Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Although a sparse lymphocytic infiltrate is relatively common in mesenchymal neoplasms, few histologic types of soft tissue tumors characteristically contain prominent inflammatory cells. In some such cases, the presence of an inflammatory infiltrate can be a diagnostic clue, whereas in other cases the infiltrate may obscure the neoplastic component and potentially lead to an erroneous diagnosis of either a reactive process or even a lymphoma. The types of inflammatory cells that can be prominent in soft tissue tumors are diverse, and they include lymphocytes, plasma cells, neutrophils, eosinophils, histiocytes, and mast cells. In many cases, the specific inflammatory cell types may help generate a differential diagnosis ( Boxes 10.1–10.3 ). In contrast, histiocytes are usually not diagnostically useful in isolation (with rare exceptions), but may be seen in combination with other inflammatory cell types. An infiltrate of eosinophils is characteristic of only a small number of neoplasms, most of which are either hematopoietic (notably, Langerhans cell histiocytosis [LCH] and cutaneous and systemic mastocytosis) or vascular in nature (especially epithelioid endothelial lesions such as epithelioid hemangioma). A prominent eosinophilic infiltrate may occasionally be seen in isolated examples of soft tissue tumors apparently as an idiosyncratic phenomenon (which may be more distracting than helpful). Finally, mast cells are often prominent in myxoid soft tissue tumors and in select other mesenchymal neoplasms (e.g., synovial sarcoma and desmoid fibromatosis) and are mentioned when appropriate in other chapters, but they will not be discussed further in this chapter.
Inflammatory myofibroblastic tumor
Inflammatory leiomyosarcoma
Follicular dendritic cell sarcoma
Fibroblastic reticular cell sarcoma
Interdigitating dendritic cell sarcoma
Indeterminate cell histiocytosis
Rosai-Dorfman disease
Histiocytic sarcoma
Well-differentiated inflammatory liposarcoma
Angiomatoid fibrous histiocytoma
Myxoinflammatory fibroblastic sarcoma
Thymoma
Dedifferentiated liposarcoma (inflammatory malignant fibrous histiocytoma)
Undifferentiated pleomorphic sarcoma with prominent inflammation
Rosai-Dorfman disease
Histiocytic sarcoma
Epithelioid inflammatory myofibroblastic sarcoma
Pseudomyogenic hemangioendothelioma
Myxoinflammatory fibroblastic sarcoma
Undifferentiated carcinoma
Anaplastic large-cell lymphoma
Langerhans cell histiocytosis/Langerhans cell sarcoma
Systemic mastocytosis/mastocytoma/mast cell sarcoma
Epithelioid hemangioma (and other epithelioid endothelial neoplasms)
Hodgkin lymphoma
The prototypical examples of soft tissue tumors with prominent inflammatory cells are inflammatory myofibroblastic tumor (IMT) and so-called inflammatory malignant fibrous histiocytoma (MFH). Most examples of IMT contain a prominent lymphoplasmacytic infiltrate, interspersed between the myofibroblastic spindle cells, although some cases may be notable for eosinophils and occasionally neutrophils (see also Chapters 4 and 16 ). In contrast, inflammatory MFH classically contains a prominent infiltrate of neutrophils and foamy histiocytes; this infiltrate is usually much more abundant than the neoplastic cellular component. Most examples of inflammatory MFH represent an unusual histologic variant of dedifferentiated liposarcoma (see also Chapters 7 and 12 ). These tumor types will be covered only briefly in this chapter.
Histiocytic and dendritic cell tumors often contain frequent inflammatory cells, which can be a helpful clue to their diagnosis. Such tumors arise not only in lymph nodes but may also present at extranodal sites such as skin, soft tissue, and the gastrointestinal tract. This group of rare tumors will be discussed in detail in this chapter.
There is also a group of mass-forming idiopathic fibroinflammatory lesions that are not uncommonly confused with mesenchymal neoplasms (e.g., sclerosing mesenteritis and idiopathic retroperitoneal fibrosis). A select subset of these lesions will be discussed briefly at the end of the chapter, with an emphasis on differential diagnosis.
IMT usually affects children and young adults and is especially rare in adults older than 50 years of age (see Chapters 4 and 16 ). Although the most common anatomic sites are the lung, abdominal cavity, and retroperitoneum, nearly any site may be involved. Up to one third of patients (predominantly children) present with a systemic syndrome of fever, weight loss, anemia, hypergammaglobulinemia, and an elevated erythrocyte sedimentation rate, which usually remits following surgical excision of the tumor.
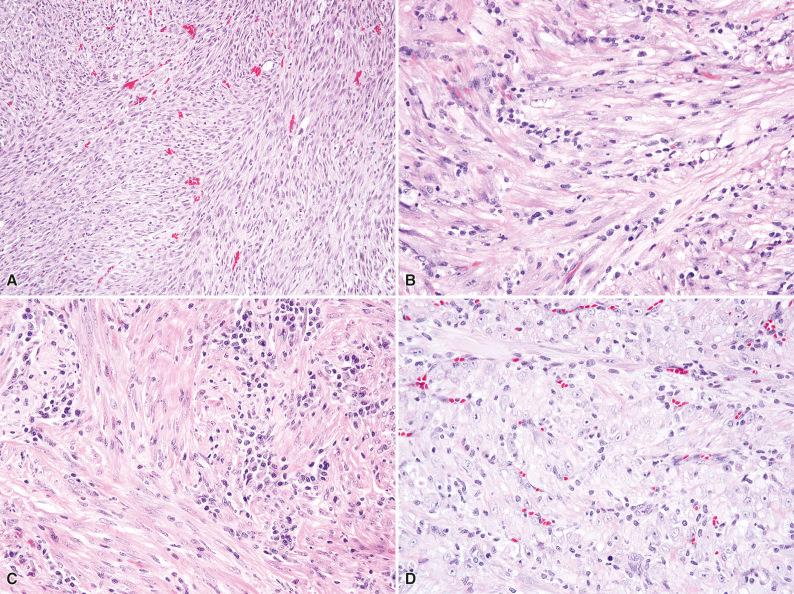
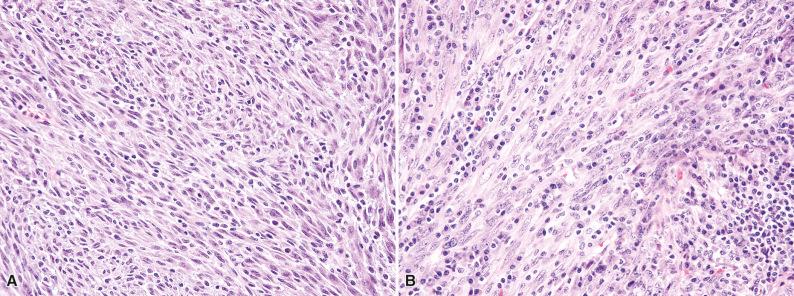
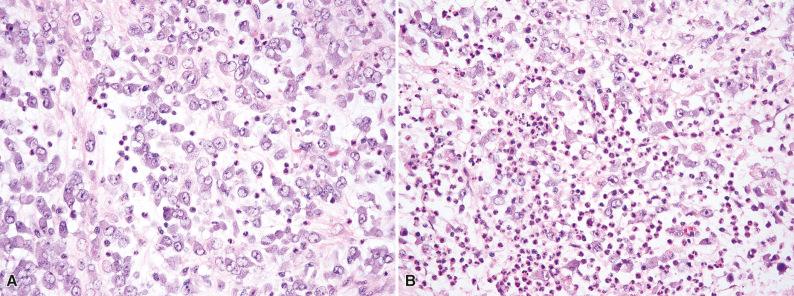
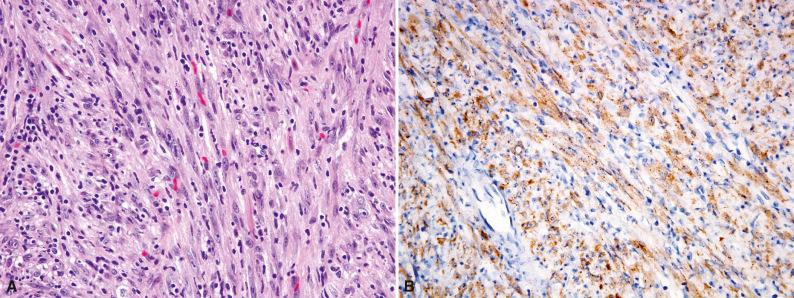
Histologically, IMT is composed of variably cellular fascicles of uniform plump spindle cells with elongated, tapering nuclei, vesicular chromatin, small nucleoli, and palely eosinophilic cytoplasm with ill-defined cell borders ( Fig. 10.1 ). The presence of more than mild nuclear atypia or pleomorphism should suggest an alternative diagnosis (i.e., a sarcoma). Areas of myxoid stroma with prominent vessels or hyalinized collagenous stroma are often seen. About 50% of tumors contain a population of polygonal ganglion-like myofibroblasts with eccentric nuclei and eosinophilic to amphophilic cytoplasm (see Fig. 10.1D ). Most IMTs contain a prominent infiltrate of plasma cells and lymphocytes ( Fig. 10.2 ), which is a helpful clue to the diagnosis; eosinophils and neutrophils are less often encountered. A distinctive variant of IMT with a predilection for the mesentery and omentum is dominated by epithelioid cells with vesicular nuclei and large nucleoli, in a prominent myxoid stroma (epithelioid inflammatory myofibroblastic sarcoma). This variant characteristically contains a prominent infiltrate of neutrophils ( Fig. 10.3 ).
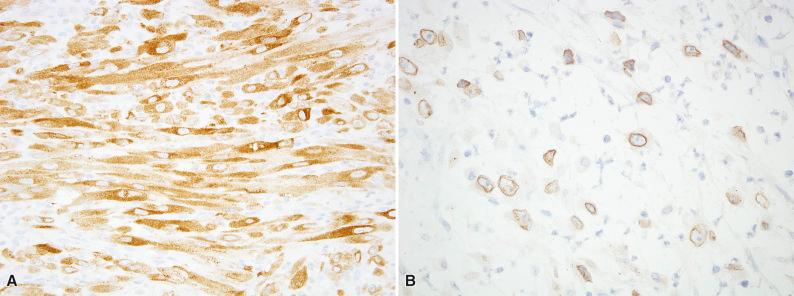
By immunohistochemistry, IMTs show a similar phenotype as other myofibroblastic tumors. Smooth muscle actin (SMA) is positive in 80% of IMTs, desmin in 60% of tumors, and keratins in about one third of cases. About 50% of IMTs show reactivity for anaplastic lymphoma kinase (ALK), which reflects the presence of ALK gene rearrangements. The pattern of ALK staining is determined by the fusion partner; most tumors show diffuse cytoplasmic staining ( Fig. 10.4A ), whereas epithelioid inflammatory myofibroblastic sarcoma usually shows a nuclear membrane pattern (see Fig. 10.4B ), less often a cytoplasmic pattern with perinuclear accentuation. Around 5% to 10% of IMTs are positive for ROS1 ( Fig. 10.5 ), which correlates with the presence of ROS1 gene rearrangements.
About 50% of IMTs contain translocations involving the ALK gene, with diverse fusion partners (see Chapter 18 for more details). Epithelioid inflammatory myofibroblastic sarcomas usually harbor an RANBP2-ALK fusion (correlating with a nuclear membrane pattern of ALK staining); a small subset harbors an RRBP1-ALK fusion. Of the IMTs that lack ALK gene rearrangements, 5% to 10% harbor ROS1 gene fusions. Rare IMTs harbor ETV6 rearrangements (including ETV6-NTRK3 ) ; individual cases with PDGFRB or RET fusions have also been reported.
The differential diagnosis of IMT may include various spindle cell sarcomas (especially leiomyosarcoma and low-grade myofibroblastic sarcoma), dedifferentiated liposarcoma, desmoid fibromatosis, gastrointestinal stromal tumor (GIST), and dendritic cell sarcomas. When IMT is positive for ALK or ROS1, the diagnosis is relatively straightforward; ALK and ROS1-negative IMTs pose particular diagnostic problems. Even low-grade spindle cell sarcomas show a greater degree of nuclear atypia and variability than IMT; this is one of the most important features to help exclude the diagnosis of IMT. In contrast, prominent plasma cells favor IMT over various sarcoma types.
Leiomyosarcomas generally contain cells with broader nuclei and more brightly eosinophilic cytoplasm than IMT. Inflammatory leiomyosarcoma is a rare variant that may easily be confused with IMT. The presence of prominent foamy histiocytes and areas of the tumor with typical histologic features of leiomyosarcoma (i.e., tight fascicles, broad and blunt-ended nuclei, brightly eosinophilic cytoplasm) are helpful diagnostic clues. SMA, desmin, and keratin expression are shared by IMT and leiomyosarcoma; caldesmon expression (when present) favors leiomyosarcoma, and reactivity for ALK or ROS1 supports IMT. Unlike IMT, low-grade myofibroblastic sarcoma typically arises in somatic soft tissues, rarely contains a prominent inflammatory infiltrate, and shows more notable nuclear atypia and infiltrative margins. Morphologically low-grade areas of dedifferentiated liposarcoma may closely mimic IMT. However, dedifferentiated liposarcoma affects older individuals and characteristically shows intratumoral heterogeneity, including pleomorphic areas, which is not a feature of IMT. Dedifferentiated liposarcoma may express SMA and desmin but is negative for ALK and ROS1. Identification of the well-differentiated adipocytic component confirms the diagnosis of dedifferentiated liposarcoma.
IMT with stromal hyalinization may be confused with desmoid fibromatosis, particularly cases that arise in the mesentery. However, desmoid tumors characteristically contain longer fascicles than IMT, and although a lymphocytic infiltrate may be present, plasma cells are usually absent. Aberrant nuclear staining for β-catenin can help confirm the diagnosis of desmoid fibromatosis. IMT may mimic spindle cell GIST, given its propensity to arise in the abdomen. In contrast to IMT, GIST has a syncytial appearance with fibrillary cytoplasm and lacks the plump myofibroblastic nuclei of IMT. Some GISTs contain prominent stromal lymphocytes, but the presence of plasma cells favors IMT. This differential diagnosis may easily be resolved with immunohistochemistry, because spindle cell GISTs are nearly always positive for KIT and DOG1, whereas ALK and ROS1 are negative.
Several dendritic cell neoplasms are also composed of spindle cells with a prominent chronic inflammatory infiltrate. Follicular dendritic cell sarcoma typically shows a more storiform or whorled growth pattern and a more syncytial appearance than IMT. Expression of CD21, CD35, and podoplanin (D2-40) confirms the diagnosis of follicular dendritic cell sarcoma in this differential diagnosis. Interdigitating dendritic cell sarcoma is exceedingly rare and usually arises in lymph nodes. This tumor type is diffusely positive for S-100 protein, whereas SMA, desmin, and ALK are negative.
IMT belongs to the group of mesenchymal neoplasms of intermediate biologic potential, because of the significant risk of local recurrence and low rate of metastasis (<5%). Intraabdominal and retroperitoneal tumors are particularly prone to recur locally (at least 25% risk), whereas the chance of recurrence of pulmonary IMT is much lower (about 5%). Complete surgical excision without adjuvant therapy is appropriate treatment. Histologic features do not correlate with clinical behavior, with rare exceptions. The distinctive intraabdominal variant of IMT with epithelioid morphology (epithelioid inflammatory myofibroblastic sarcoma) pursues an aggressive clinical course. Patients with aggressive, recurrent or metastatic IMTs (including epithelioid inflammatory myofibroblastic sarcoma) with ALK or ROS1 rearrangements may be effectively treated with targeted kinase inhibitor therapies.
Usually affects children and young adults
Lung, abdominal cavity, and retroperitoneum most common sites
Fascicles of uniform plump spindle cells with vesicular chromatin and palely eosinophilic cytoplasm
Pleomorphism is not a feature
Prominent infiltrate of lymphocytes and plasma cells is typical
Fifty percent positive for ALK and harbors ALK gene rearrangement
Five percent to 10% positive for ROS1 and harbors ROS1 gene rearrangement
Intermediate biologic potential: significant risk of local recurrence (lung: 5%; intraabdominal: at least 25%), but low metastatic rate (<5%)
Inflammatory leiomyosarcoma is a rare leiomyosarcoma variant with a prominent inflammatory infiltrate, which may obscure the neoplastic cell population and may easily be mistaken for other tumor types. This lesion was originally buried within the inflammatory MFH category. This variant has a peak incidence in young adults, and it may also affect children, with no gender predilection. Inflammatory leiomyosarcoma has a predilection for somatic soft tissue, particularly the extremities and trunk.
Histologically, the tumor is composed of spindle cells with a variably fascicular or storiform architecture, in large areas masked by prominent inflammation ( Fig. 10.6 ). The inflammatory component is often dominated by histiocytes, including aggregates of foam cells (see Fig. 10.6B ); lymphocytes are also prominent (see Fig. 10.6C ). Occasional cases may contain a neutrophilic infiltrate. In areas with less prominent inflammation, the typical cytoarchitectural features of leiomyosarcoma can be identified.
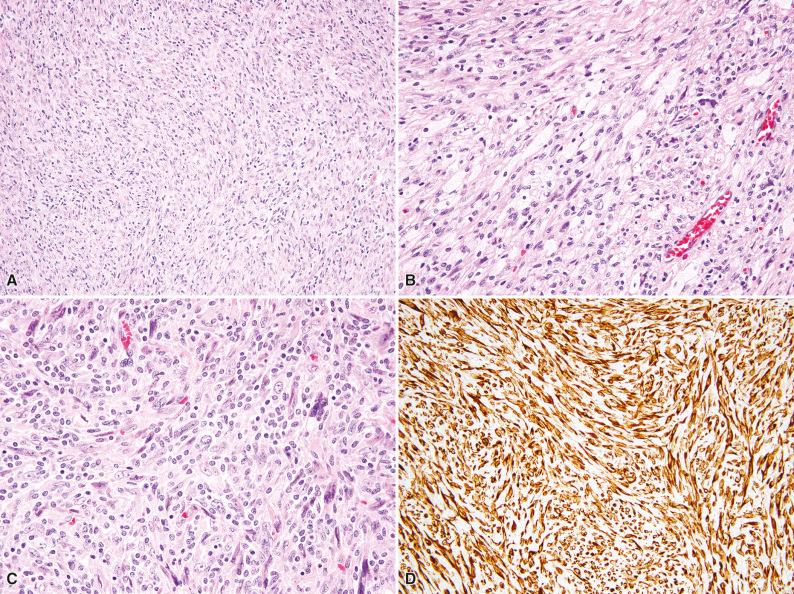
By immunohistochemistry, most cases of inflammatory leiomyosarcoma are positive for SMA and desmin (see Fig. 10.6D ), and they may also express caldesmon. By cytogenetics, hyperhaploidy (karyotype with 24–34 chromosomes), a rare finding in tumors, has been identified in nearly all cases studied ; the gene expression signature of inflammatory leiomyosarcoma is distinct from conventional leiomyosarcoma.
The differential diagnosis of inflammatory leiomyosarcoma includes IMT, dendritic cell tumors, and so-called inflammatory MFH (i.e., dedifferentiated liposarcoma). In contrast to inflammatory leiomyosarcoma, the inflammatory infiltrate in IMT often includes prominent plasma cells, and the degree of nuclear atypia is usually greater in inflammatory leiomyosarcoma. There is significant immunophenotypic overlap between these tumor types, although strong, diffuse staining for desmin and caldesmon expression favor leiomyosarcoma, whereas ALK or ROS1 expression confirms the diagnosis of IMT. Follicular dendritic cell sarcoma rarely contains prominent foamy histiocytes and is positive for CD21 and CD35. Interdigitating dendritic cell sarcoma is strongly positive for S-100 protein and lacks smooth muscle marker expression. Dedifferentiated liposarcoma has a predilection for the retroperitoneum and abdominal cavity of older adults and rarely arises in somatic soft tissue. When dedifferentiated liposarcoma shows the inflammatory MFH pattern, neutrophils usually predominate, and the tumor cells are scattered pleomorphic cells within the dense inflammatory background; the cellular, fascicular spindle cell component of inflammatory leiomyosarcoma is absent. Thorough sampling usually reveals heterogeneous histologic patterns in the dedifferentiated component, as well as areas of well-differentiated liposarcoma. Dedifferentiated liposarcoma often also expresses SMA and desmin; detection of MDM2 and CDK4 overexpression by immunohistochemistry or MDM2 amplification by FISH is helpful to confirm the diagnosis.
Although experience is limited, inflammatory leiomyosarcoma appears to pursue a less aggressive clinical course than conventional leiomyosarcoma. The metastatic rate seems to be relatively low.
Tumors composed of histiocytes (phagocytic cells) and dendritic cells (antigen-presenting cells) are rare. Such tumors typically arise at lymphoid tissue–rich anatomic sites (e.g., lymph nodes, tonsils, and spleen), but many of them can also occur in the abdominal cavity, soft tissues, and skin, among other locations.
In a broad sense, dendritic cell tumors can be separated into those that are hematopoietic in origin (bone marrow derived from the myeloid/monocytic lineage, such as histiocytic sarcoma) and those that are mesenchymal (stromal) in origin ( Box 10.4 ). The former category also includes the relatively common LCH, its exceedingly rare malignant counterpart Langerhans cell sarcoma, indeterminate cell histiocytosis, and interdigitating dendritic cell sarcoma. The plasticity of myeloid-derived histiocytic and dendritic cell tumors is illustrated by the occasional occurrence of clonally related metachronous (or synchronous) lymphoid neoplasms (such as T-lymphoblastic lymphoma and LCH harboring identical T-cell receptor gene rearrangements, and follicular lymphoma or chronic lymphocytic leukemia and histiocytic or interdigitating dendritic cell sarcomas harboring identical immunoglobulin gene rearrangements and/or translocations). Stromal-derived dendritic cell tumors include follicular dendritic cell sarcoma and the poorly characterized and exceptionally rare fibroblastic reticular cell sarcoma.
Langerhans cell histiocytosis
Langerhans cell sarcoma
Interdigitating dendritic cell sarcoma
Indeterminate cell histiocytosis
Histiocytic sarcoma
Rosai-Dorfman disease
Follicular dendritic cell sarcoma
Fibroblastic reticular cell sarcoma
The most common dendritic cell tumor in this group overall is LCH, which is familiar to and well recognized by surgical pathologists. LCH presenting as a solitary lesion is usually clinically benign. The most common malignant dendritic cell tumor is follicular dendritic cell sarcoma. This rare tumor type may mimic other more common mesenchymal (and nonmesenchymal) neoplasms histologically, arise at anatomic sites where other tumor types would be clinically much more likely (e.g., gastrointestinal tract, retroperitoneum, and mediastinum), and metastasize to the liver and lungs. Thus, follicular dendritic cell sarcoma continues to be underrecognized by pathologists and is not uncommonly misdiagnosed. Because histiocytic and dendritic cell tumors often contain a prominent inflammatory infiltrate (which is a helpful diagnostic clue), they will be discussed in this chapter.
Follicular dendritic cells normally reside in (and provide architectural support for) lymphoid follicles and present antigens to B lymphocytes. As such, follicular dendritic cell sarcomas typically arise at lymphoid tissue–rich anatomic sites. Among dendritic cell tumors, follicular dendritic cell sarcoma is most likely to be confused with other soft tissue tumors.
Follicular dendritic cell sarcoma affects adults over a wide age range, with a peak in young to middle-aged adults, with no gender predilection. Although it may develop primarily in lymph nodes (most commonly in the abdomen, axilla, mediastinum, and neck), it more often arises at extranodal sites, such as the gastrointestinal tract, mediastinum, and tonsils. Follicular dendritic cell sarcoma arises in a background of hyaline-vascular Castleman disease in 5% to 10% of cases. There are reports of follicular dendritic cell sarcoma associated with myasthenia gravis and other autoimmune disorders in rare cases.
The very rare inflammatory pseudotumor-like variant of follicular dendritic cell sarcoma shows a female predominance and occurs almost exclusively in the liver and spleen.
Grossly, most follicular dendritic cell sarcomas are between 3 and 12 cm in greatest dimension, with a mean size of 5 to 7 cm. The cut surface is fleshy in appearance.
Histologically, follicular dendritic cell sarcoma is composed of ovoid to spindled cells with vesicular chromatin, small nucleoli, and moderate amounts of palely eosinophilic cytoplasm with ill-defined cell borders, arranged in a storiform, whorled (meningioma-like), or fascicular growth pattern ( Fig. 10.7 ). Some tumors show epithelioid cytomorphology (see Fig. 10.7C ). Scattered multinucleated cells are common. Prominent admixed lymphocytes and occasional plasma cells are typically seen. Although most cases show relatively bland, uniform cytomorphology, occasional examples show notable nuclear atypia and may contain pleomorphic cells (see Fig. 10.7D ).
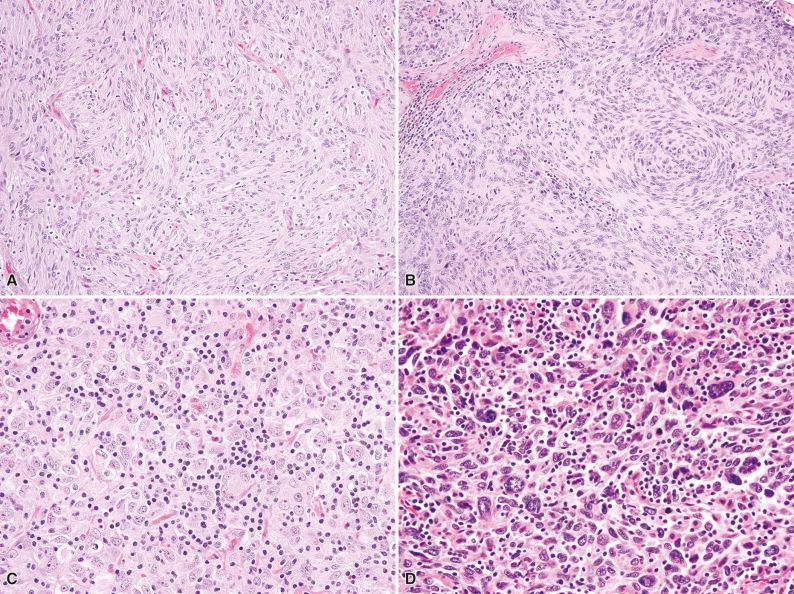
The inflammatory pseudotumor-cell variant of follicular dendritic cell sarcoma shows a loose fascicular and sheet-like growth pattern of spindle cells with more variable cytomorphology. These cells are admixed with a prominent chronic inflammatory infiltrate, which may occasionally obscure the neoplastic cells ( Fig. 10.8 ).
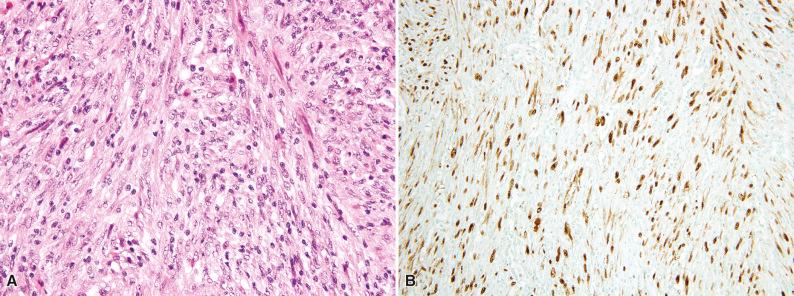
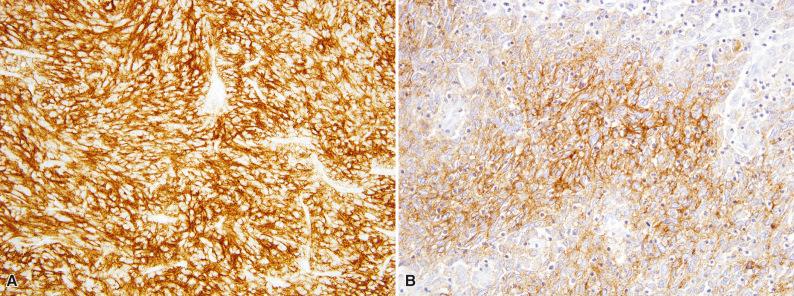
Follicular dendritic cell sarcoma is usually positive for the normal follicular dendritic cell markers CD21, CD23, and/or CD35 ( Fig. 10.9 ). Clusterin, podoplanin (D2-40), and CXCL13 are also highly expressed in follicular dendritic cell sarcoma. Epithelial membrane antigen (EMA) is at least focally positive in about 50% of cases (see Fig. 10.9B ), and S-100 protein is weakly expressed in about 10% of cases. The interspersed lymphocytes may be either CD20-positive B cells or CD3-positive T cells. The rare cases associated with myasthenia gravis contain immature (TdT-positive) T cells. Approximately 50% of follicular dendritic cell sarcomas show strong and diffuse expression of PD-L1. In contrast to conventional follicular dendritic cell sarcoma, Epstein-Barr virus–encoded RNA (EBER) is positive in the inflammatory pseudotumor-like variant (see Fig. 10.8B ).
Few studies have investigated the genetics of follicular dendritic cell sarcoma. Preliminary data include recurrent mutations in NF-κB regulatory genes ( NFKBIA , CYLD , and TNFAIP3 ), BRAF V600E mutations, loss of tumor suppressor genes ( CDKN2A and RB1 ), and copy number gain at chromosome 9p24, including the PDCD1LG1 (PD-L1) and PDCD1LG2 (PD-L2) loci in a subset of cases. However, a unifying pathogenetic basis for this neoplasm has not yet been identified.
The differential diagnosis of follicular dendritic cell sarcoma includes thymoma, interdigitating dendritic cell sarcoma, metastatic undifferentiated (e.g., nasopharyngeal) carcinoma, and GIST.
When follicular dendritic cell sarcoma arises in the mediastinum, it may easily be confused with thymoma because of its whorled growth pattern, spindle cell morphology, and admixed lymphocytes. However, keratins and p63 are diffusely positive in thymomas, whereas follicular dendritic cell markers (CD21, CD35) are consistently negative. When follicular dendritic cell sarcoma arises in lymph nodes or the spleen, interdigitating dendritic cell sarcoma is a diagnostic consideration ( Table 10.1 ). Interdigitating dendritic cell sarcoma shows a paracortical distribution in lymph nodes and lacks the storiform and whorled growth pattern of follicular dendritic cell sarcoma. Unlike follicular dendritic cell sarcoma, interdigitating dendritic cell sarcoma is diffusely positive for S-100 protein, usually also expresses CD45RO, and is negative for CD21, CD35, and other follicular dendritic cell markers.
| LCA | CD45RO | CD68 | CD163 | S-100 | CD1a | CD21/CD35 | Other | |
|---|---|---|---|---|---|---|---|---|
| Histiocytic sarcoma | ± | + | + | + | ± | − | − | PU.1 (SPI1), lysozyme, CD4, CD31 positive |
| Rosai-Dorfman disease | + | + | ± | ± | + | − | − | — |
| Langerhans cell histiocytosis/Langerhans cell sarcoma | ± | ± | ± | − | + | + | − | Langerin positive |
| Follicular dendritic cell sarcoma | − | − | − | − | ~10% | − | + | D2-40, clusterin, CXCL13 positive; ~50% focal EMA positive |
| Inflammatory pseudotumor-like follicular dendritic cell sarcoma | − | − | − | − | − | − | + | EBER+ |
| Interdigitating dendritic cell sarcoma | ± | + | ± | − | + | − | − | — |
| Indeterminate cell histiocytosis | ± | ± | ± | − | + | + | − | Langerin negative |
| Fibroblastic reticular cell sarcoma | − | − | − | − | − | − | − | Keratin, desmin, SMA variably positive |
EMA-positive follicular dendritic cell sarcomas involving lymph nodes (particularly in the neck) may be mistaken for metastatic undifferentiated or sarcomatoid carcinomas, especially because follicular dendritic cell markers are not usually included in typical immunohistochemical panels. However, keratins are nearly always negative in follicular dendritic cell sarcoma. Metastatic nasopharyngeal undifferentiated carcinoma is also positive for p63 and EBER; Epstein-Barr virus markers are only positive in the inflammatory pseudotumor-like variant of follicular dendritic cell sarcoma of the spleen and liver. Given its pale cytoplasm, indistinct cell borders, and propensity to metastasize to the liver, follicular dendritic cell sarcomas that arise in the gastrointestinal tract may be mistaken for KIT-negative GISTs. However, a dense lymphocytic infiltrate is uncommon in GIST. CD21 and CD35 are also negative in GIST, whereas DOG1 and CD34 are consistently negative in follicular dendritic cell sarcoma.
The inflammatory pseudotumor-like variant of follicular dendritic cell sarcoma may closely mimic IMT. The anatomic site (spleen or liver) is a helpful clue to the diagnosis. In addition, unlike follicular dendritic cell sarcoma, IMT is usually positive for SMA. Many tumors also express desmin, whereas 50% of tumors are positive for ALK (and harbor ALK gene rearrangements). CD21 and CD35 are negative in IMT.
Follicular dendritic cell sarcoma has a variable clinical course. Tumors arising in lymph nodes are often clinically indolent, with a small risk of metastasis (about 10%). In contrast, extranodal tumors (especially intraabdominal examples) have significant metastatic potential (>50%). The most common metastatic sites include the liver, lung, lymph nodes, and bones. Large tumor size, a high mitotic rate, marked pleomorphism, and extensive necrosis may be adverse prognostic features. Complete surgical excision should be attempted. The role of radiation therapy and chemotherapy has not been well established.
Arise in lymph nodes or at extranodal sites (e.g., gastrointestinal tract, mediastinum, tonsil)
Ovoid, spindled, or epithelioid cells with vesicular chromatin and pale, syncytial cytoplasm
Storiform and whorled growth pattern
Prominent infiltrate of lymphocytes is typical
Positive for CD21, CD23, CD35, podoplanin (D2-40), clusterin, and CXCL13
Variable expression of epithelial membrane antigen (EMA) and S-100 is a diagnostic pitfall
Extranodal tumors have significant metastatic potential
Inflammatory pseudotumor-like variant arises in liver and spleen, resembles inflammatory myofibroblastic tumor, and is positive for Epstein-Barr virus–encoded RNA (EBER)
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here