Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Spinal cord stimulation (SCS), which emerged as a direct clinical application of the gate control theory, has been in use since the early 1970s. It should now be regarded as a mainstream specific treatment of certain forms of neuropathic as well as ischemic pain, but not for non-ischemic nociceptive pain. Although it is an interventional form of therapy, it carries very few serious side effects. The anti-ischemic effect of SCS, exploited far more in Europe than in the United States, provides an opportunity to modify the underlying ischemic condition rather than merely producing palliation of the pain. The effect on ischemia also illustrates that SCS may exert influences on bodily functions other than pain. Our knowledge of the mechanisms of action of SCS has expanded considerably in the last decade.
In the slipstream of its rapidly accelerating application for movement disorders, intracerebral stimulation, currently referred to as deep brain stimulation, for relief of pain now appears to be in a state of resurgence.
For more than 2 decades, motor cortex stimulation has been found to be effective against some otherwise extremely therapy-resistant pain conditions: central post-stroke pain and trigeminal deafferentation pain.
In experienced hands, the long-term success rate of SCS for chronic neuropathic pain varies between 50 and 70%, whereas for selected ischemic pain conditions, in particular, angina pectoris, it remains steadily above 80%. Health care providers demand solid evidence of efficacy and a positive cost–benefit ratio for all types of treatments. Recently, a few randomized controlled trials of SCS, involving neuropathic as well as ischemic pain, have provided a base for classifying this treatment modality as having a relatively high degree of evidence of efficacy and a favorable cost–benefit profile.
Contrary to common belief, the first trials involving modern neurostimulation for the treatment of pain did not originate from the well-known gate control theory published by Melzack and Wall in 1965. In the 1960s, trials of electric stimulation of the sensory thalamus via implanted electrodes for the treatment of chronic neuropathic pain by had already commenced at the Hôpital St. Anne in Paris. This approach was based on an older theory implying that chronic pain originated from an imbalance between the epicritic and the protopathic components of the afferent influx of impulses. Already in 1906, Head and Thompson had proposed that discriminative sensations, such as touch, normally exert an inhibitory influence on the impulses subserving pain. They further postulated that facilitation or inhibition of sensory impulses occurs in the posterior horn before they are relayed to secondary neurons ( ). It was hypothesized that sensory thalamic stimulation would compensate for the deficit in sensory inflow after, for example, a nerve injury by artificially enhancing epicritic (non-painful) afferents ( ).
Although the basic concept of gate control, as formulated by , was not entirely novel, it emerged from experiments using modern electrophysiological techniques and represented a new view of pain and pain modulation. The gate theory was subsequently much criticized but its simplicity has made it useful as a framework for understanding the dynamics of pain generation and pain control. Its impact on modern pain research cannot be overrated, as also expressed by editorial titled “Gate Control Theory of Pain Stands the Test of Time.” Its clinical implications have been of paramount importance since it triggered the development of various forms of electrical stimulation as new treatment modalities.
The first trials involving low-intensity stimulation of peripheral nerves via implanted electrodes were performed by , and the first trials involving spinal cord stimulation (SCS) were conducted by .
Almost simultaneously, transcutaneous electrical nerve stimulation (TENS) was introduced. However, at that time this method was deployed merely to screen candidates for SCS.
A further and more specialized strategy for the activation of selected groups of large-diameter fibers was the development of peripheral nerve stimulation via implanted electrodes ( , ) and stimulation of the trigeminal ganglion and retroganglionic rootlets in patients with painful trigeminal neuropathy ( ). Stimulation of major nerve trunks has survived as a major technique in some centers and has in recent years developed into a rapidly growing field, including occipital nerve stimulation ( ), stimulation of facial branches of the trigeminal nerve ( ), and more recently, also “field stimulation,” or the application of current to thin, often unidentified skin branches of sensory nerves (e.g., ). This expanding field, however, is outside the scope of this chapter.
A second major breakthrough was the demonstration by of an endogenous pain-controlling system in the region of the cerebral aqueduct—the periaqueductal gray (PAG). This discovery triggered the first attempts to electrically activate this region with indwelling electrodes ( ) in the hope of reproducing the very powerful analgesia observed in Reynold’s experimental animals. This mode of stimulation later developed into a clinical therapy that is complementary to stimulation of the sensory thalamus and primarily used for severe chronic nociceptive and mixed pain conditions.
Based partly on the progress of brain-imaging techniques, new targets appeared in the 2000s, such as stimulation of the posteromedial hypothalamus for severe cluster headache ( , ).
A third venture, reminding us of the complexity of the endogenous control systems of the central nervous system (CNS), appeared with the report of that electrical activation of the cortical motor strip could induce pain relief in patients with one of the most therapy-resistant neuropathic pain conditions, central post-stroke pain.
Since SCS has evolved as the dominant form of central neurostimulation for pain, the major part of this chapter is devoted to a description and discussion of this mode of therapy, including patient selection, technical developments, outcomes, and complications. Stimulation of the motor cortex has emerged as a novel promising treatment of pain conditions that are otherwise very difficult to manage. Although the use of intracerebral stimulation for pain has decreased in the past 20 years and the technique is no longer approved by the Food and Drug Administration (FDA) for pain therapy in the United States, long-term favorable outcome of such procedures have been documented by a few recent studies, thus justifying a re-evaluation of this treatment modality.
It has been estimated that each year more than 30,000 SCS implantations (of these, >18,000 new systems) are performed world-wide, a reflection of the growing awareness that SCS may be an effective treatment for neuropathic pain, a condition for which there are few alternative therapies. A further reason for the renewed interest in SCS is the rapid development of new hardware and electronics, as well as its application for nociceptive pain in patients with peripheral vascular disease (PVD; more specifically, ischemic pain in peripheral arterial occlusive disease [PAOD]), vasospastic conditions (e.g., Raynaud’s syndrome), and refractory angina pectoris. However, in these conditions the pain relief that can be obtained with SCS is probably secondary to its effect on tissue ischemia.
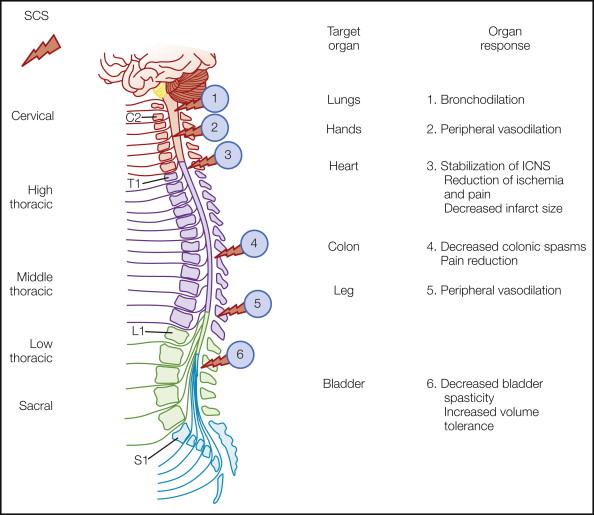
Experimental evidence from the laboratory paired with clinical observations clearly demonstrate that SCS applied to different sites on the neuraxis exerts fundamentally different effects on various target organs or parts of the body ( Fig. 41-1 ). Different parts and types of SCS equipment are illustrated in Figures 41-2 through 41-5 .
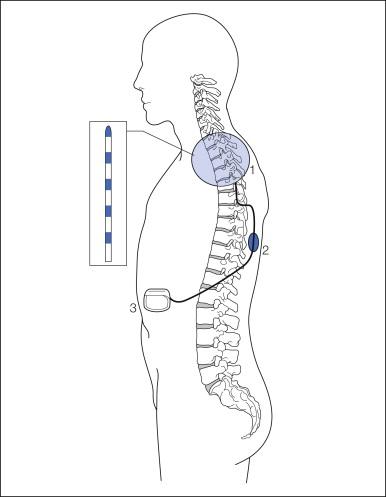
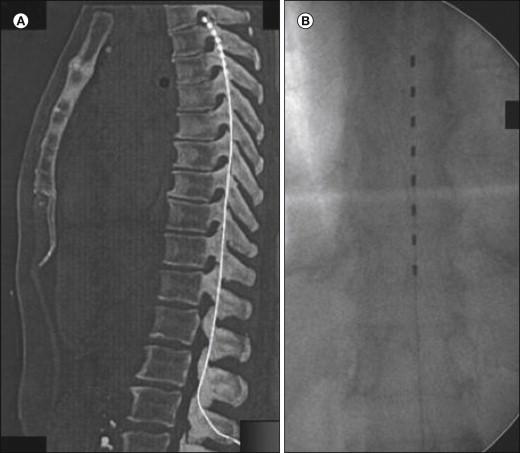
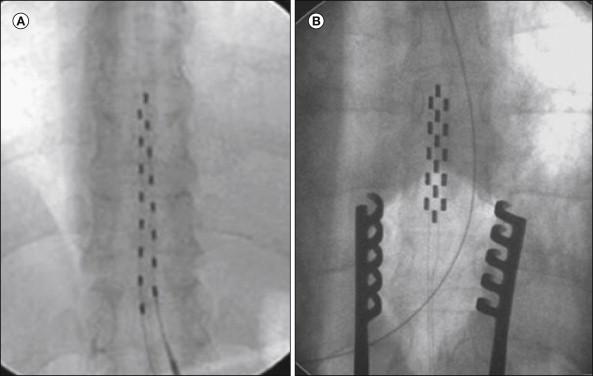

Implantation of SCS systems has developed into a routine procedure performed in many pain centers. Percutaneous leads are implanted under local anesthesia with the patient in the prone or sometimes in the sitting position. Intraoperative fluoroscopy is always used to help guide the lead to the intended position where stimulation evokes paresthesias, which should cover the painful region before the electrode is anchored subcutaneously. In patients with neuropathic or ischemic pain, trial stimulation is usually performed for a period of some days up to weeks. In contrast, in patients with angina pectoris, the whole system, including the neurostimulator, is usually implanted during the same session. For some indications, especially those with both midline pain and pain components projecting into an extremity, dual leads may be necessary to obtain satisfactory coverage with paresthesias over the entire painful area. If the lead has a tendency to migrate or if complete paresthesia coverage of the painful region cannot be obtained with multipolar catheter–type electrodes, a plate electrode implanted via a small laminotomy may be useful (see Figs. 41-4B and 41-5 A and B). In some centers such electrodes are in fact the first choice. The implantation can be performed under local or general anesthesia, but it has recently been reported that it is also possible to apply test stimulation under spinal anesthesia to ascertain whether paresthesia coverage is adequate ( , ). A variety of stimulation devices from different manufacturers are displayed in Figure 41-5 . Increasing the number of stimulation poles has multiplied the variety of possible stimulation patterns; lately, fully automated SCS stimulator hardware and software have been developed to facilitate the search for optimal pole combinations for a specified pain distribution ( ).
The recent introduction of truly dual-channel systems, whereby stimulus parameters, including amplitude, can be varied at different electrode poles independently, has permitted a more sophisticated approach to targeting by “steering” the electrical fields electronically (see Fig. 41-4 A and B).
The earlier devices consisted of passive radiofrequency receivers in combination with an external stimulator and an antenna placed on the skin overlying the receiver.
Fully implantable pulse generators (IPGs) are less obtrusive and can be operated with a simple remote control. However, they require replacement every 3–5 years (depending on use) when the battery is depleted. In conditions needing higher amplitudes, multiple active electrode poles, and several stimulation programs, drainage of current may be so high that devices with double-battery capacity or rechargeable systems are recommended (see Fig. 41-5 A).
It should be added that in patients with marked and extensive allodynia/dysesthesia, such as is often the case with complex regional pain syndromes (CRPSs), surgery within or close to the affected area carries a high risk of exacerbating the condition. This even includes small incisions, and care must therefore be taken when choosing the site for the IPG.
Knowledge about optimal rostrocaudal placement has been acquired largely through accumulated experience. produced useful “probability maps” of cathode placement—the threshold for cathodal stimulation is lower than that for anodal stimulation—for different anatomical targets, for example, C2–4 for the shoulder; C4–6 for the hand; T7–8 for the anterior aspect of the thigh; and T11–L1 for the posterior aspect of the thigh, leg, and perineum. For angina, the active electrode poles are placed between C7 and T2, usually slightly to the left (the cardiac sensory afferents enter the cord at T1–5). For PAOD affecting the lower limb, T10–11 has been the most commonly effective level. T8–9 is the level most likely to stimulate the low back region. Afferents enter the dorsal columns laterally and have lower activation thresholds than do the more medial, deeper fibers from lower segments ( ). To be most effective, the difference between the perception threshold for stimulation and the amplitude at which it becomes unpleasant (discomfort threshold) should be as large as possible so that consistent, comfortable stimulation can be achieved despite changes in posture. The use of narrowly spaced electrode poles and guarded cathodes (i.e., a cathode surrounded by anodes) increases the chance of including “difficult” targets such as the low back area and perineum by permitting the recruitment of deeper and smaller fibers without “overstimulating” others ( ).
It has been claimed that dual leads, today often with a total of 16 electrode poles, are required to cover difficult painful areas, such as the low back region ( , ). However, there is controversy about the necessity for multiple leads and a mass of contacts. stated that “in experienced hands,” double leads are not significantly better than a single four-polar lead. Moreover, it is possible to use a single octopolar lead crossing the midline at about the T10–11 level to enable both legs to be covered, as well as the axial regions ( ).
In recent years, stimulation directed onto the sacral roots either by a retrograde positioning technique from a lumbar entrance or in anterograde fashion through the sacral foramina has been used with some success for pain from the bladder, perineum, and legs ( ).
The frequency is rarely outside the range of 50–120 Hz, usually 60–100 Hz, and the pulse width is between 100 and 500 μsec. The effective amplitude varies but should be set to produce comfortable paresthesias, usually in the range of 2–6 V for “constant-voltage” systems. Patterns of use vary widely from 1 or 2 hours per day for periods of 30 minutes, sometimes not every day, to almost continuous use. Continuous, 24-hour stimulation is not recommended because it leads to rapid battery depletion; if necessary, the neurostimulator should be exchanged for a rechargeable model.
Although SCS was a direct spin-off from the gate control theory of Melzack and Wall published in 1965, ideas about how pain may be alleviated with SCS differ fundamentally between its application for neuropathic and ischemic pain conditions. Based on the original idea that antidromic stimulation of the large fibers in the dorsal columns (therefore also referred to as dorsal column stimulation) may activate the proposed gating mechanisms in the dorsal horn (DH), SCS would also be effective in suppressing pain of a nociceptive nature—both acute and chronic. This, however, is a paradox because SCS has been found to be preferentially effective for neuropathic forms of pain (for review see ; ; ; ; ; ).
Present knowledge about the spinal mechanisms involved in the effects of SCS when applied for neuropathic pain and for pain associated with PAOD and angina pectoris is schematically summarized in Figure 41-6 A–C.
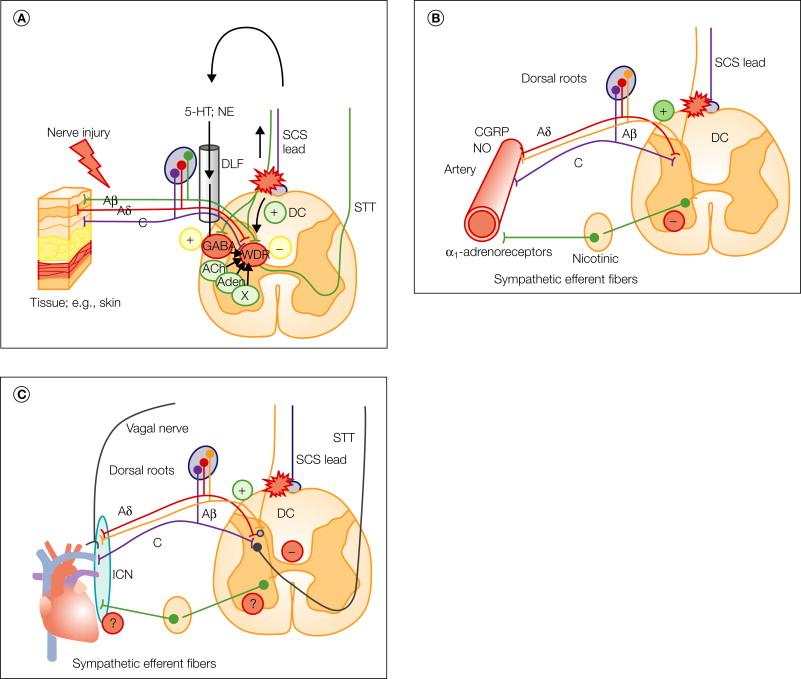
SCS applied to dorsal column axons may activate the fibers in both directions—orthodromically and antidromically. Most of our own research has focused on antidromic activation of the DH circuitry via dorsal column collaterals. It has recently been demonstrated by recordings from peripheral sensory nerves in patients that SCS does actually produce retrograde activation ( ).
In neuropathic pain, the hyperexcitability demonstrated by multimodal wide–dynamic range (WDR) cells in the DHs ( ; ; ) seems to be related to increased basal release of excitatory amino acids (e.g., glutamate) and dysfunction of the local spinal γ-aminobutyric acid (GABA) system ( ). In experiments on animal models of neuropathy, SCS has been found to inhibit the hyperexcitability of WDR cells and to induce increased release of GABA in the DHs, with a subsequent decrease in the interstitial glutamate concentration ( , ). Release of GABA is observed solely in animals in which SCS alleviates the symptoms ( ). Activation of the GABA B receptor seems to play a pivotal role in suppression of glutamate release ( ).
The crucial role of GABA in the mode of SCS action was further demonstrated by the finding that intrathecal (IT) injection of the GABA B receptor agonist baclofen could transform rats not responding to SCS into responders ( ). This observation later developed into clinical use in patients with a failing SCS effect, and the beneficial effects of such “drug-enhanced spinal stimulation therapy” have proved to be stable over a period of many years ( ).
Another transmitter recently found to play an important role in the effects of SCS is acetylcholine (ACh). The effects of cholinergic activation were first noted in a study of “enhanced spinal stimulation” using clonidine in subeffective IT doses in rats not responding to SCS per se ( ). Subsequent animal studies demonstrated augmented release of ACh in the DH with SCS, and parallel behavioral studies revealed that the effect was primarily dependent on activation of the muscarinic M 4 receptor ( ). It was also found that a subeffective IT dose of a muscarinic receptor agonist could transform animals not responding to SCS into responders ( ).
In patients, IT infusion of clonidine, which partly exerts its pain-relieving effects via cholinergic mechanisms, also proved useful clinically as an adjunct to SCS when stimulation alone was ineffective—another example of “drug-enhanced spinal stimulation” ( ).
Activation of descending inhibitory pathways via a brain stem loop has been proposed as the principal mechanism of SCS by Saadé and collaborators (e.g., ; ). This idea is consistent with recent data indicating important involvement of the descending serotonergic pain-controlling system in the SCS mode of action ( ). Though not yet clearly demonstrated, it is likely that descending noradrenergic pathways participate as well. In fact, enhancement of the SCS effect in animal studies has been demonstrated with the IT administration of antidepressants with noradrenalin reuptake inhibitory action ( ).
The different physiological mechanisms active when SCS is used for neuropathic pain and that have been explored up to date are schematically summarized in Figure 41-6 A. However, a cascade release of neuroactive substances is probably induced or modulated by SCS both in the DHs and in other sites (e.g., in the brain stem), and multiple, as yet unknown mechanisms are thereby activated.
SCS is often effective for both types of CRPSs (see the later section Results ). CRPS type 1 is a poorly understood pain condition, and recently it has been called into question whether it should in fact be classified as a form of neuropathic pain despite its typical neuropathic characteristics ( ). SCS has yet not been studied in animal models of CRPS type 1, and the putative mechanisms operating when applied for this condition can only be hypothesized ( ). Several possible alternatives have been proposed: (1) by exerting a direct inhibitory action on hyperexcitable central neuronal circuits ( , ); (2) by decreasing the sympathetic efferent output acting on de novo activated adrenoreceptors on the damaged sensory neurons ( , ); and (3) by reducing peripheral ischemia by direct antisympathetic action and/or antidromic activation with subsequent peripheral release of vasoactive substances such as calcitonin gene–related peptide (CGRP), nitric oxide, and substance P ( ; ; ; ).
Despite the lack of evidence that SCS can alleviate acute nociceptive pain, ischemic extremity pain, being mainly nociceptive, can nonetheless be treated effectively in selected cases. The primary effect of stimulation is probably resolution of the tissue ischemia that seems to be the primary event ( Fig. 41-6 B), either by increasing/redistributing blood flow to the ischemic tissues or by decreasing tissue oxygen demand. Experimental studies favor the notion that SCS induces peripheral vasodilatation by suppressing efferent sympathetic activity and thereby resulting in diminished peripheral vasoconstriction and secondary relief of pain ( ). More recent evidence indicates that antidromic mechanisms also involving small-diameter fibers may be activated by SCS at low intensities and that this may result in peripheral release of CGRP and subsequent peripheral vasodilatation ( , , ). Recent animal studies have further demonstrated that the SCS effect relates to the activity level of the sympathetic system ( , ).
The mechanisms of the beneficial effect of SCS in patients with chest pain (angina pectoris) secondary to coronary ischemia are only fragmentarily known. Although early animal data demonstrated direct inhibitory effects of SCS on cardiac nociception ( ), it was later clearly demonstrated in clinical studies that resolution of cardiac ischemia remains the primary factor ( ). Some researchers favor a stimulation-induced increase in flow or redistribution of cardiac blood supply, whereas others interpret the reduction in coronary ischemia (decreased ST changes, reversal of lactate production) as mainly being due to decreased cardiomyocyte oxygen demand (for review see ). Experimental studies have hitherto been unable to demonstrate a local increase in flow or redistribution of blood in the myocardium by SCS; instead, pre-emptive SCS seems to induce protective changes in the myocardium that make it more resistant to critical ischemia (e.g., ). Recent studies have indicated that SCS-induced local catecholamine release in the myocardium could trigger protective changes in cardiomyocytes related to the biochemical mechanisms behind “ischemic preconditioning” ( ).
SCS also appears to exert rhythm control in the heart ( ). With ischemia, the intrinsic cardiac nervous system is profoundly activated. If this activity persists, it may result in spreading dysrhythmias and lead to more generalized ischemia. SCS stabilizes the activity of these intrinsic ganglia, especially during ischemic challenge, and may in this way protect the heart from more severe ischemic threats from generalized dysrhythmia ( ). However, the exact mechanisms remain to be discovered.
SCS, as with other forms of electrical stimulation for pain, requires active participation of patients because the stimulation equipment has to be partly handled by them. This form of therapy implies heavy demands on the treating physician and team. Everyone who takes on the responsibility of subjecting patients to SCS treatment must be prepared to supply almost lifelong continuing support since these patients typically suffer from a chronic multifactorial disease with pain as the major, but not the only reason for their incapacity. Another reason for continuing physician–patient contact is that in many cases the stimulation parameters and the electrode couplings have to be modified over time.
A prerequisite for successful SCS treatment is a thorough pain analysis. Clinical experience, as documented in a large number of publications, indicates that SCS is predominantly effective for some forms of chronic neuropathic pain and for pain in certain ischemic syndromes. Therefore, it is of particular importance to establish the existence and relative importance of neuropathic pain components in so-called mixed pain conditions (e.g., co-existing neuropathic and nociceptive pain, differentiation between radiating neuropathic and referred components).
Though not essential in every case, it is recommended that the pretreatment evaluation in general include a psychological examination performed by a psychologist or a pain-oriented psychiatrist. It is well documented that psychological factors correlate with the outcome of SCS (e.g., , ), although their predictive role is not yet completely clear ( , ).
A psychological examination is also performed to detect patients with important psychological components of their pain, major personality disorders, and deficient capacity to collaborate and communicate their pain problems. In particular, drug-seeking behavior or abuse has to be taken into account in the selection of patients.
TENS has been advocated as a screening test by some authors, but others have found no correlation with the subsequent response to SCS ( , ). It should be emphasized that the presence of allodynia/dysesthesia may make electrode placement for stimulation in the painful area impossible. Nevertheless, in our experience a positive response to TENS seems to indicate the likelihood of a favorable outcome of SCS, and recent data support its predictive usefulness ( ).
Abnormalities in cutaneous sensibility are common with neuropathic pain syndromes. Total numbness in the painful area indicates complete denervation, which may be due to extensive lesion of nerve roots or ganglia. If so, the corresponding dorsal column fibers may also have degenerated and there is no substrate for the stimulation. Of particular interest is the significance of the presence of allodynia. This appears to be a somewhat controversial issue in that some claim that allodynia as the cause of evoked pain is often effectively alleviated by SCS whereas others maintain that it is unlikely to be affected ( , ).
Quantitative sensory testing has been suggested as a possible predictive instrument for the outcome of SCS, and it has been demonstrated that the results of some sensory tests relate to the outcome of SCS ( , ). There have also been trials involving the use of various neurophysiological tests to predict the outcome of SCS, and in particular the so-called R-III reflex and somatosensory evoked potentials (SSEPs) have been found to be of some value ( , , ).
Test stimulation of the spinal cord, either via temporary electrodes or via a temporary, percutaneous extension connected to potentially permanent electrodes, has considerable intuitive appeal. It has become widely adopted, is strongly advocated by many practitioners, and is a prerequisite for reimbursement in some countries. However, data on the predictive value of trial stimulation for long-term outcomes are conflicting, but in neuropathic pain conditions and other instances in which the outcome is in doubt, a test period of at least 1 week is generally recommended. It should be noted that trial stimulation is not usually performed in patients with angina pectoris (for discussion see ).
In cases of PAOD with ischemic pain, it usually takes a few days with daily “bolus stimulation” for several hours before clear signs of a stimulation-induced effect may appear. Besides reported pain reduction tests such as Tcp o 2 , skin temperature and flow measures from the affected limb are recommended. Note that pain components from ischemic ulcers and borders of gangrenous zones generally do not directly respond to SCS. Ischemic pain in vasospastic conditions often responds almost instantly when stimulation treatment is started.
The response of patients with angina pectoris and PVD to SCS is relatively straightforward because reliable objective measures of ischemia, mobility, and other factors are available to support pain estimates. Assessing the outcome in patients with neuropathic pain syndromes is much more problematic. A common experience is to find that patients’ evaluation of the effectiveness of SCS will wane over the years but that if a technical failure occurs, they demand rapid resolution and often indicate that they had forgotten how bad the original pain was. As with any treatment aimed at pain relief and improved function, unrealistic expectations by the patient will influence the reported outcome.
In the majority of studies, outcomes have been assessed by the implanting surgeon or the responsible physician, thereby leading to possible bias. External reviewers have been used in a few studies only, and these results were generally less favorable. It should be emphasized that postal and even telephone follow-up is not a substitute for “face-to-face” consultation and should be avoided if possible.
Most reports of SCS use the notation “percent pain relief,” notwithstanding the fact that pain intensity is non-quantitative and probably non-linear. Calculations by an observer based on serial visual analog scale (VAS) scores may be better than the patient’s own estimate, but the non-linearity and other possible shortcomings of the VAS do not justify calculations that yield results such as “mean pain relief.” In a prospective study, found that only 26% of 40 patients with leg pain reported 50% or greater pain relief, but nonetheless, 70% said that SCS helped them and they would recommend it. In another study, only 55% reported at least 50% relief, but 90% were able to stop or reduce their analgesic medication ( ). Thus, the criterion of 50% or greater pain relief can be misleading, and complementary, more holistic ratings, as well as more objective measures such as activity level, return to work, reduction of pain medication, and others, are preferable. More recently, it has been suggested that a reduction in VAS score to 30% instead of 50% pain relief could be applied and represents a useful degree of pain alleviation ( ).
In the past decade there has been an increasing tendency toward applying evidence-based criteria for the evaluation of various forms of therapy, and this is also the case with SCS. A number of evaluative reviews and meta-analyses covering the SCS literature up to 2006 are now available. Somewhat different evidence classification systems have been used, and although the rankings of quality of evidence are similar, comparisons of the conclusions drawn in different reviewing publications may be somewhat confusing. A commonly used system, also used by the Cochrane Collaboration, is the scale for quality assessment developed by , which involves a range of scores between 0 (lowest quality) and 5 (high quality). Studies with a score of 3 or higher are classified as being of high quality. The lowest level of evidence corresponds to case reports, case series, and expert opinion, whereas the highest relates to randomized control led trials (RCTs).
The great majority of publications on SCS for neuropathic pain have very low levels of evidence because they are observational and case series studies, most of them retrospective. However, a few RCTs have recently been conducted. It should be realized that the presence of paresthesias as a prerequisite for pain relief precludes placebo- or sham stimulation–controlled studies. On the other hand, it has been found that SCS that is subliminal for paresthesias may induce vasodilatation in the skin ( ). Recently, SCS subliminal for paresthesias was compared with truly blinded placebo stimulation in a study of SCS for angina pectoris ( ). A later study found that paresthetic SCS reduced the number of angina episodes more effectively than did subliminal stimulation ( ).
The likelihood of a beneficial response of various neuropathic and ischemic pain conditions to SCS is listed in Table 41-1 . It should be noted that with proper selection, the highest success rates are obtained for vasculopathic pain, angina pectoris, and pain caused by lesions of nerve roots and peripheral nerves, as well as CRPS type 1. It also appears that midline neuropathic pain and mixed pain with an axial distribution have a much lower probability of responding and that pain conditions caused by central lesions are generally unresponsive.
| SUCCESS >> FAILURE | SUCCESS > FAILURE | SUCCESS VARIABLE | FAILURE > SUCCESS | FAILURE >> SUCCESS |
|---|---|---|---|---|
| Angina pectoris | CRPS type 1 | Amputation: phantom pain | Perianal, genital pain | Central, post-stroke pain |
| PVD: vasospastic | CRPS type 2 | Intercostal neuralgia | Partial cord lesions | Complete cord lesions |
| PVD: occlusive | Peripheral nerve damage Diabetic neuropathy Brachial plexus damage (partial) |
Post-herpetic neuralgia | Complete root avulsion | |
| Lumbosacral and cervical rhizopathy | Low back pain combined with neuropathy | |||
| Cauda equina damage | ||||
| Amputation: stump pain |
A systematic literature review of SCS for neuropathic pain was performed by the . It covered publications from 2000 to 2005; the primary outcome for the review was pain relief, and secondary outcomes were functional status and quality of life (QoL). Not more than two RCTs and two prospective non-randomized controlled trials were retrieved. It was concluded that good evidence exists to support the effectiveness of SCS in decreasing the pain associated with lumbosacral rhizopathy, low back pain, CRPS type 1, and post-herpetic neuralgia.
The most common indication for SCS is generally referred to as the failed back surgery syndrome (FBSS), but this term is a misnomer because it is not a pain diagnosis and it tells only that the patient has been subjected to spinal surgery, usually in the low back region. The precise term, according to the International Association for the Study of Pain taxonomy, is lumbar spinal or radicular pain after failed spinal surgery. However, this type of pain may also be a sign of spinal degenerative disease without surgery on the spine. In the United States, between 600,000 and 1.1 million such patients undergo spinal surgery each year, and it is estimated that about a third of these patients report persistent pain.
In many of the recent studies on SCS performed for a pain syndrome labeled FBSS, the effect on the irradiating pain in a leg (or legs) is recorded separately from that on the low back pain component ( ). It is common experience that the latter form of pain is much more difficult to influence, and this is at least partly due to the fact that it is difficult to produce paresthesias that will cover the axial lumbar region. Moreover, this pain is presumably of a predominantly nociceptive nature, and there is no evidence that SCS can directly alleviate such pain. A few studies have specifically addressed the possibility of relieving lumbar axial pain (“low back pain”). It has been claimed that the use of dual, 4-polar, 8-polar, or even 16-polar electrode leads enhances the possibility of producing paresthesias that also cover axial structures ( , ), but this was not confirmed in a prospective, controlled trial ( ; see also ). Nevertheless, in several studies it appeared that about half the patients selected for permanent implantation after trial stimulation also enjoyed good relief of the pain component in the low back region ( , , ). For example, in a study involving 41 patients, no less than 69% were satisfied and reported fair to excellent relief of their low back pain, most of them with dual electrode leads ( ). It should, however, be noted that all these studies were case series with promising results but representing a low degree of evidence.
performed a systematic review of publications, most of them from North America and Europe, from 1995–2002 on the use of SCS for “chronic back and leg pain.” Seventy-two case series from 1995–2002 were identified and subjected to a meta-analysis. These studies included 3427 implanted patients; 62% of them had experienced greater than 50% pain relief, and relative to the total number subjected to trial stimulation, 48% reported this benefit. Furthermore, it should be noted that a somewhat larger proportion, 70%, of the implanted patients expressed satisfaction with the treatment, and there was also significant improvement in health-related QoL. The maximal follow-up time was 10 years. The review included one RCT ( ), in which patients with radicular pain after lumbosacral spine surgery were randomized to SCS or reoperation. An external reviewer performed the outpatient follow-up assessment. A total of 45 patients with a mean follow-up of about 3 years were available, and it appeared that SCS was more successful (9 of 19 patients) than reoperation (3 of 26 patients) ( P < 0.01). Patients initially randomized to SCS were significantly less likely to cross over than those randomized to reoperation. This study was scored as having a high level of evidence.
Recently, a prospective randomized controlled multicenter trial of the effectiveness of SCS (the “PROCESS study”) with a recruitment of 100 patients from a total of 12 centers has been reported. The patients suffered from radiating leg pain (lumbosacral rhizopathy) predominating over pain in the low back region. They were randomized to SCS combined with conventional medical management (CMM) or to CMM alone. In the intention-to-treat analysis at 24 months’ follow-up, 37% of the SCS patients and 2% of the CMM patients ( P = 0.0031) achieved 50% or greater pain relief in the legs ( ). In addition, with regard to back pain relief, QoL, and functional capacity, the SCS patients compared favorably with the CMM group. Interestingly, it was found that between 6 and 12 months, 32 of the 48 CMM patients crossed over to SCS. If instead the patients who actually received SCS were compared with those who did not, the percentages were 47 and 7%, respectively ( P = 0.02). Thus, SCS proved to clearly be more beneficial than CMM. The poor outcome of CMM alone is in accordance with the well-known fact that pharmacotherapy is effective only in at most 30–40% of patients with chronic neuropathic pain ( ). This RCT has been evaluated as representing class II (out of IV) evidence (according to European guidelines; ).
This type of pain is regarded by many to be the prime non-ischemic indication for SCS with the highest probability of obtaining satisfactory and long-lasting pain relief ( , , , ). On the basis of the continuing experience with SCS since the early 1970s, the authors of this chapter also share this view. However, others have reported relatively modest results in such cases in comparison to pain originating from the spinal roots (e.g., ). Nerve injury associated with pain, both spontaneous and evoked, may follow accidental trauma, surgery, entrapment, inflammation, and metabolic disorders (polyneuropathy). This form of pain may occur as a result of both injury to one major nerve (e.g., ulnar nerve entrapment) and partial injury to distal nerve branches (e.g., incision pain).
The syndromes now labeled CRPS (type 1, equivalent to reflex sympathetic dystrophy [RSD], and type 2, formerly termed causalgia), which may also respond favorably to SCS, are a special form of peripheral nerve injury pain (see below).
The most extensive study, with regard to both number of cases and length of follow-up, on SCS for pain caused by peripheral nerve injury was reported by , a co-operative study from Toulouse and Zürich consisting of 152 patients. Of the 132 patients reported from Zürich, 90% had a satisfactory long-term (2–20 years) outcome evaluated on the basis of combined estimates of pain relief, physical activity, QoL, and consumption of analgesics. Another relatively large study is that reported by . Of 49 patients characterized as having “peripheral deafferentation pain,” 36 responded favorably to trial stimulation. They were monitored for 5.5 years and substantial pain relief (75%) was reported in 57% of the patients. In a meta-analysis of 11 studies from the 1980s on pain secondary to peripheral nerve injury, often denoted as RSD or causalgia, 70% of the patients originally subjected to trial SCS stimulation were recorded as having a favorable long-term outcome ( ; see also ). In contrast to these relatively favorable results, , in a series of 30 patients with pain associated with peripheral neuropathy, recorded not more than 14 who enjoyed long-term pain relief.
The varying nature and symptomatology of CRPS make interpretation and comparison of the outcomes of SCS treatment difficult. Half of a group of 18 patients with type 1 had good pain relief, with one-third reporting no benefit ( ). In contrast, reported that, of 12 patients in whom RSD was diagnosed, 8 had excellent pain relief and 4 described good results at a mean follow-up of 41 months. SCS for CRPS type 1 has been subjected to an intention-to-treat RCT described in a series of publications by . Originally, 54 patients were assigned to SCS together with physical therapy (PT) ( n = 36) or to PT alone ( n = 18). After 6 months, patients in the PT group were given the choice to cross over to SCS. At 2 years’ follow-up, pain intensity, assessed by VAS scores, decreased by 3.6 cm in the SCS group versus 0.2 cm in the PT group. However, after 5 years’ assessment, a comparison of patients who actually received SCS (irrespective of the original randomization) and those undergoing PT only disclosed that the 20 SCS plus PT patients available at follow-up had somewhat, albeit not statistically significant greater, pain relief ( P = 0.06). Moreover, 7 of these 20 patients with implantation reported “much improvement” as compared with 2 of the 13 patients treated with PT only. No less than 90% of the patients with an SCS implant indicated that they had responded positively to the treatment. Despite this somewhat disappointing outcome, the authors concluded that they, for several reasons, remain confident that SCS is worthwhile for chronic CRPS type 1, and they refer to the fact that after 5 years there was also still a high degree of patient satisfaction. A similar long-term tendency toward some decline in the efficacy of SCS has been recorded by .
analyzed data from 25 case studies on SCS for CRPS with a mean follow-up of 33 months. They found that 67% of the patients were reported to have greater than 50% pain relief. A similar result was reported by the European Task Force for Evaluation of Neurostimulation ( ), but the evidence quality has been ranked as low. A literature review was also performed by . They considered 15 studies worthy of analysis, including 1 RCT (referred to above), 2 prospective observational studies, and 12 retrospective observation studies. They concluded that although the observational studies were of poor quality, available evidence from the literature examined suggests that SCS is effective in the management of CRPS. A small recent study of SCS for CRPS 1 also confirmed the usefulness of this therapy in adolescent cases ( ). In a few non-controlled studies it has been claimed that temporary pain relief following a sympathetic nerve block is a reliable predictor of a beneficial effect of SCS in patients with CRPS 1 ( , ), although others have questioned the relationship between sympathetic activity and the pain ( , , ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here