Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Spinal, epidural, and caudal blocks are collectively referred to as central neuraxial blocks . Significant procedural, physiological, and pharmacological differences exist between the techniques, but all blocks result in some combination of sympathetic, sensory, and motor blockade. Spinal anesthesia requires a small amount of drug to produce rapid, profound, reproducible, but finite neuraxial blockade. In contrast, epidural anesthesia typically progresses more slowly, is commonly prolonged using a catheter, and requires a comparatively larger volume of local anesthetic, which may be associated with systemic side effects and complications unknown to spinal anesthesia. Combined spinal and epidural techniques blur some of these differences but add flexibility to clinical care.
Central neuraxial blocks are widely used in surgery, obstetrics, acute postoperative pain management, and the field of chronic pain. A single-injection spinal or epidural is commonly used to provide anesthesia for surgery to the lower abdomen, pelvic organs (e.g., prostate), and lower limbs, as well as for cesarean deliveries. Continuous catheter-based epidural infusions are most often used for obstetric labor analgesia and to provide postoperative pain relief after major thoracoabdominal or lower limb surgery. When epidural analgesia is used as a component of enhanced recovery after surgery protocols, this must be balanced against possible motor block and a reduction in mobility. Caudal blocks are mostly performed for surgical anesthesia and analgesia in children (also see Chapter 34 ) and for therapeutic analgesia in adults with chronic pain (also see Chapter 44 ). Indwelling long-term spinal catheters may be inserted for chronic malignant and nonmalignant pain.
The impact of neuraxial anesthesia and analgesia on perioperative morbidity and mortality is a challenging and ongoing matter of investigation. In brief, when compared with systemic opioid analgesia, epidural analgesia is superior and improves respiratory outcomes in adults undergoing noncardiac surgery. However, recent evidence suggests cardiovascular complications such as myocardial infarction may be increased when epidural analgesia is combined with general anesthesia. Overall, all outcome benefits appear to be greatest when neuraxial anesthesia is used alone rather than combined with general anesthesia.
The spinal cord is continuous with the medulla oblongata proximally and terminates distally in the conus medullaris as the filum terminale (fibrous extension) and cauda equina (neural extension) ( Fig. 17.1 ). This distal termination varies from L3 in infants to the lower border of L1 in adults.
![Fig. 17.1, Terminal spinal cord and cauda equina. (From Bridenbaugh PO, Greene NM, Brull SJ. Spinal [subarachnoid] blockade. In Cousins MJ, Bridenbaugh PO, eds. Neural Blockade in Clinical Anesthesia and Management of Pain. Philadelphia: Lippincott-Raven; 1998:203–242.) Fig. 17.1, Terminal spinal cord and cauda equina. (From Bridenbaugh PO, Greene NM, Brull SJ. Spinal [subarachnoid] blockade. In Cousins MJ, Bridenbaugh PO, eds. Neural Blockade in Clinical Anesthesia and Management of Pain. Philadelphia: Lippincott-Raven; 1998:203–242.)](https://storage.googleapis.com/dl.dentistrykey.com/clinical/SpinalEpiduralandCaudalAnesthesia/0_3s20B9780323796774000176.jpg)
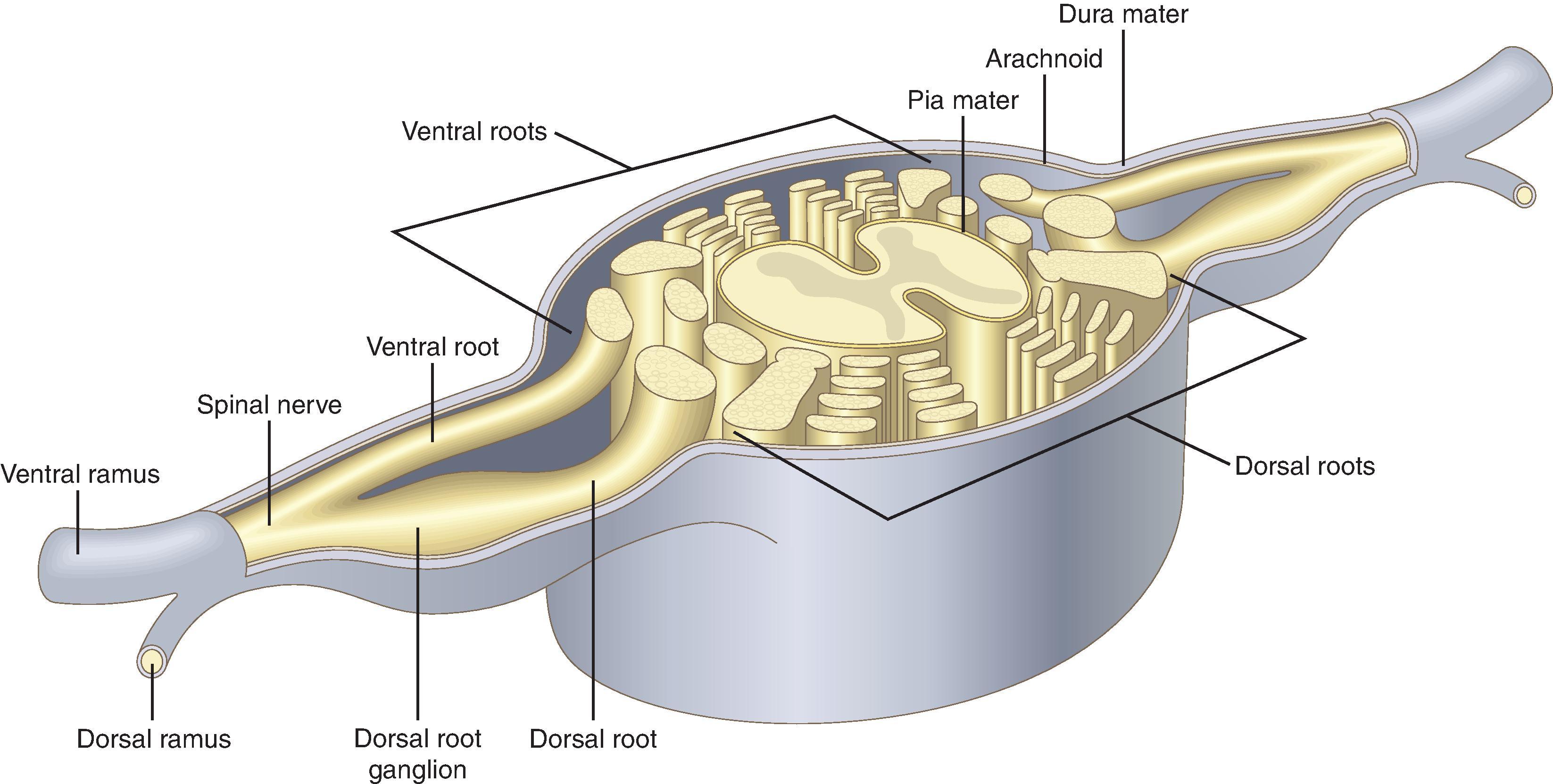
The spinal cord lies within the bony vertebral canal and is surrounded by three membranes: from innermost to outermost, the pia mater, the arachnoid mater, and the dura mater ( Fig. 17.2 ). The pia mater is a highly vascular membrane that closely invests the spinal cord and brain. The arachnoid mater is a delicate, nonvascular membrane and is the principal barrier to drugs crossing into (and out of) the cerebrospinal fluid (CSF). The dura mater is a tough fibroelastic membrane. CSF resides in the subarachnoid (or intrathecal ) space between the pia mater and the arachnoid mater.
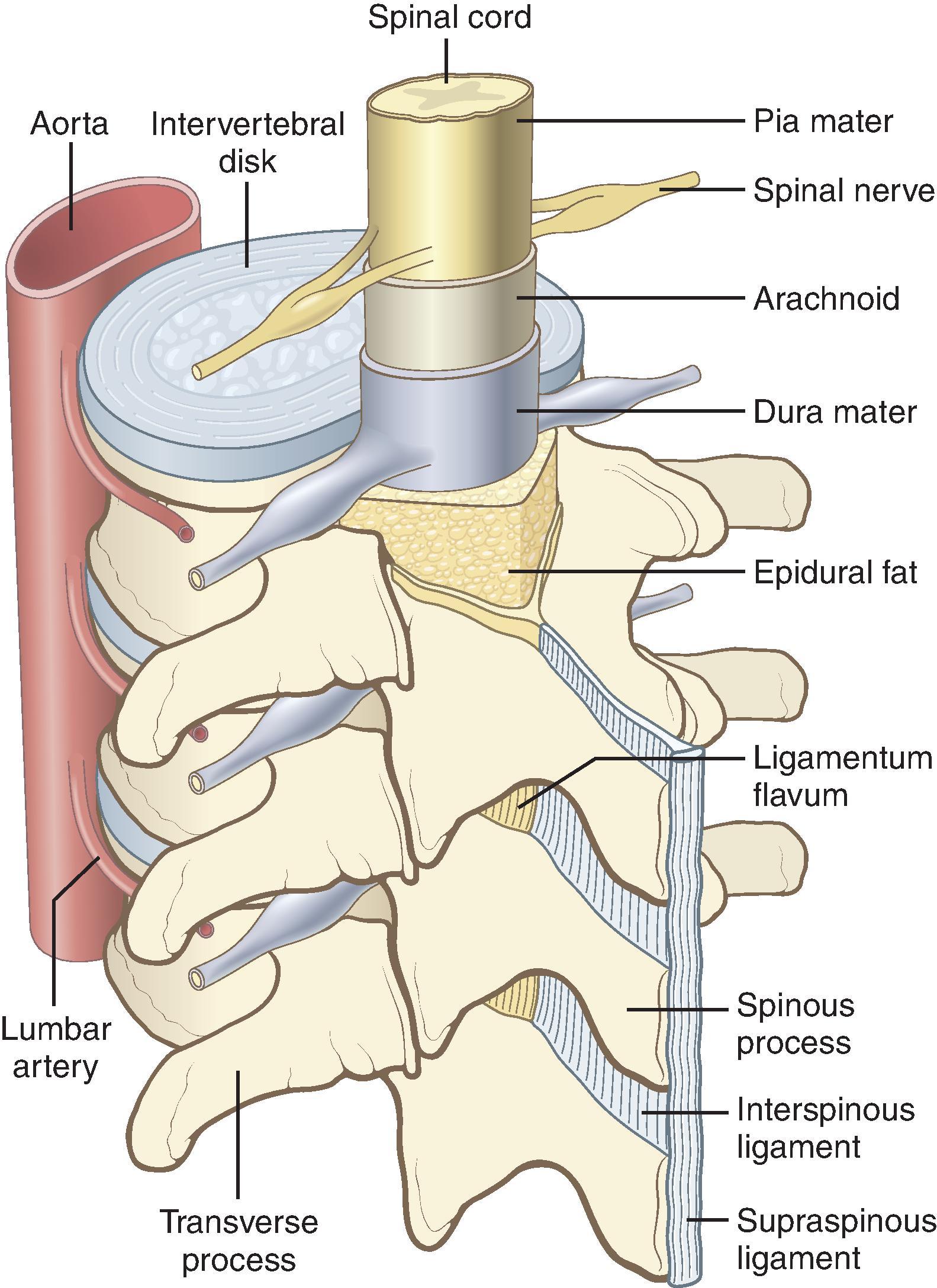
Surrounding the dura from the foramen magnum to the sacral hiatus is the epidural space. The epidural space is bounded anteriorly by the posterior longitudinal ligament, laterally by the pedicles and intervertebral foramina, and posteriorly by the ligamentum flavum. Contents include nerve roots, fat, areolar tissue, lymphatics, and blood vessels.
The ligamentum flavum (Latin for “yellow ligament”) also extends from the foramen magnum to the sacral hiatus and is composed of right and left ligamenta flava, which join to form an acute midline angle (see Fig. 17.2 ). At some vertebral levels (particularly in the cervical and high thoracic spine), midline gaps in the continuity of the ligamentum flavum may exist. Ligament thickness, distance from the ligament to the dura, and skin-to-dura distance also show significant interindividual variability. The vertebral canal is triangular and largest in cross-sectional area at the lumbar levels, whereas it is circular and smallest in area at the thoracic levels. Immediately posterior to the ligamentum flavum are either the lamina of vertebral bodies or the interspinous ligaments (which connect the spinous processes). Finally there is the supraspinous ligament, which extends from the seventh cervical vertebra to the sacrum and attaches to the vertebral spinous processes (see Fig. 17.2 ).
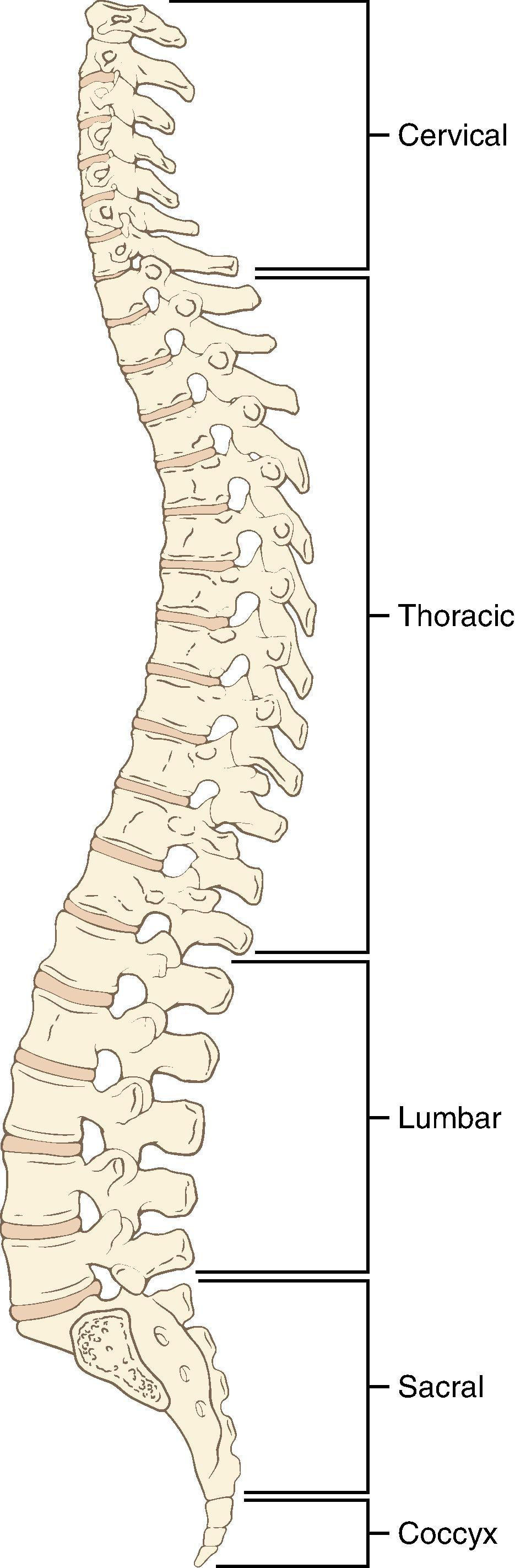
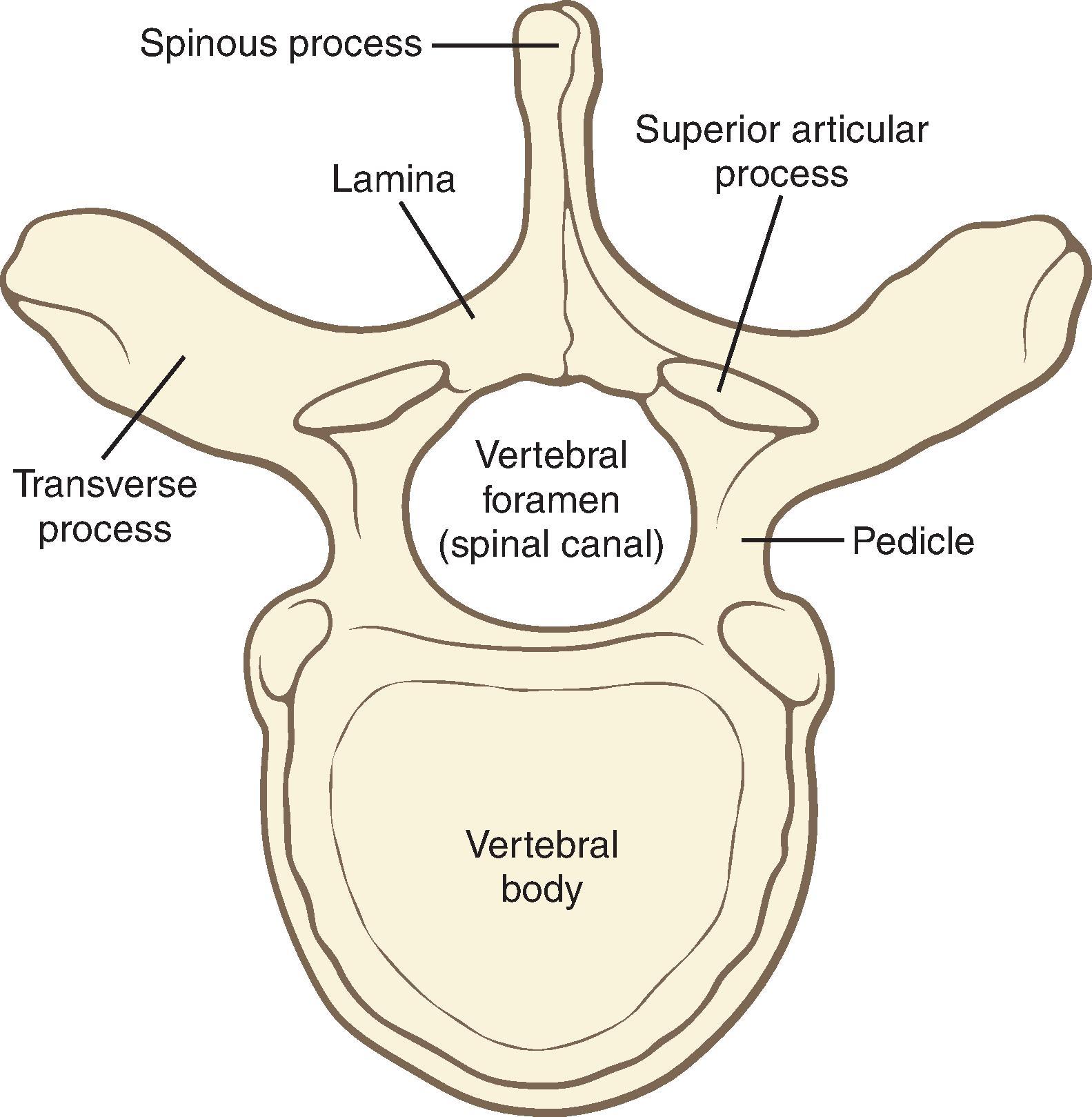
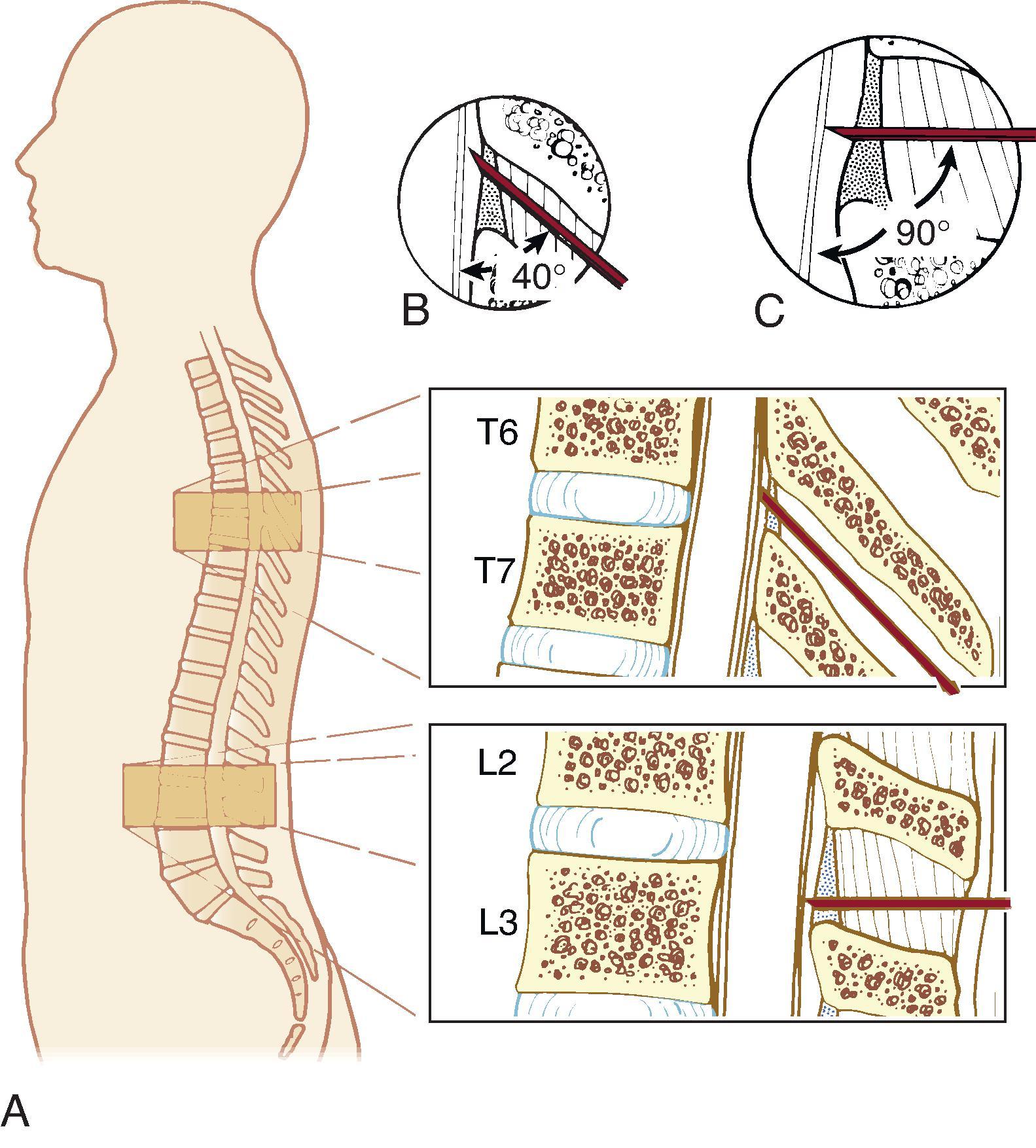
There are 7 cervical, 12 thoracic, and 5 lumbar vertebrae and a sacrum ( Fig. 17.3 ). The vertebral arch, spinous process, pedicles, and laminae form the posterior elements of the vertebra, and the vertebral body forms the anterior element ( Fig. 17.4 ). The vertebrae are joined anteriorly by fibrocartilaginous joints with central discs containing the nucleus pulposus and posteriorly by the zygapophyseal (facet) joints. Thoracic spinous processes are angulated more steeply caudad as opposed to the almost horizontal angulation of the lumbar spinous processes. These differences are clinically important for needle insertion and advancement ( Fig. 17.5 ).
The sacral canal contains the terminal portion of the dural sac, which typically ends at S2 in adults and lower in children. The sacral canal also contains a venous plexus.
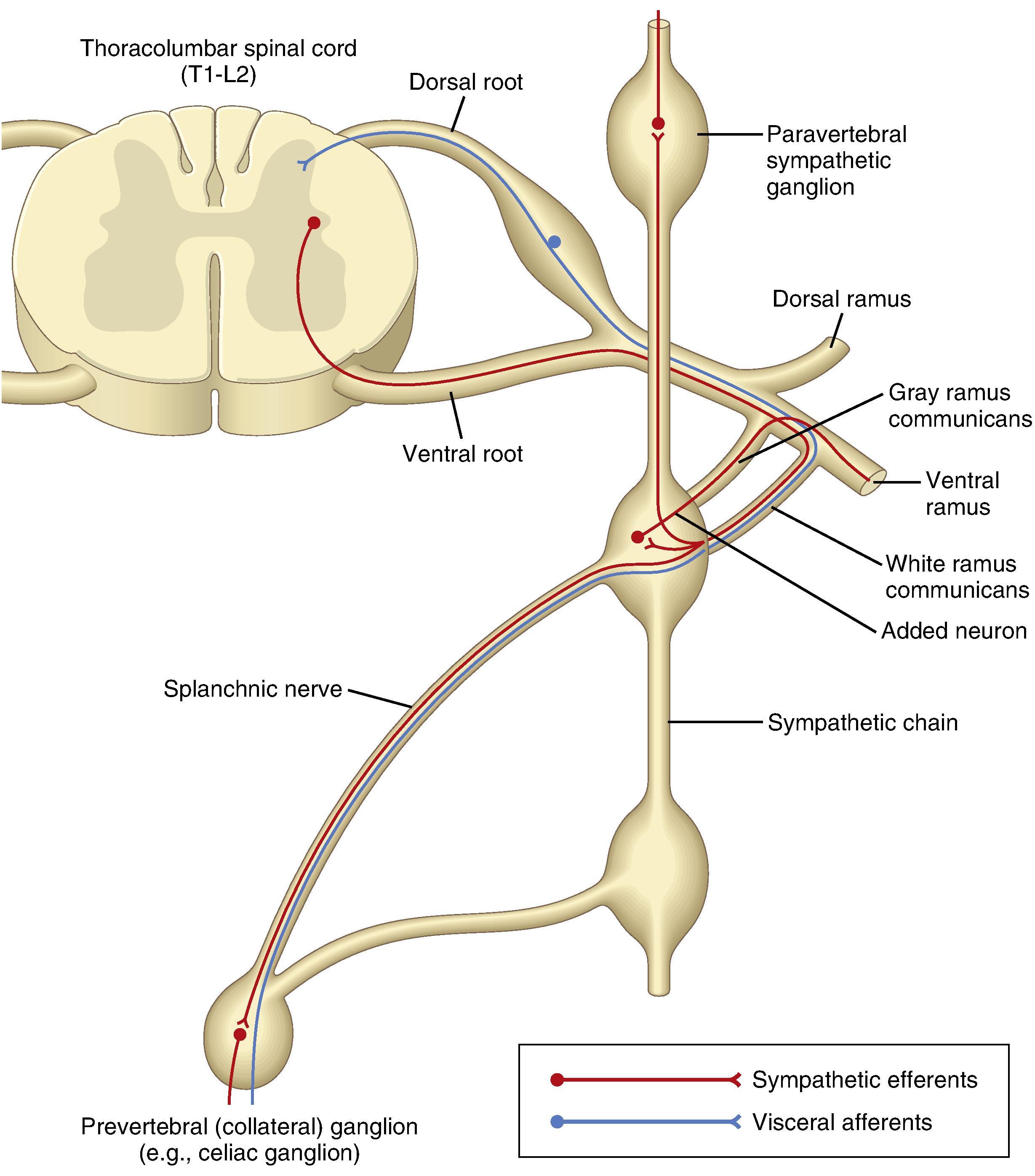
Dorsal (afferent) and ventral (efferent) nerve roots merge distal to the dorsal root ganglion to form spinal nerves ( Fig. 17.6 ). There are 31 pairs of spinal nerves (8 cervical, 12 thoracic, 5 lumbar, 5 sacral, and 1 coccygeal). The nerves pass through the intervertebral foramen, ensheathed by the dura, arachnoid, and pia, which, respectively, become the epineurium, the perineurium, and the endoneurium. Preganglionic sympathetic fibers originate in the intermediolateral gray columns between T1 and L2 and pass via the ventral nerve root to the paravertebral sympathetic ganglia and more distant plexuses ( Fig. 17.7 ).
Two posterior spinal arteries supply the posterior one-third of the spinal cord, whereas the anterior two-thirds of the spinal cord are supplied by a single anterior spinal artery ( Fig. 17.8 ). One of the largest anastomotic feeder arteries to the anterior system is the artery of Adamkiewicz, which arises from the aorta and enters an intervertebral foramen between T7 and L4 on the left. Infarction in the territory of the anterior spinal artery leads to anterior spinal artery syndrome, classically manifesting as motor weakness and loss of pain and temperature sensation below the affected spinal level, but with retention of proprioception and vibratory sense because of the preserved blood supply to the dorsal columns. Ischemia and infarction may result from profound systemic hypotension, thrombosis or emboli, vasculopathy, trauma, or as a consequence of surgery to the aorta.
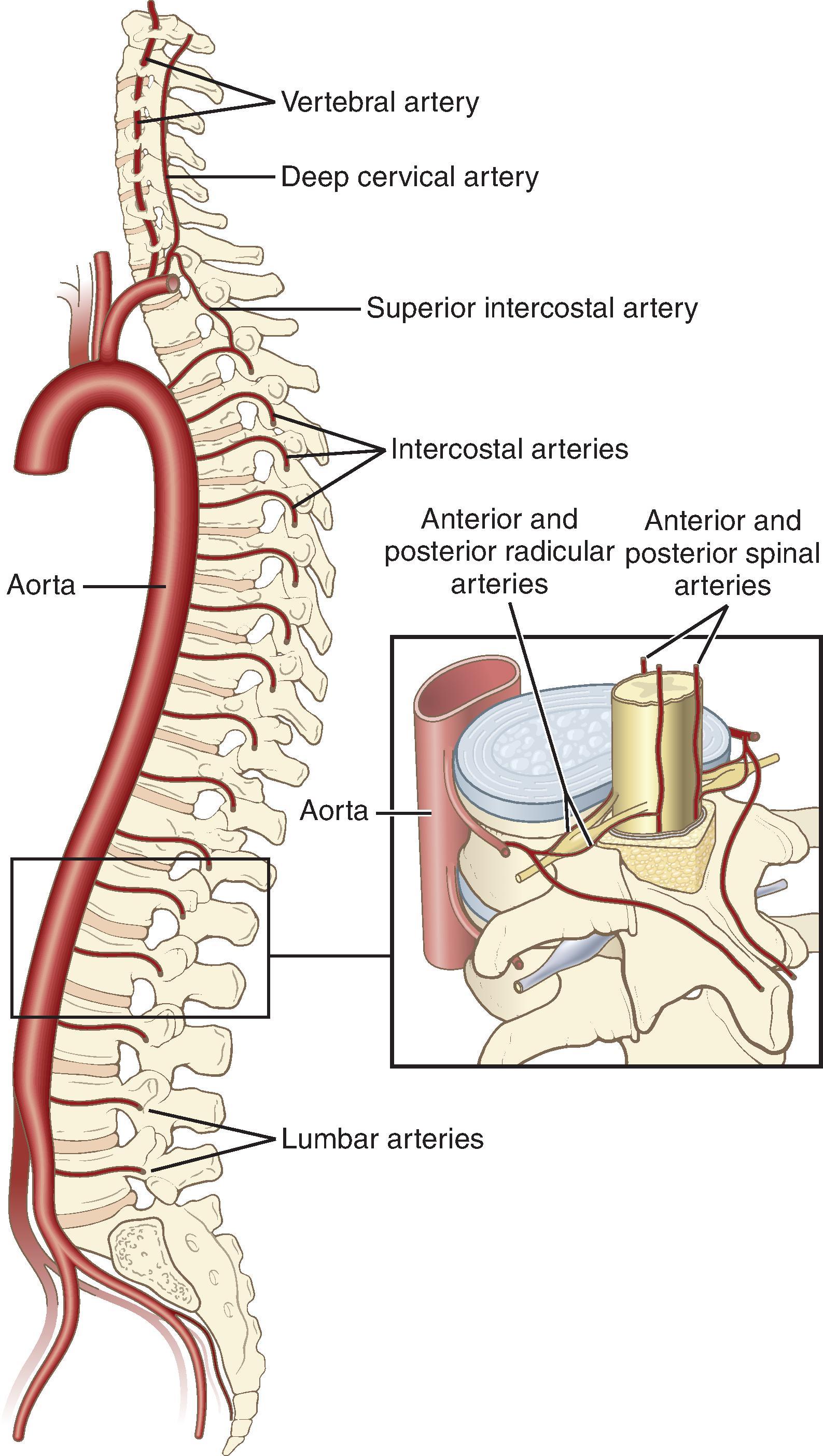
Longitudinal anterior and posterior spinal veins communicate with segmental anterior and posterior radicular veins before draining into the internal vertebral venous plexus in the medial and lateral components of the epidural space. These drain into the azygous system.
Variations exist in size and structure of the spinal nerve roots and CSF volume, both of which may contribute to variability in spinal block quality, height, and regression time. Similarly, the epidural space is more segmented and less uniform than previously believed, which may be a factor in the unpredictability of drug spread. Finally, contents of the epidural space also vary and can influence the volume of local anesthetic required.
Administration of local anesthetics to the neuraxis disrupts nerve transmission within the spinal cord, the spinal nerve roots, and the dorsal root ganglia. Nerves in the subarachnoid space are easily anesthetized, even with a small dose of local anesthetic, compared with the extradural nerves, which are often ensheathed by dura mater (the “dural sleeve”). The speed of neural blockade depends on the size, surface area, and degree of myelination of the nerve fibers exposed to the local anesthetic. Small preganglionic sympathetic fibers (B fibers, 1 to 3 μm, moderately myelinated) are most sensitive to local anesthetic blockade. Unmyelinated polymodal (“slow” pain) C fibers (0.3 to 1 μm) are blocked more readily than the myelinated A-delta pinprick sensation fibers (1 to 4 μm) responsible for cold sensation, which in turn are blocked more readily than heavily myelinated A-beta fibers (5 to 12 μm) conducting touch sensation. Large myelinated A-alpha motor fibers (12 to 20 μm) are the most resistant to local anesthetic blockade. Clinical onset of spinal blockade usually follows this pattern, with block regression occurring in the reverse order. Maximum block height varies according to each sensory modality, termed differential sensory block . Therefore cold sensation (also an approximate level of sympathetic blockade) is most cephalad and is, on average, one to two spinal segments higher than the level of pinprick anesthesia, which in turn is one to two segments higher than anesthesia to touch.
Local anesthetic injected directly into the CSF diffuses from areas of high concentration toward other segments of the spinal cord. Local anesthetic diffuses through the pia mater and penetrates through the spaces of Virchow-Robin (extensions of the subarachnoid space accompanying the blood vessels that invaginate the spinal cord from the pia mater) to reach the deeper dorsal root ganglia. A portion of the subarachnoid drug diffuses outward to enter the epidural space, and some is taken up by the blood vessels of the pia and dura maters.
Drug penetration and uptake are directly proportionate to the drug mass, CSF drug concentration, contact surface area, lipid content (high in spinal cord and myelinated nerves), and local tissue vascular supply, but inversely related to nerve root cross-sectional area.
Epidural drug uptake and distribution are more complex. Some of the injected local anesthetic (<20%) traverses the dura into the CSF and some is absorbed into the plasma compartment, but the majority spreads by bulk flow longitudinally and circumferentially within the epidural space and is taken up by epidural fat. Factors that may enhance the distribution of local anesthetic within the epidural space are small epidural space cross-sectional area (resulting in greater spread in the thoracic levels), decreased epidural space compliance, decreased epidural fat content, decreased local anesthetic leakage through the intervertebral foramina (e.g., in the elderly and those with spinal stenosis), and increased epidural venous engorgement (e.g., during pregnancy). The direction of drug spread also varies with the vertebral level. Spread is mostly cephalad in the lumbar and low thoracic region, but caudad after a high thoracic injection.
No local anesthetic drug metabolism takes place in the CSF. Regression of neural blockade arises primarily from a decline in CSF and/or epidural drug concentration resulting from vascular absorption. Local anesthetics with higher lipid solubility (e.g., bupivacaine) form a depot within epidural fat, thereby slowing this process.
Neuraxial anesthesia causes blockade of the sympathetic and somatic (sensory and motor) nervous systems. The physiologic effects of epidural anesthesia are similar to those of spinal anesthesia, with the exception that local anesthetic blood levels reach concentrations sufficient enough to produce systemic effects on their own.
Blockade of the peripheral (T1–L2) and cardiac (T1–T4) sympathetic fibers reduces systemic vascular resistance (SVR) and, to a much lesser extent, cardiac output, commonly resulting in arterial hypotension after spinal or epidural blockade. The degree to which arterial blood pressure decreases with either spinal or epidural technique depends on multiple factors.
The vasodilatory changes depend on both baseline sympathetic tone (i.e., higher baseline sympathetic nervous system activity in older patients equates to a greater SVR reduction and consequent hypotension compared with younger patients) and the extent of the sympathectomy (i.e., the height of the block). The sympathectomy typically extends two to six dermatomes cephalad to the sensory block level with spinal anesthesia but the same level with epidural anesthesia.
Cardiac output is the product of heart rate and stroke volume, and it is generally either maintained or slightly decreased during the onset of spinal anesthesia. Venous and arterial vasodilation reduces preload (venous return) and afterload (SVR), respectively. Because 75% of the total blood volume resides in the venous system, the venodilation effect predominates and stroke volume is reduced. Despite a compensatory baroreceptor-mediated sympathetic response (vasoconstriction and increased heart rate) above the level of blockade, the reduction in venous return and right atrial filling reduce signal output from intrinsic atrial and great vein chronotropic stretch receptors, thereby increasing parasympathetic activity. The two opposing responses result in a minimal change in heart rate unless neuraxial anesthesia is extended to the T1 level when blockade of the cardioaccelerator fibers (in addition to a marked reduction in venous return) may result in severe bradycardia and even asystole. The Bezold-Jarisch reflex can also cause profound bradycardia and circulatory collapse after spinal anesthesia, especially in the presence of hypovolemia, when a small end-systolic left ventricular volume may trigger a mechanoreceptor-mediated bradycardia.
Spinal anesthesia–induced hypotension may decrease cerebral blood flow (CBF) in older patients and those with preexisting hypertension. However, in studies that demonstrated a decrease in cerebral perfusion there was no postoperative change in cognitive function in any of the patients. Nevertheless avoiding hypotension would seem prudent. Spinal anesthesia has been shown to result in significant patient sedation in the absence of systemic sedatives caused by decreased afferent stimulation of the reticular activating system. Intravenous sedative and hypnotic drug doses should be reduced accordingly in the context of concomitant neuraxial blockade.
Alterations in pulmonary variables during lumbar and low thoracic neuraxial block are usually of little clinical importance. A decrease in vital capacity follows a reduction in expiratory reserve volume related to paralysis of the abdominal muscles necessary for forced exhalation and cough rather than a decrease in phrenic or diaphragmatic function. These changes are more marked in obese patients and those with severe respiratory disease. In the postoperative setting of thoracoabdominal surgery the analgesic benefits of neuraxial techniques render improvements in respiratory function that outweigh the modest changes described earlier arising from the blockade itself.
Neuraxial blockade from T5 to L1 disrupts splanchnic sympathetic innervation to the gastrointestinal tract, resulting in increased colonic mucosal blood flow and motility because of unopposed parasympathetic (vagal) activity. These local effects may be opposed by the direct arterial blood pressure–dependent effect on intestinal perfusion caused by thoracic epidural-induced hypotension. Correction of systemic hypotension by vasopressor therapy (e.g., norepinephrine) reverses impaired colonic perfusion. The impact of neuraxial analgesia on clinically relevant gastrointestinal outcomes such as anastomotic healing and postoperative ileus remains an area of ongoing research and some controversy.
Despite a predictable decrease in renal blood flow accompanying neuraxial blockade, this decrease is of little physiologic importance. The belief that neuraxial blocks frequently cause urinary retention is questionable (see “Complications”).
Single-injection spinal anesthesia is useful for procedures of known duration involving the lower limbs, perineum, pelvis, or lower abdomen. It is especially useful when patients wish to remain conscious or when one or more comorbid conditions, such as severe respiratory disease or an airway that may be difficult to manage, increases the risks of general anesthesia. Epidural anesthesia allows for more prolonged surgical anesthesia by catheter-based local anesthetic delivery. Indwelling catheter-based spinal anesthesia is less conventional, but may be useful when insertion of an epidural catheter is challenging or in the setting of severe cardiac disease when the reliability of a single-shot spinal anesthetic must be combined with the hemodynamic stability of low-dose incremental adminstration.
Spinal or epidural local anesthetics, along with other additives such as opioids, provide excellent-quality, long-lasting intraoperative and postoperative analgesia for labor and delivery (also see Chapter 33 ), hip or knee replacement (also see Chapter 32 ), laparotomy, thoracotomy (also see Chapter 27 ), and even after cardiac surgery (also see Chapter 26 ). Neuraxial local anesthetics may also be used in the management of chronic pain (also see Chapter 44 ).
Absolute contraindications to neuraxial blockade are patient refusal, localized infection at the needle insertion site and allergy to any of the drugs to be administered. A patient's inability to maintain stillness during needle puncture (which could expose neural structures to traumatic injury), as well as increased intracranial pressure (which may predispose to brainstem herniation) are also considered strong contraindications to a neuraxial technique.
Relative contraindications can be approached by system and must be weighed against the potential benefits of neuraxial blockade.
Although preexisting central or peripheral neurologic deficit has never been definitively demonstrated to increase susceptibility to injury after neuraxial anesthesia or analgesia (the double-crush phenomenon), the risk–benefit ratio of performing neuraxial techniques should be considered carefully in patients with preexisting central or peripheral neurologic diseases. Chronic low back pain without neurologic deficit is not a contraindication to neuraxial blockade.
There is an association between the presence of spinal stenosis and nerve injury after neuraxial techniques. When suspected nerve injury occurs in patients with underlying spinal stenosis, the relative causative contributions of neuraxial needling, local anesthetic maldistribution, surgical positioning, and natural history of cord or nerve root impingement from the stenosis itself are often unclear. Asymptomatic and undiagnosed spinal stenosis is relatively common in the elderly, and the vast majority undergo neuraxial blockade without incident.
Previous spine surgery does not predispose patients to an increased risk of neurologic complications. However, in the presence of scar tissue, adhesions, hardware, or bone grafts, needle access to the CSF or epidural space and epidural catheter insertion may be challenging or impossible. In addition, the resultant spread of local anesthetic in the CSF or epidural space in particular can be unpredictable and incomplete.
Because of the demyelinating pathology, patients with multiple sclerosis (MS) may be more sensitive to neuraxial local anesthetics and exhibit a prolonged motor and sensory blockade. There is no conclusive evidence that neuraxial blockade itself worsens MS symptoms, and neuraxial techniques are not absolutely contraindicated in this patient group.
Depending on the severity of the neural tube defect, the potential for traumatic needle injury to the spinal cord may be increased. The spread of local anesthetic in the CSF and epidural space (if present) can also be markedly variable. In any of these circumstances a careful evaluation of neurologic status must first be undertaken and noted along with documentation of the discussion of the risks and benefits.
The potential rapid and significant reduction in SVR after spinal anesthesia risks dangerous decreases in coronary perfusion in afterload-dependent patients. In the presence of aortic stenosis, neuraxial anesthesia must be considered on an individual patient basis in the context of disease severity, left ventricular function, and case urgency. Invasive continuous blood pressure monitoring, concurrent vasopressor infusion, and/or a catheter-based neuraxial anesthetic with repeated small doses of local anesthetic facilitate hemodynamic control.
An exaggerated hypotensive response because of vasodilatory effects may occur, and euvolemia should be targeted before and during neuraxial anesthesia to minimize relative hypotension.
Catastrophic cases of epidural (or subdural) hematoma and permanent paralysis after neuraxial techniques in patients on various anticoagulant medications, including low-molecular-weight heparin (LMWH), have occurred. The American Society of Regional Anesthesia and Pain Medicine (ASRA) guidelines regarding neuraxial techniques (including catheter removal) in patients receiving antiplatelet, heparin, anti–factor Xa, and direct oral anticoagulation agents should be consulted before practitioners undertake a neuraxial technique. Herbal medications can have an impact on coagulation pathways, and the time from herbal discontinuation to normal hemostasis is 24 hours for ginseng, 36 hours for ginkgo, and 7 days for garlic. It is not currently deemed necessary to discontinue these medications before surgery or anesthesia, although they may have a confounding impact if other anticoagulant medications are administered.
Patients who have received therapeutic fibrinolytic or thrombolytic therapy within the preceding 48 hours should not undergo spinal or epidural techniques because of the risk of epidural hematoma.
Hemorrhagic complications after neuraxial techniques in patients with known hemophilia, von Willebrand disease, or idiopathic thrombocytopenic purpura are infrequent when factor levels are more than 0.5 IU/mL for factor VIII, von Willebrand factor, and ristocetin cofactor activity, or when the platelet count is more than 50 × 109/L before block performance. The minimum safe factor levels and platelet count for neuraxial blockade remain undefined in both the obstetric and general populations, and an individual risk–benefit assessment is required in all cases.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here