in certain circumstances, as for instance in biofilms. Indeed, the association of clinical outcome with agr dysfunction and SCC mec type was observed in a study from South Korea, in which MRSA bacteremia-associated mortality was highest with types II/III, in which agr dysfunction is significantly increased.
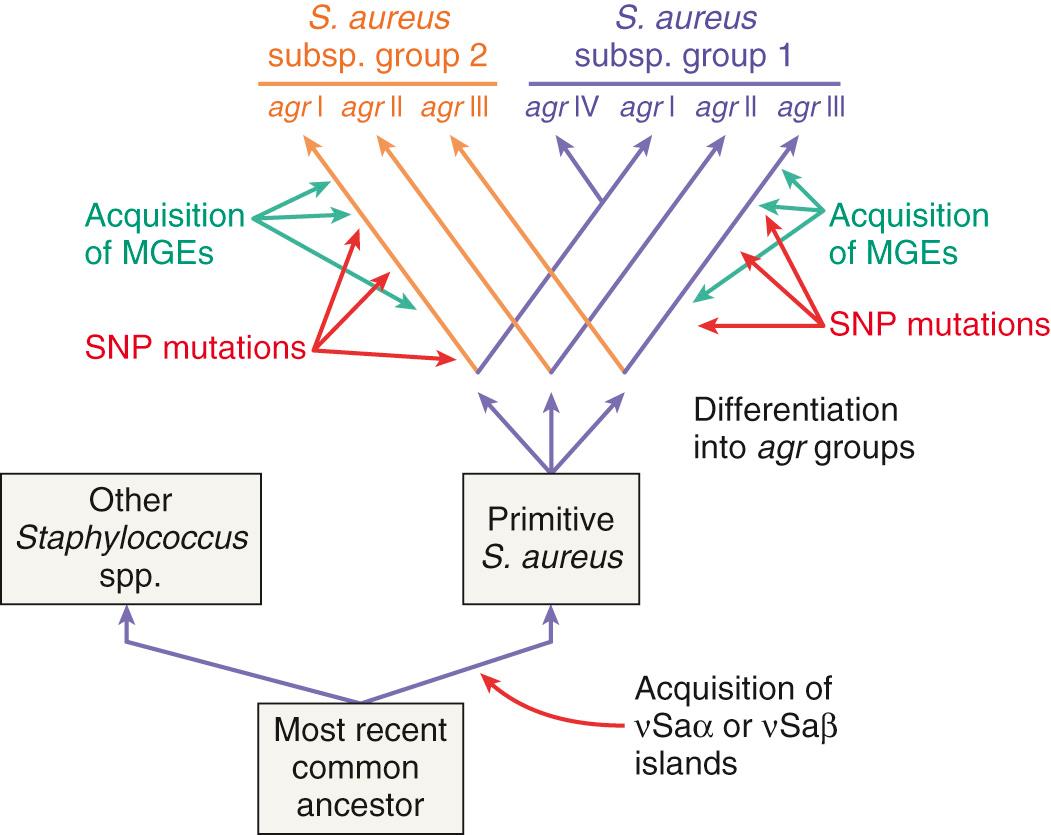
Using whole-genome sequencing, McAdam and colleagues traced back the evolution of notoriously virulent CC30 to over 100 years ago. In the late 1800s, CC30 first segregated into phage type 80/81 and Southwest Pacific clades, which encoded PVL and produced severe community- and hospital-acquired infections due to penicillin-resistant (but methicillin-susceptible) strains in the mid 1900s. In the mid 1950s, a third hospital-related clade emerged (EMRSA-16), which was devoid of PVL but had acquired a new methicillin-resistance MGE named staphylococcal cassette chromosome mec (SCC mec; see later discussion). All three clades gave downstream variants related to further SNPs or MGE acquisitions. Moreover, the CC30 evolution demonstrates acquisition, loss, and reacquisition of PVL and other MGEs, underlying the virulence plasticity of this particular organism. It will be important to understand whether the success of CC30 is due to a peculiar ability to capture useful MGEs or rather to contemporary environmental conditions that favored clonal expansion (e.g., in the hospital), or perhaps both.
S. aureus is also an animal pathogen that raises particular concerns in livestock and cattle. Human and animal S. aureus strains tend to segregate in different lineages. However, cross-species jumps exist and need to be considered. Companion animals and veterinary providers have been shown to share strains. Two outbreaks have been described in Israel involving horses and veterinary personnel.
Another example is the swine-related MRSA CC398, which appeared to colonize swine husbandries since the early 2000s. Price and colleagues proposed that CC398 was first transferred from human to swine. Indeed, CC398 is also present in humans and had been devoid of the SCC mec cassette (and thus was methicillin susceptible). The swine CC398 has lost the ϕSa3 β-hemolysin–disrupting prophage, which is present in the human CC398. As mentioned, in human strains prophage ϕSa3 disrupts the β-hemolysin gene and simultaneously imports virulence factors, including the IEC carrying Sak, CHIPS, and SCIN (discussed under “ Immune Evasion ” earlier). In swine CC398, the loss of prophage ϕSa3 restores the β-hemolysin gene, which may be important for skin or mucosal colonization in animals. The swine CC398 also acquired a new SCC mec cassette that may carry useful determinants for survival in swine husbandries, including antibiotic use. Thus, CC398 was first a human MSSA. It was transferred to swine with the parallel loss of ϕSa3 and acquisition of a new SCC mec. The loss of prophage ϕSa3 seems critical in this evolution. This raises the question as to whether reacquisition of prophage ϕSa3 by swine CC398 could promote its reestablishment in humans, carrying along a new SCC mec cassette. Indeed, cases of invasive human infection with swine-CC398 MRSA are increasingly reported. Likewise, a linear increase of 66% of human CC398 MRSA cases occurred in Denmark from 2004 to 2011; one-third of these patients reported no livestock exposure. Most important, an epidemiologic evaluation in the Republic of Ireland has revealed an elaborate pattern of cases mixing human and animal disease, wherein a few human CC398 MRSAs did not carry the ϕSa3 prophage IEC cluster, thus resembling typical animal strains, whereas some animal CC398 MRSAs did carry the ϕSa3 prophage IEC cluster, thus resembling human strains. In this line, a large study in Iowa found higher rates of S. aureus carriage in swine workers, with more than a third carrying livestock-associated strains that did cause infection in humans. This strongly supports the likelihood constant of interspecies passage.
Similar scenarios were described with the ST5 S. aureus strain that invades poultry and with the notoriously virulent human CC8 S. aureus strain that infects cows. As in swine CC398, the bovine version of CC8 first lost the ϕSa3 prophage and then acquired a new composite SCC element, which is as yet devoid of the methicillin resistance mecA gene but carries a new LPXTG surface protein that might be responsible for colonization of the bovine mammary gland. Thus, gene trafficking and genome evolution should be apprehended globally.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here