Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The overwhelming majority of human tumors showing melanocytic differentiation occur in the skin and represent benign melanocytic nevi, their variants, and malignant melanomas. However, a small percentage of tumors showing melanocytic differentiation occur in unusual locations, such as the meninges (melanocytoma), in association with nerves, and in visceral and somatic soft tissue locations. This chapter addresses several unusual soft tissue and visceral tumors showing melanocytic differentiation, including malignant melanotic schwannian tumor (melanotic schwannoma), melanotic neuroectodermal tumor of infancy, clear cell sarcoma, and perivascular epithelioid cell neoplasms. It also covers a very rare visceral sarcoma, malignant gastrointestinal neuroectodermal tumor, which does not show melanocytic differentiation but was historically confused with conventional clear cell sarcoma. Spindle cell/desmoplastic melanoma, which frequently enters the differential diagnosis of malignant peripheral nerve sheath tumor and neurofibroma, is discussed in Chapter 27 .
A rare form of pigmented neural tumor usually arising from the sympathetic nervous system was described in 1932 by Millar as “malignant melanotic tumor of ganglion cells,” discussed under the term melanocytic schwannoma by Fu et al., and redesignated “psammomatous melanotic schwannoma” by Carney, who noted its association with Carney complex. Melanotic schwannoma is a distinctive neoplasm of adult life that differs significantly from classic schwannoma, despite the similarity in the names. The tumor usually arises from the spinal or autonomic nerves near the midline. However, a number of cases have been reported in the stomach and in bone and soft tissues. Unusual sites include the heart, bronchus, liver, and skin. In some series, more than 50% of patients with the tumor have evidence of Carney complex , which includes myxomas of the heart, skin, and breast, spotty pigmentation caused by lentigenes, blue nevus and the distinctive epithelioid blue nevus, endocrine overactivity manifested by Cushing disease (pigmented nodular adrenal disease), acromegaly (pituitary adenoma), or sexual precocity (Sertoli cell tumor). However, other series of melanotic schwannoma have noted an association with Carney complex in 5% or less of affected patients. The tumor typically develops at an earlier age (average 22.5 years) in patients with Carney complex than in those without the syndrome (average 33.2 years). About 20% of patients with melanotic schwannomas have multiple tumors and an even higher probability that other manifestations of Carney complex will be present. The symptoms related specifically to the tumor depend on its location and rate of growth, but most frequently are pain and neurologic symptoms in the affected part. Most striking is a case in which the patient lost sympathetic nerve function in the ipsilateral lower extremity.
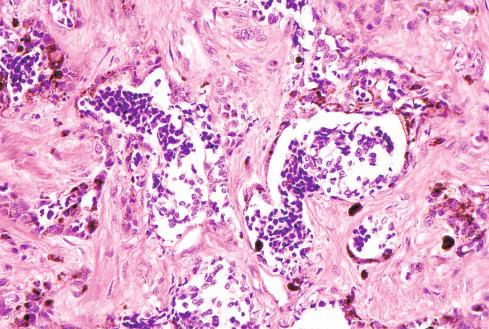
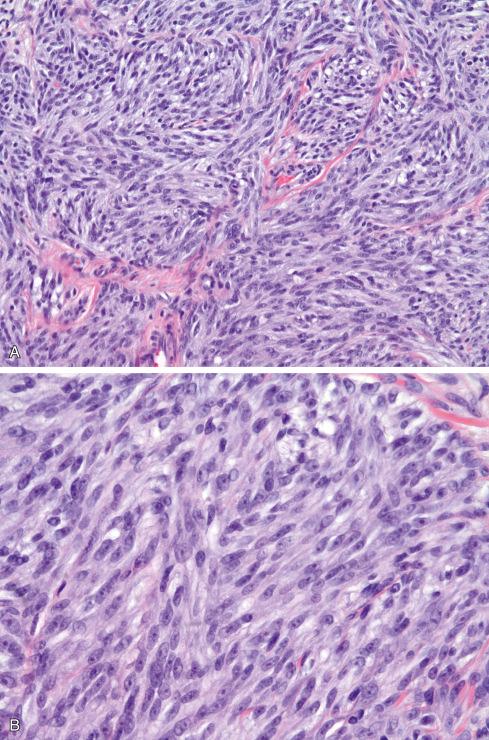
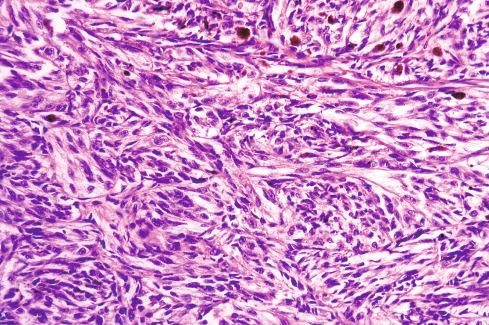
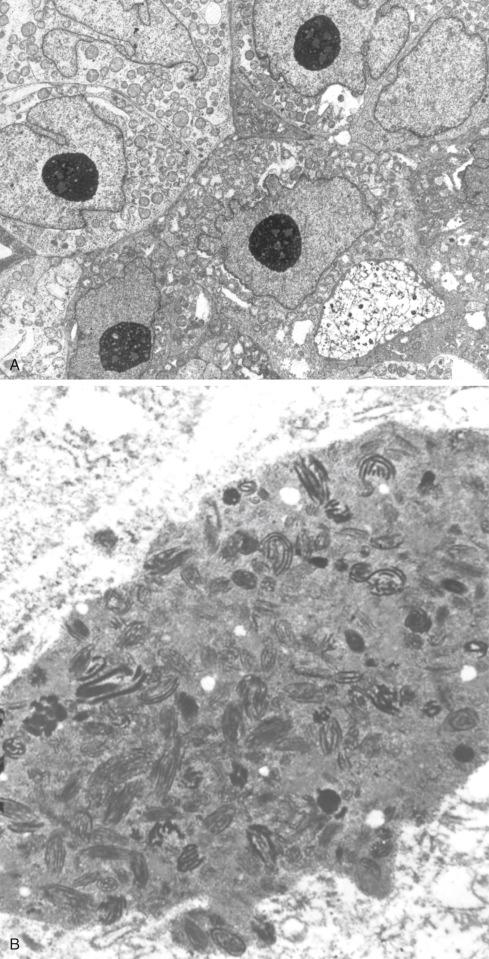
The tumors are usually circumscribed or encapsulated and vary from black-brown to gray-blue. The neoplastic cells grow in fascicles or sheets, vary in shape from polygonal to spindled, and blend gradually from one to another ( Figs. 29.1 to 29.5 ). This feature, coupled with the ill-defined borders of the cytoplasm, often imparts a syncytial quality to the tumors that is somewhat reminiscent of a schwannoma. It is often difficult to make out cellular detail in these tumors because of the heavy pigment deposits ( Figs. 29.2 and 29.5 ). Usually, there are at least focal areas with little or no pigment, so the character of the cells can be evaluated. Likewise, the nuclei may display clear intranuclear cytoplasmic (pseudo) inclusions characteristic of Schwann cells ( Fig. 29.2 .) Nuclear hyperchromatism may be marked, and the nucleoli are often prominent ( Fig. 29.6 ). Occasionally, there is vague palisading or formation of whorled structures so that the tumor resembles a schwannoma or neurofibroma. Psammoma bodies are present in most cases, although extensive sampling may be required to identify them ( Fig. 29.3 ). There are no clinical differences between psammomatous and nonpsammomatous malignant melanotic schwannian tumors, with both showing a variable association with Carney complex, loss of PRKAR1A expression, and similar clinical behavior.
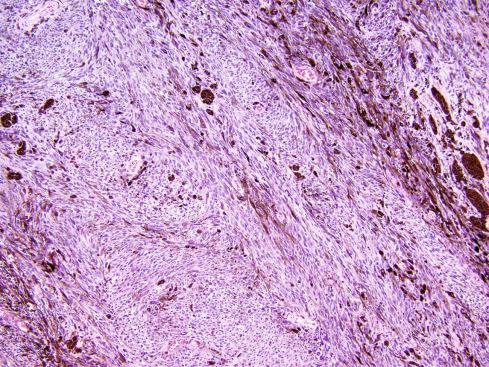
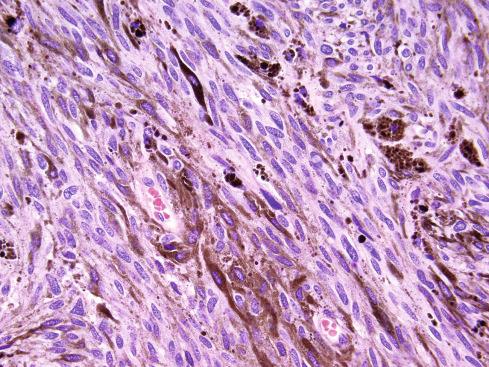
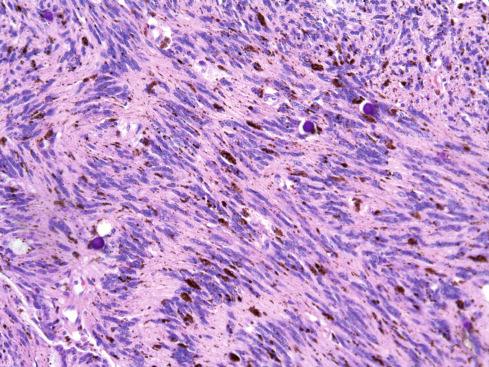
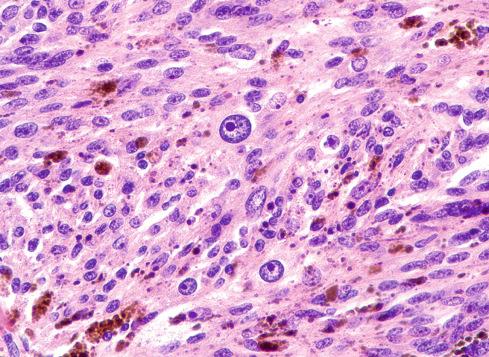
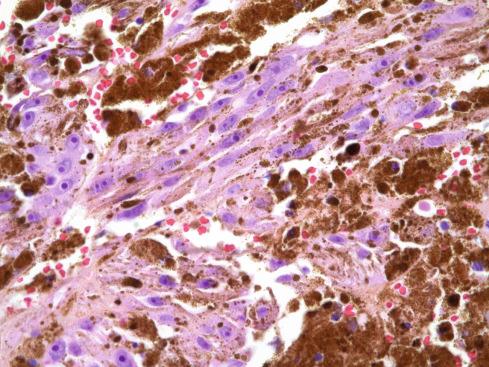
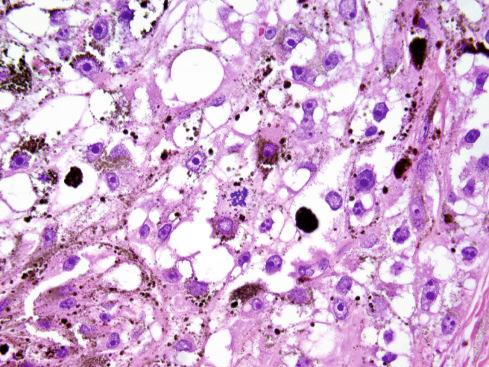
The melanin pigment may be coarsely clumped or finely granular and varies from area to area. Tinctorially, it is similar to dermal melanin and stains positively with Fontana and negatively for iron and periodic acid–Schiff (PAS). In this respect, it differs from the faint and focal pigment seen in conventional schwannomas, which is neural melanin. On immunohistochemistry (IHC), these tumors strongly express S-100 protein, SOX10, and various melanocytic markers, including HMB-45, melan-A, and tyrosinase. Ultrastructurally, there is a spectrum of maturation, including premelanosomes and melanosomes, strongly suggesting that the pigment is synthesized by the tumor cell. Except for the presence of melanosomes, the cells resemble Schwann cells with elaborate cytoplasmic processes that interdigitate or spiral in the manner of mesaxons.
Genetic studies show malignant melanotic schwannian tumors to have clearly different gene expression profiles than conventional schwannomas or melanomas, supporting their classification as a distinct entity. Torres-Mora et al. first demonstrated loss of expression of the Carney complex–associated gene, PRKAR1A , in malignant melanotic schwannian tumors both in patients with and in those without other stigmata of Carney complex. A recent series showed PRKAR1A mutations in all of 14 studied cases, only one of which occurred in a patient with known Carney complex.
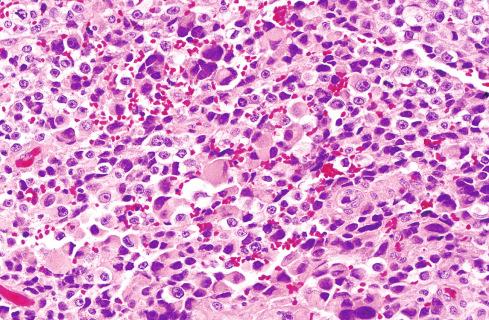
The biologic behavior of these tumors is difficult to predict, and metastases can occur in the absence of overt malignant features. Neither tumor size nor ploidy predicts malignant behavior. It was once thought that most of these lesions had a benign, indolent course. Metastases, for example, were reported in only 13% of patients with melanotic schwannomas and Carney complex. A review of approximately 60 cases in the literature has disclosed metastasis in 26%. Furthermore, only 53% of patients followed for more than 5 years were disease free, suggesting that long-term follow-up is required to fully judge metastatic risk. Torres-Mora et al. found a local recurrence rate of 35% and a metastatic rate of 44%, with a greater risk for metastases in tumors showing a mitotic rate of greater than 2 figures per 10 high-power fields (hpf). Of the 14 cases studied by Wang et al., four (33%) metastasized and two resulted in patient death. Malignant melanotic schwannian tumors should thus be considered malignant neoplasms with an uncertain risk for aggressive behavior, rather than “unpredictable” but benign tumors. For this reason the term “malignant melanotic schwannian tumor” has been proposed to replace “melanotic schwannoma.” Tumors showing significant mitotic activity may have a particularly high risk for aggressive clinical behavior. When metastases develop, they, too, abound with melanin pigment ( Fig. 29.7 ).
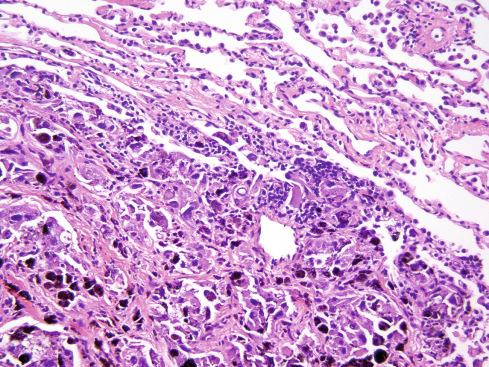
The usual problem in differential diagnosis is distinguishing this tumor from a metastatic malignant melanoma . Primary melanotic schwannomas usually do not have the degree of nuclear atypia or mitotic activity expected in a metastatic melanoma. The peculiar syncytial quality of the cells and when present, psammomatous calcification, are important features of melanotic schwannoma that metastatic melanomas lack. PRKAR1A loss is not a feature of conventional melanoma.
First described in 1918 by Krompecher, melanotic neuroectodermal tumor of infancy, a rare tumor of disputed histogenesis, has been referred to as congenital melanocarcinoma, melanotic adamantinoma, retinal anlage tumor, melanotic progonoma, and pigmented epulis of infancy. Although most current studies support a neural crest origin of this tumor, there is little evidence that the tumor specifically represents retinal anlage. Therefore, the preference is for the less fanciful term melanotic neuroectodermal tumor of infancy .
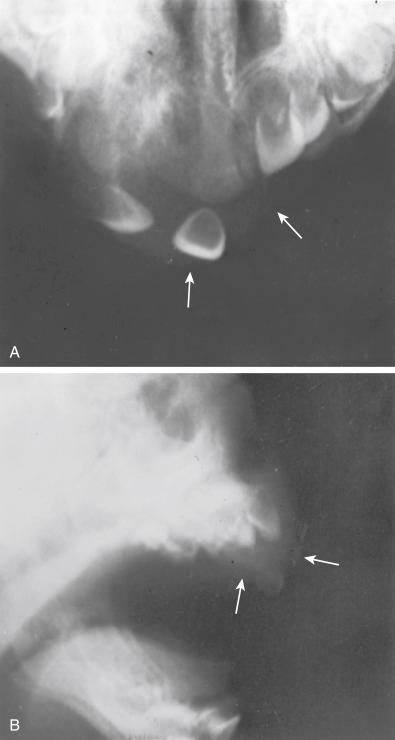
The tumor usually develops during the first year of life and presents as a protruding mass in the upper or lower jaw. The skin or mucosa is tightly stretched over the lesion, but it is rarely, if ever, ulcerated. Radiographically, the tumor is a cystic radiolucent lesion with a capacity for local destruction and displacement of the developing teeth ( Fig. 29.8 ). Patients with this tumor in unusual sites, such as the anterior fontanelle, epididymis, mediastinum, and brain, develop symptoms referable to those sites. The few cases reported in the uterus and shoulder and those in adults should be disregarded because they represent different lesions altogether. One of these tumors has been encountered in the soft tissues of the extremity, and it has also been reported in long bone.
Grossly, the tumor ranges in color from slate gray to blue-black, depending on the amount of melanin pigment. It is composed of irregular alveolar spaces lined by cuboidal cells containing varying amounts of melanin pigment ( Fig. 29.9 ). In addition, small, round, less well-differentiated cells resembling those of neuroblastoma lie in the alveolar space or as isolated nests in a fibrous stroma ( Fig. 29.10 ). Neurofibrillary material resembling glial tissue may be seen in association with these cells in the alveolar spaces. In two exceptional cases, glial tissue was found outside the epithelial islands: (1) a tumor arising in the brain in which the entire stroma was glial, and (2) a tumor arising in a glial heterotopia of the oropharynx.
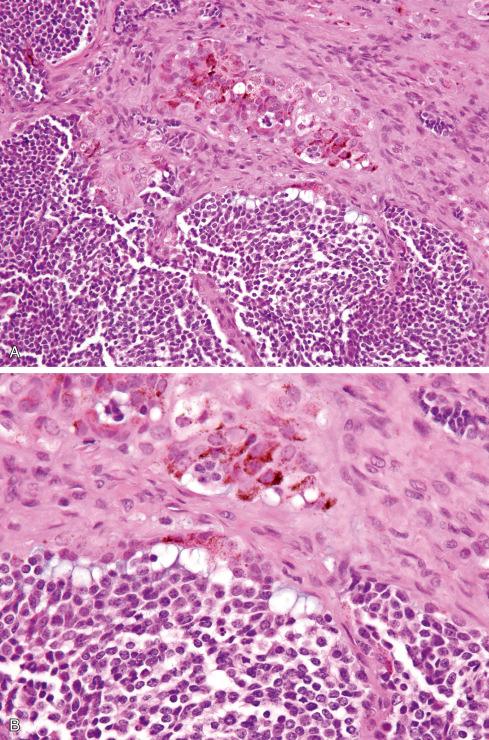
The cuboidal cells have the electron microscopy features of epithelial and melanocytic cells. They are bounded by basal laminae and elaborately interdigitate laterally with neighboring cells, forming desmosomes. Both mature and immature melanosomes similar to those of melanocytes and melanoma cells are present in the cytoplasm. Functionally, they share certain properties of the melanocyte in that melanization of these cells may be increased by agents that induce similar changes in melanocytes of animals. On IHC the cuboidal cells express keratin and melanocytic markers, such as HMB-45. Neuron-specific enolase (NSE), CD57, and synaptophysin are variably present in the epithelial cells and the small neuroblastic element.
The round, less well-differentiated cells contain few organelles but are believed to be neuroblastic by virtue of their elongated cell processes, dense-core vesicles, and intracytoplasmic neurofilamentous material. Their association with glial-like areas, and in one case with ganglioneuromatous areas, provides further support for this contention.
Traditionally, this tumor was considered benign, but in a large series from the Armed Forces of Pathology (AFIP), almost half those with follow-up information recurred, and 5% to 10% of cases reported in the literature have produced metastasis. One case, a stillborn, was noted to have multiple metastases at delivery. A second case, a tumor of the epididymis, produced micrometastases in regional lymph nodes, and two others metastasized as primitive neuroblastic tumors devoid of melanin. Although metastasis is a relatively uncommon event, attempts to eradicate the tumor at the initial surgery are endorsed. Unfortunately, to date, it has not been possible to predict recurrence or metastasis in this disease. Radiotherapy and chemotherapy have been tried in patients with unresectable or recurrent disease, with varying efficacy.
The histogenesis of this tumor has been controversial. The concepts of a congenital melanoma and an odontogenic tumor are now obsolete for various reasons. The former does not account for the primitive neuroblastic component, and the latter does not consider tumors at sites where there are no odontogenic rests. To date, the most appealing theory is that the tumor is derived from neural crest. This concept allows latitude in the distribution of the lesions, accounts for the presence of pigmented and neuroblastic elements, and explains the rare tumor associated with increased levels of vanillylmandelic acid. It does not seem necessary to compare this tumor specifically to the developing retina; in fact, the embryologic evidence against this possibility has been extensively summarized. It seems more probable that this tumor merely reflects a primitive stage or degree of differentiation common to many types of pigmented neuroepithelium. No specific genetic aberration has been identified in these tumors.
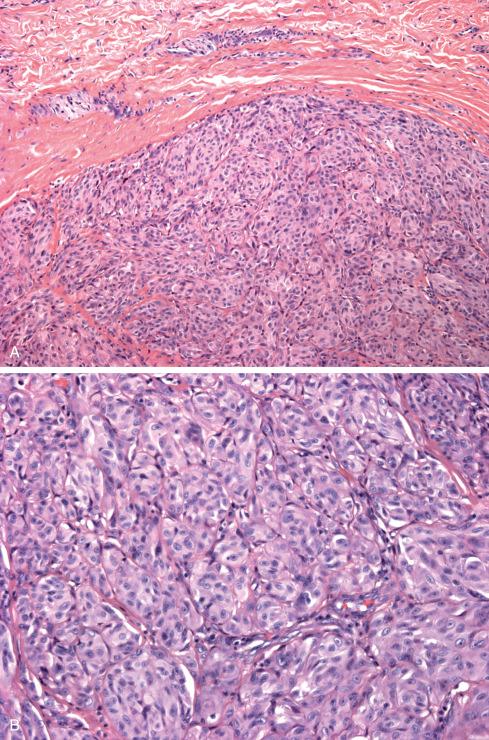
Described by Enzinger in 1965, clear cell sarcoma is a rare melanin-producing soft tissue sarcoma. Although unfortunately it has also been referred to as “malignant melanoma of soft parts,” it is clinically, genetically, and biologically distinct from cutaneous melanoma, despite certain histologic similarities; use of this term is discouraged. In contrast to cutaneous melanoma, clear cell sarcomas invariably arise in the deep soft tissue of the distal extremities, and 70% possess a consistent balanced translocation t(12;22)(q13;q12) that is not found in melanoma and is believed to be an early, if not primary, event in tumorigenesis. This translocation fuses EWSR1 on chromosome 22 with ATF1 , a member of the CREB transcription factor family on chromosome 12. This results in four fusion transcripts that are differentially expressed among tumors. The fusion protein mimics the action of melanocyte-stimulating hormone by binding to and constitutively activating the promoter for MiTF, the melanocyte master transcription factor. Evidence suggests that MiTF is a critical oncogenic target because it not only mediates melanin production in these tumors but also EWSR1-ATF1 –induced tumor growth. The EWSR1-ATF1 fusion also results in downstream activation of MET (c-Met), a finding that suggests a potential role for MET inhibitors such as crizotinib in the treatment of clear cell sarcoma
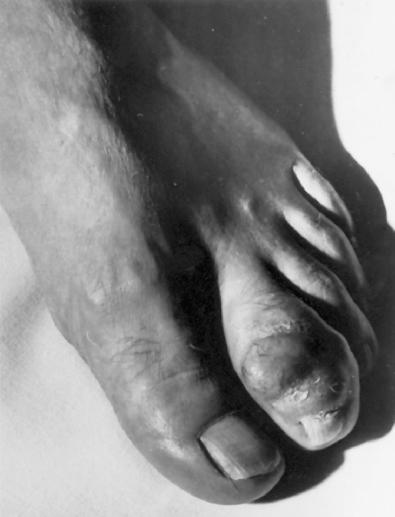
Clear cell sarcoma mainly affects young adults between ages 20 and 40 (median: about 30 years). Approximately 40% of cases occur on the foot and ankle ( Fig. 29.11 ), with another 30% on the knee, thigh, and hand. The head and neck region and the trunk are distinctly unusual sites ( Table 29.1 ). Clear cell sarcomas present as a slowly enlarging, occasionally painful mass, which is usually present about 2 years at the time of diagnosis, although a significant percentage has been present for 5 years or longer. They arise in deep soft tissue, and unless the lesion is extremely large or distal, the overlying skin and dermis are usually not involved. Exceptionally, however, genetically confirmed clear cell sarcoma may occur in the skin.
| Location | No. of Patients | (%) |
|---|---|---|
| Head and neck | 1 | 0.8 |
| Trunk | 3 | 2.1 |
| Upper extremity | 31 | 22.0 |
| Lower extremity (foot 28; knee 21; heel 15; ankle 11) | 106 | 75.1 |
| total | 141 | 100.00 |
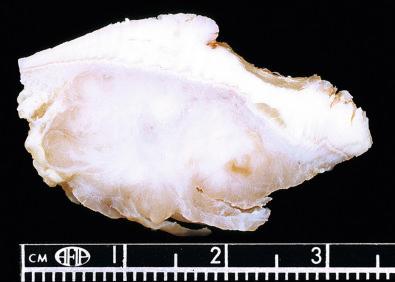
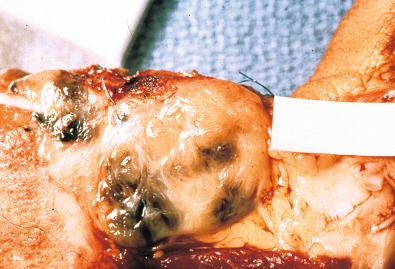
Macroscopically, the tumor consists of a lobulated or multinodular gray-white mass firmly attached to tendons or aponeuroses, which averages between 2 and 6 cm ( Fig. 29.12 ). The cut surface may be marred by focal hemorrhage, necrosis, or cystic change. In a small proportion of cases, the melanin may be prominent enough to be visualized as foci of dark-brown or black discoloration ( Fig. 29.13 ).
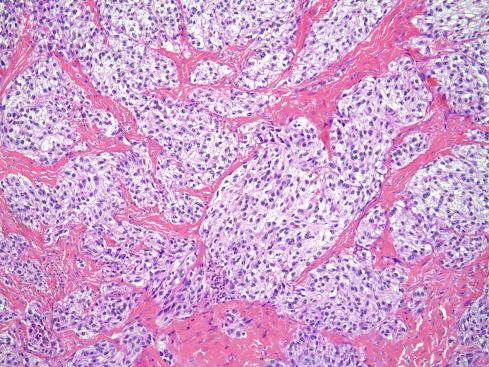
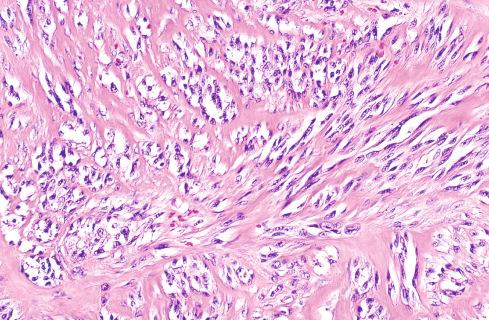
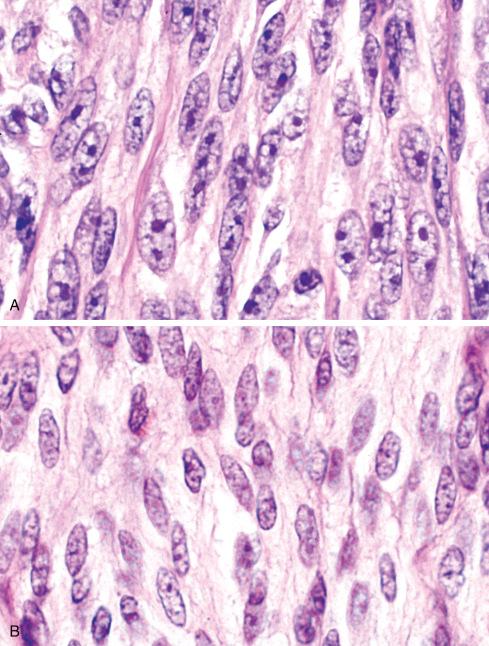
Histologically, tumors consist of compact nests or fascicles of predominantly fusiform or spindled cells with clear cytoplasm bordered and defined by a delicate framework of fibrocollagenous tissue contiguous with adjacent tendons or aponeuroses ( Figs. 29.14 to 29.19 ). The cells have highly distinctive features consisting of nuclei with a vesicular nuclear chromatin pattern and prominent basophilic nucleoli reminiscent of malignant melanoma. The cytoplasm varies from clear to weakly eosinophilic ( Figs. 29.15 and 29.16 ) and contains large amounts of intracellular glycogen. Clear cells and eosinophilic cells coexist in different portions of the same neoplasm with focal transitions between the two. A highly characteristic feature is the multinucleated tumor giant cells with 10 to 15 peripherally placed nuclei ( Fig. 29.16A ). In general, clear cell sarcomas are neither pleomorphic nor mitotically highly active, although this observation does not necessarily apply to recurrent or metastatic lesions, which appear more pleomorphic ( Fig. 29.20 ).
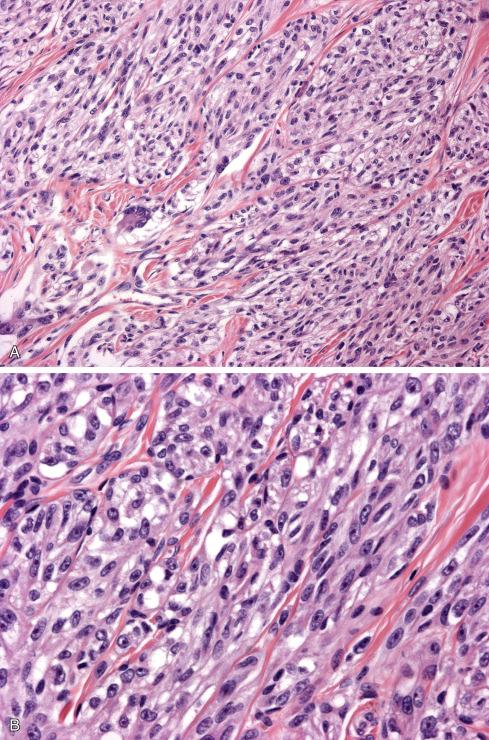
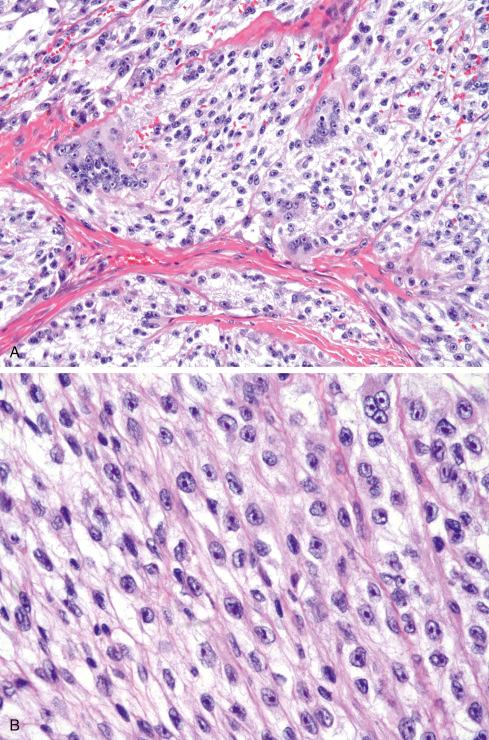
Melanin is present in more than 50% of clear cell sarcomas but is usually not abundant enough to be seen on hematoxylin-eosin stain. It can be detected with appropriate histochemical (Fontana or Warthin-Starry) and immunohistochemical stains as well as ultrastructurally by electron microscopy ( Fig. 29.22 ). Virtually all clear cell sarcomas diffusely express S-100 protein and SOX10 ( Fig. 29.21 ), and most also express antigens associated with melanin synthesis (HMB-45, melan-A, Mel-CAM, MiTF). NSE, CD57, and LN3 have also been noted in these lesions.
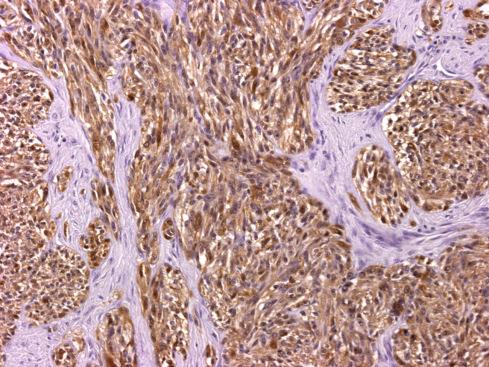
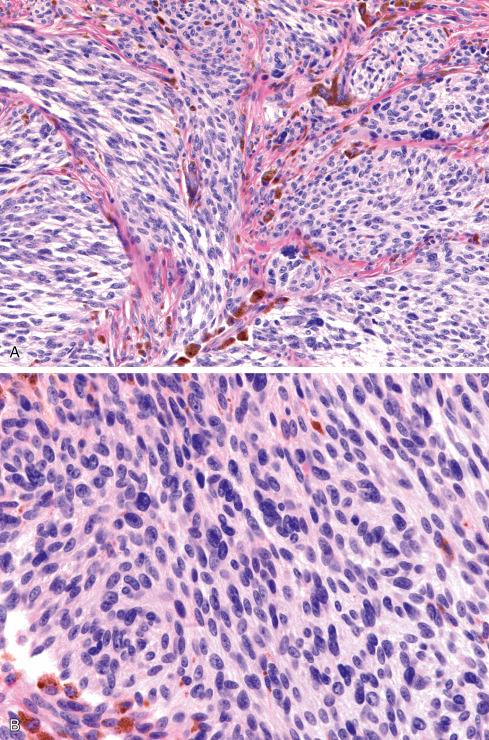
Because cytologic features are crucial in the diagnosis of clear cell sarcoma, care must be exercised to evaluate optimally preserved areas only. Poorly preserved or degenerated clear cell sarcomas having shriveled cells that cling to the fibrous bands are easily misconstrued as a round cell sarcoma , particularly alveolar rhabdomyosarcoma. In well-preserved material, however, the differential diagnosis typically includes, on the one hand, sarcomas with a predominant fascicular growth pattern, such as fibrosarcoma, synovial sarcoma, and malignant peripheral nerve sheath tumor, and on the other hand, melanin-producing tumors such as cellular blue nevus and nodular malignant melanoma. The distinctive cytologic features of clear cell sarcoma, including prominent melanoma-like nucleoli, clear cytoplasm, and immunophenotypic profile, set this lesion apart from the spindle cell sarcomas noted previously.
The distinction of clear cell sarcoma from other melanin-producing lesions can be more problematic and may require correlation of the histologic, clinical, and molecular data. In general, clear cell sarcomas originate in deep structures, rarely involve the dermis, and have a predominantly and relatively uniform spindle cell appearance that contrasts with the epithelioid appearance of nodular melanomas. However, in ambiguous situations, molecular genetic analysis is highly recommended because the t(12;22) that characterizes clear cell sarcoma has not been identified in malignant melanoma. BRAF V600E mutations, often found in conventional melanomas, can also occasionally be identified in clear cell sarcomas, and BRAF testing is not useful in this differential diagnosis. Cellular blue nevus can occur in a similar age and location and have certain common histologic features, including spindled cells and giant cells with clear cytoplasm. Cellular blue nevi typically are dermal-based lesions with a peripheral zone that resembles a neurofibroma by virtue of the interdigitation of slender, pigmented dendritic cells with surrounding collagen ( Fig. 29.23 ). The cells lack atypia and have small, pinpoint nucleoli, in contrast to the macronucleoli of clear cell sarcoma ( Fig. 29.24 ). Recurrent cellular blue nevi, however, can acquire more atypical cytologic features, so a distinction from clear cell sarcoma is not always possible. In these situations, review of the original material or molecular genetic analysis is essential. The recently described paraganglioma-like dermal melanocytic tumor , while having cells with a clear to eosinophilic cytoplasm, also has zellballen-like nests of cells of distinctly low nuclear grade ( Fig. 29.25 ). These lesions, based in the dermis, rarely extend to deep structures. At this point, all have behaved in a benign fashion. Dermal perivascular epithelioid cell neoplasms , later discussed in depth, typically show lesser degrees of nuclear atypia than do clear cell sarcoma, lack S-100 protein expression, and coexpress muscle and melanocytic markers. An unusual cutaneous melanocytic tumor resembling clear cell sarcoma, but showing CRTC1-TRIM11 fusion, has recently been reported as well.
Clear cell sarcoma is an extremely rare tumor for which it has been difficult to amass outcome and therapeutic data in a large patient population. For example, only 28 pediatric patients were referred to the Italian and German Soft Tissue Sarcoma Cooperative Group from 1980 to 2000. Nevertheless, there is agreement based on the largest studies that this is a high-grade sarcoma. Recurrences, which reflect the adequacy of initial surgery, range from 14% to 39%, whereas metastases to lung or lymph node develop in approximately one-half of patients within an interval of 2 to 8 years. Late metastases after 10 to 20 years have been reported in patients with repeated local recurrences. Because patients who develop local recurrences or regional lymph node metastases eventually develop distant metastases, controlling local disease and long-term surveillance are clearly needed. Given the risk for regional lymph node metastases in this disease, there has been recent interest in performing a sentinel lymph node biopsy. Picciotto et al. described the feasibility of identifying and excising sentinel nodes, but data are insufficient for a therapeutic recommendation.
Clear cell sarcoma has traditionally been considered an ungradable sarcoma, and thus several studies have attempted to identify other prognostic factors. Size and necrosis have proved to be the most robust prognostic factors, with tumors greater than 5 cm having a significantly worse outcome than smaller tumors. Other factors, such as age, location, depth, and proliferation index, are independent prognostic factors. Radical surgery is the mainstay of therapy, and chemotherapy has proved to have little efficacy.
Malignant gastrointestinal neuroectodermal tumor (MGNET), also known as clear cell sarcoma-like tumor of the gastrointestinal tract, and originally described as “osteoclast-rich tumor of the gastrointestinal tract with features resembling clear cell sarcoma of soft parts,” is extremely rare, with fewer than 30 reported cases. A case morphologically corresponding to this entity had been previously published by Alpers and Beckstead as a “malignant neuroendocrine tumor of the jejunum with osteoclast-like giant cells.” Since the description of this tumor by Zambrano et al., fewer than 50 cases have been reported. Reporting the largest series to date, Stockman et al. coined the term “malignant gastrointestinal neuroectodermal tumor” to describe these rare lesions. It now seems clear that MGNET differs significantly from soft tissue clear cell sarcoma, and we recommend use of this term to emphasize these differences.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here