Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Since antiquity, scientists, philosophers, writers, and religious scholars from all cultures and continents have repeatedly raised two fundamental questions—(1) what is sleep? and (2) why do we sleep?—without satisfactory answers.
Some 2000 years ago, Lucretius postulated that sleep is an absence of wakefulness. Macnish, in 1830, proposed a variation of Lucretius’s concept, defining sleep as “suspension of sensorial power in which the voluntary functions are absent but the involuntary functions, such as circulation, respiration, and other functions controlled by the autonomic nervous system, remain intact.” Sleep, however, is not simply an absence of wakefulness, nor is it simply suspension of sensorial power; it results from a combination of passive withdrawal of afferent stimuli to the brain and activation of certain neurons in selective brain areas.
Three basic physiological processes of life consist of wakefulness , nonrapid eye movement (NREM) sleep , and rapid eye movement (REM) sleep , with independent functions and controls. The invention of the electroencephalogram (EEG) in 1929, fundamental physiological studies to understand consciousness, sleep, and wakefulness in the 1930s and 1940s, and the discovery of REM sleep by ushered in the golden age of sleep medicine. In the second half of the last century, substantial advances were made in our scientific understanding of sleep and its disorders, and sleep medicine was established as an important clinical discipline. Scientific progress in sleep medicine has accelerated markedly in recent years, particularly in our appreciation of the glymphatic system and the adverse consequences of poor or insufficient sleep. Neurologists are in a unique position to screen for sleep disorders for three key reasons: (1) sleep disturbances occasionally precede the diagnosis of key neurological conditions (insomnia in the case of Alzheimer dementia and REM sleep behavior disorder in the case of Parkinson disease [PD]); (2) sleep disorders, when untreated/unrecognized often increase the severity of the primary neurological disease (i.e. untreated sleep apnea in the setting of epilepsy) and worsens quality of life for both patients and bedpartners/caregivers; (3) sleep disorders when identified and managed represent a unique window of opportunity in lessening the disease burden and may in the future help delay and even reverse the progression of a neurodegenerative condition. Neurologists must be aware of the importance of disorders and ask their patient at least one question about their satisfaction with, or quality of, their sleep.
This chapter is intended to provide a brief overview and definition of sleep covering the following attributes of sleep: architecture, requirements, function, ontogeny, dreams, the neurobiology of sleep and wakefulness, and review chronobiology and sleep-wake circadian rhythm. Discussion will focus on physiological changes in normal sleep and will transition to a focus on sleep deprivation, excessive sleepiness, and causes/consequences of excessive daytime sleepiness. This chapter will review the classification of sleep disorders, outline a pragmatic approach to a patient with sleep complaints, review clinical spectrum of sleep disturbances, underlying pathophysiogy, best practices for assessment,and management. For more in-depth information about sleep disorders, the reader is referred to the growing number of sleep medicine textbooks ( ; ; ; ; ; ; ).
This review of sleep medicine is written during these unprecedented times due to the COVID-19 pandemic and the associated sleep disorders related to anxiety, depression, and poor sleep quality related to physical and social isolation, quarantine, anxiety, stress, or financial losses. As new data are emerging, neurologists are encouraged to appreciate the patterns and emergence of sleep disturbances, insomnia, sleep disordered breathing, nightmares, fatigue, exhaustion, and complex nocturnal behaviors. Current data highlights that fatigue, central hypersomnolence, and REM sleep behavior disorder might be conferred by severe acute respiratory syndrome coronavirus 2 ( SARS-CoV- 2 ) infection per se, while rising rates of insomnia (“coro/covidnosomnia”) might be related mainly to confinement, anxiety, and psychosocial factors ( ).
Sleep is defined on the basis of both behavioral and physiological criteria ( Table 101.1 ). The behavioral criteria include lack of mobility or slight mobility, closed eyes, a characteristic species-specific sleeping posture, reduced response to external stimulation, quiescence, increased reaction time, elevated arousal threshold, impaired cognitive function, and a reversible unconscious state. Physiological criteria (see Sleep Architecture and Sleep Stages, later) are based on EEG, electro-oculography (EOG), and electromyography (EMG) findings as well as other physiological changes in ventilation and circulation.
 |
The moment of sleep onset is characterized by gradual shift of EEG wave rhythms, cognition, and mental processing. The onset of sleep occurs even prior to entrance to stage N1 NREM sleep and is defined as subjective heaviness and ptosis of the eyelids; clouding of the sensorium; and a decrement in logical or rational perception of visual and auditory stimuli and reduced capacity to respond with appropriate logic. The term predormitum was originally coined by to describe this moment of sleep onset. The moment of sleep offset, or awakening, is also a gradual process similar to the moment of sleep onset, but is pathologically prolonged in the setting of sleep disturbances such as sleep apnea or sleep deprivation, leading to grogginess and significant cognitive performance decrements, an impairment defined as sleep inertia ( ).
Sleep is divided into two independent states: NREM and REM sleep based on physiological correlates. NREM sleep is further divided into three stages, primarily on the basis of EEG criteria. NREM and REM sleep alternate, with each cycle lasting for approximately 90–100 minutes. Four to six such cycles are noted during a normal sleep period. The first two cycles are dominated by slow-wave sleep (stage N3), but in later cycles, these stages are noted only briefly and sometimes not at all. Conversely, the duration of the REM sleep cycle increases from the first to the last cycle, and toward the end of the night, the longest REM cycle may last as long as 1 hour. Thus, the first third of a normal sleep episode is dominated by slow-wave sleep, and REM sleep dominates the last third as illustrated in Fig. 101.1, A–C .
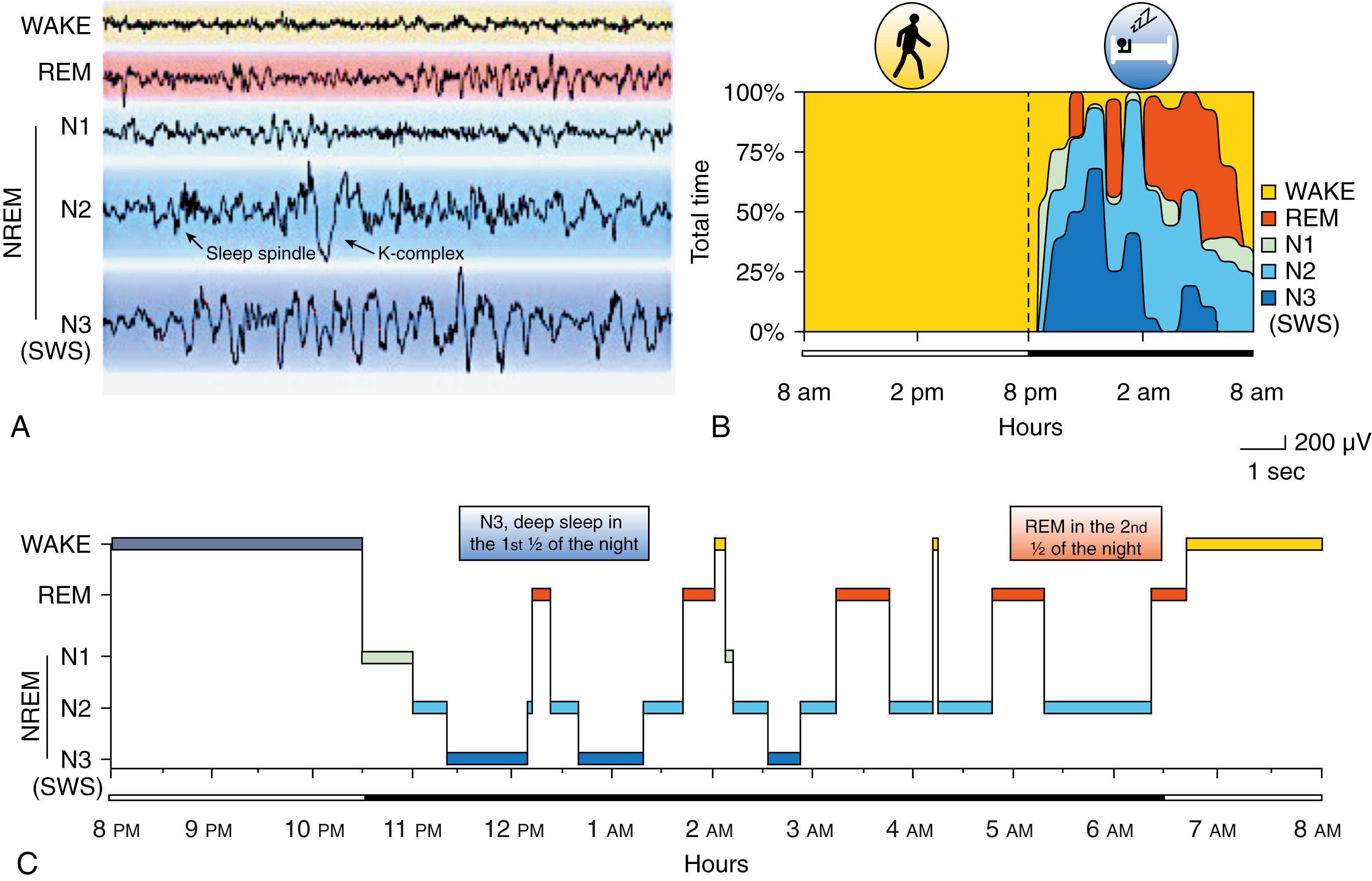
The hypnogram is a pictorial representation of the sleep stages of sleep from sleep onset to offset and is helpful following sleep study staging and interpretation in serving as a fingerprint of a patient’s sleep patterns at baseline and prior to and following treatment.
In their 1968 criteria, Rechtschaffen and Kales (RK) divided NREM sleep into stages 1, 2, 3, and 4. In 2007, this staging was modified slightly by an American Academy of Sleep Medicine (AASM) Task Force, and NREM sleep is now divided into three stages: N1, N2, and N3 (slow-wave sleep). In the modified staging criteria adopted by the AASM ( ), the traditional RK stage 1 NREM sleep was labeled N1, RK stage 2 was labeled N2, and RK stages 3 and 4 were combined into one stage, N3. Sleep scoring and staging is a procedure whereby the sleep recording is broken up into 30-second segments, “ epochs ,” of a polysomnographic (PSG) tracing with a paper or monitor speed of 10 mm/sec.
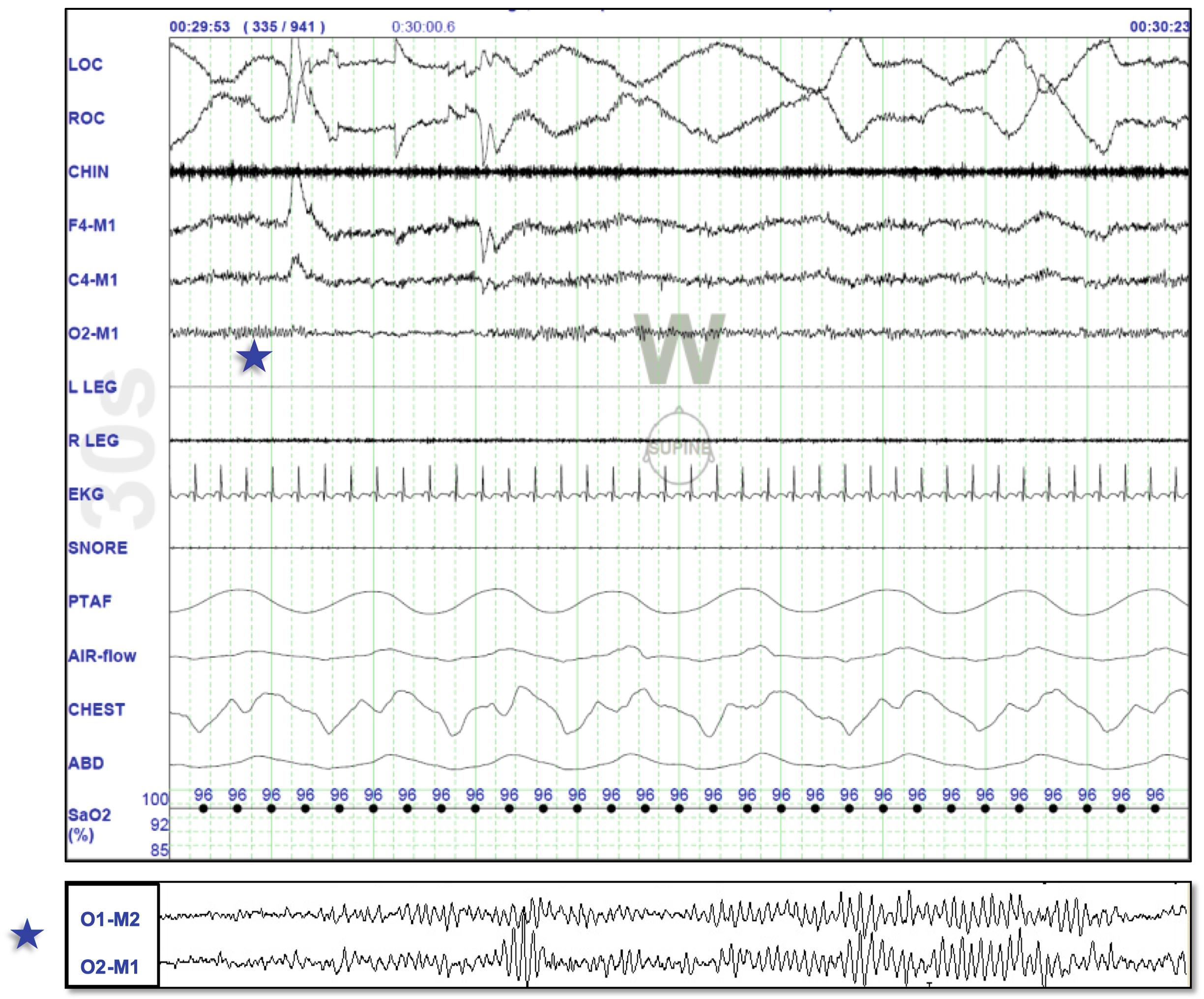
Stage Wake ( Fig. 101.2 ): Stage W is scored when more than 50% of the epoch consists of alpha EEG frequency. Chin EMG is relatively high tone reflecting the high-amplitude muscle contractions and movement artifacts. From stage W, patients typically proceed to stage N1, but infrequently they may enter REM sleep or stage N2 sleep directly, if the pressure to do so is high (reflecting a state of severe pathological sleep deprivation such as in observed in narcolepsy).
NREM Sleep : Accounts for the highest proportion (75%–80%) of sleep time in adults and is made up of stages N1, N2 (light sleep), and N3 (deep or slow-wave sleep).
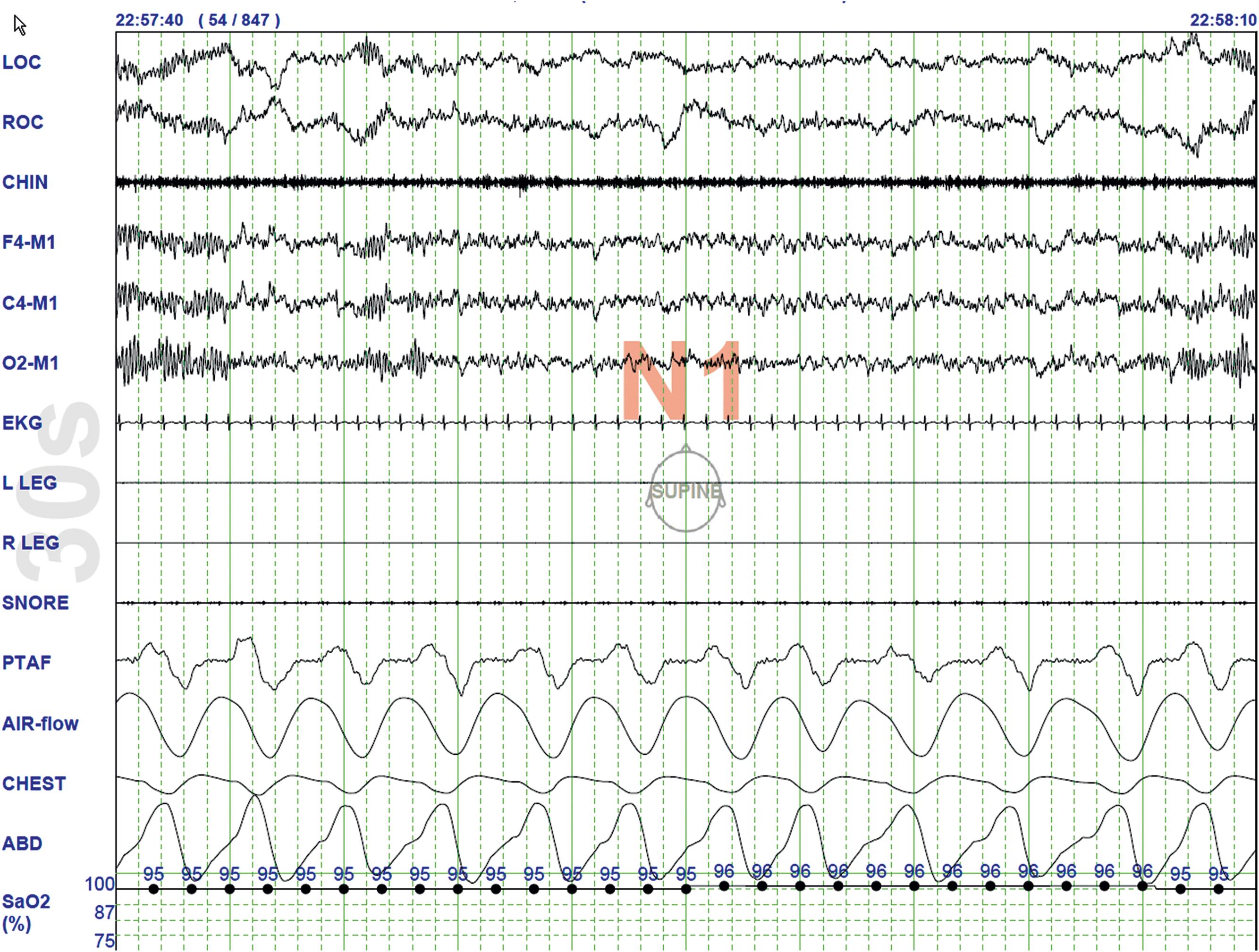
Stage N1 Sleep ( Fig. 101.3 ): Makes up approximately 3%–to-8% of total sleep time. It is demarcated by a significant shift of alpha rhythms (8–13 Hz), a characteristic of wakefulness, which diminishes to less than 50% of the epoch. N1 sleep is demarcated by the emergence of a mixture of slower theta rhythms (4–7 Hz) and beta waves (>13 Hz). The EMG activity drops slightly, and slow rolling eye movements may be recorded. Vertex sharp waves are noted toward the end of stage N1 sleep.
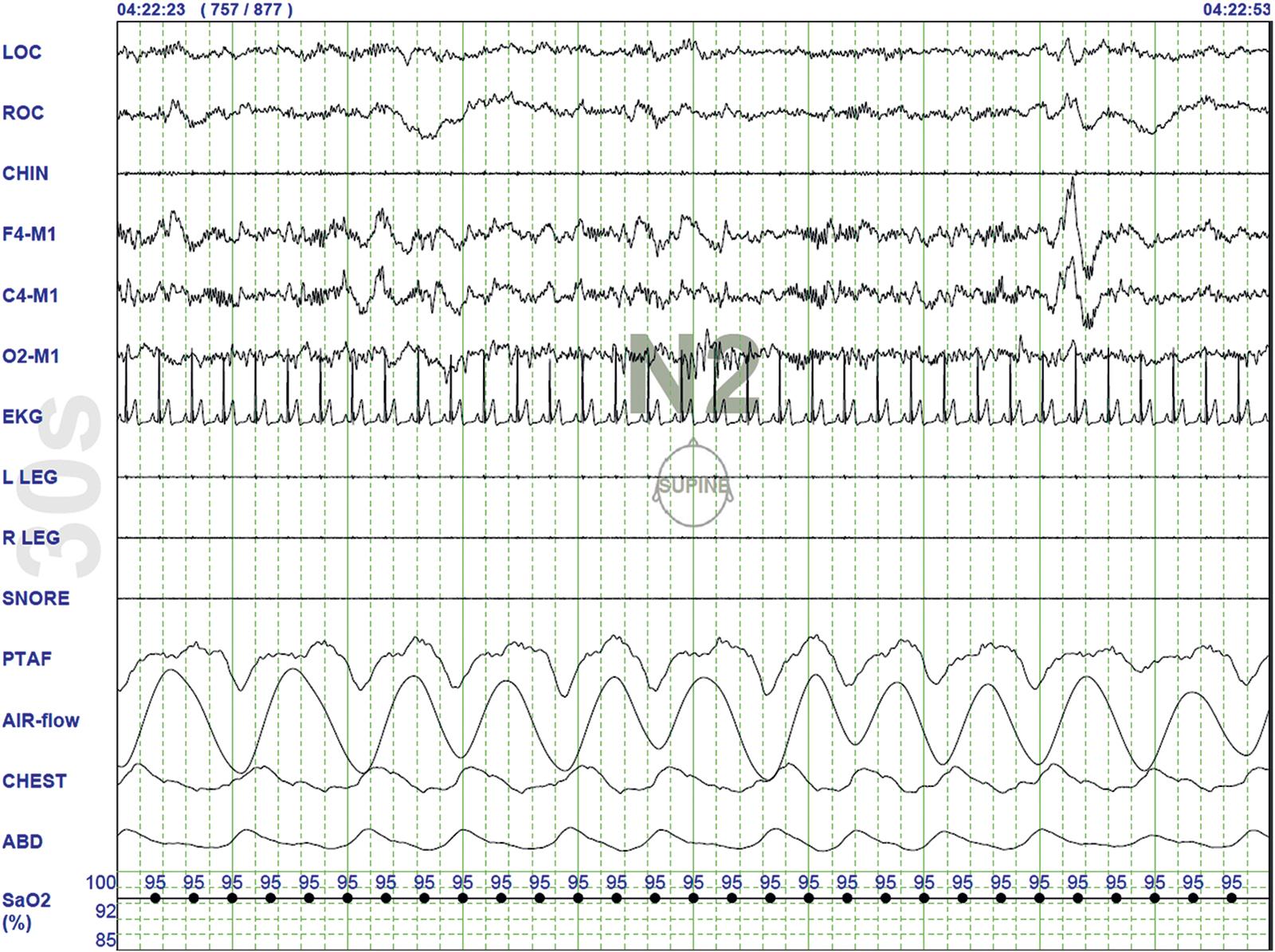
Stage N2 sleep ( Fig. 101.4 ): Begins after approximately 10–12 minutes of stage N1 sleep. The characteristic EEG findings of stage N2 sleep include sleep spindles (12–18 Hz, most often 14 Hz) and biphasic K complexes intermixed with vertex sharp waves. The EEG recording contains theta activity and fewer than 20% slow waves (0.5–2 Hz). Stage N2 sleep lasts for approximately 30–60 minutes.
Stage N3 sleep ( Fig. 101.5 ) : N3 or slow wave sleep comprises 15%-to–25% of total sleep. The patient is less responsive to environmental stimuli. It is defined by the presence of a minimum of 20 percent delta waves ranging from 0.5–to-2 Hz and having a peak-to-peak amplitude >75 μV. It is an important sleep stage as it is the most restorative form of sleep, but also where one is most likely to observe disorders of arousal (none-REM parasomnias) such as somnambulism and sleep terrors (Previous criteria defined stage N3 with 20–50 percent delta waves and a stage N4 with greater than 50 percent delta waves. However, at present time N2 and N4 are combined as stage N3.
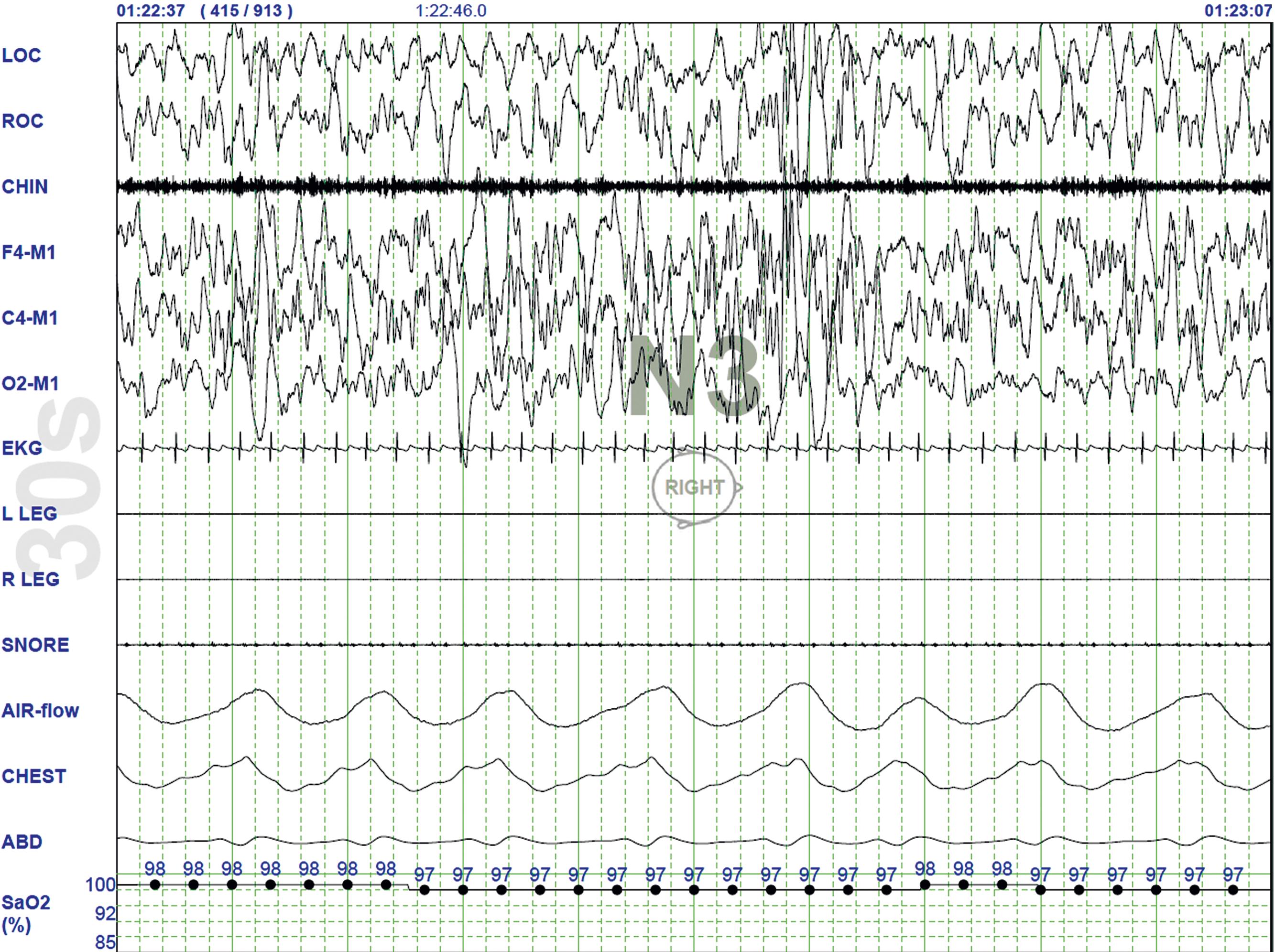
R or REM Sleep ( Fig.101.6 ): The first REM sleep (R sleep) episode is noted 60–90 minutes after the onset of sleep. REM sleep accounts for 20%–25% of sleep time. Based on EEG, EMG, and EOG characteristics, REM sleep can be subdivided into two stages: tonic-REM and phasic-REM. However, this subdivision is not recognized in the recently modified staging. The EEG tracings during REM sleep are characterized by fast rhythms and theta activity, some of which may have a sawtooth appearance. A desynchronized EEG, atonia of the major muscle groups, and depression of monosynaptic and polysynaptic reflexes are characteristics of the tonic stage . Phasic REM sleep is characterized by REMs in all directions, as well as phasic swings in blood pressure and heart rate, irregular respiration, spontaneous middle-ear muscle activity, and tongue movements. A few periods of apnea or hypopnea may arise during REM sleep.
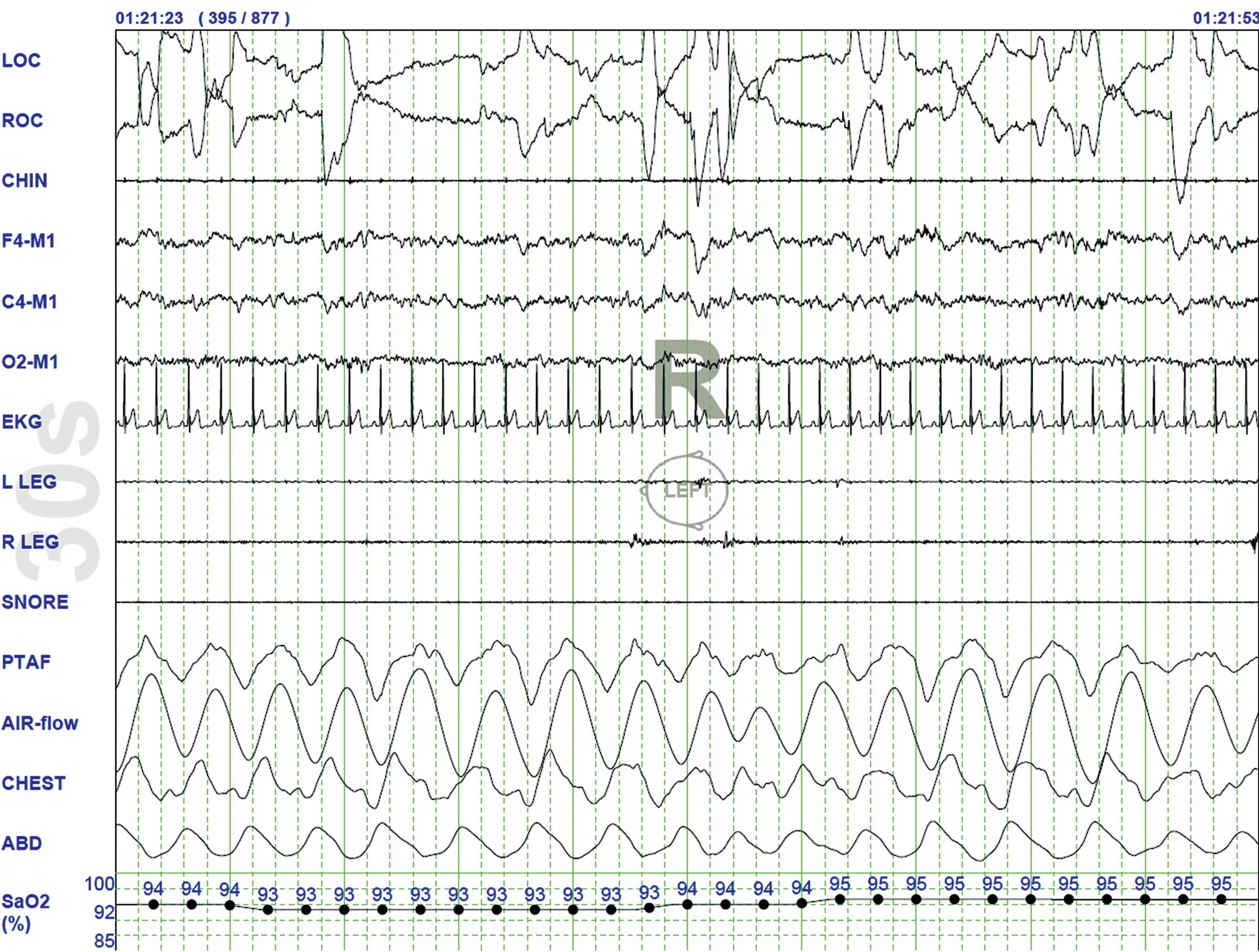
Under normal circumstances, one observes an orderly progression from wakefulness to sleep onset to NREM sleep proceeding to REM sleep. A relaxed wakefulness is characterized by a behavioral state of quiescence and a physiological state of alpha and beta frequencies on the EEG recording, waking eye movements, and increased muscle tone. Deviation from this progression may be seen in conditions of sleep state instability such as in narcolepsy, characterized by short sleep onset of REM sleep, or sleep fragmentation related to conditions such as sleep-disordered breathing (SDB). Certain medications (such as antidepressants) may reduce and sometimes eliminate normal appearance of slow-wave or REM sleep.
Table 101.2 summarizes EEG sleep stage frequencies and Table 101.3 highlights the proportion of each sleep stage during NREM and REM sleep states.
 |
| Sleep State | % Sleep Time |
|---|---|
| NREM sleep | 75–80 |
| N1 | 3–8 |
| N2 | 45–55 |
| N3 | 13–23 |
| REM sleep (stage R) | 20–25 |
| Tonic stage | Continuous |
| Phasic stage | Intermittent |
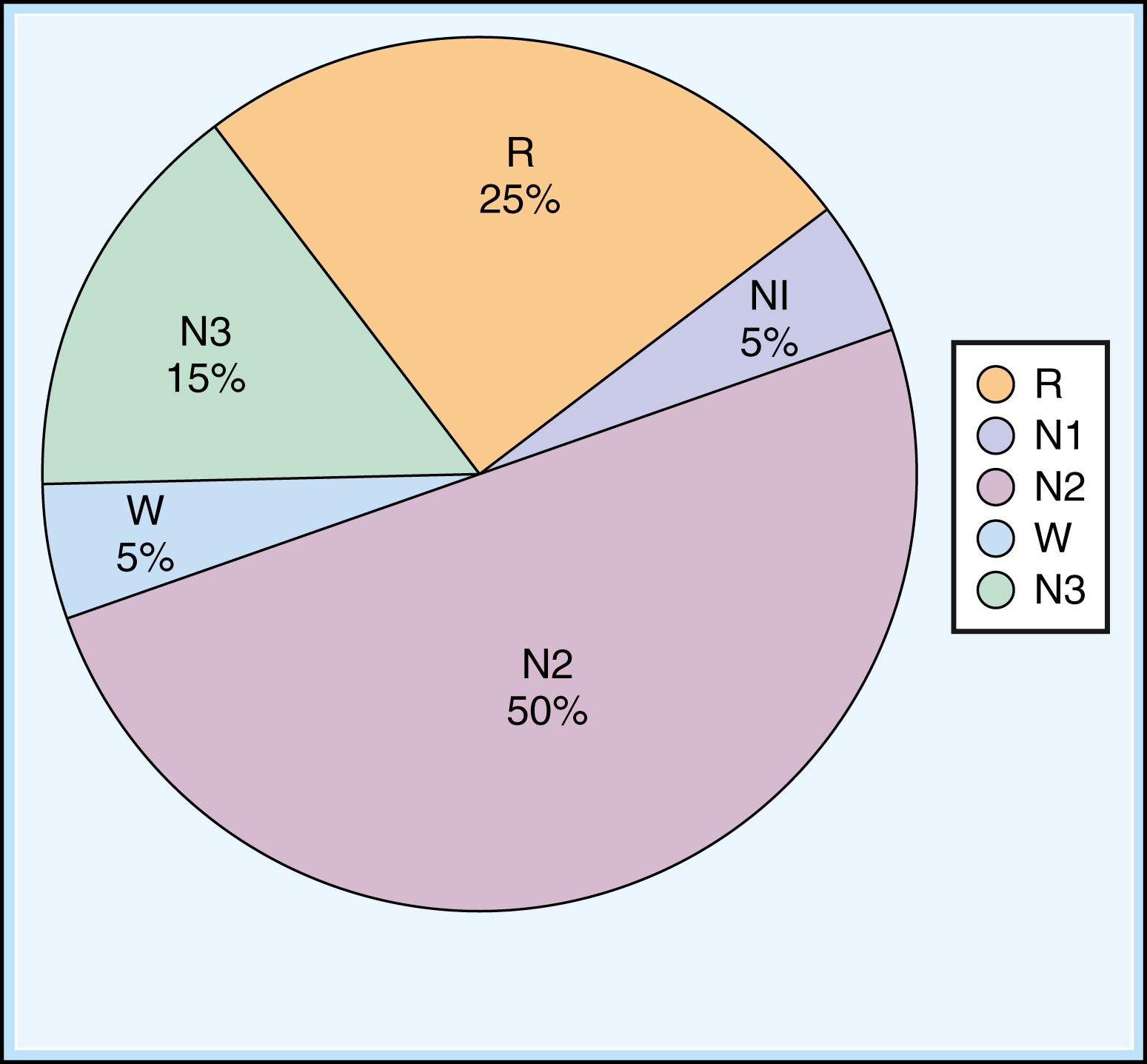
Fig. 101.7 illustrates the percent sleep stage distribution during the night. Sleep staging and scoring address normal adult sleep and the macrostructure ( ![]() eBox 101.1 ) and microstructure ( eBox 101.2 ) of sleep. In patients with sleep disorders such as sleep apnea, parasomnias, or nocturnal seizures disrupting sleep, such assessments may be difficult.
eBox 101.1 ) and microstructure ( eBox 101.2 ) of sleep. In patients with sleep disorders such as sleep apnea, parasomnias, or nocturnal seizures disrupting sleep, such assessments may be difficult.
Sleep states and stages
Sleep cycles
Sleep latency
Sleep efficiency (ratio of total sleep time to total time in bed, expressed as a percentage)
Wake after sleep onset (WASO)
Arousals
Cyclic alternating pattern
Sleep spindles
K complexes
Sleep microstructure consists of momentary dynamic phenomena defined as arousals (see ![]() eBox 101.2 ).
eBox 101.2 ).
An arousal is a shift in EEG frequency, and an arousal index is defined as the number of arousals per hour of sleep; up to 10 can be considered a normal arousal index, while a higher index signifies sleep fragmentation.
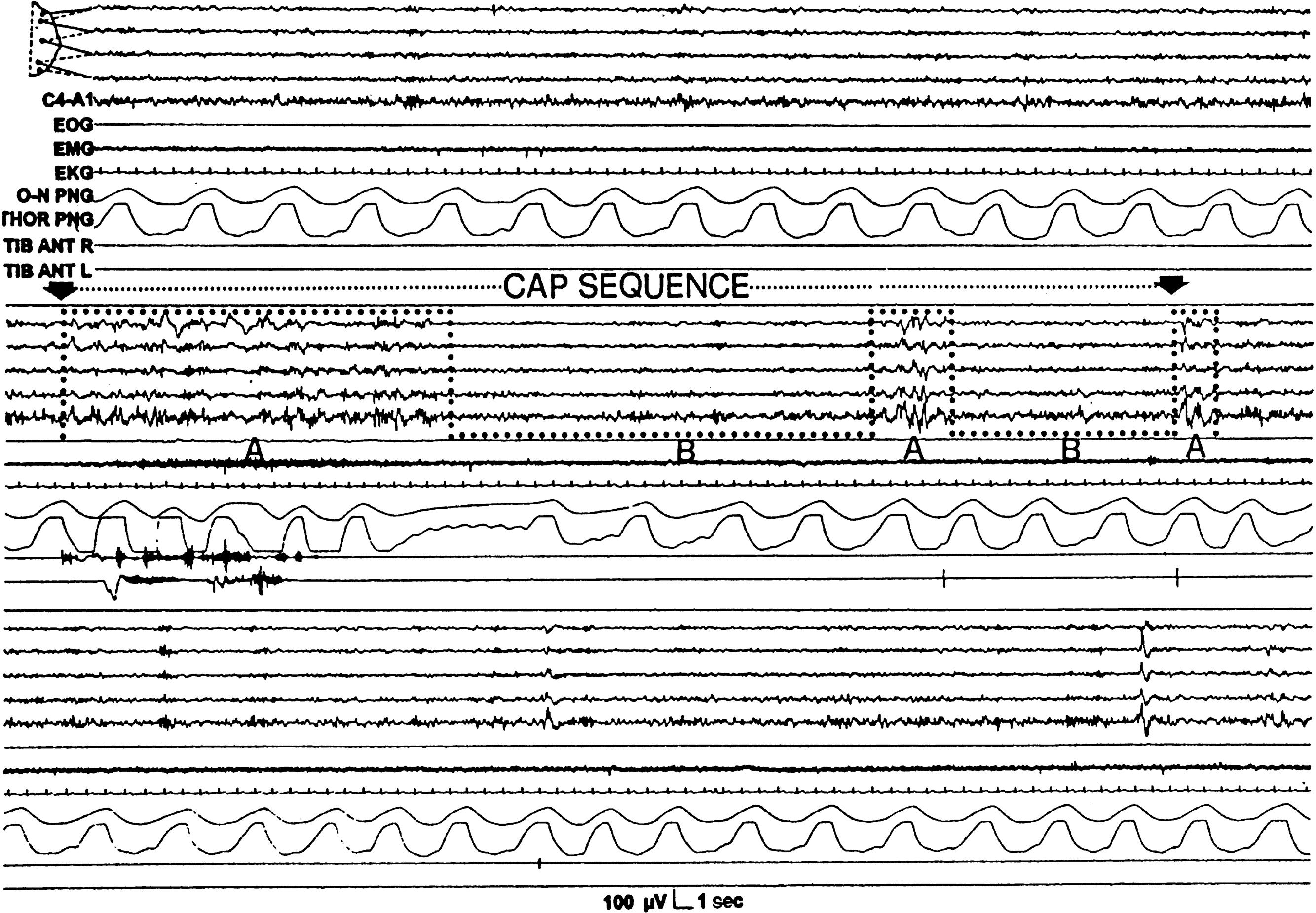
Cyclic alternating pattern (CAP) consist of repetitive cortical EEG pattern that is noted mainly during NREM sleep, lasts for 2–60 seconds, and implies predisposition toward sleep instability. A phase of CAP is marked by increased EEG potentials, with contributions from both synchronous high-amplitude slow and desynchronized fast rhythms in the EEG recording. A CAP cycle consists of an unstable phase (phase A) and a relatively stable phase (phase B) ( ![]() eFig. 101.8 ). During phase A, heart rate, respiration, blood pressure, and muscle tone increase. The rates of CAP cycles and arousals increase in both older individuals and a variety of sleep disorders such as parasomnias. A period without CAP is thought to indicate a state of sustained stability and may be used to understand normal and abnormal sleep.
eFig. 101.8 ). During phase A, heart rate, respiration, blood pressure, and muscle tone increase. The rates of CAP cycles and arousals increase in both older individuals and a variety of sleep disorders such as parasomnias. A period without CAP is thought to indicate a state of sustained stability and may be used to understand normal and abnormal sleep.
The evolution of EEG and wake-sleep states from the fetus, preterm infant, term infant, preschooler, adolescent, to adult follows in an orderly manner depending on the maturation of the central nervous system (CNS). Neurological, environmental, and genetic factors, as well as comorbid medical or neurological disorders, will have significant effects on such ontogenetic changes. Sleep requirements change dramatically from infancy to old age. Newborns have a polyphasic sleep pattern with 16 hours of sleep per day. The sleep requirement decreases to approximately 10 hours per day by age 3–5 years. In preschool children, sleep assumes a biphasic pattern. Adults exhibit a monophasic sleep pattern, with an average duration of 7.5–8 hours per night.
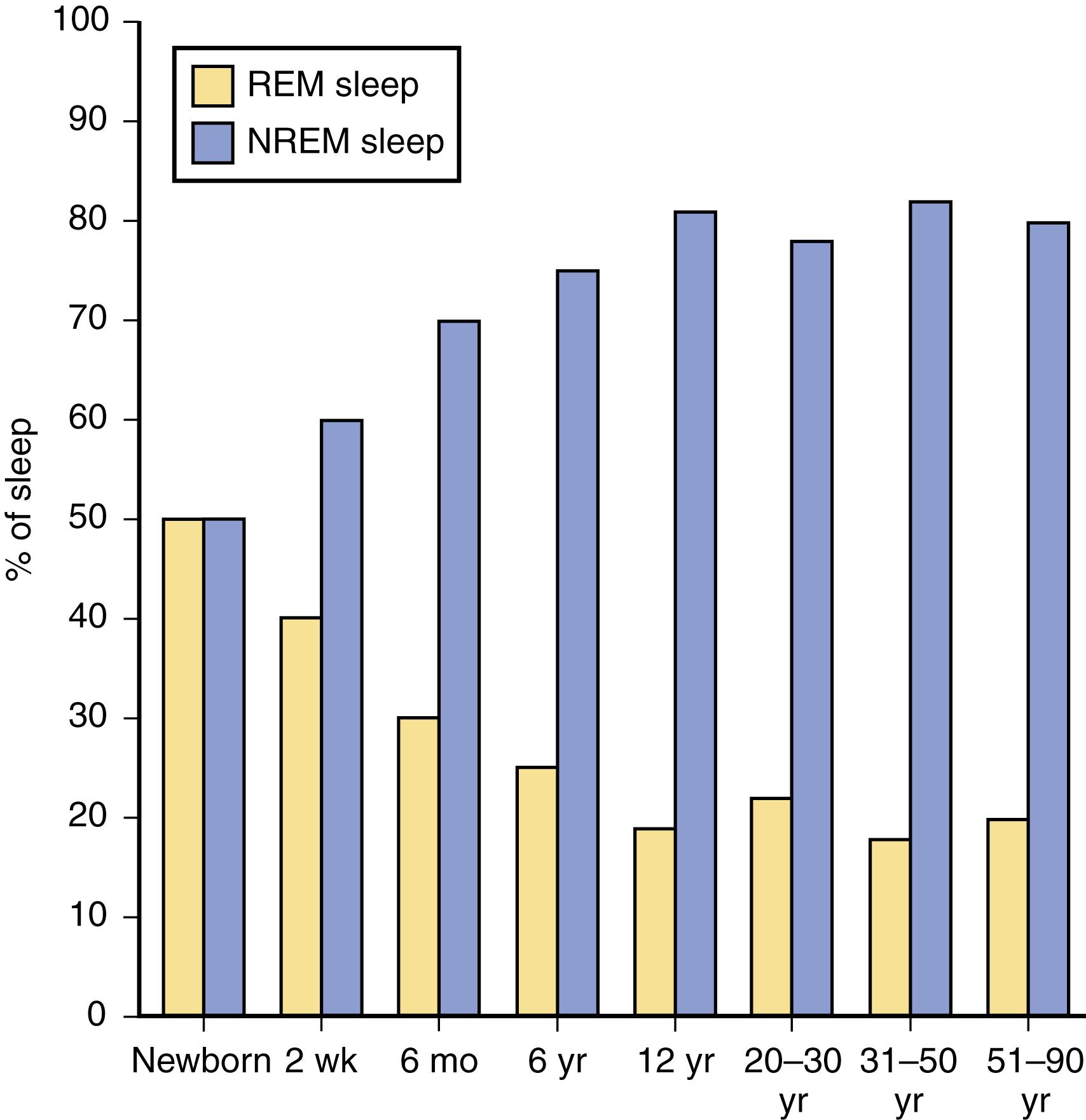
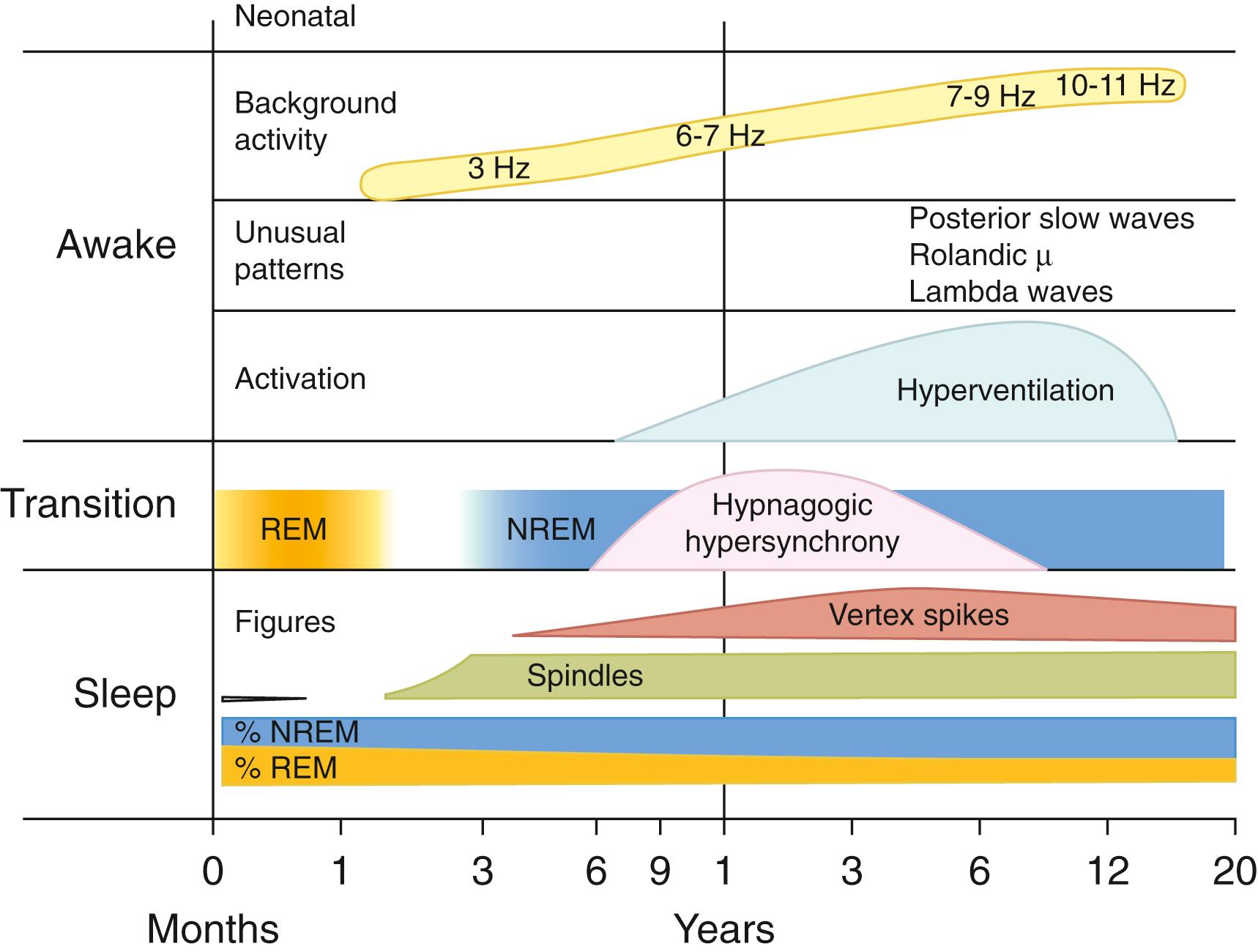
The newborn infant spends approximately 50% of the time in REM sleep, but by age 6 years this time is decreased to the normal adult pattern of 25% ( Fig. 101.9 ). On falling asleep, a newborn baby goes immediately into REM sleep, or active sleep, which is accompanied by restless movements of the arms, legs, and facial muscles. In premature babies, it is often difficult to differentiate REM sleep from wakefulness. By age 3 months, the NREM-REM cyclical pattern of adult sleep is established. However, the duration of the NREM-REM cycle is shorter in infants, lasting for approximately 45–50 minutes and increasing to 60–70 minutes by age 5–10 years, and to the normal adult cyclical pattern of 90–100 minutes by age 10 years. Sleep spindles begin to appear at about 3 months of age; K complexes are seen by about 6 months ( Fig. 101.10 ).
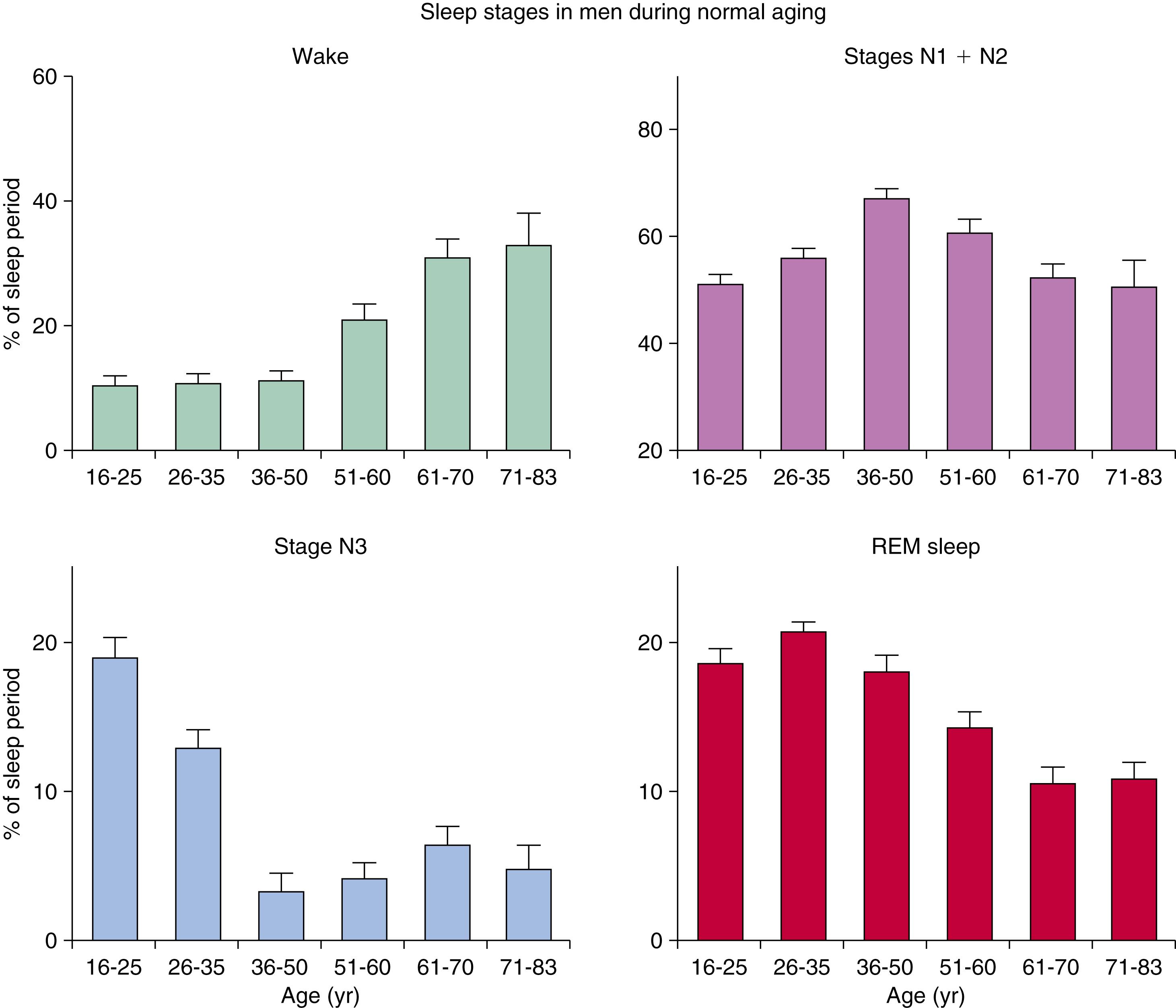
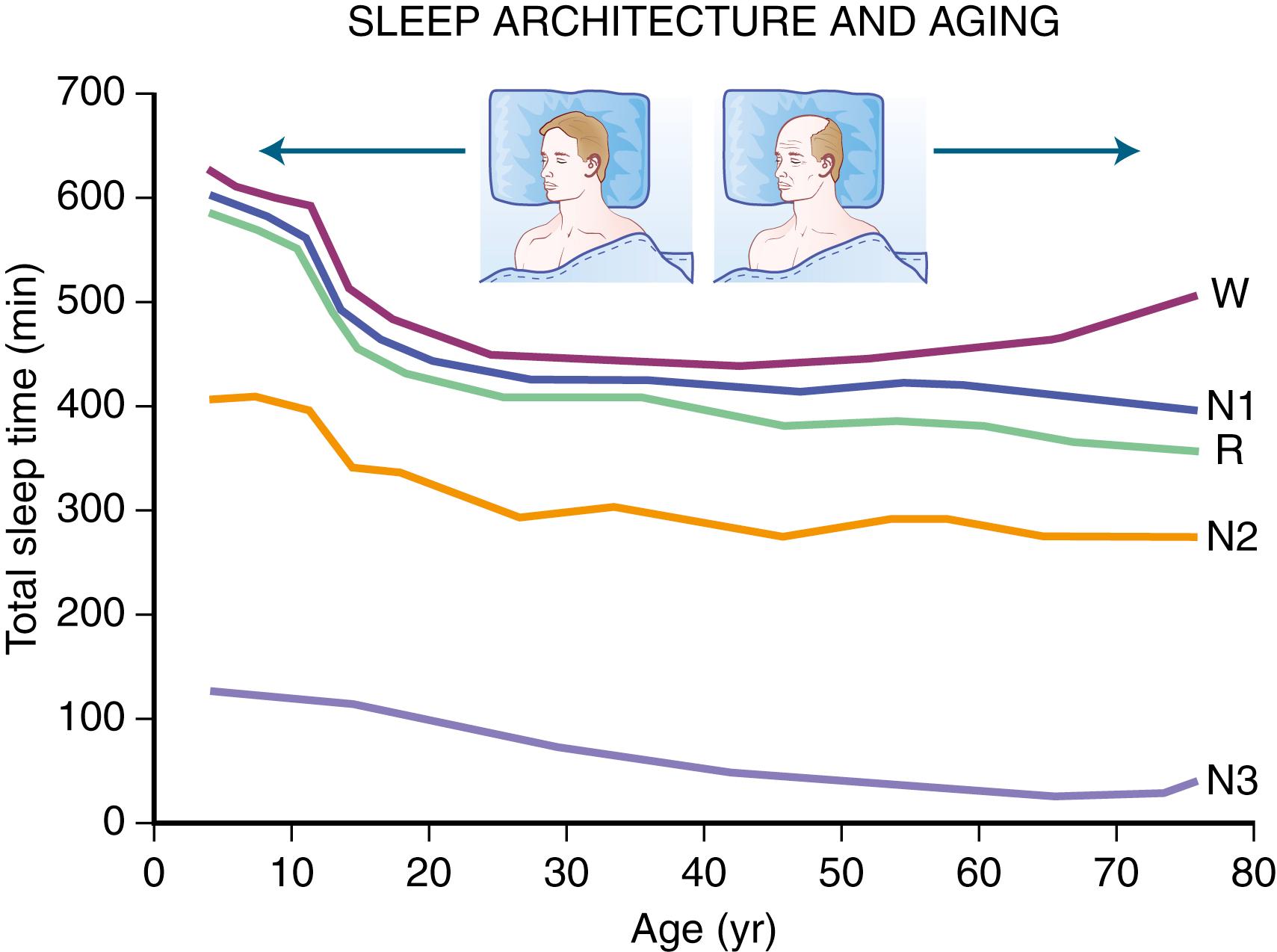
A characteristic feature of sleep in old age is marked attenuation of the amplitude of slow waves; therefore, during scoring of slow sleep, which depends not only on the rate but also on the amplitude of slow waves, the percentage of slow waves decreases, time awake increases, and light sleep (stage N1) increases ( Fig. 101.11 ). The other characteristic feature during old age is repeated awakenings throughout the night, including early-morning awakenings. Older people spend more time awake in bed than younger people and undergo deterioration in the quality of sleep: Sleep is lighter and more fragmented with increasing age, with reductions of slow-wave sleep. Total sleep time and sleep efficiency decrease. The percentage of slow-wave sleep is reduced and is accompanied by an increase in the percentage of non-REM N1 and N2 sleep. An increase in sleep latency and time spent awake after sleep onset also occurs. While sleep need is similar to younger people, the ability to sleep is impaired. Fig. 101.12 schematically shows the evolution of sleep state distribution in newborns, infants, children, adults, and older adults as a function of time.
Depending on the sleep habit, two groups of “ circadian phenotypes ” are recognized: evening types and morning types. Evening types (“owls”) have difficulty getting up early and feel tired in the morning; however, they feel fresh and energetic toward the end of the day. These people perform best in the evening; they go to sleep late and wake up late. In contrast, the morning types (“larks”) wake up early, rested and refreshed, and work efficiently in the morning. The body temperature rhythm shows two different curves in these two types of people. The body temperature reaches the evening peak an hour earlier in morning types than in evening types. Morning and evening types are most likely determined by genetic factors. Katzenberg and colleagues (1998), using the 19-item Horne-Östberg Questionnaire to determine “morningness/eveningness” in human circadian rhythms, discovered a CLOCK gene polymorphism associated with human diurnal preference.
![]() Additional text available at http://expertconsult.inkling.com .
Additional text available at http://expertconsult.inkling.com .
Sleep need is determined by heredity rather than by different personality traits or other psychological factors. Social or biological factors may also play a role.
Sleep requirement is defined as the optimal amount of sleep required to remain alert and fully awake and to function adequately throughout the day. Sleep requirement for an average adult is approximately 7.5–8 hours regardless of environment or cultural differences. The 2015 Join Consensus Statement from the AASM and Sleep Research Society (SRS) recommended that adults should sleep 7 or more hours per night on a regular basis to promote optimal health. The recommendations went on to highlight that sleeping less than 7 hours per night regularly is associated with greater predisposition to adverse health outcomes, including weight gain, insulin resistance, cardiovascular disease, stroke, impaired immune function, increased pain, impaired performance, increased errors, psychiatric conditions such as depression, and greater risk of accidents ( ). Fig. 101.13 illustrates the Join Consensus Statement from the AASM and SRS sleep duration recommendations from infancy to older age.
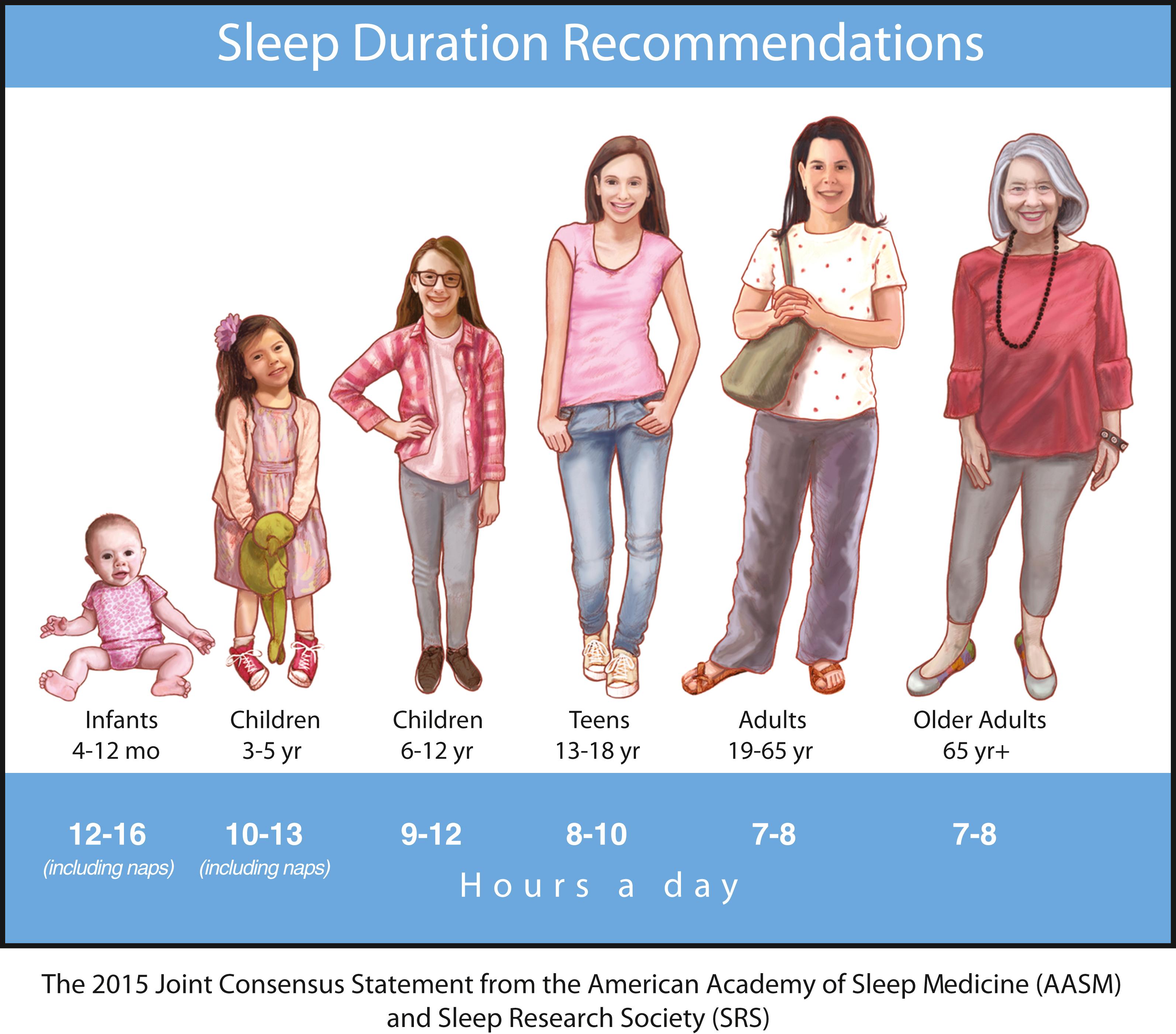
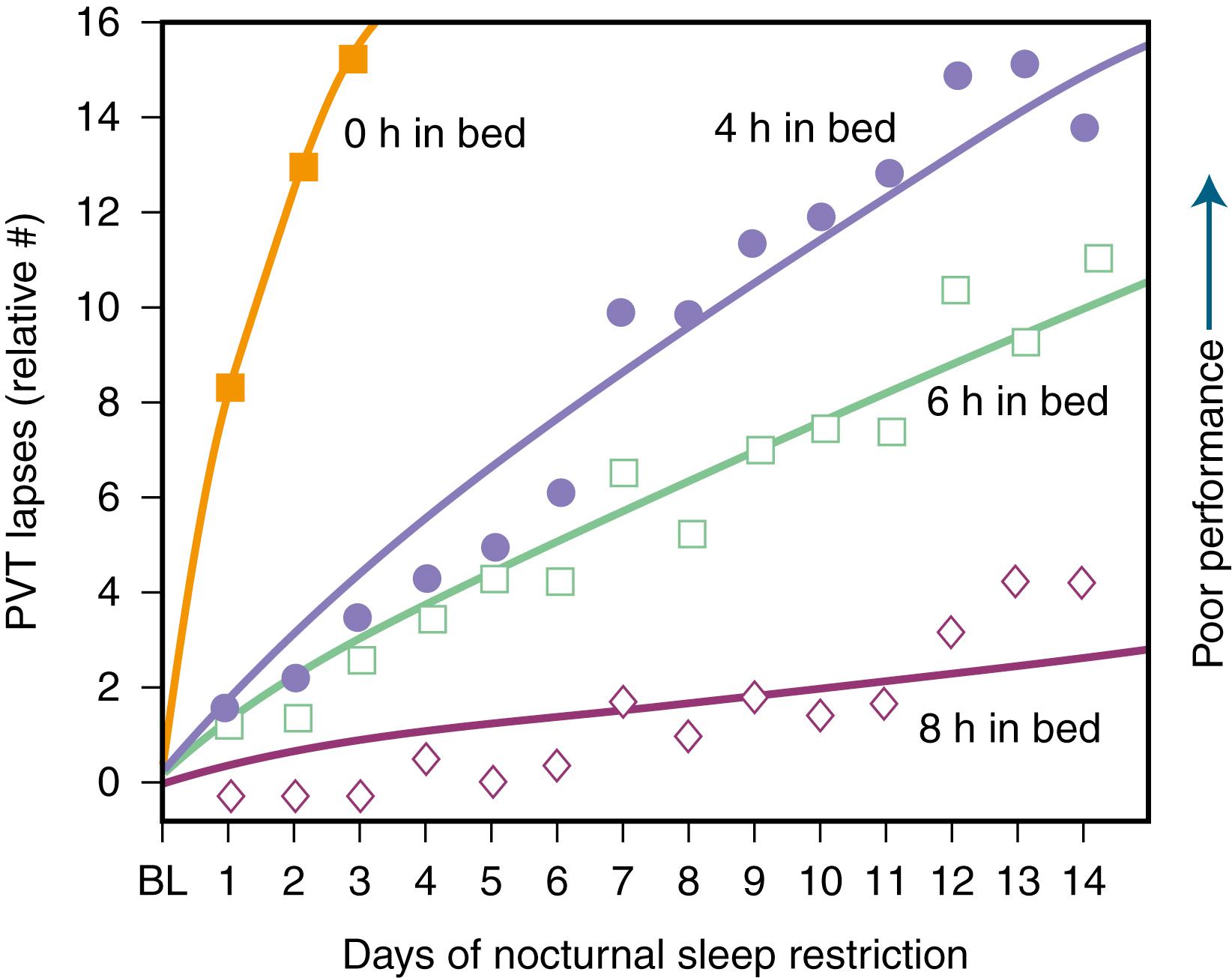
Modern society appears to be chronically sleep deprived. Data demonstrate detrimental loss of vigilance with progressive sleep loss, confirming the possibility that sleep deprivation’s consequences are serious. Fig. 101.14 shows psychomotor vigilance task (PVT) performance lapses under varying doses of daily sleep, confirming the data of serious lapses when sleep is curtailed beyond 8 hours. A poll by the National Sleep Foundation (NSF) in 2000, 2001, and 2002 indicated that the average sleep duration for Americans had fallen to 6.9–7.0 hours. Across the board, over the last half of the 20th century, sleep duration has diminished by 1.5–2 hours leading to an epidemic of sleep deprivation, where most people are in bed for only 5–6 hours per night on a regular basis. A more recent Sleep in America poll from the NSF found that a majority of the public (65%) reported that getting enough sleep makes them a “more effective person,” yet 41% admit to rarely taking into account how much sleep they need in planning for the next day and ranked sleep second to last when asked which of five items were most important to them personally ( ).
![]() Additional text available at http://expertconsult.inkling.com .
Additional text available at http://expertconsult.inkling.com .
![]() Additional text available at http://expertconsult.inkling.com .
Additional text available at http://expertconsult.inkling.com .
Sigmund Freud called dreams the “royal road to the unconscious,” representing repressed feelings that are psychologically suppressed in the unconscious mind. Modern sleep scientists, however, interpret dreams in anatomical and physiological terms. Dream research has taken a new direction since the existence of REM sleep was first observed by Aserinsky and Kleitman in 1953. It is believed that approximately 80% of dreams occur during REM sleep and 20% during NREM sleep.
REM sleep is characterized by highly emotionally charged, complex, and bizarre dreams, whereas NREM dreams are more realistic and rational. People are generally oriented when awakening from REM sleep but are somewhat disoriented and confused when awakening from NREM sleep. Also, it is easier to recall REM dreams than NREM dreams and easier to recall dreams if the subject is awakened immediately after the onset of REM dreams. During REM sleep, nerve cells, synapses, and the fibers connecting these cells become activated first in the brainstem, then the signals are transmitted to the cerebral hemisphere, which synthesizes the signals, creating colorful (most of our dreams take place in natural color) or black and white images during dreams.
The neurobiological significance of dreams remains unknown. Some suggestions include activation of the neural networks in the brain, restructuring and reinterpretation of data stored in memory, and removal of unnecessary and useless information from the brain of a dreamer. Dream-enacting behavior (DEB) associated with abnormal movements during sleep, in the absence of muscle atonia, constitutes an important REM parasomnia known as REM sleep behavior disorder (RBD).
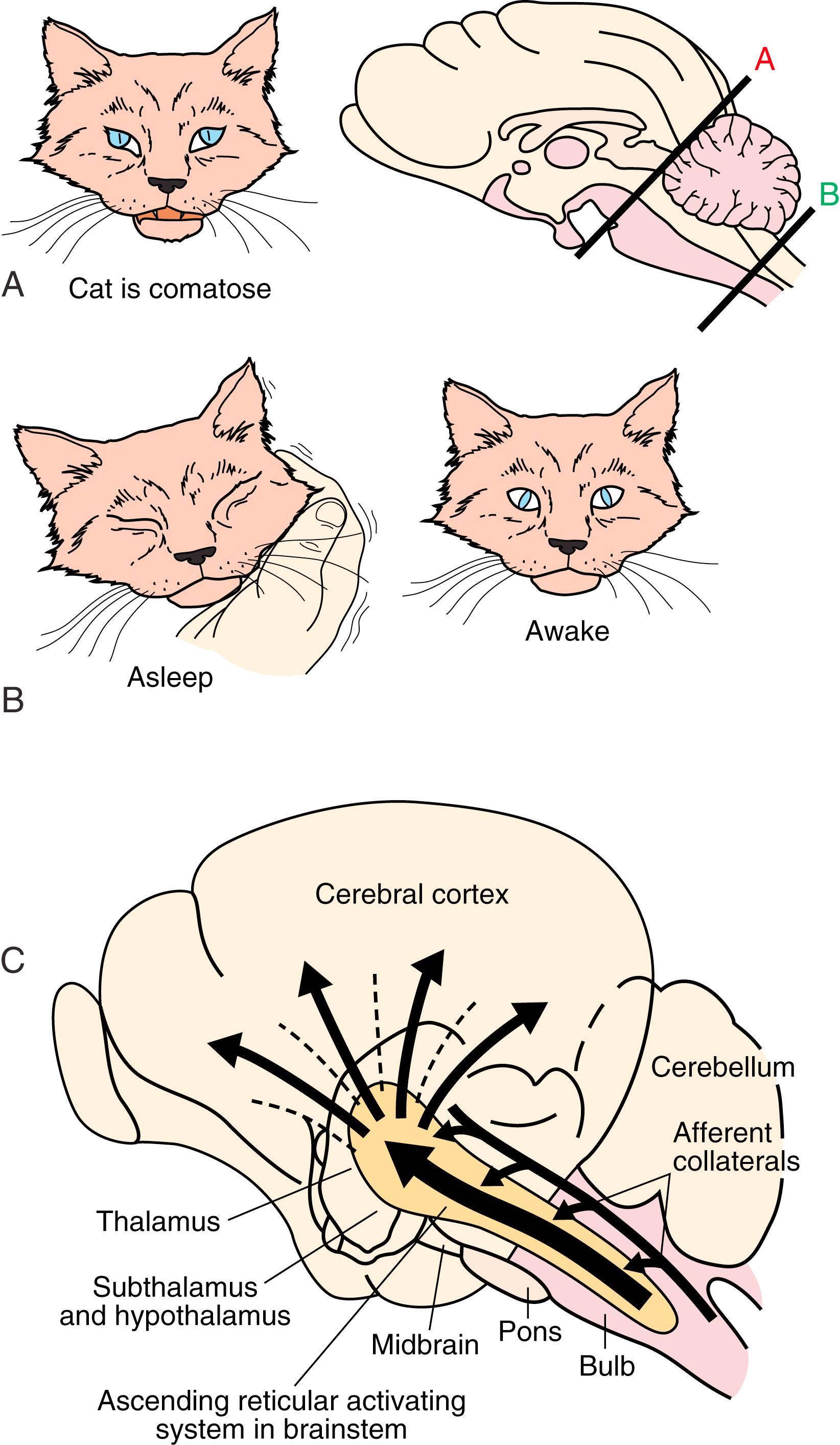
In the 1930s, Bremer discovered evidence of an ascending arousal system necessary for cortical arousal when he demonstrated that transection of the brainstem at the midcollicular level (i.e., cerveau isolé ), but not the spinomedullary junction (i.e., encéphale isolé ), produced coma in anesthetized cats. Bremer hypothesized that the resulting reduction in “cerebral tone” following the cerveau isolé was due to interruption of ascending sensory inputs—that is, a passive “deafferentation theory” of sleep ( ). Wakefulness is controlled by the ascending reticular activating system (ARAS) containing glutamatergic, cholinergic, aminergic, and hypocretinergic neurons ( ). Projections from the ARAS terminating in the thalamus and thalamocortical projections to widespread areas of the cerebral cortex produce cerebral cortical activation during wakefulness. Extrathalamic projections from the brainstem reticular neurons terminate in the posterior hypothalamus and the basal forebrain regions; the latter project to the cerebral cortex (basocortical projections) to maintain wakefulness
Reciprocal interaction between sleep-promoting neurons in the region of the NTS and wake-promoting neurons within the ARAS of the brainstem independent of the reciprocal interaction of the neurons of the forebrain also plays a role in the generation of NREM sleep. The latter is the old reticular passive hypothesis , or the disfacilitation hypothesis , of sleep. In this model, sleep is said to result from a cascade of disfacilitation within the brainstem. The passive theory originated in the two classic preparations in cats by , noted earlier in the chapter: cerveau isolé and encéphale isolé. Bremer found that in cerveau isolé (e.g., midcollicular transection), all specific sensory stimuli were withdrawn, and the animals were somnolent, whereas in encéphale isolé (transection at C1 vertebral level disconnecting the entire brain from the spinal cord), these specific stimuli maintained activation of the brain and animals were awake ( ![]() eFig. 101.15, A and B ). postulated that withdrawal of generalized activation from ARAS is responsible for somnolence in cerveau isolé preparations, whereas activation of the midbrain reticular neurons causing direct excitation of the thalamocortical projections results in EEG desynchronizations and behavioral arousal (see
eFig. 101.15, A and B ). postulated that withdrawal of generalized activation from ARAS is responsible for somnolence in cerveau isolé preparations, whereas activation of the midbrain reticular neurons causing direct excitation of the thalamocortical projections results in EEG desynchronizations and behavioral arousal (see ![]() eFig 101.15 , C ). These observations support the later suggestion of Steriade and colleagues (2005) that at the onset of NREM sleep, there is a deafferentation of the brain due to blockage of afferent information, first at the thalamic level, causing the waking “open” brain to be converted to a “closed” brain owing to thalamocortical inhibition. This passive reticular theory, however, was challenged by the experiments of , producing the midpontine pretrigeminal section, which was only a few millimeters below the section that produced cerveau isolé. In this midpontine preparation, there were persistent EEG and behavioral signs of alertness, suggesting that structures located in the brainstem between cerveau isolé and midpontine pretrigeminal preparations are responsible for wakefulness. Thus, an active inhibitory role of the lower brainstem hypnogenic neurons in the region of the NTS on the upper brainstem ARAS was clearly demonstrated by this preparation. Both active and passive theories partly explain the generation of NREM sleep, but the contemporary theory favors the activation of ventrolateral preoptic (VLPO) neurons and inhibition of posterior hypothalamic neurons containing histamine and hypocretin. Reduced activity of the hypocretin projections to the locus coeruleus noradrenergic, midline raphe serotonergic, mesopontine dopaminergic, and tuberomammillary hypothalamic histaminergic cells may also decrease the level of arousal, causing sleepiness.
eFig 101.15 , C ). These observations support the later suggestion of Steriade and colleagues (2005) that at the onset of NREM sleep, there is a deafferentation of the brain due to blockage of afferent information, first at the thalamic level, causing the waking “open” brain to be converted to a “closed” brain owing to thalamocortical inhibition. This passive reticular theory, however, was challenged by the experiments of , producing the midpontine pretrigeminal section, which was only a few millimeters below the section that produced cerveau isolé. In this midpontine preparation, there were persistent EEG and behavioral signs of alertness, suggesting that structures located in the brainstem between cerveau isolé and midpontine pretrigeminal preparations are responsible for wakefulness. Thus, an active inhibitory role of the lower brainstem hypnogenic neurons in the region of the NTS on the upper brainstem ARAS was clearly demonstrated by this preparation. Both active and passive theories partly explain the generation of NREM sleep, but the contemporary theory favors the activation of ventrolateral preoptic (VLPO) neurons and inhibition of posterior hypothalamic neurons containing histamine and hypocretin. Reduced activity of the hypocretin projections to the locus coeruleus noradrenergic, midline raphe serotonergic, mesopontine dopaminergic, and tuberomammillary hypothalamic histaminergic cells may also decrease the level of arousal, causing sleepiness.
The existence of REM sleep-generating neurons in the pontine cat has been established by transection experiments (see ![]() eFig. 101.15 ) through different regions of the midbrain, pons, and medulla ( ). A transection at the junction of the pons and midbrain produced all the physiological findings compatible with REM sleep in the section caudal to that transection, whereas in the forebrain region rostral to the section, the recording showed no signs of REM sleep. After transection between pons and medulla, structures rostral to the section showed signs of REM sleep, but structures caudal to the section had no signs of REM sleep. After transection at the junction of the spinal cord and medulla, REM sleep signs were noted in the rostral brain areas. Finally, transection at the pontomesencephalic and pontomedullary junctions produced an isolated pons that showed all the signs of REM sleep. The pons is therefore sufficient and necessary to generate all the signs of REM sleep.
eFig. 101.15 ) through different regions of the midbrain, pons, and medulla ( ). A transection at the junction of the pons and midbrain produced all the physiological findings compatible with REM sleep in the section caudal to that transection, whereas in the forebrain region rostral to the section, the recording showed no signs of REM sleep. After transection between pons and medulla, structures rostral to the section showed signs of REM sleep, but structures caudal to the section had no signs of REM sleep. After transection at the junction of the spinal cord and medulla, REM sleep signs were noted in the rostral brain areas. Finally, transection at the pontomesencephalic and pontomedullary junctions produced an isolated pons that showed all the signs of REM sleep. The pons is therefore sufficient and necessary to generate all the signs of REM sleep.
The neuroanatomical substrates for wakefulness and sleep (REM and NREM) are located primarily within the diencephalon and brainstem ( , ; ; ; ; ; ). Fig. 101.16 summarizes the determination of sleep-wake states according to the specific site and the corresponding neurotransmitter responsible for either wakefulness or sleep. Neuronal networks for achieving wake/sleep are both interconnected and redundant.
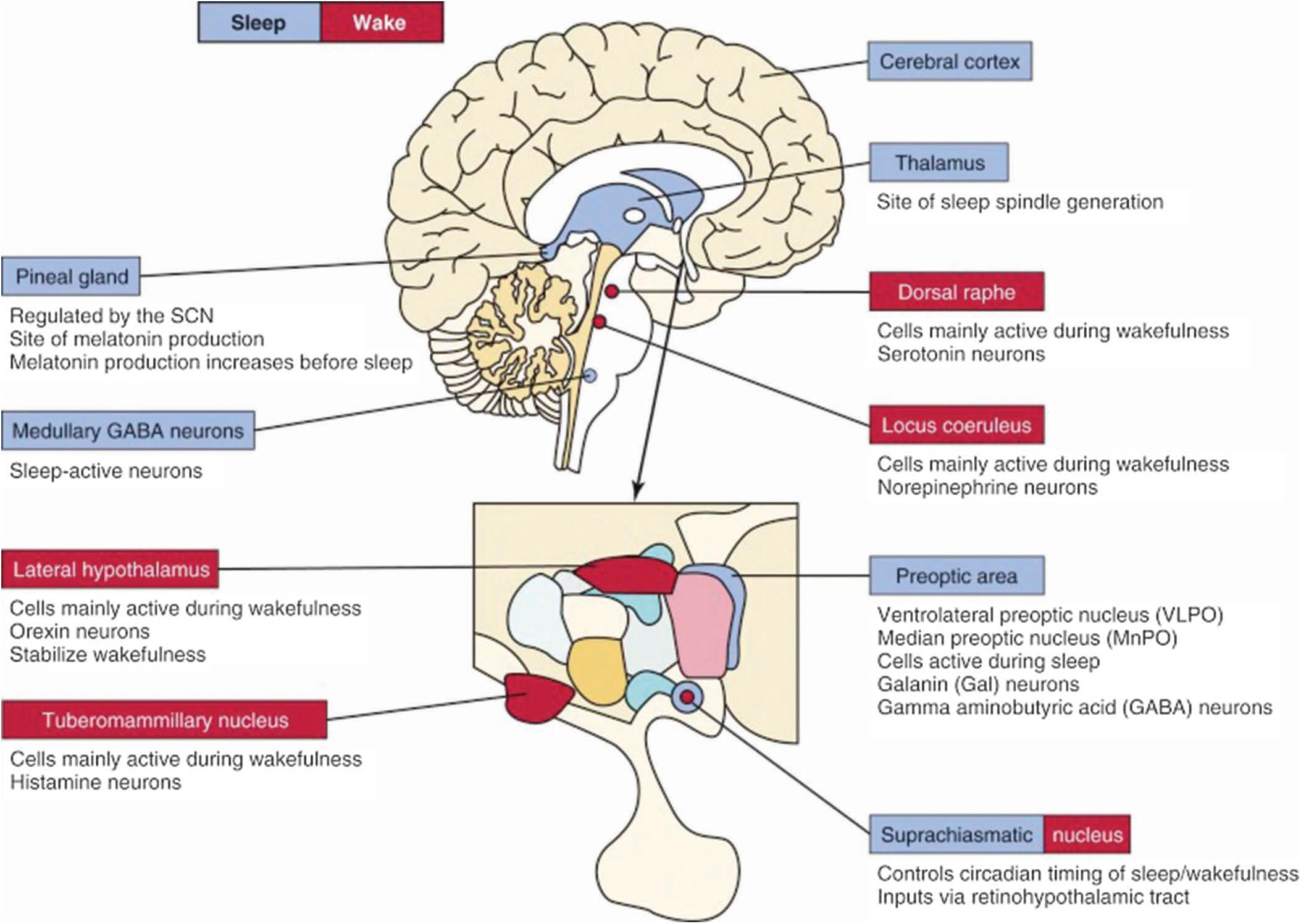
Brain pathways regulating wakefulness use the cholinergic, noradrenergic (AKA NE=norepinephrine), dopaminergic, and histaminergic neurons Fig. 101.17 , A . The cholinergic neurons fire at the highest rate during wakefulness and REM sleep but decrease their rates of firing at the onset of NREM sleep. Wakefulness-promoting aminergic neurons include noradrenergic neurons in the locus coeruleus, serotonergic neurons in the dorsal raphe of the brainstem, histaminergic neurons in the tuberomammillary nucleus of the hypothalamus, dopaminergic neurons in the ventral tegmental area, substantia nigra, and ventral periaqueductal area.
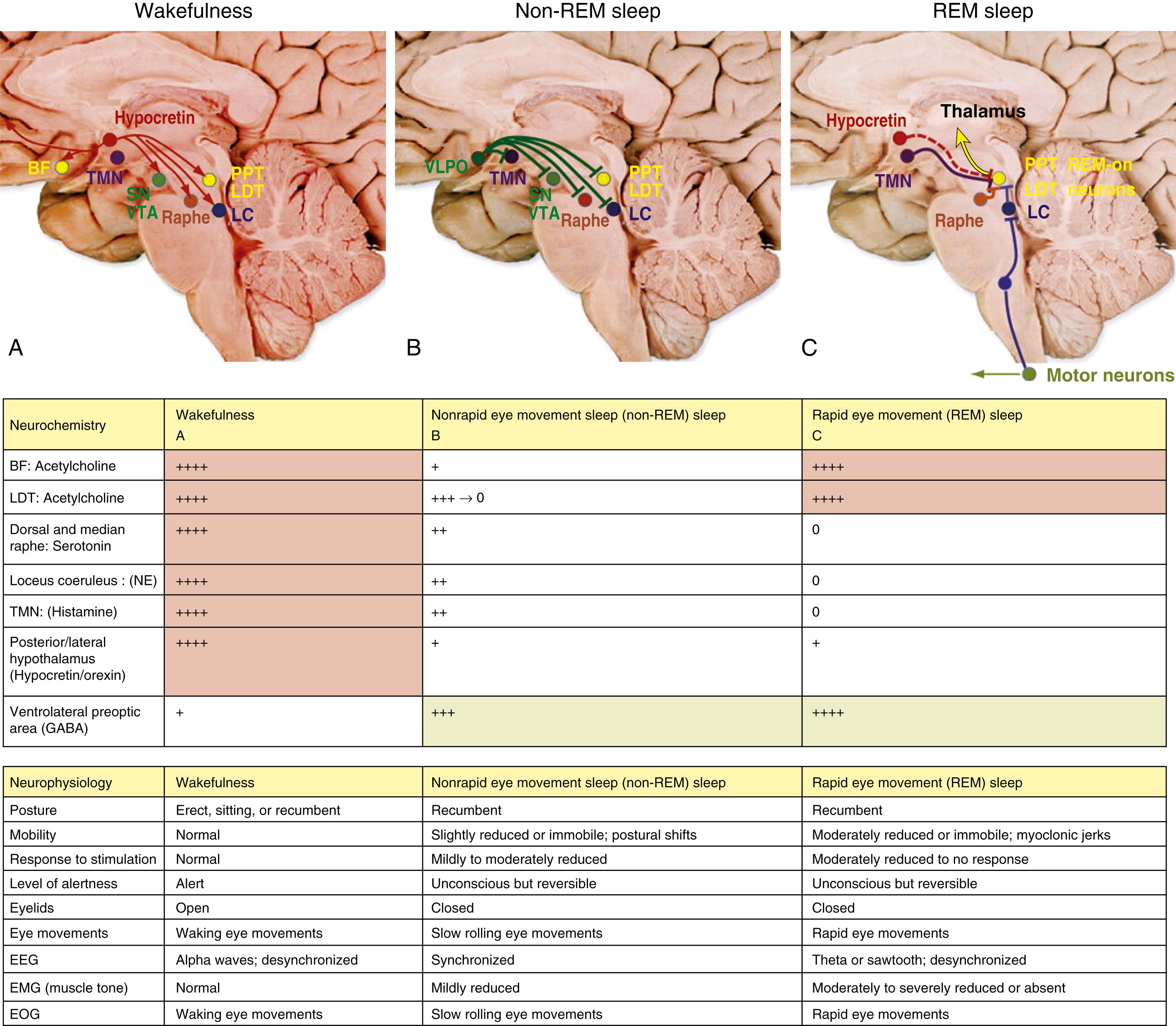
After a period of uncertainty regarding the role of dopamine, it has recently been confirmed that the midbrain dopaminergic system (A8–A10), particularly the dopaminergic neurons in the ventral periaqueductal gray (vPAG), play an active role in maintaining wakefulness through their widespread reciprocal connections with sleep/wake regulatory systems of neurons ( ). Norepinephrine-containing locus coeruleus neurons show their highest firing rates during wakefulness, their lowest during REM sleep, and intermediate rates during NREM sleep. Pharmacological studies suggest that posterior hypothalamic histaminergic neurons also help maintain wakefulness.
The excitatory amino acids, glutamate and aspartate, are intermingled within the ARAS and are present in many neurons projecting to the cerebral cortex, forebrain, and brainstem. These excitatory amino acids are maximally released during wakefulness. The discovery of hypothalamic hypocretin neurons and their widespread CNS projections has directed attention to the role of the hypocretin system in sleep/wake regulation. In 1998, deLecea and coauthors described two neuropeptides in the lateral hypothalamus and perifornical region that were termed hypocretin 1 and hypocretin 2 . Independently in the same year, Sakurai and colleagues (1998) described two neuropeptides in the same region, which they named orexin A and orexin B (corresponding to hypocretin 1 and hypocretin 2, respectively).
It was shown thereafter that these hypocretin systems have widespread ascending and descending projections to the locus coeruleus, dorsal raphe, ventral tegmental area, tuberomammillary nuclei of the posterior hypothalamus, laterodorsal tegmental (LDT) and pedunculopontine tegmental (PPT) nuclei, VLPO neurons in the hypothalamus, basal forebrain, limbic system (hippocampus and amygdala), cerebral cortex, thalamus (intralaminar and midline nuclei), and autonomic neurons (nucleus tractus solitarius [NTS], dorsal vagal nuclei, and intermediolateral neurons of the spinal cord). Hypocretin systems promote wakefulness mainly through excitation of tuberomammillary histaminergic, locus ceruleus noradrenergic, and midline raphe serotonergic neurons as well as dopaminergic neurons. Reduced activity of hypocretin systems may be partly responsible for inducing sleepiness. These systems also suppress REM sleep through activation of the aminergic neurons (REM-off), which in turn inhibit REM-on neurons in the LDT/PPT nuclei. Brainstem arousal centers were identified and characterized, and support was later provided for the concept of sleep-promoting circuitry in the anterior hypothalamus/preoptic area, where the VLPO nucleus contains sleep-active cells that contain the inhibitory neurotransmitters γ-aminobutyric acid (GABA) and galanin (Gal), which promote sleep ![]() eFig. 101.7 .
eFig. 101.7 .
Fig. 101.17 summarizes the neuroanatomical substrates of wakefulness, REM and NREM sleep highlighting the neuroanatomy, neurochemistry, and corresponding physiological activity during polysomnography.
In the first quarter of the last century during the great epidemic of encephalitis lethargica, von Economo noted that patients with encephalitis lethargica who had excessive sleepiness had pathological alterations in the posterior hypothalamus, whereas those with severe insomnia had predominant lesions in the anterior hypothalamus. These findings immediately suggested the existence of sleep/wake centers in the hypothalamus. Sleep-promoting neurons are thought to reside in the VLPO and the median preoptic neurons (MnPn) of the anterior hypothalamus ( ) and in the region of the NTS in the medulla. The hypothalamic preoptic neurons (VLPO and MnPn) as well as melanin concentrating hormone (MCH) neurons ( ) intermingled with hypocretin neurons in the lateral hypothalamus are sleep-promoting neurons. VLPO neurons consist of two subgroups, clustered and diffuse, depending on their distribution pattern. The tightly clustered neurons project to the tuberomammillary nuclei and promote NREM sleep, whereas diffusely distributed neurons project to the aminergic nuclei in the locus coeruleus and the dorsal raphe region of the brainstem, participating in REM sleep. The VLPO, MnPn, and MCH neurons fire actively during NREM sleep, and their lesion induces insomnia. The GABA- and Gal-containing VLPO and MnPn neurons project to inhibit the arousal systems in the brain stem (locus coeruleus and dorsal raphe), forebrain cholinergic, and posterolateral hypothalamic hypocretinergic and histaminergic nuclei, which in turn inhibit VLPO, MnPn, and MCH neurons.
The contemporary theory for the mechanism of NREM sleep suggests a reciprocal interaction between two antagonistic neurons in the VLPO area of the anterior hypothalamus and wake-promoting neurons in the tuberomammillary nuclei of the posterior hypothalamus, basal forebrain, and mesopontine tegmentum ( ; ).
There are three main models to explain the mechanism of REM sleep. The earliest and most generally well known is the McCarley-Hobson reciprocal interaction model ( ![]() eFig. 101.18 ), based on the reciprocal interaction of REM-on and REM-off neurons ( ). Neurons in the PPT and LDT nuclei in the pontomesencephalic region are cholinergic and are REM-on cells, which are responsible for REM sleep, showing highest firing rates at this stage. The REM-off cells are located in the locus coeruleus and dorsal raphe nuclei. These cells are aminergic neurons and are inactive during REM sleep. Histaminergic neurons in the tuberomammillary region of the posterior hypothalamus can also be considered REM-off cells. Thus, the cholinergic REM-on and aminergic REM-off cells are all located within the transections in the pons. LDT-PPT cholinergic neurons promote REM sleep through pontine reticular formation (PRF) effector neurons, which in turn send feedback loops to LDT-PPT neurons. Cholinergic neurons of the PPT and LDT projecting to the thalamus and basal forebrain regions, as well as to the PRF, are responsible for activation and generation of REM sleep. Aminergic cells seem to play a permissive role in the appearance of the REM sleep state. In the latest modification of the reciprocal interaction model, McCarley suggested that in addition to cholinergic excitation of PRF, a reduction of GABA inhibition in the PRF also may play a role in REM sleep generation. For example, GABA levels in the PRF (as measured by microdialysis technique) are lowest during REM and intermediate between wakefulness and REM and NREM sleep. Also, injection of GABA antagonists (e.g., bicuculline) into rostral PRF produced REM sleep in cats and rats. There is evidence of GABA-ergic inhibition of locus coeruleus/dorsal raphe nuclei (REM-off neurons). The source of GABA-ergic neurons is probably both local (e.g., a subgroup of PRF GABA-ergic neurons) and distant (e.g., GABA-ergic neurons in the ventrolateral periaqueductal gray matter).
eFig. 101.18 ), based on the reciprocal interaction of REM-on and REM-off neurons ( ). Neurons in the PPT and LDT nuclei in the pontomesencephalic region are cholinergic and are REM-on cells, which are responsible for REM sleep, showing highest firing rates at this stage. The REM-off cells are located in the locus coeruleus and dorsal raphe nuclei. These cells are aminergic neurons and are inactive during REM sleep. Histaminergic neurons in the tuberomammillary region of the posterior hypothalamus can also be considered REM-off cells. Thus, the cholinergic REM-on and aminergic REM-off cells are all located within the transections in the pons. LDT-PPT cholinergic neurons promote REM sleep through pontine reticular formation (PRF) effector neurons, which in turn send feedback loops to LDT-PPT neurons. Cholinergic neurons of the PPT and LDT projecting to the thalamus and basal forebrain regions, as well as to the PRF, are responsible for activation and generation of REM sleep. Aminergic cells seem to play a permissive role in the appearance of the REM sleep state. In the latest modification of the reciprocal interaction model, McCarley suggested that in addition to cholinergic excitation of PRF, a reduction of GABA inhibition in the PRF also may play a role in REM sleep generation. For example, GABA levels in the PRF (as measured by microdialysis technique) are lowest during REM and intermediate between wakefulness and REM and NREM sleep. Also, injection of GABA antagonists (e.g., bicuculline) into rostral PRF produced REM sleep in cats and rats. There is evidence of GABA-ergic inhibition of locus coeruleus/dorsal raphe nuclei (REM-off neurons). The source of GABA-ergic neurons is probably both local (e.g., a subgroup of PRF GABA-ergic neurons) and distant (e.g., GABA-ergic neurons in the ventrolateral periaqueductal gray matter).
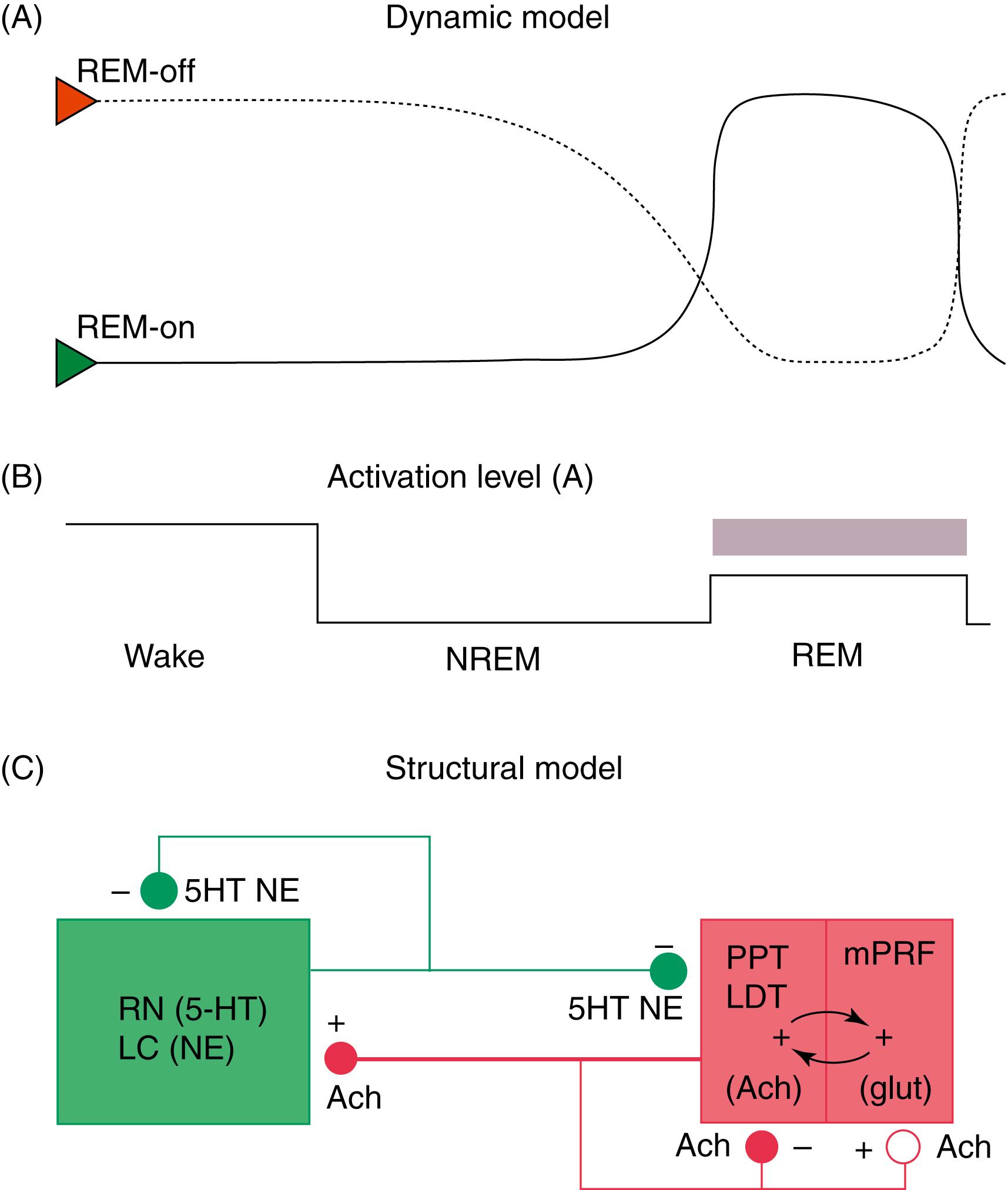
In the model proposed by Lu and coworkers (2006), there is reciprocal interaction between GABA-ergic REM-off neurons in ventrolateral periaqueductal gray matter (vlPAG) and lateral pontine tegmentum and GABA-ergic REM-on neurons in the sublaterodorsal nucleus (SLD; corresponding to the dorsal subcoeruleus or peri-locus coeruleus alpha in cats) and a dorsal extension of the SLD termed the precoeruleus (PC). These mutually inhibitory neuronal populations (SLD GABA-ergic REM-on and GABA-ergic REM-off neurons in the vlPAG- lateral pontine tegmentum) serve as a flip-flop switch. Ascending glutamatergic projections from PC neurons to medial septum are responsible for EEG hippocampal theta rhythm during REM sleep. Descending glutamatergic projections from ventral SLD directly to spinal interneurons, apparently without a relay in the medial medulla, inhibit spinal ventral horns by both glycerinergic and GABA-ergic mechanisms. Cholinergic and aminergic neurons play a modulatory role and are not part of the flip-flop switch. The question arises as to how two mutually inhibitory neuronal populations (e.g., GABA-ergic REM-on and GABA-ergic REM-off neurons) stabilize the flip-flop switch permitting NREM-REM cycling. It has been suggested that inhibitory external projections from extended ventrolateral preoptic neurons (eVLPO) and excitatory projections from hypocretinergic and aminergic neurons to the REM-off neurons stabilize the switch, permitting NREM-REM cycling. suggested that this model is based on C-fos labeling, only without electrophysiological recordings. Furthermore, this model does not address how REM sleep duration progressively increases with the progression of the night. A third promising model has recently been proposed by , in which at the onset of REM sleep, SLD glutamatergic REM-on neurons are activated with deactivation of REM-off GABA-ergic VLPAG and mesopontine tegmentum. Ventral SLD glutamatergic neurons using both a direct pathway to the spinal cord and an indirect pathway through the ventromedial medulla activate glycinergic and GABA-ergic inhibitory interneurons causing hyperpolarization of motor neurons and REM atonia, a hallmark of the REM sleep state. Dorsal SLD sends ascending glutamatergic neurons causing cerebral cortical activation through projections to thalamocortical neurons.
![]() Additional text available at http://expertconsult.inkling.com .
Additional text available at http://expertconsult.inkling.com .
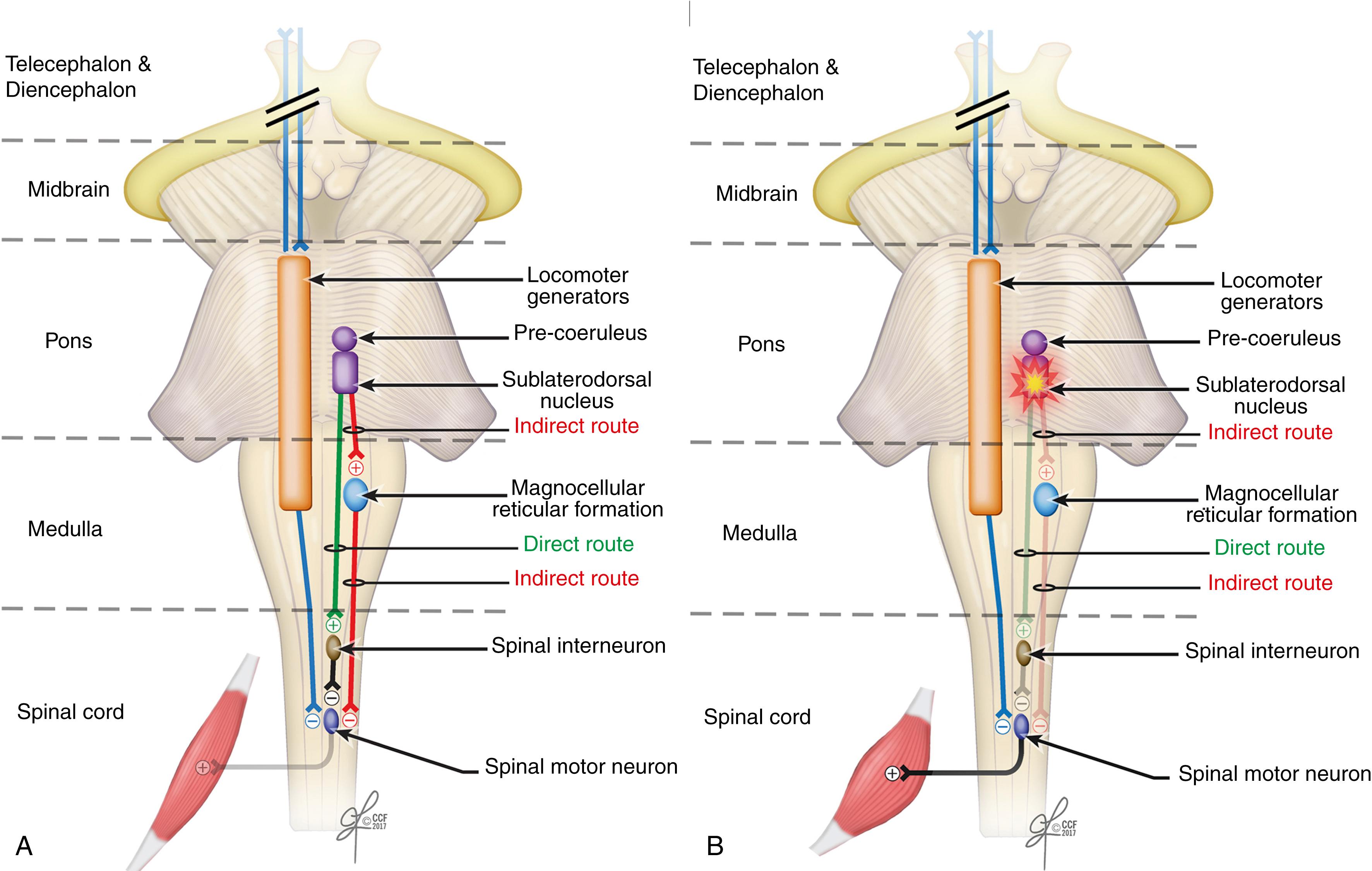
Muscle hypotonia or atonia during REM sleep is thought to depend on inhibitory postsynaptic potentials generated by dorsal pontine interneurons sending descending axons as depicted in Fig. 101.19 . The pathway from the peri-locus coeruleus (Pre-Coeruleus, in the diagram) alpha region ventral to the locus coeruleus, to the lateral tegmental reticular tract, and then to the medial medullary region (demarcated as the magnocellular reticular formation) and the reticulospinal tract projecting to the spinal interneuron of the spinal cord controls REM sleep-induced muscle atonia. An experimental lesion (☆) in the peri-locus coeruleus alpha region and in the medial medullary region (Panel B) produced REM sleep without muscle atonia (RSWA). In addition to DEB associated with RSWA, it is thought that a structural or functional alteration of the pathway maintaining muscle atonia during REM sleep is most likely responsible.
Advances in neuroimaging studies, including positron emission tomography (PET) and single-photon emission computed tomography (SPECT) scans, have been able to visualize dramatic changes in function in cortical and subcortical neuronal networks in different sleep states and stages, advancing our understanding of the functional neuroanatomy of sleep/wakefulness ( ). PET scans have shown marked activation of the amygdala and the anterior cingulate region (part of the limbic system) during REM sleep, which is, of course, generated by brainstem neurons. In contrast, in NREM sleep, neuroimaging techniques have shown declining function in the thalamocortical circuits, including the association cortex of the frontoparietal and temporal lobes. Thus, the brainstem, hypothalamic, and forebrain sleep/wake-promoting neurons modulate functions of widespread forebrain cortical areas, keeping in balance cortical and subcortical circuits to control sleep/wake.
![]() Additional text available at http://expertconsult.inkling.com .
Additional text available at http://expertconsult.inkling.com .
Many important questions regarding the mechanism of sleep remain unanswered. Why do VLPO neurons fire at sleep onset? What initiates the cascade of disfacilitation in brainstem wake-promoting neurons? What initiates activation of LDT neurons and PPT neurons at REM onset? What causes activation of wake-promoting neurons at sleep offset? What maintains NREM-REM sleep cycling?
The following speculative summary attempts to answer some of these questions. VLPO excitation at NREM sleep onset is initiated by adenosine (a sleep-promoting factor in the forebrain region, accumulated during prolonged wakefulness) and suprachiasmatic nucleus (SCN), as well as by reciprocal inhibition of aminergic and orexin wake-promoting neurons; progressive inhibition of aminergic REM-off neurons, causing disinhibition of REM-on cholinergic neurons and initiating REM sleep; and simultaneous cascade of disfacilitation of the brainstem arousal system due to decreased environmental afferent stimuli, culminating in blockades at the thalamic level. Physiological facilitation (or disinhibition) after a certain period (perhaps determined in the case of sleep/wake regulation by the SCN regulatory neurons connected anatomically to sleep/wake neurons) will be followed by inhibition (or disfacilitation), and thus the cycle will begin again.
Marked impairment of the arousal and cognition systems may result in coma or severe sleepiness. The reversibility of this state of awareness differentiates sleep from coma. There are also physiological and metabolic differences between sleep and coma. Coma is a passive process (loss of function), whereas sleep is an active state resulting from physiological interactions of various systems in the brainstem and cerebral cortex. Metabolic depression of the cerebral cortex and brainstem characterizes coma and stupor, whereas in sleep, oxygen use and metabolic rhythm remain intact. By disrupting the arousal system or stimulating the sleep-promoting neurons, focal neurological lesions may also cause excessive sleepiness. For example, lesions of the brainstem, thalamus, hypothalamus, and periaqueductal region may produce excessive sleepiness, stupor, and coma. These regions may also affect REM-generating neurons in the pons and cause various REM sleep alterations, so lesions in these structures may cause symptomatic narcolepsy.
![]() Additional text available at http://expertconsult.inkling.com .
Additional text available at http://expertconsult.inkling.com .
The 18th-century French astronomer de Mairan (1731) first directed our attention to the existence of circadian rhythm when he noted that in a heliotrope plant, the leaves closed at sunset and opened at sunrise even when the plant was kept in darkness (see ). This observation clearly pointed to a 24-hour rhythm controlled by an internal clock. The existence of such a circadian rhythm in human beings and other animals was confirmed toward the last half of the last century. The term circadian rhythm originates from the Latin circa , meaning “about,” and dies , meaning “day.” Human circadian rhythm generally has a cycle length close to 24 hours (approximately 24.2 hours) ( ). The existence of circadian rhythms independent of environmental stimuli has been clearly demonstrated by experimental isolation of humans from all environmental time cues (the German term, Zeitgeber , or “time giver”), as in a cave or underground bunker, to study free-running rhythms.
The paired SCN, the paired nuclei above the hypothalamus, function as the body clock to control circadian rhythm ( Fig. 101.20 ). The recent discovery of anatomical projections from SCN to the lateral hypothalamic neurons containing hypocretin (wake-promoting) and anterior hypothalamic VLPO containing sleep-promoting neurons suggested that the SCN may also affect sleep regulation and homeostasis independent of circadian rhythm generation ( ) ( ![]() ).
).
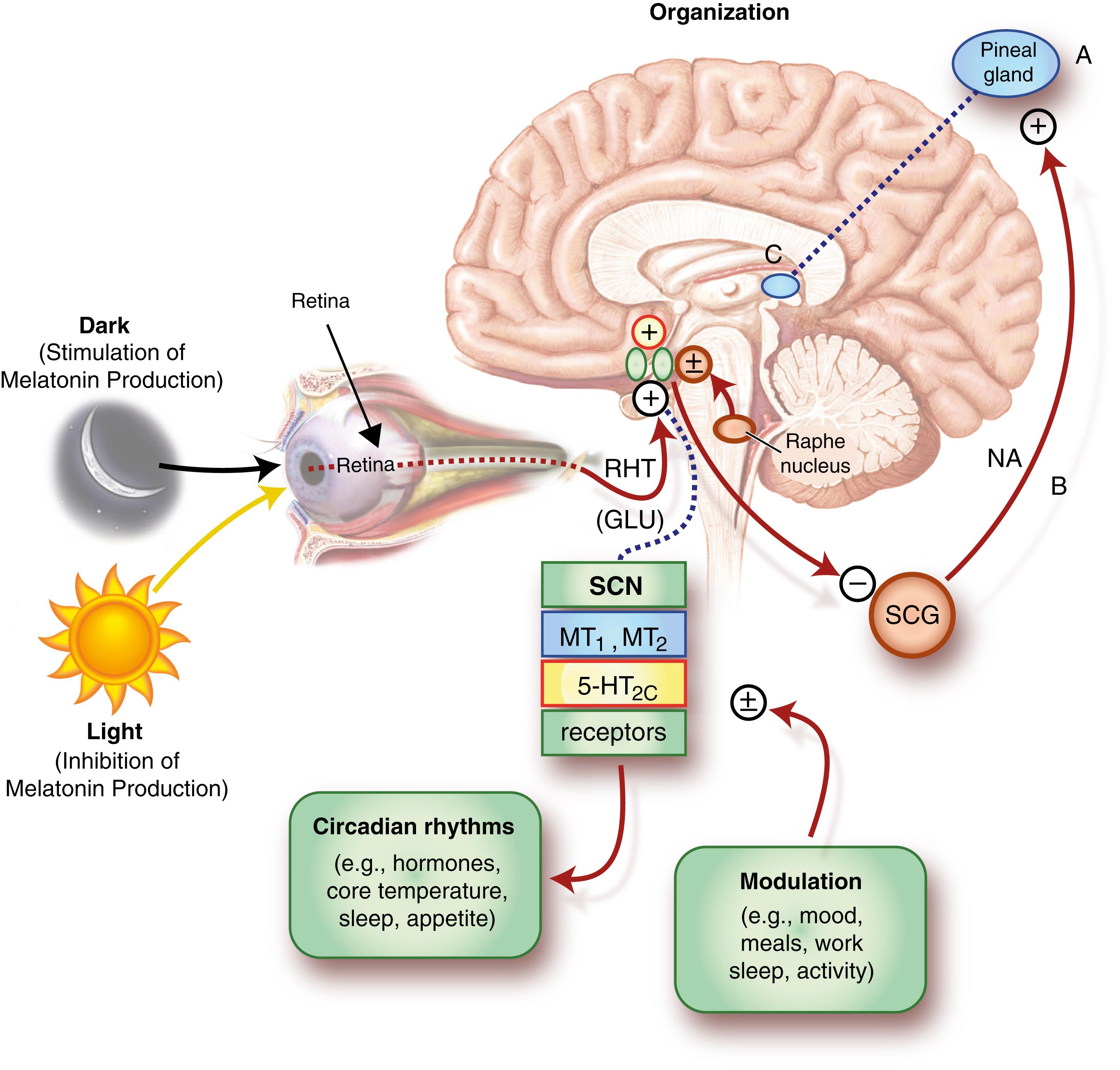
Circadian Oscillators in the Epithalamus.
(From Guilding, C., Hughes, A.T.L., Piggins, H.D., 2010. Neuroscience , 169[4] . Copyright © 2010 IBRO.)
The existence of environment-independent autonomous rhythms suggests that the human body also has an internal biological clock. The SCN receives photic information from the retinohypothalamic tract, which sends signals to multiple synaptic pathways in other parts of the hypothalamus, the superior cervical ganglion, and the pineal gland where melatonin is produced and released (A). Through a polysynaptic projection, the SCN inhibits the activity of the superior cervical ganglia (SCG), which supply the pineal gland with an excitatory, noradrenaline (NA)-containing input (B). This mechanism allows light to suppress the production and release of melatonin from the pineal gland and, subsequently, melatonin secretion is enhanced in the dark period ( ). Melatonin is a critical modulator of human circadian rhythm for entrainment by the light/dark cycle. The melatonin level rises fairly abruptly in the evening and then reaches its maximum level between 3:00 am and 5:00 am , after which it decreases to low levels during the daytime. Melatonin reciprocally activates the SCN at two melatonin receptors (C): melatonin type 1 (MT 1 ) and melatonin type 2 (MT 2 ) receptors. Serotonergic input from the raphe nucleus modulates the SCN through actions at serotonin (also known as 5-hydroxytryptamine; 5-HT) receptor 5-HT 2C . Daily activity and behaviors likewise influence output from the SCN—the neuronal master clock for coordinating circadian rhythms—including key physiological processes such as the sleep/wake cycle, body temperature, and neuroendocrine secretion. The SCN also receives key signals vital for metabolism ( Fig. 101.21 ) and derives “time of day” information from environmental light cues captured by specialized cells within the retina.
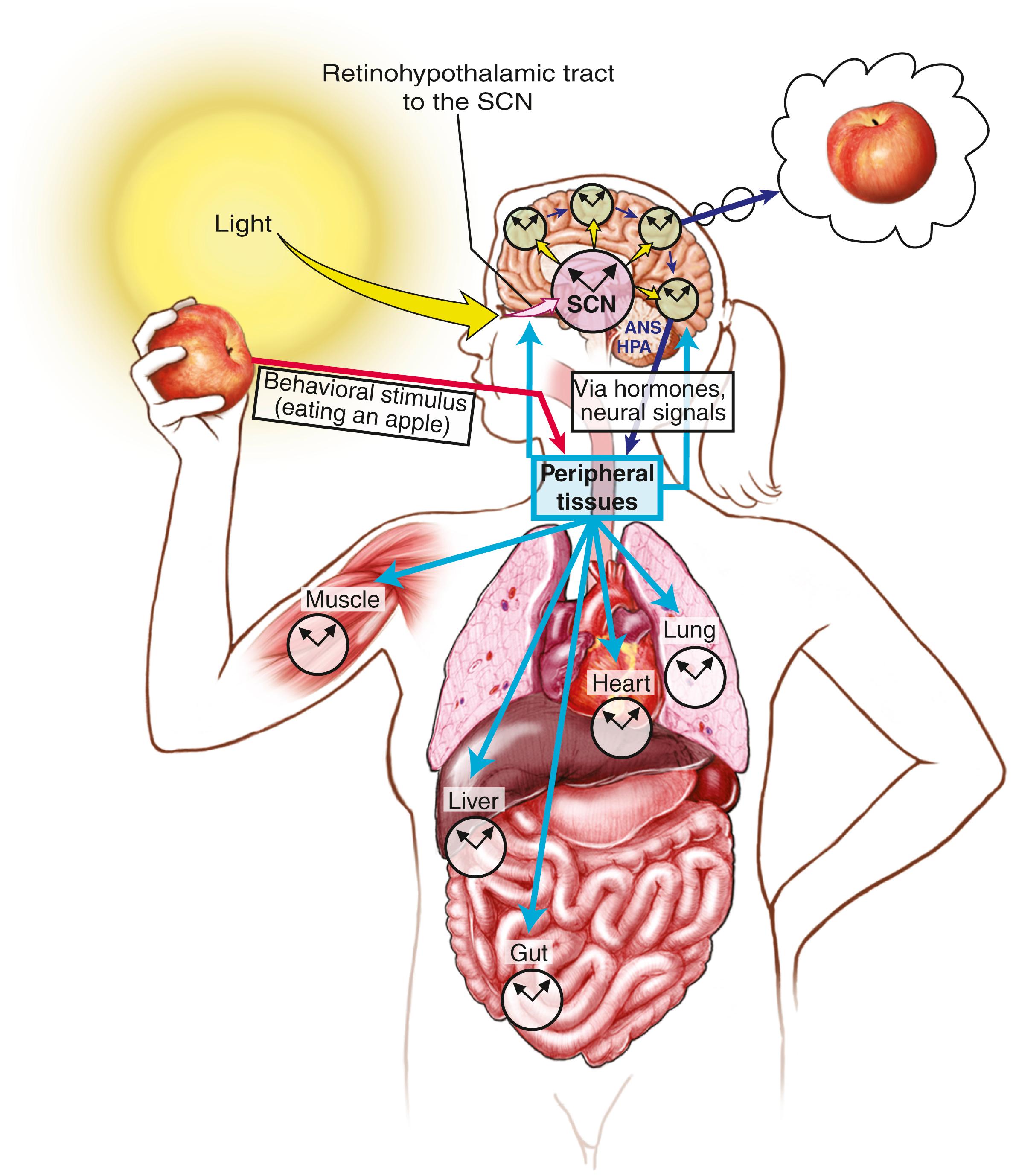
Sleep scientists have begun to identify the molecular basis of the mammalian circadian clock. A total of eight or nine genes (e.g., CLOCK, PER, Bmal) and their protein products have been identified within the circadian clock system; understanding of these is still evolving ( ). Remarkable progress has been made in the past few years in the key components of the circadian clock in both fruit flies (Drosophila) and mammals. Dysfunction of circadian rhythm results in some important human sleep disorders. The molecular mechanism of two human circadian rhythm disorders—advanced sleep phase syndrome (ASPS) and delayed sleep phase syndrome (DSPS)—has been uncovered by applying gene sequencing techniques. ASPS occur due to a mutation of the PER2 gene (a human homolog of the period 2 gene in Drosophila ) causing advancing of the clock (i.e., alteration of the circadian timing of sleep propensity). DSPS is due to polymorphism of the PER3 gene.
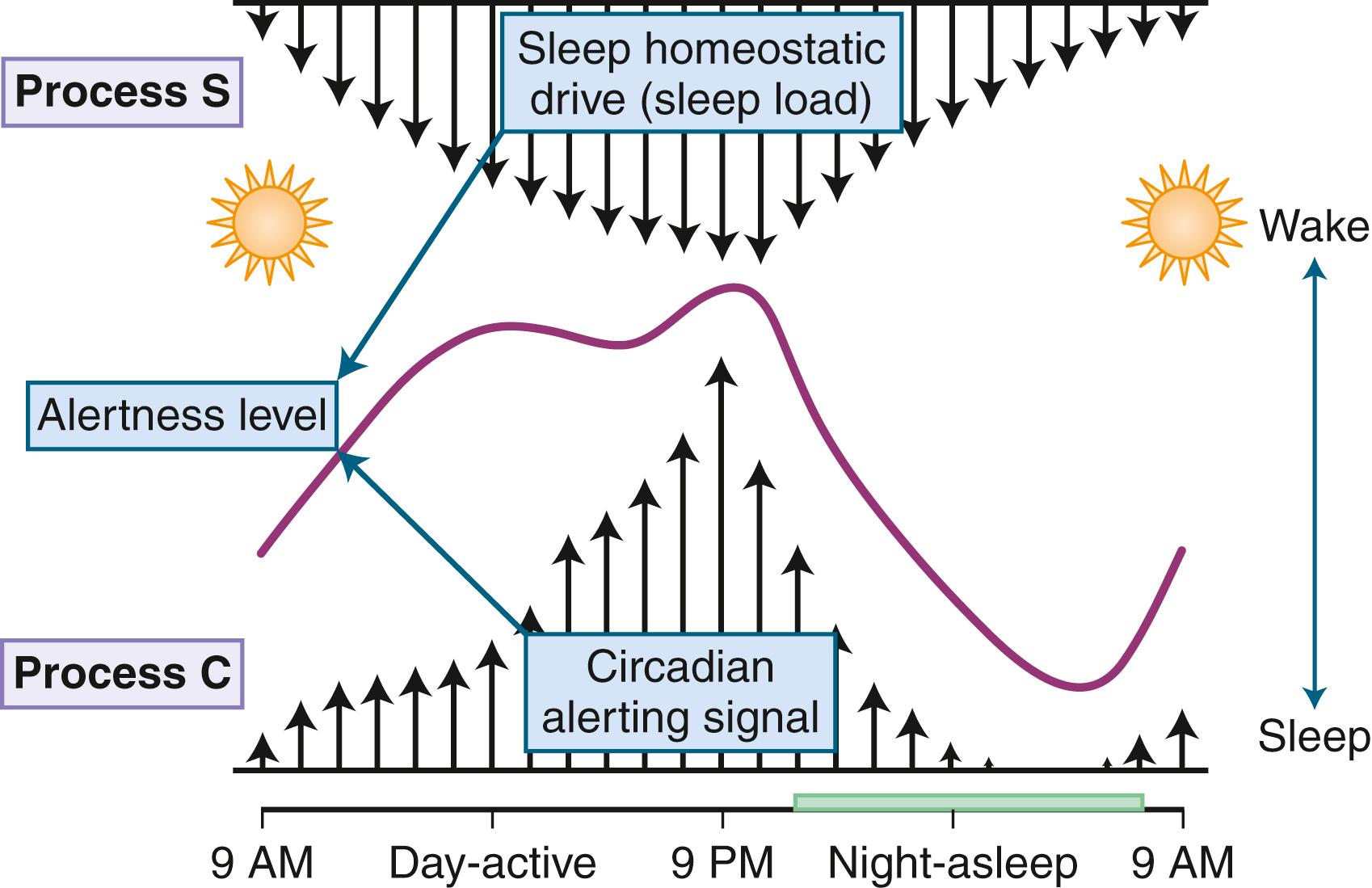
Sleep and wakefulness are controlled by both homeostatic and circadian factors. The duration of prior wakefulness determines the propensity to sleepiness (homeostatic factor), whereas circadian factors determine the timing, duration, and characteristics of sleep. There are two types of sleepiness: physiological and subjective ( ). Physiological sleepiness is the body’s propensity to sleepiness. There are two highly vulnerable periods of sleepiness: 2:00–6:00 am , particularly 3:00–5:00 am , and 2:00–6:00 pm , particularly 3:00–5:00 pm . The propensity to physiological sleepiness (e.g., midafternoon and early-morning hours) depends on circadian factors. The highest number of sleep-related accidents have been observed during these periods. Subjective sleepiness is the individual’s perception of sleepiness; it depends on several external factors such as a stimulating environment and ingestion of coffee and other caffeinated beverages.
Physiological sleepiness depends on two processes: homeostatic factor and circadian phase. Homeostatic factor refers to a prior period of wakefulness and sleep debt. After a prolonged period of wakefulness, there is an increasing tendency to sleep. The recovery from sleep debt is aided by an additional amount of sleep, but this recovery is not linear. Thus, an exact number of hours of sleep are not required to repay sleep debt; rather, the body needs an adequate amount of slow-wave sleep for restoration. The interaction between the circadian and the homeostatic drive producing alertness is depicted in Fig. 101.22 . The circadian factor determines the body’s propensity to maximal sleepiness between 3:00 am and 5:00 am . The second period of maximal sleepiness (3:00–5:00 pm ) is not as strong as the first. Sleep and wakefulness and the circadian pacemaker have a reciprocal relationship: the biological clock can affect sleep and wakefulness, and sleep and wakefulness can affect the clock. There are two variables that seem to play a role in regulating the timing of sleep. First is the homeostatic sleep drive, which increases as the day progresses and the longer a person is awake. The second is timing information from the SCN. In this two-process model, the SCN promotes wakefulness by stimulating arousal networks. The activity of the circadian system appears to oppose that of the homeostatic sleep drive, and thus the alerting mediated by the SCN increases during the day. The propensity to be awake or asleep at any time is related to the homeostatic sleep drive and the opposing SCN alerting signal. At normal bedtime, both the alerting drive and the sleep drive are at their highest level. The two melatonin receptors, MT1 and MT2 receptor subtypes, are directly involved in the regulation of sleep and circadian regulation. Stimulation of MT1 receptors is believed to decrease the alerting signal from the SCN, while MT2 stimulation is thought to be involved in synchronizing the circadian system.
Various sleep factors have been identified, but their role in maintaining homeostasis has not been clearly established ( ). Several cytokines, such as interleukin-1, interferon-α, and tumor necrosis factor, promote sleep. Other sleep factors increase in concentration during prolonged wakefulness and infection. It has been shown that adenosine in the basal forebrain can fulfill the major criteria for the neural sleep factor that mediates the somnogenic effect of prolonged wakefulness by acting through adenosine A1 and A2A receptors. Several other endogenous compounds may serve as sleep factors, including delta sleep-inducing peptides, muramyl peptides, cholecystokinins, arginine vasotocin, vasoactive intestinal peptides, growth hormone-releasing factors, and somatostatins. Finally, neurologists need to be mindful of how the circadian clock regulates how the body responds to an illness, such as COVID-19. It is particularly relevant these days to appreciate the role of chronotherapy when matching immunotherpy to the body’s circadian rhythm in impacting recovery from illness.
The function of sleep remains the greatest biological mystery of all time. There are several theories about the function of sleep ( ![]() eBox 101.3 ), but none are satisfactory ( ; ; ). Sleep deprivation experiments in animals have clearly shown that sleep is necessary for survival, but from a practical point of view, complete sleep deprivation for a prolonged period cannot be conducted in humans. Sleep deprivation studies in humans have shown an impairment of performance, which demonstrates the need for sleep. The performance impairment of prolonged sleep deprivation results from a decreased motivation and frequent “microsleeps.” Overall, human sleep deprivation experiments have proven that sleep deprivation causes sleepiness and impairment of performance, vigilance, attention, concentration, and memory. Sleep deprivation may also cause some metabolic, hormonal, and immunological effects. Sleep deprivation causes immune suppression; even partial sleep deprivation reduces cellular immune responses. Studies from Van Cauter’s ( ) group include a clearly documented elevation of cortisol level following even partial sleep loss, suggesting an alteration in hypothalamic-pituitary-adrenal axis function. This has been confirmed even in chronic sleep deprivation that causes impairment of glucose tolerance. Glucose intolerance may contribute to memory impairment as a result of decreased hippocampal function. Chronic sleep deprivation may also cause a decrement of thyrotropin concentration, increased evening cortisol level, and sympathetic hyperactivity, which may serve as risk factors for obesity, hypertension, and diabetes mellitus. Further data from the same group demonstrated that increase in ghrelin levels associated with short sleep reduced leptin. These differences in leptin and ghrelin may induce increased appetite, possibly explaining the increased body mass index (BMI) observed with short sleep duration in the study populations. The authors correctly indicate that in Western societies, where chronic sleep restriction is common and food is ubiquitous, alterations in appetite regulatory hormones with sleep curtailment may contribute to obesity.
eBox 101.3 ), but none are satisfactory ( ; ; ). Sleep deprivation experiments in animals have clearly shown that sleep is necessary for survival, but from a practical point of view, complete sleep deprivation for a prolonged period cannot be conducted in humans. Sleep deprivation studies in humans have shown an impairment of performance, which demonstrates the need for sleep. The performance impairment of prolonged sleep deprivation results from a decreased motivation and frequent “microsleeps.” Overall, human sleep deprivation experiments have proven that sleep deprivation causes sleepiness and impairment of performance, vigilance, attention, concentration, and memory. Sleep deprivation may also cause some metabolic, hormonal, and immunological effects. Sleep deprivation causes immune suppression; even partial sleep deprivation reduces cellular immune responses. Studies from Van Cauter’s ( ) group include a clearly documented elevation of cortisol level following even partial sleep loss, suggesting an alteration in hypothalamic-pituitary-adrenal axis function. This has been confirmed even in chronic sleep deprivation that causes impairment of glucose tolerance. Glucose intolerance may contribute to memory impairment as a result of decreased hippocampal function. Chronic sleep deprivation may also cause a decrement of thyrotropin concentration, increased evening cortisol level, and sympathetic hyperactivity, which may serve as risk factors for obesity, hypertension, and diabetes mellitus. Further data from the same group demonstrated that increase in ghrelin levels associated with short sleep reduced leptin. These differences in leptin and ghrelin may induce increased appetite, possibly explaining the increased body mass index (BMI) observed with short sleep duration in the study populations. The authors correctly indicate that in Western societies, where chronic sleep restriction is common and food is ubiquitous, alterations in appetite regulatory hormones with sleep curtailment may contribute to obesity.
Facilitation of waste clearance of the central nervous system via the glymphatic system
Synaptic neuronal network integrity
Body and brain tissue restoration
Energy conservation
Adaptation
Memory reinforcement and consolidation
Gene expression in sleep/wakefulness
Thermoregulation
The restorative theory suggests that sleep is needed to restore cerebral function after periods of waking. The findings of increased secretion of anabolic hormones (e.g., growth hormone, prolactin, testosterone, and luteinizing hormone) and decreased levels of catabolic hormones (e.g., cortisol) during sleep, as well as the subjective feeling of being refreshed after sleep, may support the theory of body and brain tissue restoration by sleep. The role of NREM sleep in restoring the body is further supported by the presence of increased slow-wave sleep after sleep deprivation. The critical role of REM sleep for CNS development in young organisms and increased protein synthesis in the brain during REM sleep may support the theory of restoration of brain function by REM sleep. Although data remain scant and controversial, studies of brain basal metabolism that suggest an enhanced synthesis of macromolecules such as nucleic acids and proteins in the brain during sleep provide an argument in favor of the restorative theory of sleep.
The energy conservation theory is somewhat inadequate. The fact that animals with a high metabolic rate sleep longer than those with slower metabolism has been cited in support of this theory. It should, however, be noted that during 8 hours of sleep, only 120 calories are conserved.
The adaptation theory suggests that sleep is an instinct that allows creatures to survive under a variety of environmental conditions.
Some recent advances have been made in understanding the molecular mechanisms of memory consolidation during sleep ( ). Studies have revealed that new memories can be strengthened by sleep. In other words, sleep can rescue memories that are lost during wakefulness during the daytime, and consolidated memories can be reconsolidated when they are reactivated. Memory is stored in two stages: short-term memory, which is labile, and long-term memory, which is stable and consolidated, requiring synthesis of new ribonucleic acid (RNA) and proteins by the neurons. Recent studies have clearly shown a significant contribution of sleep to memory consolidation. It has been suggested that memory consolidation occurs during both slow-wave and REM sleep. There is a further suggestion that REM-NREM sleep cycling is also important for memory consolidation.
The theory of maintenance of synaptic and neuronal network integrity is an emerging concept that is concerned with the primary function of sleep. Intermittent stimulation of neural network synapses is necessary to preserve CNS function ( ).
Gene expression ( ) studied by deoxyribonucleic acid (DNA) microarray technique identified sleep- and wakefulness-related genes (brain transcripts) subserving different functions (e.g., energy metabolism, synaptic excitation, long-term potentiation, and response to cellular stress during wakefulness and protein synthesis, memory consolidation, and synaptic downscaling during sleep). Fig. 101.23 provides a basis for the synaptic homeostasis theory.
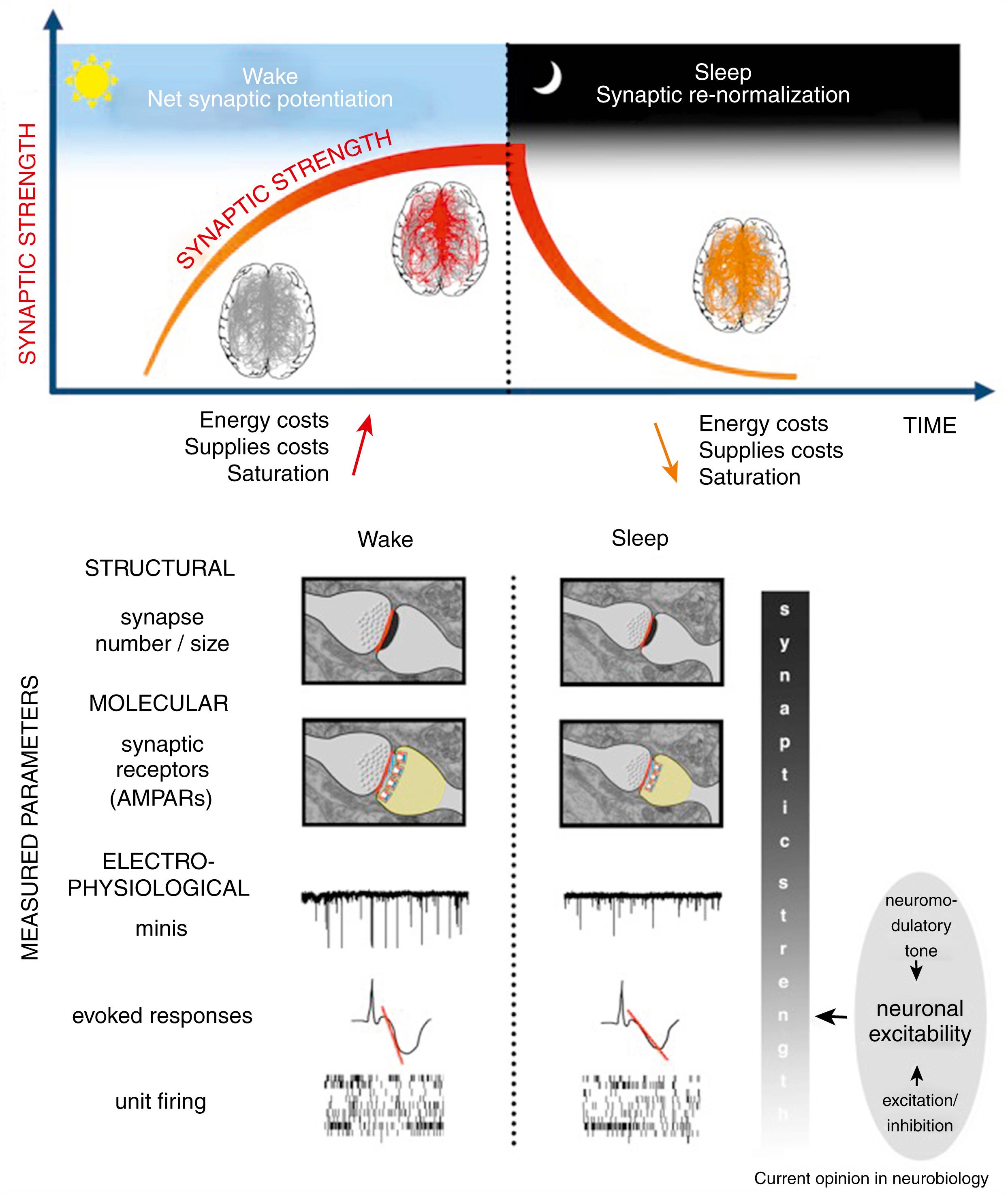
The top panel of the figure illustrates schematically how a net increase in synaptic strength occurs during wakefulness when many circuits in the brain become potentiated (red lines in the brain schematic), resulting in overall cellular and systems costs, followed by synaptic renormalization during sleep. This is that time when most, if not all, circuits undergo synaptic down-selection (green lines). The bottom portion of the figure highlights how parameters are used to test SHY. The color gradient in the column labeled “synaptic strength” highlights that while objectively verifiable indicators of structural and molecular measures were more likely to reveal a state of synaptic strength, electrophysiological measures such as firing rates and evoked responses can be strongly affected by other factors that modulate intrinsic neuronal excitability, including the levels of neuromodulators and the overall balance between excitation and inhibition. However, these cannot be used alone to infer synaptic strength. In the upper panels (structural and molecular), the red line indicates the axon to spine interface (ASI), and the yellow area outlines the head of the spine. Excitatory glutamatergic receptors (AMPARs) are shown as squared boxes close to the ASI. Minis = miniature excitatory synaptic currents ( ; ).
The thermoregulatory function theory is based on the observation that thermoregulatory homeostasis is maintained during sleep, whereas severe thermoregulatory abnormalities follow total sleep deprivation. The preoptic anterior hypothalamic neurons participate in thermoregulation and NREM sleep. These two processes are closely linked by preoptic anterior hypothalamic neurons but are clearly separate. Thermoregulation is maintained during NREM sleep but is suspended during REM sleep. Thermoregulatory responses such as shivering, piloerection, panting, and sweating are impaired during REM sleep. There is a loss of thermosensitivity in the preoptic anterior hypothalamic neurons during REM sleep.
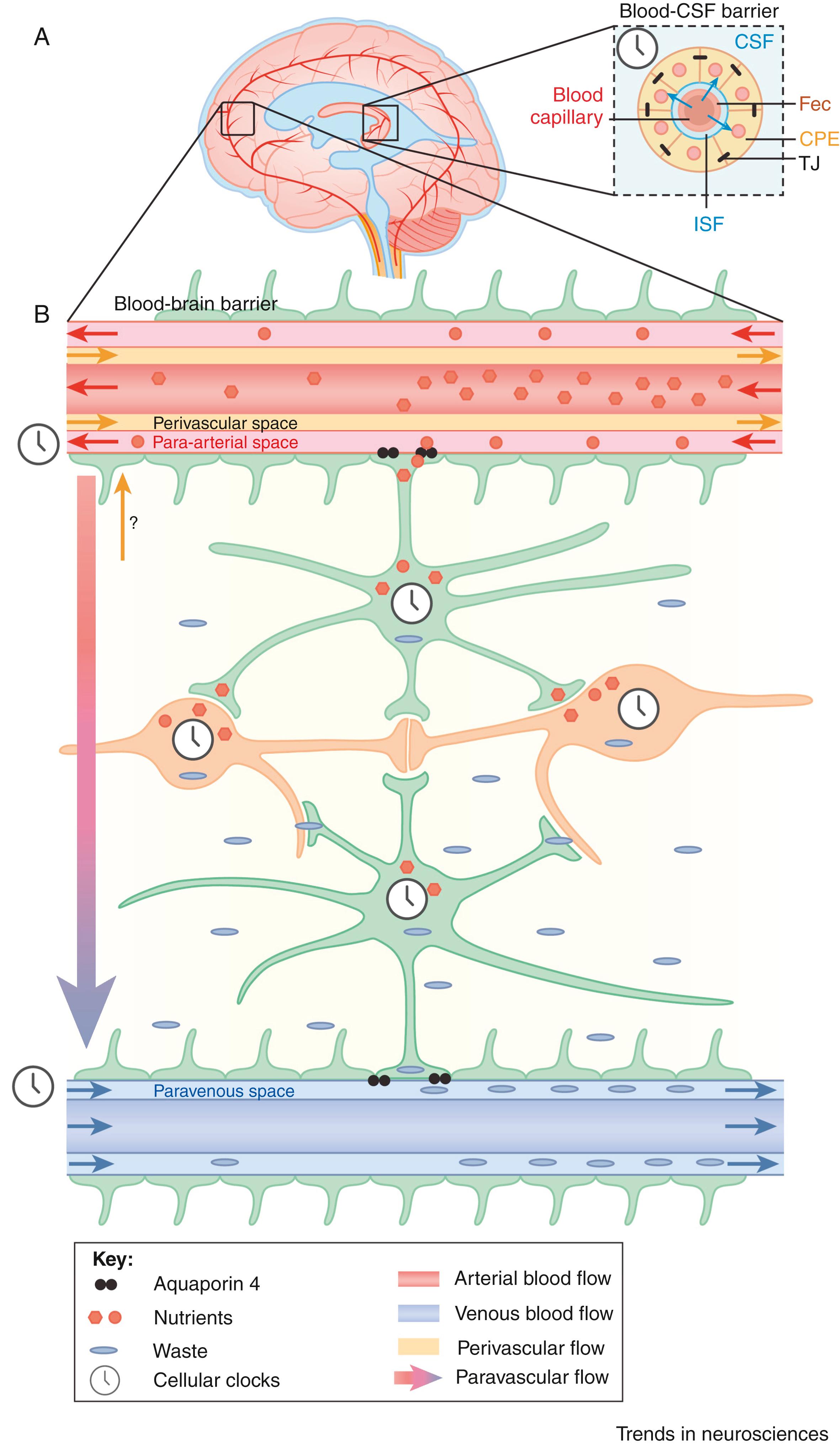
A fascinating new theory proposes a sleep state-dependent enhanced brain flushing as depicted by enhanced CSF flow through a relatively newly discovered drainage system, the so-called glymphatic system ( ). In mice, data revealed that enhanced CSF-flow, during sleep state, increased the removal of β-amyloid metabolites. The glymphatic system allows for CSF circulation through the brain, exchanging fluid allowing for enhanced clearance waste products across the blood–brain barrier into the nearby blood vessels for further transportation, as illustrated in Fig. 101.24 ( ). Emerging new data shows that the glymphatic (paravascular) system mediates liquid flux through the brain, highlights that the circadian clock probably plays a critical role in this context, and likely holds new research paradigms to further elucidate the role of sleep-state depended CSF-flow in delaying and preventing neurodegenerative disease-associated metabolites during sleeping, including alpha-synucleinopathies and tauopathies ( ). The glymphatic fluid transporting system could provide an additional source of apoE and then delivers it to brain via the periarterial space. By inference, when the glymphatic fluid flow and interstitial fluid (ISF) clearance system fails in this essential physiological role, one would presume a high likelihood of apoE isoform-specific disorders chronically ( ).
A constellation of physiological changes are observed in humans, which are distinctly different in three states of existence, namely wakefulness, NREM sleep, and REM sleep that are different from those noted during wakefulness ( ). This section outlines physiological changes observed during NREM and REM sleep in somatic and autonomic nervous systems and includes changes in the respiratory, cardiovascular, and gastrointestinal systems; endocrine, renal, and sexual function; and thermoregulation ( eTable 101.4 ).
| Physiology | Wakefulness | NREM sleep | REM sleep |
|---|---|---|---|
| Parasympathetic activity | ++ | +++ | ++++ |
| Sympathetic activity or variable (++) | ++ | + | Decreases |
| Heart rate | Normal sinus rhythm | Bradycardia | Brady/tachyarrhythmia |
| Blood pressure | Normal | Decreases | Variable |
| Cardiac output | Normal | Decreases | Decreases further |
| Peripheral vascular resistance | Normal | Normal or decreases slightly | Decreases further |
| Respiratory rate | Normal | Decreases | Variable; apneas may occur |
| Alveolar ventilation | Normal | Decreases | Decreases further |
| Upper airway muscle tone | ++ | + | Decreases or absent |
| Upper airway resistance | ++ | +++ | ++++ |
| Hypoxic and hypercapnic ventilatory responses | Normal | Decreases | Decreases further |
| Cerebral blood flow | ++ | + | +++ |
| Thermoregulation | ++ | + | − |
| Gastric acid secretion | Normal | Variable | Variable |
| Gastric motility | Normal | Decreases | Decreases |
| Swallowing | Normal | Decreases | Decreases |
| Salivary flow | Normal | Decreases | Decreases |
| Migrating motor complex (a special type of intestinal motor activity) | Normal | Slow velocity | Slow velocity |
| Penile or clitoral tumescence | Normal | Normal | Increases markedly |
Firing rates of many neurons in the CNS decrease during NREM sleep but increase during REM sleep.
During sleep, the autonomic nervous system undergoes several changes that may have implications for the pathophysiology of autonomic failure and sleep disorders in humans. Most of the autonomic changes involve respiration, circulation, thermoregulation, and the pupils (e.g., pupilloconstriction during sleep). During NREM sleep, there is an overall decrease in sympathetic activity and tonic increase in parasympathetic activity, which increases further during tonic REM sleep. In addition, during phasic tonic REM sleep, sympathetic activity decreases. Sympathetic activity, however, during phasic REM sleep, increases intermittently, which results in swings of blood pressure and heart rhythm, causing bradytachyarrhythmias.
Two systems—metabolic (or automatic) and voluntary (or behavioral)—control respiration during sleep and wakefulness. Both metabolic and voluntary systems operate during wakefulness, whereas only the metabolic system operates during NREM sleep. The wakefulness stimuli that act through the ascending reticular activity system (ARAS) also act as tonic stimuli to ventilation. Activity decreases in the respiratory neurons in the parabrachial and Kölliker-Fuse nuclei in the pons, the nucleus tractus solitarious (NTS), nucleus ambiguus, and nucleus retroambigualis in the medulla. Respiratory muscle activity decreases slightly during NREM sleep but markedly during REM sleep. A marked decrement or even temporary suppression of intercostal muscle tone occurs during REM sleep, whereas tonic activity of the diaphragm diminishes, and phasic activity continues. Muscle tone in the upper airway decreases in NREM sleep and disappears in REM sleep, resulting in an increase in upper airway resistance. The decreased sensitivity of the respiratory neurons to carbon dioxide, inhibition of the reticular activating system, and alteration of metabolic control of respiratory neurons during sleep result in a decrement of tidal volume, minute ventilation, and alveolar ventilation. In normal individuals, diminished alveolar ventilation causes arterial carbon dioxide tension (PaCo 2 ) to rise by 2–8 mmHg, the arterial oxygen tension (PaO 2 ) to decrease by 3–10mmHg, and oxygen saturation (SaO 2 ) to decrease by less than 2% during sleep. These blood gas changes are noted despite a fall in oxygen consumption and carbon dioxide production during sleep. Both hypercapnic and hypoxic ventilatory responses decrease during REM and NREM sleep, with a more marked decrease during REM sleep. These decrements result from a combination of factors: fewer functional medullary respiratory neurons during sleep, decreased sensitivity of the central chemoreceptors subserving medullary respiratory neurons, and increased resistance in the upper airway. Arousal responses also decrease, particularly during REM sleep. The voluntary respiratory control system may be active during some portion of REM sleep. Respiration is therefore vulnerable during sleep in normal individuals. Mild respiratory irregularity with few apneic episodes (apnea index <5) may occur at sleep onset and during REM sleep. In disease states, however, particularly PD and multiple system atrophy ( ), neuromuscular and brainstem disorders, and chronic obstructive pulmonary disease (COPD), apneas may become more frequent, more prolonged, and pathologically more significant.
Heart rate, blood pressure, cardiac output, and peripheral vascular resistance decrease during NREM sleep and decrease still further during REM sleep. During phasic REM, blood pressure and heart rate are unstable because of phasic vagal inhibition and sympathetic activation caused by alterations in brainstem neural activity. Heart rate and blood pressure fluctuate during REM sleep. Cerebral blood flow and cerebral metabolic rate for glucose and oxygen decrease by 5%–23% during stages 1–4 of NREM sleep, whereas these values increase by 10%–41% above waking levels during REM sleep. These data indirectly suggest that NREM sleep is the state of resting brain, with reduced neuronal activity, decreased synaptic transmission, and depressed cerebral metabolism. The data also are consistent with the assumption that REM sleep represents an active brain state with increased neuronal activity and increased brain metabolism. The largest increases during REM sleep are noted in the hypothalamus and the brainstem structures, and the smallest increases are in the cerebral cortex and white matter.
Because of all the hemodynamic and sympathetic alterations, REM sleep, which is prominent during the third part of the night’s sleep, could initiate increased platelet aggregation, plaque rupture, and coronary artery spasm. These increases may act as triggering mechanisms for thrombotic events, causing myocardial infarction (MI), ventricular arrhythmias, or even sudden cardiac death.
Gastric acid secretion shows a variable response during sleep in normal individuals, but patients with duodenal ulcers show a striking increase in acid secretion and no inhibition of secretion during the first 2 hours of sleep. Swallowing is suppressed during sleep, and there is prolonged acid clearance; these factors are important in the pathogenesis of esophagitis caused by nocturnal gastroesophageal reflux ( ). Esophageal motility is also reduced during sleep. Results of studies of intestinal motility during sleep are contradictory. A special pattern of motor activity called the migrating motor complex shows a circadian rhythm in its propagation, with the slowest velocity during sleep.
Profound changes in neuroendocrine secretions are found during sleep ( ![]() eFig. 101.25 ). Growth hormone secretion exhibits a pulsatile increase during NREM sleep in the first one-third of the night. Prolactin secretion also rises 30–90 minutes after the onset of sleep. Sleep inhibits cortisol secretion. Secretion of thyroid-stimulating hormone reaches a peak in the evening and then decreases throughout the night.
eFig. 101.25 ). Growth hormone secretion exhibits a pulsatile increase during NREM sleep in the first one-third of the night. Prolactin secretion also rises 30–90 minutes after the onset of sleep. Sleep inhibits cortisol secretion. Secretion of thyroid-stimulating hormone reaches a peak in the evening and then decreases throughout the night.
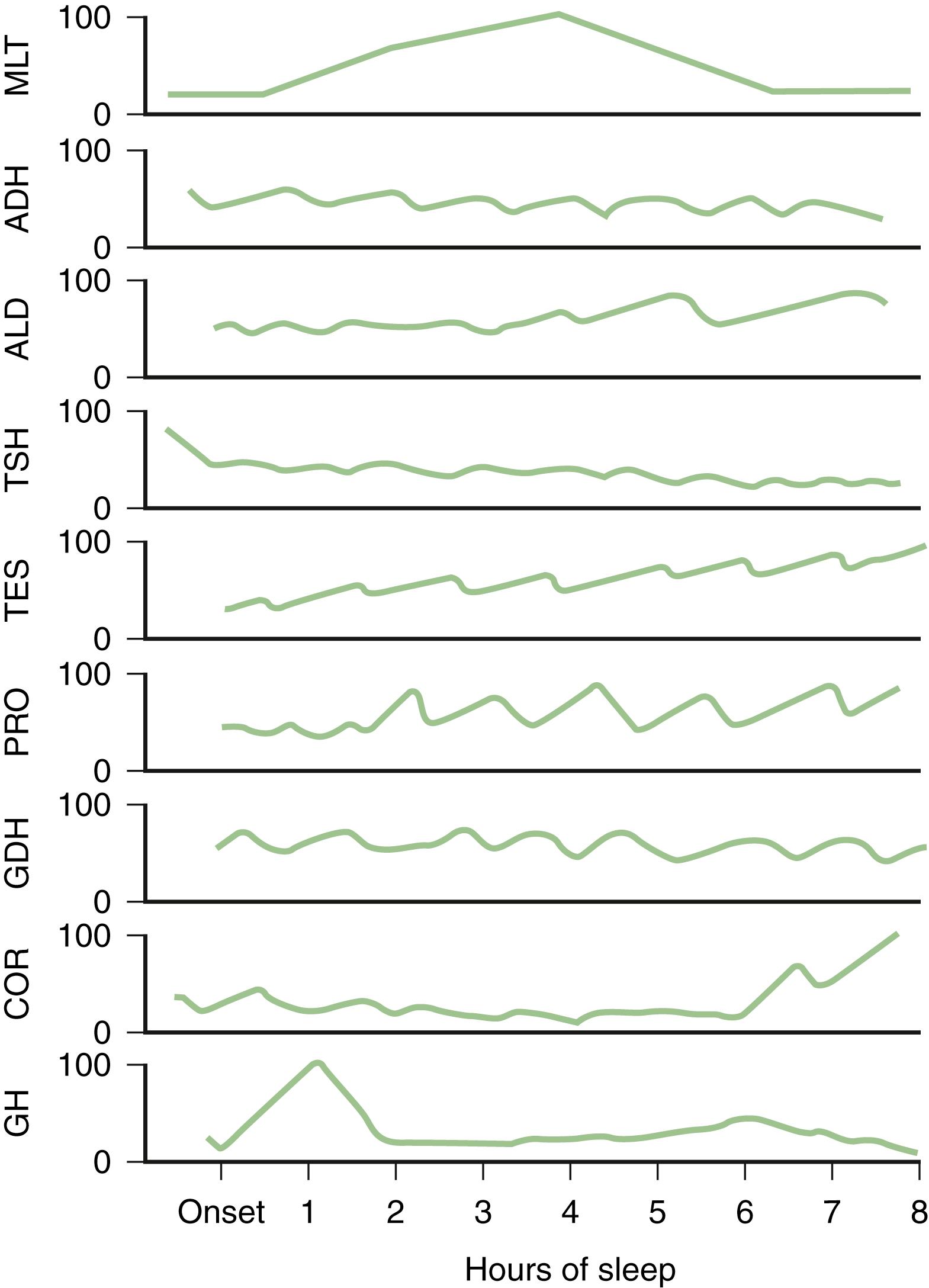
Testosterone levels in adult men continue to be highest during sleep, but no clear relationship has been demonstrated between levels of gonadotropic hormones and the sleep/wake cycle in children or adults. During puberty, gonadotropin levels increase in sleep. Melatonin, which is synthesized and released by the pineal gland and derived from serotonin, begins to rise in the evening, attaining maximal values between 3:00 am and 5:00 am , and decreases to low levels during the day.
Other endocrine changes include a maximum rise of aldosterone just before awakening in the early hours of the morning and a marked decrease of plasma renin activity during REM sleep.
The most striking finding is increased penile tumescence in men and increased clitoral tumescence in women during REM sleep.
Body temperature has been linked intimately to the sleep-wake cycle, but it follows a circadian rhythm that is independent of the sleep-wake rhythm. At the onset of sleep, body temperature begins to fall; it reaches its lowest point during the third sleep cycle. Thermosensitive neurons are located within the sleep promoting anterior hypothalamic preoptic neurons. Warm-sensitive neurons show increased firing rates at sleep onset causing peripheral vasodilation, heat loss, fall of body temperature, and increased EEG slow-wave activity during sleep. Thermoregulation is maintained during NREM sleep but is nonexistent in REM sleep, and experimental animals become poikilothermic. Thus, physiological responses (e.g., shivering, panting, sweating, and piloerection) to thermal stimuli are depressed or absent during REM sleep.
![]() Additional text available at http://expertconsult.inkling.com .
Additional text available at http://expertconsult.inkling.com .
Sleepiness is determined by both circadian and homeostatic factors. The circadian phase is determined by the suprachiasmatic nuclei, and it works in a biphasic manner. There are two phases when an individual has the maximal sleepiness propensity: an intense phase that occurs around 3:00–5:00 am and another, which is less intense, around 3:00–5:00 pm . In addition, there are two forbidden, or dead, zones when the individual is wide awake and unable to sleep, noted around late morning and early evening. Homeostasis refers to a balance between sleep and wakefulness. After prolonged wakefulness, there is an intense desire to sleep. REM sleep is influenced by the circadian rhythm, whereas slow-wave sleep is influenced by homeostasis. Much is known about the physiology of circadian rhythm, but the neurobiology of the homeostatic influences remains largely unknown. Excessive sleepiness, therefore, may result from both circadian dysrhythmia and disruption of homeostasis of the body.
A large segment of the population working different shifts and having irregular sleep/wake schedules is chronically sleep deprived ( ). The at-risk groups include doctors, nurses, firefighters, interstate truck drivers, police officers, overnight train drivers, and high school and college students. Comparing a survey conducted at the beginning of the last century and another toward the end of the century, modern America is sleep deprived by approximately 1.5 hours of sleep nightly ( ). This does not necessarily mean sleep requirement is smaller today, it simply means Americans are getting less sleep. However, there may have been a sampling error in these two surveys; for example, 2000 people were sampled in the earlier survey versus 311 in the later one. Increased environmental light and sound, industrialization, increasing numbers of people working in various shifts, and the advent of television and radio are cited as some of the factors responsible for this reduction of total sleep hours. , however, argued that most people are not chronically sleep deprived but have the capacity to take more sleep. In one epidemiological study, an average prevalence of sleepiness in Western society was estimated to be 5%–15% of the total population ( ).
Experiments have been conducted to study the consequences of total, partial, and selective sleep deprivation. These experiments have shown clearly that in animals, sleep is necessary for survival, but studies of complete sleep deprivation for prolonged periods (e.g., weeks to months) cannot be conducted in humans from a practical point of view. Experiments in rats, using a carousel device, have provided evidence that sleep is essential for survival. All rats deprived of sleep for 10–30 days died after having lost weight despite increasing food intake. The rats also lost temperature control. It took longer for rats deprived of REM sleep to die than it did those deprived of slow-wave sleep.
![]() Additional text available at http://expertconsult.inkling.com .
Additional text available at http://expertconsult.inkling.com .
Observational studies focusing on sleep deprivation have revealed frequent occurrence of “microsleep” episodes (i.e., brief episodes of NREM sleep) and a decrement of performance that may have been caused by the loss of motivation. Sleep deprivation increases the daytime tendency to sleep, as proven by multiple sleep latency tests in such subjects. The percentage of slow-wave sleep increases considerably during the recovery sleep period after sleep deprivation. The REM sleep percentages also increase during the recovery sleep after a prolonged period of sleep deprivation, but this increment of REM sleep percentage was not shown after short periods of sleep deprivation for 4 days. These facts might suggest that different mechanisms regulate NREM and REM sleep.
![]() Additional text available at http://expertconsult.inkling.com .
Additional text available at http://expertconsult.inkling.com .
Measurements of mood and performance after partial sleep deprivation (e.g., restricting sleep to 4.5–5.5 hours for 2–3 months) showed minimal deficits in performance, which may have been related to decreased motivation. After selective REM deprivation, PSG studies showed increased REM pressure (i.e., earlier and more common onset of REM sleep during successive nights) and REM rebound (i.e., quantitative increase of REM percentages during recovery sleep). The observation of a psychotic reaction after REM deprivation as noted by Dement was proved to be inaccurate in subsequent investigations. Similar to REM deprivation, after sleep deprivation for two consecutive nights, there is an increase in slow-wave sleep during the recovery night. It is more difficult to deprive a person of slow-wave sleep than of REM sleep.
In summary, these experiments have proven conclusively that sleep deprivation causes sleepiness and impairment of performance, vigilance, attention, and concentration but does not cause permanent memory or other CNS changes.
The consequences of excessive daytime sleepiness (EDS) presented in ![]() eBox 101.4 principally affect four key domains: (1) performance and productivity at work and school, (2) higher cerebral functions, (3) quality of life and social interactions, and (4) morbidity and mortality ( ).
eBox 101.4 principally affect four key domains: (1) performance and productivity at work and school, (2) higher cerebral functions, (3) quality of life and social interactions, and (4) morbidity and mortality ( ).
Impaired performance and productivity
Impaired short-term memory, attention, conception, and cognition
Impaired quality of life
Psychological stress
Increased morbidity and mortality (i.e., increased likelihood of accidents)
Impaired performance and reduced productivity at work for shift workers, reduced performance in class for school and college students, and impaired job performance in patients with narcolepsy, sleep apnea, circadian rhythm disorders, and chronic insomnia are well-known adverse effects of sleep deprivation and sleepiness. Sleepiness and associated morbidity are worse in night shift workers, older workers, and female shift workers. Recent data indicates that chronic sleep deprivation is associated with poorer academic performance as assessed by grade point average (GPA) ( ). The ramifications of chronic sleep loss may also predict the likelihood of obtaining a college degree. Students who experienced sleep deprivation from their freshman to senior years had a lower chance of graduation than students who were not sleep deprived ( ).
Sleepiness interferes with higher cerebral functions, causing impairment of short-term memory, concentration, attention, cognition, and intellectual performance. Psychometric tests document increased reaction time in patients with excessive sleepiness. These individuals make increasing numbers of errors, and they need increasing time to reach the target in reaction time tests ( ). Sleepiness can also impair perceptual skills and new learning. Insufficient sleep and excessive sleepiness may cause irritability, anxiety, and depression. Learning disabilities and cognitive impairment due to impaired vigilance have also been described ( ).
People complaining of EDS are often under severe psychological stress. They are often wrongly perceived as dull, lazy, and downright stupid. Excessive sleepiness may cause severe marital and social problems and impaired quality of life. Individuals with this problem have serious difficulty with interpersonal relationships.
Shift workers constitute approximately 20%–25% of the work force in America (i.e., approximately 20 million people). A majority of them have difficulty with sleeping and sleepiness as a result of insufficient sleep and circadian dysrhythmia. Data illustrates the positive effects of protecting sleep and preventing sleep deprivation. A study from 2010 in healthy elderly individuals older than 75 years of age who had no sleep complaints demonstrated that allowing more time to sleep (about 7.5 hours nightly) was associated with better maintenance of physical health-related quality of life and stability of medical illness burden over 30 months ( ).
Persistent daytime sleepiness causes individuals to have an increased likelihood of accidents. Estimates by the US National Highway Traffic Safety Administration showed that approximately 56,000 police-reported crashes per year resulted from drivers who were “asleep at the wheel.” New York state police estimate that 30% of all fatal crashes along the New York State Thruway occur because the driver fell asleep at the wheel. Approximately 1 million crashes annually (one-sixth of all crashes) are thought to be produced by driver inattention or lapses. Sleep deprivation and fatigue make such lapses more likely to occur. Truck drivers are especially susceptible to fatigue-related crashes ( ). Many truckers drive during the night while they are sleepiest. Truckers also may have a high prevalence of sleep apnea. The US Department of Transportation estimates that 200,000 automobile accidents each year may be related to sleepiness. Nearly one-third of all trucking accidents that are fatal to the driver are related to sleepiness and fatigue. A general population study by Hays and colleagues (1996) involving 3962 elderly individuals reported an increased mortality risk of 1.73 in those with EDS, defined by napping most of the time.
The presence of sleep disorders (see Primary Sleep Disorders Associated with Excessive Daytime Sleepiness, later) increases the risk of crashes. Individuals with untreated insomnia, sleep apnea, and narcolepsy, as well as shift workers, have more automobile crashes than other drivers ( ). A 1991 Gallup Organization national survey found that individuals with chronic insomnia reported 2.5 times as many fatigue-related automobile accidents as those without insomnia. The same 1991 Gallup survey found serious morbidity associated with untreated sleep complaints, as well as impaired ability to concentrate and accomplish daily tasks, impaired memory, and interpersonal difficulties. Sleep deprivation has an adverse impact on cognition and leads to increases in number of failures to carry out intended actions, which may have severe consequences in safety-critical situations ( ).
A 1994 telephone survey of drivers by the New York State Task Force estimated that approximately 25% reported that they had fallen asleep at the wheel at some time. Young male drivers are especially susceptible to crashes caused by falling asleep, as documented in a study in North Carolina in 1990, 1991, and 1992 (e.g., in 55% of the 4333 crashes, the drivers were predominantly male and age 25 or younger). In the October 1995 Gallup poll, 52% of all adults surveyed said that in the past year, they had driven a car or other vehicle while feeling drowsy; 31% of adults admitted dozing off while at the wheel of a car or other vehicle, and 4% reported having had an automobile accident because of tiredness during driving. Sleep deprivation increases morbidity and mortality due to metabolic derangements and accidents. A number of national and international catastrophes involving industrial operations, nuclear power plants, and all modes of transportation have been related to sleepiness and fatigue ( ). Some of the more infamous examples are the Exxon Valdez oil spill in Alaska; the nuclear disaster at Chernobyl in the former Soviet Union; the near-nuclear disaster at Three Mile Island in Pennsylvania; the gas leak disaster in Bhopal, India, resulting in 25,000 deaths; and the Challenger space shuttle disaster. In postgraduate medical training, data has repeatedly shown a dose response relationship between average hours of sleep per night and personal stress, reports of working while professionally and personally impaired. Studies comparing extended call shift with a revised schedule allowing for sleep recovery revealed the rate of diagnostic errors was 5.6 times higher in the traditional schedule than the intervention schedule as depicted in Fig. 101.26 ( ). These reports among others have catalyzed the Institute of Medicine (IOM) to proposed new work hours schedules which were accepted by the and went into effect in July 2011.
Excessive sleepiness may result from both physiological and pathological causes ( ![]() eBox 101.5 ).
eBox 101.5 ).
Sleep deprivation and sleepiness related to lifestyle and irregular sleep/wake schedule
Primary Sleep Disorders
Obstructive sleep apnea syndrome
Central sleep apnea syndrome
Narcolepsy
Idiopathic hypersomnia
Circadian rhythm sleep disorders:
Jet lag
Delayed sleep phase state
Irregular sleep/wake pattern
Shift-work sleep disorder
Free-running (non-entrained) type
Periodic limb movement disorder
Restless legs syndrome
Behaviorally induced insufficient sleep syndrome
Inadequate sleep hygiene
Kleine-Levin syndrome (KLS)
Seasonal affective depression
Occasionally due to insomnia
Hepatic failure
Renal failure
Respiratory failure
Electrolyte disturbances
Cardiac failure
Severe anemia
Endocrine causes
Hypothyroidism
Acromegaly
Diabetes mellitus
Hypoglycemia
Hyperglycemia
Depression
Psychogenic unresponsiveness or sleepiness
Neurodegenerative disorders
Alzheimer disease
Parkinson disease
Multiple system atrophy
Myotonic dystrophy and other neuromuscular disorders causing sleepiness
CNS space-occupying tumors/lesions (i.e., tumors, demyelinating or vascular lesions) affecting the diencephalon
Post-traumatic hypersomnolence
Encephalitis lethargica and other encephalitides and encephalopathies, including Wernicke encephalopathy
Cerebral trypanosomiasis (African sleeping sickness)
secondary to sleep apnea
Benzodiazepines
Nonbenzodiazepine hypnotics (e.g., zolpidem, eszopiclone)
Sedative antidepressants (e.g., tricyclic antidepressants, trazodone)
Antipsychotics
Nonbenzodiazepine anxiolytics (e.g., buspirone)
Antihistamines
Narcotic analgesics
Beta-blockers
Toxin- and Alcohol-Induced Hypersomnolence
It is important to first differentiate sleepiness from fatigue or tiredness. Based on the behavioral definition, fatigue can be differentiated by absence of the previously mentioned criteria for the presence of sleep. Fatigue defines a state of sustained lack of energy coupled with a lack of motivation and drive, but without the behavioral criteria of sleepiness such as heaviness or drooping of the eyelids, sagging or nodding of the head, and yawning. In addition, fatigue is often a secondary consequence of sleepiness. Sleep deprivation and sleepiness due to lifestyle and habits of going to sleep and waking up at irregular hours can be considered to result from disruption of the normal circadian and homeostatic physiology. Groups who are excessively sleepy because of lifestyle and inadequate sleep include young adults and elderly individuals, workers on irregular shifts, healthcare professionals (e.g., physicians, particularly the residents, and nurses), firefighters, police officers, train drivers, pilots and flight attendants, commercial truck drivers, and those individuals with competitive drives to move ahead in life, sacrificing hours of sleep and accumulating sleep debt. Among young adults, high school and college students are particularly at risk for sleep deprivation and sleepiness. The reasons for excessive sleepiness in adolescents and young adults include both biological and psychosocial factors. Some of the causes for later bedtimes and sleep deprivation in these groups include social interactions with peers; homework in the evening; sports, employment, or other extracurricular activities; early wake-up times to start school; and academic obligations requiring additional school or college work at night. Biological factors may play a role but are not well studied. For example, teenagers may need extra hours of sleep. Also, the circadian timing system may change with sleep phase delay in teenagers. EDS associated with shift work has been described (see Primary Sleep Disorders Associated with Excessive Daytime Sleepiness, later).
Neurological causes of excessive sleepiness may include CNS tumors and vascular lesions affecting the ARAS and its projections to the posterior hypothalamus and thalamus, leading to daytime sleepiness. It should be noted that lesions of this system often cause coma rather than just sleepiness. Brain tumors (e.g., astrocytomas, suprasellar cysts, metastases, lymphomas, and hamartomas affecting the posterior hypothalamus, pineal tumors, and astrocytomas of the upper brainstem) may produce excessive sleepiness. Prolonged hypersomnia may be associated with tumors in the region of the third ventricle. “Secondary” narcolepsy with or without cataplexy with hypocretin deficiency resulting from craniopharyngioma and other tumors of the hypothalamic and pituitary regions has been described. Cataplexy associated with sleepiness, sleep paralysis, and hypnagogic hallucinations has been described in patients with rostral brainstem gliomas with or without infiltration of the walls of the third ventricle. Narcolepsy-cataplexy also has been described in a human leukocyte antigen (HLA) DR2-negative patient with a pontine lesion documented by MRI.
Other neurological causes of EDS include bilateral paramedian thalamic and sometimes other cortical and subcortical infarcts ( ), post-traumatic hypersomnolence, and multiple sclerosis (MS) ( ). Narcolepsy-cataplexy has been described in occasional patients with MS ( ) and arteriovenous malformations in the diencephalon.
EDS has been described in association with encephalitis lethargica and other encephalitides, as well as with encephalopathies, including Wernicke encephalopathy. It was noted that the lesions of encephalitis lethargica described by Von Economo in the beginning of the last century, which severely affected the posterior hypothalamic region ( ![]() eFig. 101.27 ), were associated with the clinical manifestation of extreme somnolence. These lesions apparently interrupted the ascending arousal systems projecting to the posterior hypothalamus through a variety of mechanisms including cerebrovascular accidents ( ). While encephalitis lethargica is now extinct, lesions in the diencephalon due to CNS and comorbid medial reasons may occasionally be encountered on the neurology wards. Cerebral sarcoidosis, Whipple disease, and tumors involving the hypothalamus may cause symptomatic or secondary narcolepsy, which may present with severe hypersomnolence with or without hypothalamic deficiencies.
eFig. 101.27 ), were associated with the clinical manifestation of extreme somnolence. These lesions apparently interrupted the ascending arousal systems projecting to the posterior hypothalamus through a variety of mechanisms including cerebrovascular accidents ( ). While encephalitis lethargica is now extinct, lesions in the diencephalon due to CNS and comorbid medial reasons may occasionally be encountered on the neurology wards. Cerebral sarcoidosis, Whipple disease, and tumors involving the hypothalamus may cause symptomatic or secondary narcolepsy, which may present with severe hypersomnolence with or without hypothalamic deficiencies.
Cerebral trypanosomiasis, or African sleeping sickness, is transmitted to humans by tsetse flies: Trypanosoma gambiense causes Gambian or West African sleeping sickness, and Trypanosoma rhodesiense causes East African sleeping sickness.
Certain neurodegenerative diseases such as Alzheimer disease (AD), PD, and multisystem atrophy (MSA) also may cause EDS ( ). The causes of EDS in AD include degeneration of the SCN, resulting in circadian dysrhythmia, associated sleep apnea-hypopnea, and frequent periodic limb movements in sleep (PLMS). In PD, excessive sleepiness may be due to the associated PLMS, sleep apnea, and depression. EDS in MSA associated with cerebellar-parkinsonism or parkinsonian-cerebellar syndrome and progressive autonomic deficit may be caused by the frequent association with sleep-related respiratory dysrhythmias, impaired melatonin secretion, and possible degeneration of the reticular activating arousal systems ( ; ; ; ; ).
Sleep disorders are being increasingly recognized as a feature of PD and other parkinsonian disorders. Although some studies have attributed the excessive daytime drowsiness and irresistible sleep episodes (sleep attacks) to antiparkinsonian medications ( ), sleep disturbances are also an integral part of PD ( ). In one study of 303 patients with PD, 21% reported falling asleep while driving ( ). Several studies also reported a relatively high incidence (10%–20%) of symptoms of restless legs syndrome (RLS) in patients with PD ( ; ; ). There is also increasing awareness about the relationship between parkinsonian disorders and REM sleep behavior disorder (RBD). RBD as well as its associated PSG biomarker—RSWA—may be the presenting feature of PD, MSA, and other parkinsonian disorders, long before the appearance of the motor manifestations ( ; ; ; ; ; ; ).
The following question was found to have 94% sensitivity and 87% specificity in detecting RBD: “Have you ever been told, or suspected yourself, that you seem to ‘act out your dreams’ while asleep (for example, punching, flailing your arms in the air, making running movements, etc.)?” ( ).
These and other studies provide evidence supporting the notion that dopamine activity is normally influenced by circadian factors ( ). For example, tyrosine hydroxylase levels fall several hours before waking, and their increase correlates with motor activity. The relationship between hypocretin and sleep disorders associated with PD is of considerable interest especially since recent data has linked depletion of hypothalamic hypocretin/orexin neurons as positively correlating with impaired memory in animal models of PD ( ) Myotonic dystrophy and other neuromuscular disorders may cause EDS due to associated sleep apnea-hypopnea syndrome and hypoventilation. In addition, in myotonic dystrophy, there may be involvement of the ARAS as part of the multisystem membrane effects noted in this disease.
Several systemic diseases such as hepatic, renal, or respiratory failure and electrolyte disturbances may cause metabolic encephalopathies that result in EDS. Patients with severe EDS drift into a coma. The other medical causes for EDS include congestive heart failure and severe anemia. Hypothyroidism and acromegaly also may cause EDS due to the associated sleep apnea syndrome. Hypoglycemic episodes in diabetes mellitus and severe hyperglycemia are additional causes of EDS.
A number of primary sleep disorders cause excessive sleepiness (see ![]() eBox 101.5 ). The most common cause of EDS in the general population is behaviorally induced insufficient sleep syndrome which is associated with significant adverse health and social outcomes including association with 7 of the 15 leading causes of death and has been declared to be a “public health epidemic” ( ). The next most common cause is obstructive sleep apnea syndrome (OSAS) followed with narcolepsy and idiopathic hypersomnolence. Most patients with EDS referred to the sleep laboratory have OSAS. Other causes of EDS include circadian rhythm sleep disorders, periodic limb movement disorder (PLMD), RLS, and inadequate sleep hygiene and certain CNS acting agents.
eBox 101.5 ). The most common cause of EDS in the general population is behaviorally induced insufficient sleep syndrome which is associated with significant adverse health and social outcomes including association with 7 of the 15 leading causes of death and has been declared to be a “public health epidemic” ( ). The next most common cause is obstructive sleep apnea syndrome (OSAS) followed with narcolepsy and idiopathic hypersomnolence. Most patients with EDS referred to the sleep laboratory have OSAS. Other causes of EDS include circadian rhythm sleep disorders, periodic limb movement disorder (PLMD), RLS, and inadequate sleep hygiene and certain CNS acting agents.
Many sedatives and hypnotics cause EDS. In addition to the benzodiazepine and nonbenzodiazepine hypnotics and sedative antidepressants (e.g., tricyclic antidepressants and trazodone), nonbenzodiazepine neuroleptics, antihistamines, antipsychotics, most older generation antiepileptic drugs, and narcotic analgesics including tramadol also cause EDS. Beta-blockers for treatment of hypertension may occasionally cause excessive sleepiness.
Toxin and alcohol-related hypersomnolence can occur as well. Many industrial toxins such as heavy metals and organic toxins (e.g., mercury, lead, arsenic, and copper) may cause EDS. These may sometimes also cause insomnia. Individuals working in industrial settings using toxic chemicals routinely are at risk. These toxins also may cause systemic disturbances such as alteration of renal, liver, and hematological function. There may be an impairment of nerve conduction. Chronic use of alcohol at bedtime may produce alcohol-dependent sleep disorder. Usually this causes insomnia, but sometimes the patients may have excessive sleepiness in the daytime. Many of these patients suffer from chronic alcoholism. Acute ingestion of alcohol causes transient sleepiness.
The latest edition of the International Classification of Sleep Disorders (ICSD-III) ( ) listed seven broad categories of disordered sleep, along with several subcategories under each category as well as Appendices A and B (see ![]() eBox 101.6 ). Appendix A includes sleep-related medical and neurological disorders, including sleep-related epilepsy and headache, fatal familial insomnia (FFI), as well as sleep-related laryngospasm, sleep-related gastroesophageal reflux, and sleep-related myocardial ischemia. Appendix B includes substance-induced (e.g., alcohol, opioid, cannabis, sedative, hypnotic or anxiolytic, cocaine, other stimulants, hallucinogens, nicotine, inhalants, and other psychoactive substances) sleep disorders. The seven broad categories consist of insomnia, sleep-related breathing disorders (SBDs), central disorders of hypersomnolence, circadian rhythm sleep disorders, parasomnias, sleep-related movement disorders, and other sleep disorders. The ICSD-III reflects a major change from the ICSD-II predominantly in simplification of the insomnia disorders, an expansion of the SBDs, classification of narcolepsy into type 1 and type 2, consolidating the ICSD-II idiopathic hypersomnias into single entity and recurrent hypersomnia into a singly entry with a subtype.
eBox 101.6 ). Appendix A includes sleep-related medical and neurological disorders, including sleep-related epilepsy and headache, fatal familial insomnia (FFI), as well as sleep-related laryngospasm, sleep-related gastroesophageal reflux, and sleep-related myocardial ischemia. Appendix B includes substance-induced (e.g., alcohol, opioid, cannabis, sedative, hypnotic or anxiolytic, cocaine, other stimulants, hallucinogens, nicotine, inhalants, and other psychoactive substances) sleep disorders. The seven broad categories consist of insomnia, sleep-related breathing disorders (SBDs), central disorders of hypersomnolence, circadian rhythm sleep disorders, parasomnias, sleep-related movement disorders, and other sleep disorders. The ICSD-III reflects a major change from the ICSD-II predominantly in simplification of the insomnia disorders, an expansion of the SBDs, classification of narcolepsy into type 1 and type 2, consolidating the ICSD-II idiopathic hypersomnias into single entity and recurrent hypersomnia into a singly entry with a subtype.
The third edition of the International Classification of Sleep Disorders (ICSD-III: Diagnostic and Coding Manual, 2014; American Sleep Disorders Association, Rochester, MN) includes seven major categories of sleep disorders:
Insomnia
Sleep-related breathing disorders
Central disorders of hypersomnolence
Circadian rhythm sleep-wake disorders
Parasomnias
Sleep-related movement disorders
Other sleep disorders
Insomnia
Disorders
Chronic insomnia disorder
Short-term insomnia disorder
Other insomnia disorder
Isolated symptoms and normal variants
Excessive time in bed
Short sleeper
Sleep-Related Breathing DisordersSleep-related breathing disorders are characterized by abnormal respiration during sleep; they occur in both adults and children. There are four major sleep-related breathing disorders:
Central sleep apnea syndromes
Central sleep apnea with Cheyne-Stokes
Central apnea due a medical disorder without Cheyne-Stokes breathing
Central sleep apnea due to high altitude periodic breathing
Central sleep apnea due to a medication or substance
Primary central sleep apnea
Primary sleep apnea of infancy
Primary central sleep apnea of prematurity
Treatment-emergent central sleep apnea (previously noted as “Complex sleep apnea”)
Obstructive sleep apnea syndromesThe obstructive sleep apnea syndromes include adult obstructive sleep apnea and pediatric obstructive sleep apnea.
Sleep-related hypoventilation disorders
Obesity hypoventilation syndrome
Congenital central alveolar hypoventilation syndrome
Late-onset central hypoventilation with hypothalamic dysfunction
Idiopathic central alveolar hypoventilation
Sleep-related hypoventilation due to a medication or substance
Sleep-related hypoventilation due to a medical disorder
Sleep-related hypoxemia disorder:
Sleep-related hypoxemia
Isolated symptoms and normal variants
Snoring
Catathrenia
Central Disorders of Hypersomnolence:
Narcolepsy type 1 (NT1)
Narcolepsy type 2 (HT2)
Idiopathic hypersomnia (IH)
Kleine-Levin syndrome (KLS)
Hypersomnia due to a medical disorder
Hypersomnia due to a medication or substance
Hypersomnia associated with a psychiatric disorder
Insufficient sleep syndrome
Isolated symptoms and normal variants
Long sleeper
Circadian Rhythm Sleep–Wake Disorders
Delayed sleep-wake phase disorder (DSPD)
Advanced sleep-wake phase disorder (ASPD)
Irregular sleep-wake rhythm disorder (ISWD)
Non-24-hour sleep-wake rhythm disorder
Shift work disorder (SWD)
Jet lag disorder
Circadian sleep-wake disorder not otherwise specified (NOS)
Parasomnias
NREM-related parasomnias
Disorders of arousal (From NREM Sleep)
confusional arousals
Sexsomnias
Sleepwalking
Sleep terrors
REM-related parasomnias
REM sleep behavior disorder (RBD)
Recurrent isolated sleep paralysis
Nightmare disorder
Other parasomnias
Exploding head syndrome
Sleep-related hallucinations
Sleep enuresis
Parasomnia due to a medical disorder
Parasomnia due to a medication or substance
Parasomnia, unspecified
Isolated symptoms and normal variants
Sleep talking
Sleep-Related Movement Disorders
Restless legs syndrome (RLS)
Periodic limb movement disorder (PLMD)
Sleep-related leg cramps
Sleep-related bruxism
Sleep-related rhythmic movement disorder
Benign sleep myoclonus of infancy
Propriospinal myoclonus at sleep onset
Sleep-related movement disorder due to a medical disorder
Sleep-related movement disorder due to a medication or substance
Sleep-related movement disorder, unspecified
Isolated symptoms and normal variants
Excessive fragmentary myoclonus
Hypnagogic foot tremor and alternating leg muscle activation
Sleep starts (hypnic jerks)
Other Sleep Disorders
This category includes sleep disorders that cannot be appropriately classified elsewhere in the ICSD-III, either because the disorder overlaps with more than one category or when insufficient data have been collected to firmly establish another diagnosis.
Other sleep-related symptoms or events do not meet the standard definition of a sleep disorder.
The third edition of the International Classification of Sleep Disorders (ICSD-III) includes two appendices:
Appendix A includes diagnoses that can be classified as medical or neurological disorders
Disorders
Fatal familial insomnia
Sleep-related epilepsy
Sleep-related headaches
Sleep-related laryngospasm
Sleep-related gastroesophageal reflux
Sleep-related myocardial ischemia
Appendix B is a guide to ICD-10-CM coding for substance-induced sleep disorders.
The approach to a patient with a sleep complaint must begin with a clear understanding of sleep disorders as listed in the International Classification of Sleep Disorders (ICSD). Some common sleep complaints are trouble falling asleep and staying asleep (insomnia) ; falling asleep during the day (daytime hypersomnolence) ; and inability to sleep at the right time (circadian rhythm sleep disorders) . Other common complaints are thrashing and moving about in bed with repeated leg jerking (parasomnias and other abnormal movements, including nocturnal seizures) and RLS.
Cardinal manifestations in a patient complaining of insomnia include all or some of the following: difficulty falling asleep; frequent awakenings, including early-morning awakening; insufficient or total lack of sleep; daytime fatigue, tiredness, or sleepiness; lack of concentration, irritability, anxiety, and sometimes depression and forgetfulness; and preoccupation with psychosomatic symptoms, such as aches and pains.
Cardinal manifestations of hypersomnia include EDS, falling asleep in an inappropriate place and under inappropriate circumstances, no relief of symptoms after additional sleep at night, daytime fatigue, inability to concentrate, and impairment of motor skills and cognition. Additional symptoms depend on the nature of the underlying sleep disorder (e.g., snoring and apneas during sleep witnessed by a bed partner in patients with OSAS; attacks of cataplexy, hypnagogic hallucinations, sleep paralysis, automatic behavior, and disturbed night sleep in patients with narcolepsy).
Sleeplessness and EDS are symptoms; therefore, every attempt should be made to find a cause for these complaints. Insomnia may be due to a variety of causes. The etiological differential diagnosis for EDS may include OSAS; central sleep apnea (CSA); narcolepsy; idiopathic hypersomnia; several psychiatric, neurological, and other medical illnesses; drug or alcohol abuse; and periodic hypersomnolence (Kleine-Levin syndrome [KLS]). Sometimes a patient with RLS may complain of EDS. Abnormal movements and behavior during sleep include REM and NREM sleep parasomnias and other abnormal movements (e.g., PLMS), some daytime movement disorders that persist during sleep, and nocturnal seizures.
The physician must evaluate the patient first on the basis of the history and physical examination before undertaking laboratory tests, which must be determined by the clinical diagnosis. The first step in the assessment of a sleep-wakefulness disturbance is careful evaluation of the sleep complaints. The history should include information on the patient’s entire 24 hours and must include a detailed sleep history, with a sleep questionnaire as well as a sleep log or diary ( Fig. 101.28 ). It must also include psychiatric, neurological, medical, drug-alcohol, and family and past histories. The history must be followed by a physical examination to uncover medical or neurological causes of insomnia, hypersomnia, and parasomnias ( ). Physical examination of patients with OSAS may uncover upper airway anatomical abnormalities.
It is advisable to interview the bed partner, caregiver, or parents of children to get an adequate history, particularly the history during sleep at night, which may have an effect on daytime functioning. A sleep questionnaire containing a list of pertinent questions relating to sleep complaints; sleep hygiene; sleep patterns; medical, psychiatric, and neurological disorders; and drug and alcohol use may be filled out by the patient to save time during the history taking. A sleep log kept over a 2-week period also is a valuable indicator of sleep-wake-patterns and is of fundamental importance in screening for causes of sleep deprivation. Such a log should include bedtime, rise time on weekdays and weekends, daytime naps, amount of time needed to fall sleep, number and duration of nighttime awakenings, total sleep time, and subjective feelings upon awakening.
Pertinent questions can help diagnose primary sleep disorders. For example, a history of snoring and apneas witnessed during sleep at night would suggest OSAS. Unusual movements during sleep at night may suggest PLM. A history of sleep attacks and REM-sleep intrusion phenomena such as cataplexy, hypnagogic hallucinations, sleep paralysis, and disturbed night sleep in a young adult suggests narcolepsy. Nonrefreshing sleep or no benefit from additional sleep may suggest sleep apnea syndrome and idiopathic hypersomnia. Identification of an irregular sleep-wake schedule and delayed sleep onset and awakening and inquiry into the patient’s lifestyle to uncover sleep deprivation and insufficient sleep are important for the diagnosis of sleepiness. An urge to move the limbs in the early evening may suggest RLS. A family history is important in primary sleep disorders such as narcolepsy, RLS, and advanced/delayed circadian patterns
A variety of scales have been developed to assess the subjective degree of sleepiness. The most well-known is the Epworth Sleepiness Scale (ESS), which assesses the level of sleepiness ( ) in a variety of situations. The patient is rated on eight situations with a score of 0–3 (3 being the highest chance of dozing off). The maximum score is 24, and a score greater than 10 suggests the presence of excessive sleepiness ( Box 101.7 ). This scale has been weakly correlated with multiple sleep latency test (MSLT) scores, which is the gold standard measurement at present for daytime sleepiness, and normal values do not necessarily imply the lack of clinically significant daytime sleepiness. However, it is high in vulnerable population such as people with narcolepsy, sleep apnea, and sleep-deprived residents as depicted in Fig. 101.29 ( ; ; ).
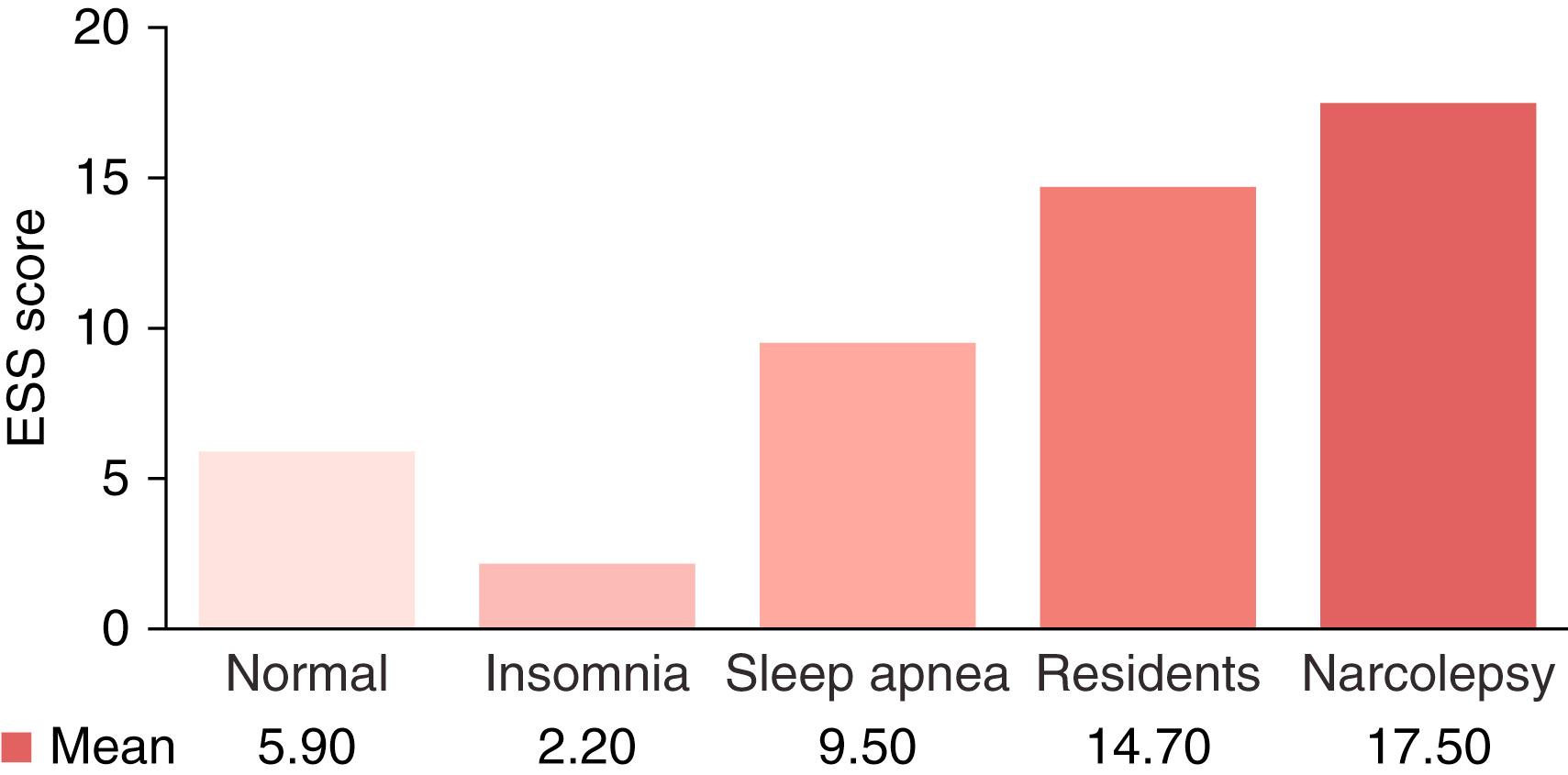
| Eight Situations | Scores ∗ |
|---|---|
| Sitting and reading | __ |
| Watching television | __ |
| Sitting in a public place (e.g., a theater or meeting) | __ |
| Sitting in car as a passenger for an hour without a break | __ |
| Lying down to rest in the afternoon | __ |
| Sitting and talking to someone | __ |
| Sitting quietly after a lunch without alcohol | __ |
| In a car, while stopped for a few minutes in traffic | __ |
∗ Scale to determine the total scores: 0, would never doze; 1, slight chance of dozing; 2, moderate chance of dozing; 3, high chance of dozing.
The Stanford Sleepiness Scale (SSS) ( ![]() eBox 101.8 ) is a seven-point scale that measures subjective sleepiness but may not be reliable in patients with persistent sleepiness. The scale may detect sleepiness as it waxes and wanes over the course of a day. Advantages include its brevity, its ease of administration, and its ability to be administered repeatedly, but normative data do not exist, making it difficult to use for clinical decision making or comparisons between persons.
eBox 101.8 ) is a seven-point scale that measures subjective sleepiness but may not be reliable in patients with persistent sleepiness. The scale may detect sleepiness as it waxes and wanes over the course of a day. Advantages include its brevity, its ease of administration, and its ability to be administered repeatedly, but normative data do not exist, making it difficult to use for clinical decision making or comparisons between persons.
| Scale Rating | Degree of Sleepiness |
| 1 | Wide awake, active, and alert |
| 2 | Awake and able to concentrate but not functioning at peak |
| 3 | Relaxed, awake, and responsive but not fully alert |
| 4 | Feeling a little foggy |
| 5 | Difficulty staying awake |
| 6 | Sleepy, prefer to lie down |
| 7 | Cannot stay awake; sleep onset is imminent |
| X | Asleep |
The Karolinska Sleepiness Scale (KSS) is another subjective measure of sleepiness somewhat similar to the SSS measuring responses on a 9-point scale (1 = very alert to 9 = very sleepy), while scores of 7 or above are considered pathologic. The KSS is useful when evaluating sleepiness in drug trials, and fatigue in flight crews. Similarly to the SSS, it lacks normative data, and diurnal pattern and the impact of stress, sleep quality, sex, illness, and age ( ).
Another scale is the visual analog scale of alertness and well-being. In this scale, subjects are asked to indicate their feelings on an arbitrary line.
Laboratory tests should be used to confirm the clinical diagnosis and must be subservient to the clinical history and physical examination. The tests to uncover suspected sleep disorders are resource intensive and are best planned in consultation with a sleep specialist; they are described in later sections of this chapter.
In this section, pertinent features of some common primary sleep disorders are described, along with a summary of sleep dysfunction in neurological, medical, psychiatric, and pediatric disorders.
Insomnia is the most common sleep disorder affecting the population and the most common disease encountered in the practice of sleep medicine ( , ). Several population surveys of adults have determined that approximately 35% of the general public has had insomnia complaints; in 10%, this is a persistent problem ( ; ; ; ). Insomnia is defined as an inability to initiate, maintain sleep, or early awakening, despite an adequate opportunity for sleep, culminating in poor sleep quality associated with a lack of feeling restored and refreshed in the morning, leading to a dissatisfaction with sleep and poor daytime functioning ( ). The ICSD-III ( ) classifies insomnia into three types: short-term insomnia disorder, chronic insomnia disorder, and other insomnia disorder. The perception of insomnia does not necessarily depend on total hours of sleep obtained, because there is considerable individual variation in sleep requirement. There is an increasing association of insomnia with age, female sex, low socioeconomic status, divorce, widowhood, separation, recent stress and depression, and drug or alcohol abuse, with higher predilection among women ( ).
This type of insomnia is characterized by short-term difficulty in initiating or maintaining sleep that results in general sleep dissatisfaction and is accompanied by daytime distress about the poor sleep quality or impairment in social, family, occupational, academic, or other important areas of functioning. The disorder and its associated symptoms occur despite having adequate time and circumstances to obtain necessary sufficient sleep ( ). In many cases of short-term insomnia lasting less than 3 months, there is an identifiable cause that serves as the precipitant. In other cases, insomnia occurs episodically, often coincident to daytime stressors that account for the insomnia ( ). Once the stressful event is removed and the patient adjusts to the event, the sleep disturbance resolves. Causes of acute insomnia are listed in Box 101.9 .
| Internal (patient-related factors) | Acute medical or surgical illnesses (including intensive care unit stays) Stimulant medications (e.g., theophylline, beta-blockers, corticosteroids, thyroxine, bronchodilators, or withdrawal of central nervous system depressant medications) |
| External (environmental factors) | A change of sleeping environment (most common cause of transient insomnia, the so-called first night effect)Jet lag Unpleasant sleep environment (excessively hot/cold room temperature) |
| Excessive environmental noise (living close to the motor way/train track/airport) | |
| Excessive environmental light exposure |
Most cases of insomnia are chronic and comorbid with other conditions ( , ). Common comorbidities include psychiatric, medical, primary sleep-related (i.e., OSAS), neurological disorders, pain syndrome, or drug and alcohol abuse. eBox 101.10 ![]() lists the causes of chronic insomnia. These comorbid insomnias were classified as secondary insomnia in the past, but this signifies causation; no definite evidence exists that these conditions are responsible for insomnia ( ).
lists the causes of chronic insomnia. These comorbid insomnias were classified as secondary insomnia in the past, but this signifies causation; no definite evidence exists that these conditions are responsible for insomnia ( ).
Idiopathic or primary insomnia
Psychophysiological insomnia
Paradoxical insomnia (sleep state misperception)
Inadequate sleep hygiene
Insomnia comorbid with psychiatric disorders
Insomnia comorbid with general medical disorder
Insomnia comorbid with neurological disorder
Insomnia due to drug or other substance abuse
In addition to short-term effects of disturbed sleep and inadequate daytime functioning, people with insomnia report impaired work performance and productivity, reduced social and physical functioning, reduced overall quality of life, and a two- to fivefold increased risk for developing subsequent depression associated with significant suicidal behavior. There are also suggestions of increased mortality and morbidity from coronary artery disease, hypertension, obesity, and diabetes mellitus ( , ; ). A longitudinal sleep laboratory follow-up study for 14 years, however, found increased mortality in men with chronic insomnia with objectively measured short sleep duration that could not be explained by comorbid type 2 diabetes mellitus or hypertension ( ). The clinically significant consequences (both short-term and long-term) of insomnia most typically develop when sleep difficulties occur at least three times per week and persist for at least 3 months. Hence those frequency and duration criteria must be met for a diagnosis of chronic insomnia disorder ( ).
The proposed basis and evolution of insomnia as a disorder of hyperarousal ( Fig. 101.30 ) and its evolution during early life to adulthood and from acute to chronic forms is summarized in Fig. 101.31 , as it transforms to chronic insomnia. Patients suffering from insomnia may suffer from a general disorder of hyperarousal due to a functional alteration in the anatomical substrates for sleep/wake states conferred by hyperactivity of wake-promoting neurons or hypoactivity of sleep-generating neurons that is genetically determined. Evidence of hyperarousal is provided by the following observations in these patients ( , 2015) and is highlighted in Fig. 101.31 :
Increased heart rate
Elevated sympathetic and decreased parasympathetic tone (as measured by spectral analysis to show heart rate variability)
Increased 24-hour and evening cortisol levels and increased 24-hour corticotropin levels (indicating abnormal hypothalamic–pituitary–adrenal axis)
Increased brain glucose metabolism
Increased EMG activity, as noted in the frontalis muscle
EEG spectral analysis showing increased beta activity
Decreased nocturnal melatonin and increased diurnal melatonin levels
Increased latencies on MSLT, despite disturbed nocturnal sleep
Neuroimaging findings of subcortical (ARAS) hyperarousal. The data support that in people with insomnia, during transition from waking to sleep, there is a state of increased cerebral metabolism due to a lack of a reduction in activity in subcortical structures compared to people without insomnia ( ).
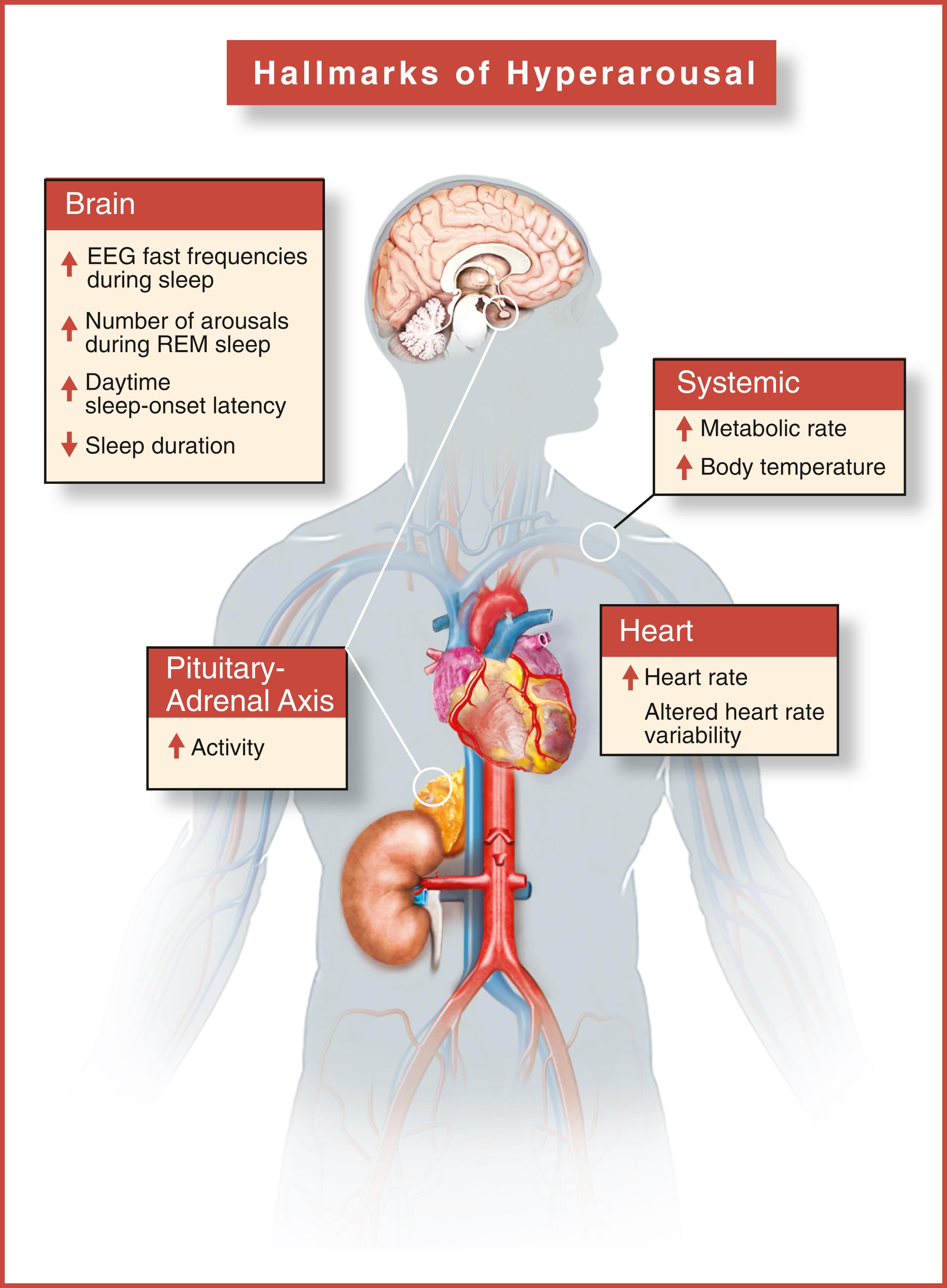
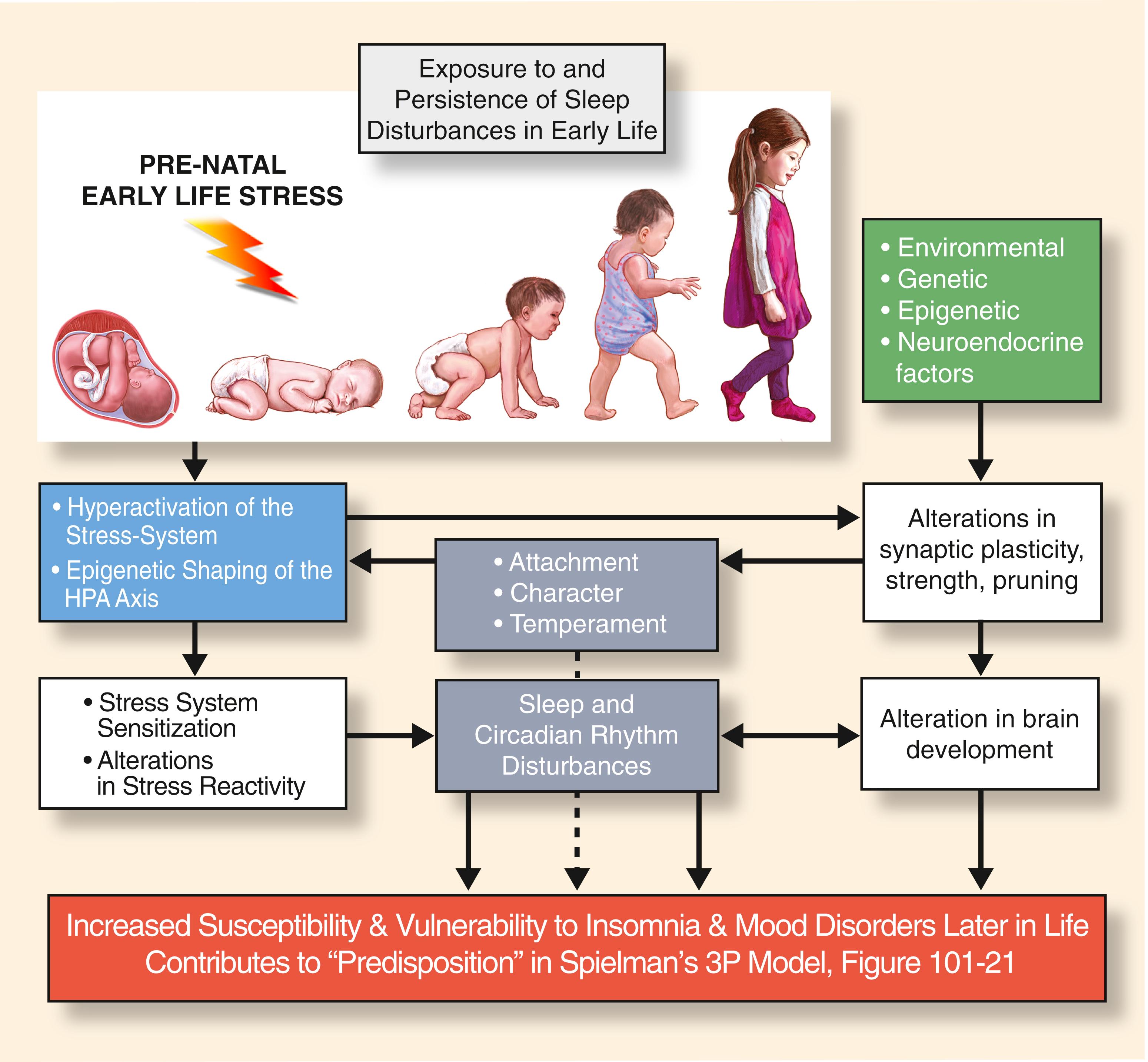
Hyperarousal dysfunction is supported by functional neuroanatomy, where PET imaging designed to measure regional brain metabolism demonstrates that patients with insomnia show increased activity during the transition from waking to sleep onset as compared to healthy subjects, suggesting that there is an overall cortical hyperarousal in insomnia ( ![]() eFig. 101.32 ). Furthermore, patients with insomnia have less reduction of activation from waking to NREM sleep in the ARAS, hypothalamus, insular cortex, amygdala, hippocampus, anterior cingulate, and medial prefrontal cortices, as illustrated in the figure ( ; ).
eFig. 101.32 ). Furthermore, patients with insomnia have less reduction of activation from waking to NREM sleep in the ARAS, hypothalamus, insular cortex, amygdala, hippocampus, anterior cingulate, and medial prefrontal cortices, as illustrated in the figure ( ; ).
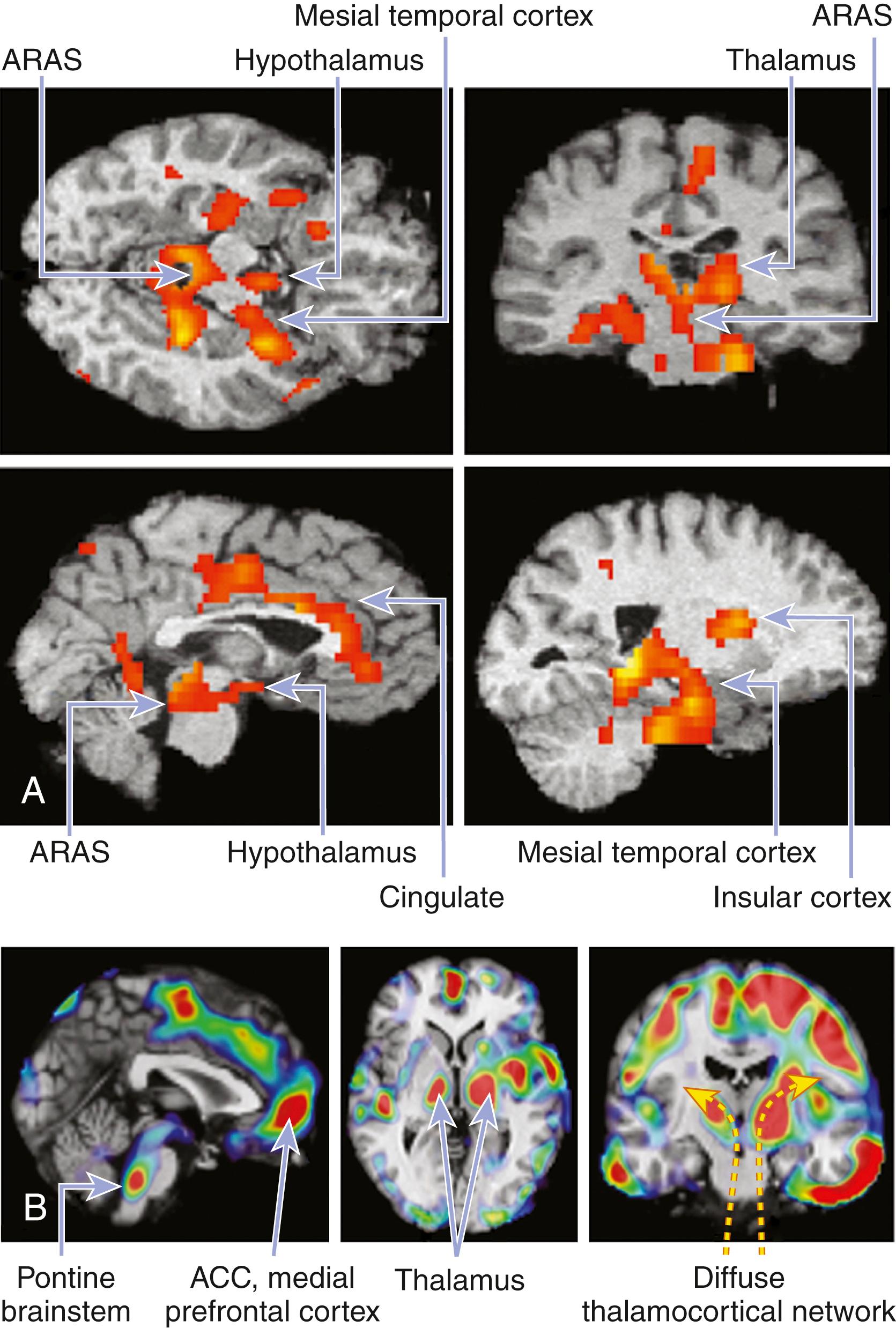
Additional insomnia subtypes mentioned in the ICSD-II are provided for historical reasons. This outline as well as insomnia criteria from the ICSD-III along with a listing of medical and neurological comorbidities are provided in Boxes 101.11 and 101.12 at http://expertconsult.inkling.com ![]() .
.
The section below covers insomnia subtypes of described in the ICSD-III ( ).
The onset of idiopathic insomnia occurs in early childhood, as highlighted in Fig.101.31 and contributing to a “Predisposition” to develop insomnia Fig. 101.33 , as demarcated by the red column. Patients generally have lifelong difficulty with initiating or maintaining sleep, or both, resulting in poor daytime functioning. Diagnosis depends on the exclusion of concomitant comorbid medical, neurological, psychiatric, or psychological disturbances. Psychophysiological insomnia may transpire during young adulthood, and the symptoms may persist for decades. This is chronic insomnia is characterized by hypervigilance and hyperarousal and it is fueled by learned sleep-preventing associations. Patients are overconcerned on sleep problems, but they may not have generalized anxiety or any other psychiatric disorders. Sometimes a family history exists. The development of conditioned responses incompatible with sleep is the predominant feature of psychophysiological insomnia. Insomnia is an event precipitated or initiated by a stressor such as acute life events such as a new job, death of a loved one, COVID19 and surgery, but it persists even after that initial stress is gone (see Fig.101.33, demarcated by the yellow column). Factors contributing to negative conditioning in the setting of insomnia are “perpetuating” factors include excessive fear and frustration about being unable to initiate and maintain sleep, and the identification of the bedroom as an arousal sign and environmental and disadvantages life-style factors that perpetuate the insomnia Fig. 101.33 , blue column including alcohol ![]() , caffeine,
, caffeine,  artificial and excessive blue light exposure from light exposure, television and electronics
artificial and excessive blue light exposure from light exposure, television and electronics 

 high temperatures and excessive environmental noise
high temperatures and excessive environmental noise  . Patients generally sleep poorly during nocturnal polysomnogram, a phenomena referred to as “the first night effect”, although occasionally patients sleep better because they are removed from their usual sleep environment (reverse first-night effect). Patients with psychophysiological insomnia confine anxiety to sleep-related issues, which differentiates them from patients with generalized anxiety disorders. New data provide good evidence to support the view that primary psychophysiological insomnia is a true conflict between the sleep system and inappropriate activation of CNS ( ).
. Patients generally sleep poorly during nocturnal polysomnogram, a phenomena referred to as “the first night effect”, although occasionally patients sleep better because they are removed from their usual sleep environment (reverse first-night effect). Patients with psychophysiological insomnia confine anxiety to sleep-related issues, which differentiates them from patients with generalized anxiety disorders. New data provide good evidence to support the view that primary psychophysiological insomnia is a true conflict between the sleep system and inappropriate activation of CNS ( ).
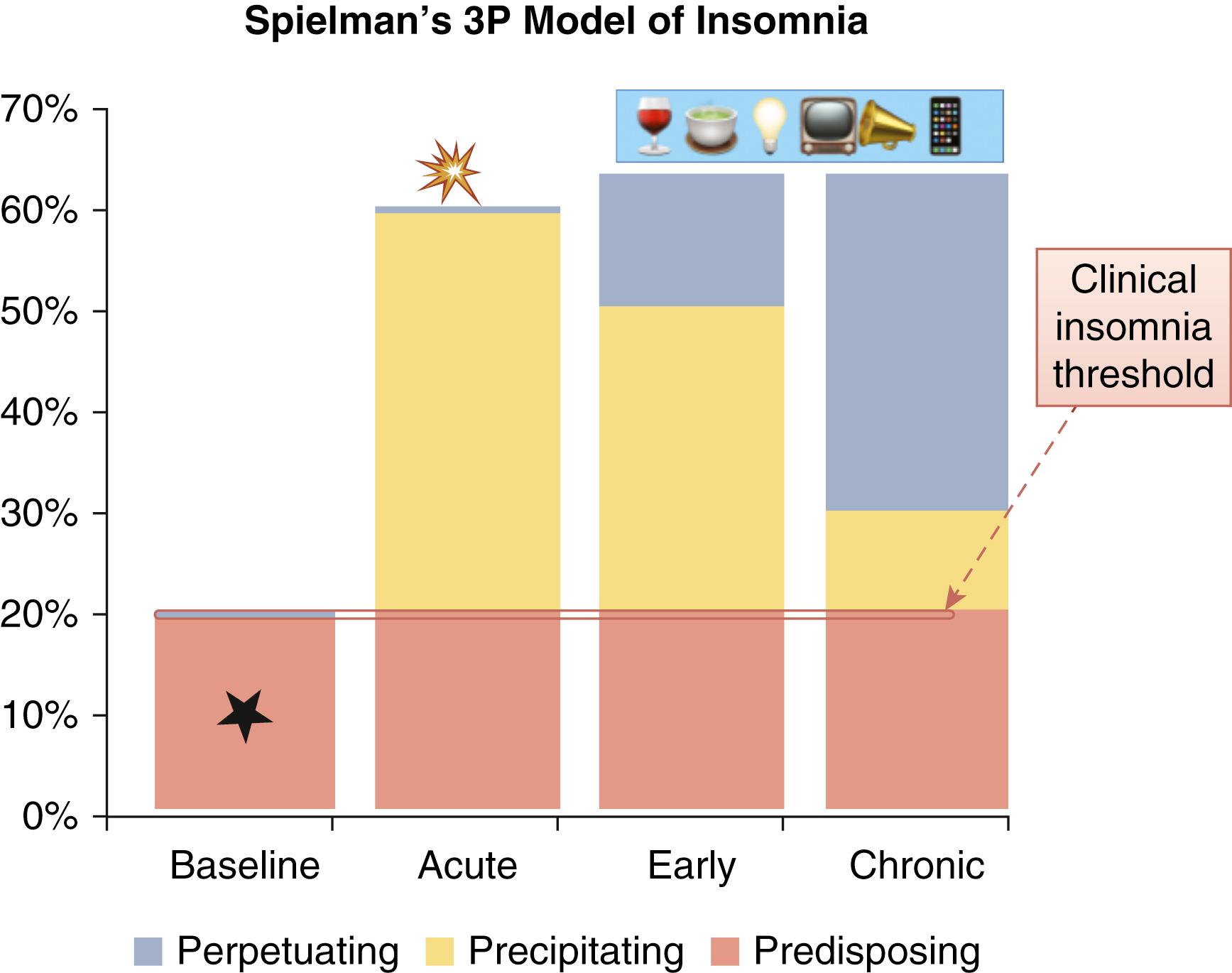
Paradoxical insomnia is a sleep state misperception characterized by subjective complaints of sleeplessness without objective evidence (e.g., PSG evidence of insomnia). Patients often complain of severe sleep disturbance but lack supportive objective evidence to corroborate the degree of sleep disturbance claimed. ( ). There is an increased propensity for these patients to underestimate the amount of sleep they are actually getting. In essence, they are thought to perceive much of the time they actually sleep as wakefulness. Actigraphy (an accelerometer which measures sleep/wake activities) or PSG recording documents normal sleep patterns in such patients.
Patients with inadequate sleep hygiene negate the good sleep measures that promote sleep. Such measures include avoidance of caffeinated beverages, alcohol, and tobacco in the evening; avoidance of intense mental activities close to bedtime (this has been seriously questioned in recent literature); avoidance of daytime naps and excessive time spent in bed awake; and adherence to a regular sleep/wake schedule.
Insomnia commonly coexists or precedes the development of a number of psychiatric illnesses. Surveys have shown that individuals with insomnia are more likely to develop new psychiatric disorders, particularly major depression, within 6–12 months. Anxiety disorders, depression, and schizophrenia are some of the major psychiatric disorders associated with insomnia.
Medical and neurological disorders comorbid with insomnia are listed in ![]() eBoxes 101.11 and 101.12 . RLS, PLMD, and circadian rhythm disorders are some of the other causes of chronic insomnia. In some patients, insomnia may coexist with sleep apnea.
eBoxes 101.11 and 101.12 . RLS, PLMD, and circadian rhythm disorders are some of the other causes of chronic insomnia. In some patients, insomnia may coexist with sleep apnea.
Ischemic heart disease
Nocturnal angina
Congestive cardiac failure
Chronic obstructive pulmonary disease
Bronchial asthma
Peptic ulcer disease
Gastroesophageal reflux disease
Rheumatic disorders, including:
Fibromyalgia
Rheumatoid arthritis
Osteoarthritis
Ankylosing spondylitis
Sjögren syndrome
Acquired immunodeficiency syndrome (AIDS)
Chronic fatigue syndrome
Lyme disease
Dermatological disorders (e.g., nocturnal pruritus)
Systemic cancer
Neurodegenerative diseases:
Parkinson disease
Alzheimer disease
Other degenerative dementias
Progressive supranuclear palsy
Multiple system atrophy
Huntington disease
Torsion dystonia
Tourette syndrome
Brain tumors
Cerebral hemispheric and brainstem strokes
Traumatic brain injury causing post-traumatic insomnia
Headache syndrome (e.g., migraine, cluster and hypnic headaches, exploding head syndrome)
Fatal familial insomnia (rare prion disease)
Propriospinal myoclonus at sleep onset
Insomnia in neurological disorders may result from the following suggested mechanisms:
Hypofunction (metabolic or structural) of the hypothalamic VLPO neurons or the lower brainstem hypnogenic neurons in the region of the NTS and dysfunction of thalamus may alter the balance between the waking and sleeping brain, causing sleeplessness.
Neurological disorders may cause pain, agitation and confusion, alteration of the sensory-motor system, and abnormal movements during sleep, which may interfere with normal progression and cycling of NREM-REM sleep.
Medications used to treat neurological illnesses (e.g., corticosteroids, antiepileptic drugs such as lamotrigine, zonisamide, and levetiracetam, dopaminergic medications, decongestants) may cause insomnia.
Certain neurological disorders may alter circadian rhythm in the SCN causing insomnia. (This is particularly true in the case of Alzheimer dementia, but also PD and traumatic brain injury [TBI].)
Neurological illnesses have a bidirectional relationship with psychiatric conditions, particularly depression and anxiety, which promote sleeplessness.
Neurological disorders such as MSA, MS and neuromuscular disorders such as myotonic dystrophy type 2 have strong association with primary sleep disorders such as SDB, resulting in sleep fragmentation, excessive sleepiness impaired quality of life.
Since the French physician Gelineau used the term narcolepsy in 1880 to describe irresistible sleep attacks and astasia, which includes all the features of cataplexy, there have been many reports of patients with narcolepsy-cataplexy syndrome ( ; ; ). The prevalence of narcolepsy is estimated to be approximately 1 in 2000 individuals in the United States, 1 in 600 people in Japan, and 1 in 500,000 individuals in Israel. Reports of adequate epidemiological studies in different parts of the world are, however, lacking ( ![]() ).
).
Thirty-Five Years of Undiagnosed Narcolepsy.
Patients with classic narcolepsy frequently go undiagnosed for decades, as did this patient. (From Kryger MH, Avidan, AY, Berry, R. Atlas of clinical sleep medicine. Second edition. ed. Philadelphia, PA: Elsevier/Saunders; 2013)
Most cases of human narcolepsy are sporadic, but some are dominant. There is, however, a 10–40 times greater prevalence of narcolepsy in families than in the general population. For example, approximately one or two first-degree relatives of narcoleptic patients, compared with 0.02%–0.18% in the general population, manifest the illness. Twin studies show that the majority of monozygotic twins are discordant, and only 25%–31% are concordant, suggesting an influence of environmental factors in the etiology of narcolepsy. From 95% to 100% of white and Japanese patients carry the HLA. It has been established that HLA-DQB1∗0602 is a marker for narcolepsy on chromosome 6 across all ethnic groups. However, cases of narcolepsy not carrying HLA-DR2 or HLA-DQ1 antigens have been reported. In addition, 12%–38% of the general population carries the same HLA alleles, but narcolepsy is present in only 0.02%–0.18% of the population. Clinically, HLA-DQB1∗0602 typing may be indicated when considering a CSF hypocretin measurement. Hypocretin levels are generally normal if the patient is HLA-negative, unless the patient has a diencephalic lesion that explains the CNS hypersomnia (narcolepsy type due to a medical condition) ( ).
The most exciting recent development in sleep medicine is the pathogenetic role played by the recently discovered hypocretin (orexin) peptidergic systems in the lateral hypothalamus. Reports of mutation of the hypocretin receptor 2 gene (HCRTR2) in dogs ( ) and pre-prohypocretin knockout mice ( ) producing the phenotype of human narcolepsy brought narcolepsy research to the forefront of the molecular neurobiology field. Most cases of human narcolepsy and cataplexy have decreased hypocretin 1 in the cerebrospinal fluid (CSF) ( ; ; ). Hypocretins are noted to be depleted in narcoleptic brains at autopsy, as can be seen in Fig. 101.34 ( ) with a proposed mechanism involving an environmental trigger which produces an autoimmune response in genetically venerable individuals ( ). In addition, mutation of the pre-prohypocretin gene has been identified in one child with severe narcolepsy ( ). Therefore, the contemporary theory for the pathogenesis of narcolepsy-cataplexy syndrome suggests that the condition results from a depletion (degeneration or autoimmune disorder) of the hypocretin neurons in the lateral and perifornical region of the hypothalamus, as can be appreciated in Fig. 101.35 . Narcolepsy-cataplexy syndrome thus can be considered a hypocretin (orexin) deficiency syndrome, which is how the condition is being referred to in the updated version of the ICSD-III ( ). The possibility of an autoimmune disorder has been raised because of the association of narcolepsy with HLA-DQB1∗0602 haplotype; however, all attempts to find evidence of autoimmune disorder in narcolepsy have so far failed ( ). There is no evidence of inflammatory processes or immune abnormalities, presence of classical autoantibodies, or an increase of oligoclonal bands in the CSF in these patients. Erythrocyte sedimentation rate, serum immunoglobulin levels, C-reactive protein, complement levels, and lymphocyte subset ratio are all found to be normal in these patients, pointing against an autoimmune disorder. On the other hand, increased levels of streptococcal antibodies in narcoleptic patients in some reports and evidence of gliosis in the lateral hypothalamus by Thannickal and colleagues (2000) indicate that further exploration for possible immune-related dysfunction in narcolepsy is needed. Finally, the presence of autoantibodies (patient serum binding to rat hypocretin neurons) in one out of nine narcolepsy-cataplexy patients keeps the autoimmune theory very much in the forefront ( ). Environmental considerations such as streptococcal infection, seasonal influenza, and more recent reports implicating the 2009 influenza pandemic A/H1N1 raise even stronger concerns for an immunological mechanism for narcolepsy. Proposed immunological mechanisms that could trigger specific destruction and subsequent elimination of hypocretin producing neurons include molecular mimicry or bystander activation and are likely a combination of genetic and environmental factors, such as upper airway infections ( ). FLOAT NOT FOUND FLOAT NOT FOUND
In the summer of 2010 concerns were raised in Scandinavia about a possible association between narcolepsy and ASO3 adjuvanted A/H1N1 2009 pandemic vaccine following observation of a potential temporal association by clinicians in sleep centers in these countries. A follow-up study of children in England depicted a comparable association of increased risk ( ). The earlier data from Finland described a 13-fold increased risk of narcolepsy following vaccination of patients aged 4–19, the majority of whom developed narcolepsy symptoms within 3 months after vaccination ( Fig. 101.36 ) and almost all within 6 months ( ; ). New data from Stanford University implicated a small epitope of pH1N1 that resembles hypocretin and is likely involved in molecular mimicry ( ). Fig. 101.37 highlights a few pathophysiological mechanisms for narcolepsy which points toward selective venerability (conferred through HLA positivity), a trigger, and a reaction leading to destruction of the vulnerable populations of orexin neurons. It is believed that the underlying pathology of narcolepsy is related to genetic, environmental, and acute triggering factors. The implications of genetic factors (specifically HL A-DQB1∗06:02 positivity) are a strong predisposition to narcolepsy. The first clinical marker of such a predisposition is a short sleep latency period before onset of REM sleep. Environmental exposures to bacterial and viral pathogens in early life might alter immune system development.
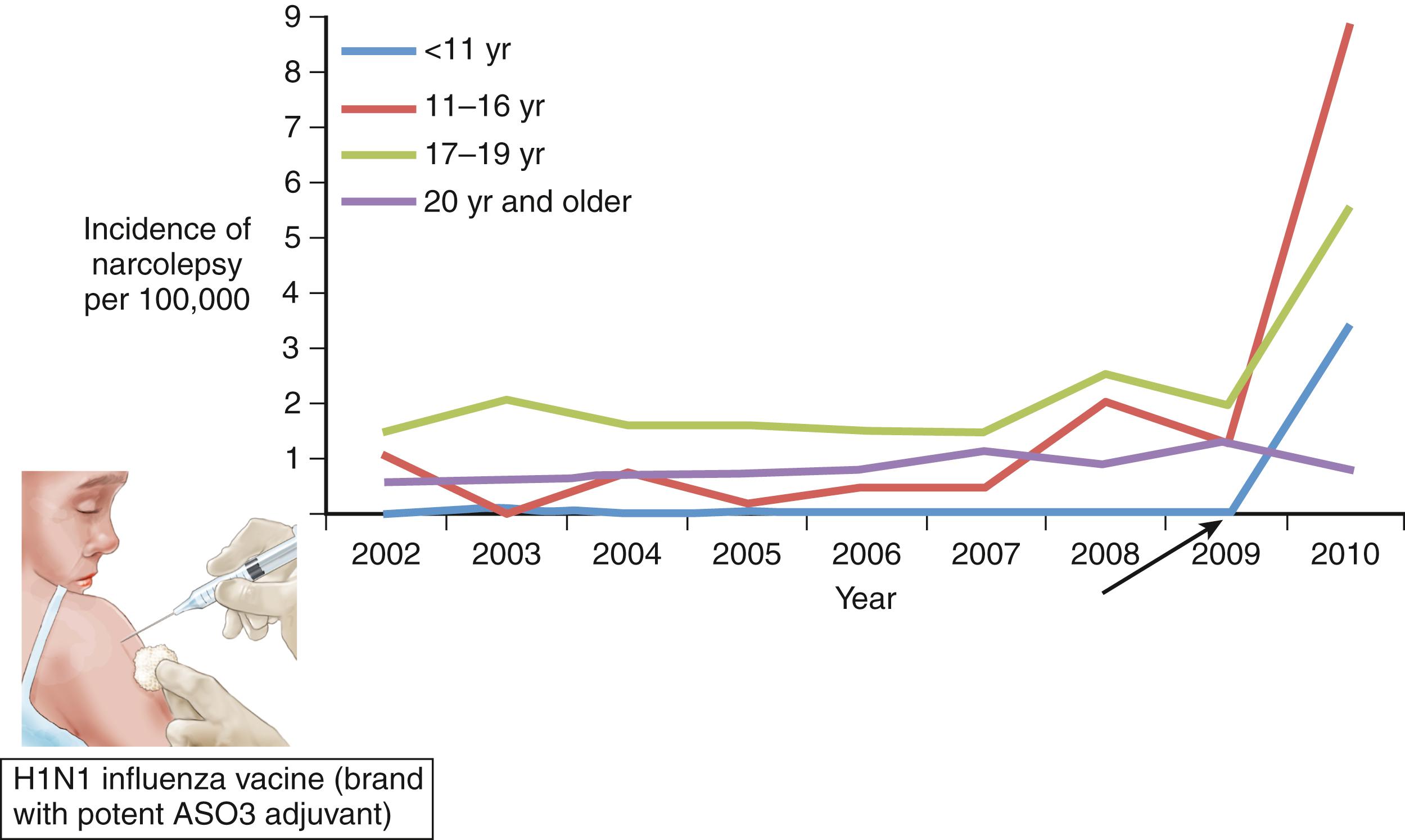
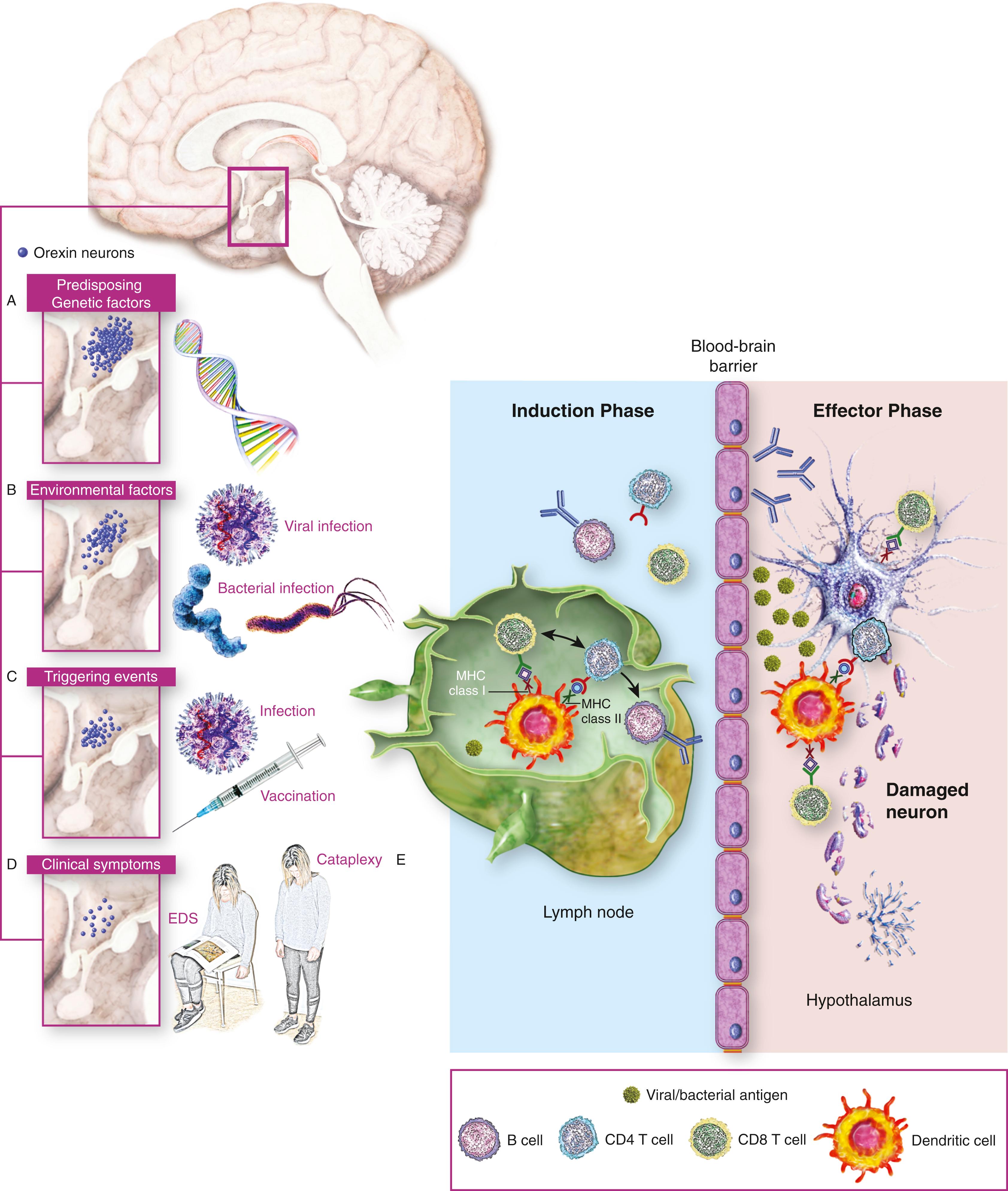
The onset of the disorder in most cases is in adolescents and young adults, with a peak incidence between ages 15 and 30. However, rare cases have been described in children younger than age 5 and adults older than age 50. The ICSD-III divides narcolepsy into two types: narcolepsy type 1 (with cataplexy, or low hypocretin levels), and narcolepsy type 2 (without cataplexy, and with normal hypocretin levels) ( ) eTable 101.6 ![]() . Secondary narcolepsy due to a known underlying CNS disorder is referred to as narcolepsy type 1 due to a medical condition (when hypocretin levels are low) or narcolepsy type 2 due to a medical condition (when hypocretin levels are normal). Diseases impacting the major clinical manifestations of narcolepsy include sleep attacks (100%), cataplexy (60%–70%), sleep paralysis (25%–50%), hypnagogic hallucinations (20%–40%), disturbed night sleep (70%–80%), and automatic behavior (20%–40%) ( ; ; ) ( Table 101.5 ).
. Secondary narcolepsy due to a known underlying CNS disorder is referred to as narcolepsy type 1 due to a medical condition (when hypocretin levels are low) or narcolepsy type 2 due to a medical condition (when hypocretin levels are normal). Diseases impacting the major clinical manifestations of narcolepsy include sleep attacks (100%), cataplexy (60%–70%), sleep paralysis (25%–50%), hypnagogic hallucinations (20%–40%), disturbed night sleep (70%–80%), and automatic behavior (20%–40%) ( ; ; ) ( Table 101.5 ).
| Major Manifestations | |
|---|---|
| Excessive sleepiness | 100% |
| Cataplexy | 70% |
| Sleep paralysis | 25%–50% |
| Hypnagogic/hypnopompic hallucinations | 20%–40% |
| Disturbed night sleep | 70%–80% |
| Automatic behavior | 20%–40% |
| Sleep apnea | Up to 30% |
| Periodic limb movements in sleep | 10%–60% |
| REM sleep behavior disorder | Up to 36% |
| Eating disorder (craving for food) | High prevalence, but exact percentage unknown, including night eating syndrome not related to obesity |
| Increased BMI or obesity | Up to 30%, higher in children diagnosed with narcolepsy (50%) |
| Increased prevalence of type 2 diabetes mellitus | Up to 10% |
The classic narcoleptic sleep attack is an irresistible desire to fall asleep in inappropriate circumstances and at inappropriate places (e.g., while talking, driving, eating, playing, or when involved in boring or monotonous circumstances) as illustrated in Fig. 101.38 . These spells last from a few minutes to as long as 20–30 minutes, and the patient generally feels refreshed on waking. There are wide variations in frequency of attacks—anywhere from daily, weekly, monthly, or every few weeks to months. Attacks generally persist throughout the patient’s lifetime, although fluctuations and rare temporary remissions may occur. Patients often show a decline in performance at school and work and encounter psychosocial and socioeconomic difficulties as a result of sleep attacks and EDS.
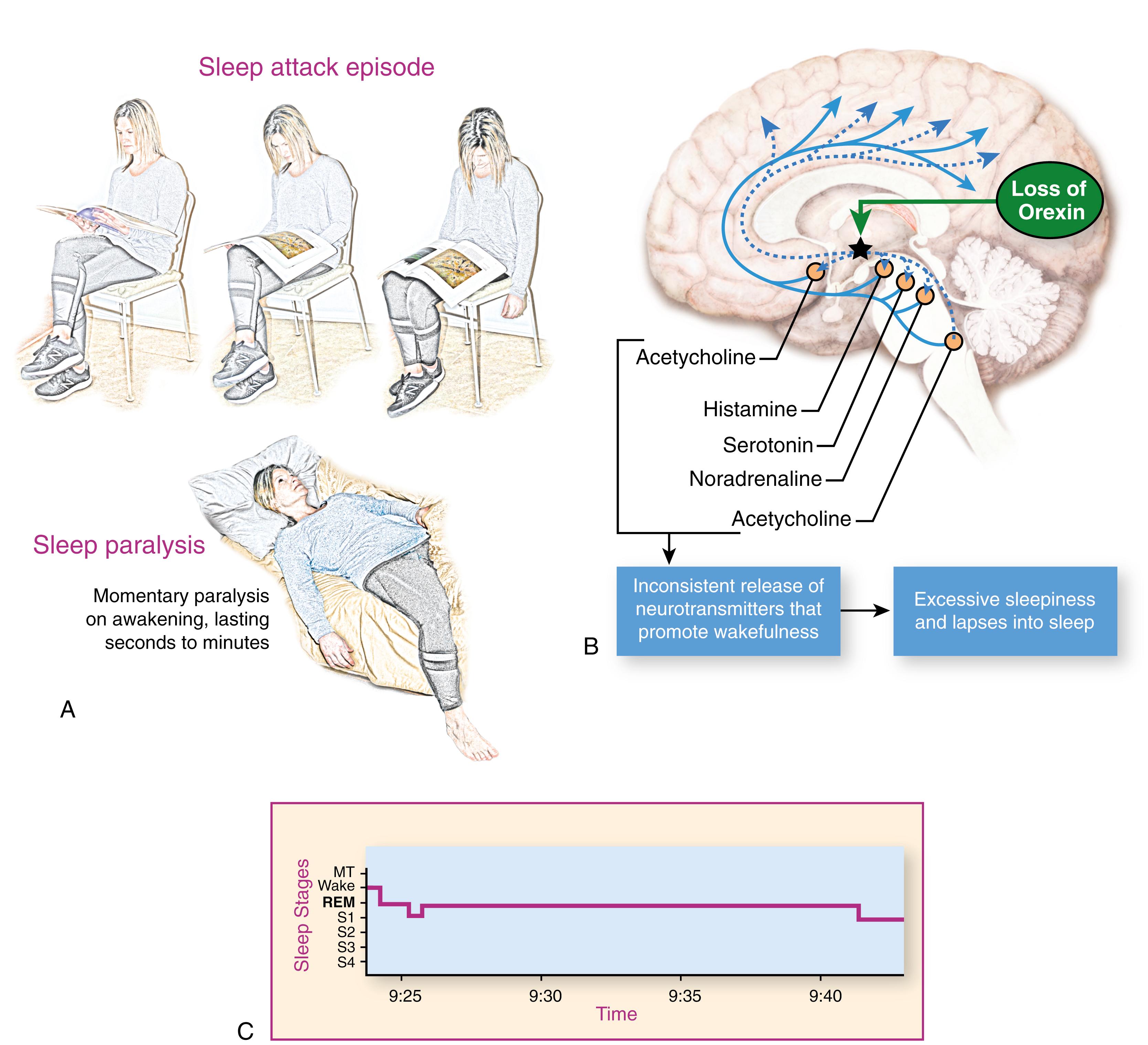
Cataplexy is defined as a sudden loss of tone in all voluntary muscles with the exception of the respiratory and ocular muscles. By definition, cataplexy is universally preceded by a prodromal of emotion leading to a transient episode of muscle weakness. The typical emotions that trigger cataplexy are positive phenomena and include laughter and excitement, rage, or anger more than 95% of the time. The attacks may be complete or partial but are rarely unilateral. Most commonly, patients momentarily have head nodding, sagging of the jaw, buckling of the knees, dropping of objects from the hands, dysarthria, or loss of voice, but sometimes they may slump or fall forward to the ground for a few seconds. The duration is usually a few seconds to a minute or two, and consciousness is retained completely during the attack. During brief cataplectic spells, neurological examination reveals flaccidity of the muscles and absence or markedly reduced muscle stretch reflexes. H reflex and F responses are decreased or absent. Generally, cataplectic spells occur months to years after the onset of sleep attacks, but occasionally cataplexy is the initial manifestation. It is a lifelong condition but is generally less severe and may even disappear in old age. Rarely, status cataplecticus occurs, particularly after withdrawal of anticataplectic medications. An EEG recording shows evidence of wakefulness during brief cataplectic spells, but if the attack lasts longer than 1 to 2 minutes, the EEG shows REM sleep. A potential pathogenetic mechanism postulated for cataplexy is illustrated in Fig. 101.39 and is based on activation, during awake state, of brainstem neuronal circuitry that normally induces skeletal muscle atonia during REM sleep. During cataplexy, muscle weakness is generated by decreased excitation of noradrenergic neurons and increased inhibition of skeletal motor neurons by GABA-releasing or glycinergic neurons ( ) ( ). The medial prefrontal cortex and amygdala contain neural networks through which positive emotions conceivably trigger cataplectic attacks ( ).
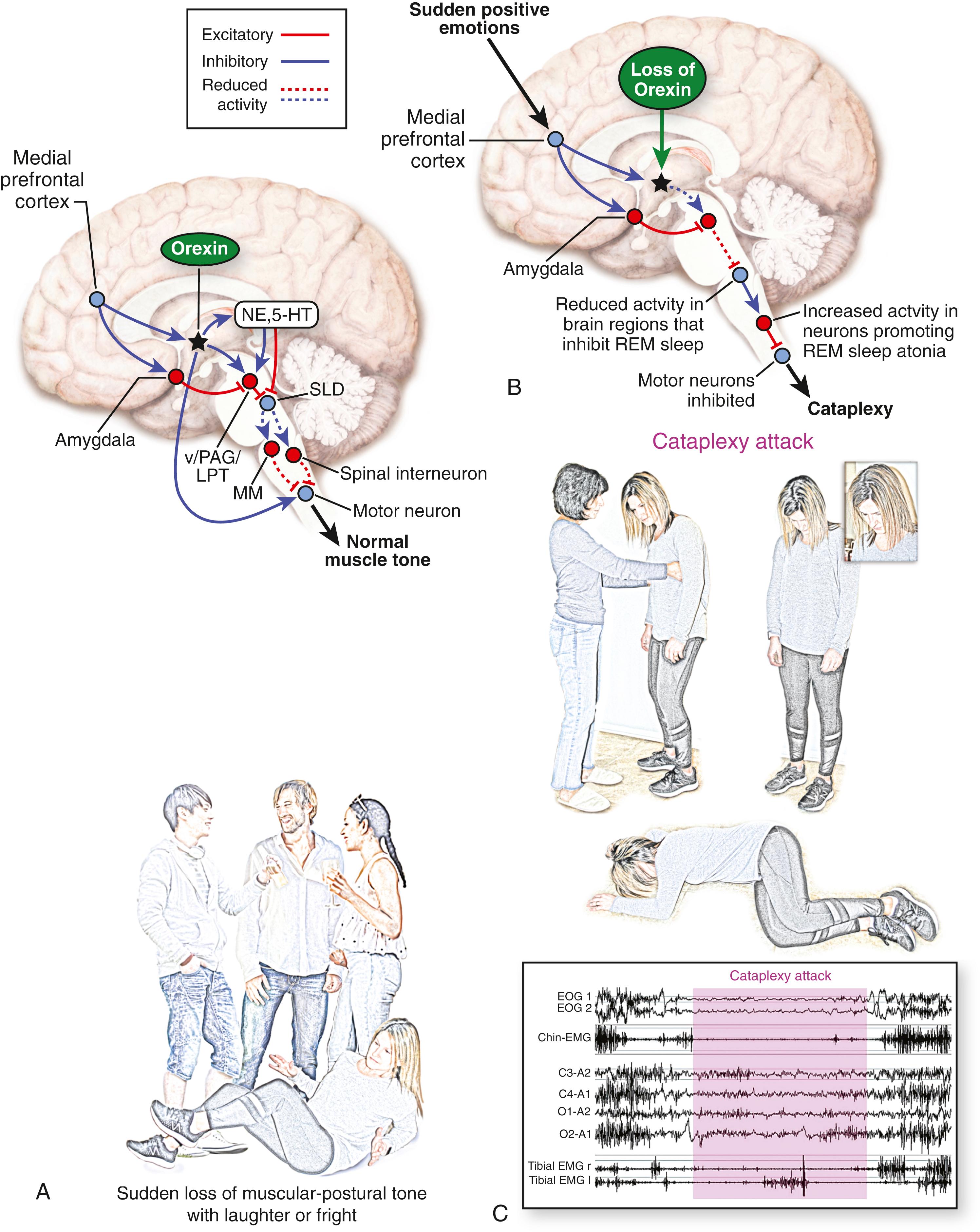
Sleep paralysis occurs in approximately 25%–50% of patients and is generally noted months to years after the onset of narcoleptic sleep attacks. The sudden apparent paralysis of one or both sides of the body or one limb occurs either during sleep onset (hypnagogic) or on awakening (hypnopompic) in the morning. The patient is unable to move or speak and is frightened, although he or she retains consciousness. The attacks last from a few minutes to 15–20 minutes.
Hypnagogic hallucinations are found in 20%–40% of narcoleptic patients. They occur either at sleep onset or on awakening in the morning and generally appear months to years after the onset of sleep attacks. Hallucinations are most commonly vivid and visual (and often fear-inducing) but are sometimes auditory, vestibular, or somesthetic in nature. In 30% of patients, three of the four major manifestations of the narcoleptic tetrad (sleep attacks, cataplexy, sleep paralysis, and hypnagogic hallucinations) occur together, and in about 10% of cases, all four major features occur together.
Disturbed night sleep is commonly noted in 70% to 80% of patients. In approximately 20%–40% of cases, automatic behavior characterized by repeated performance of a single function such as speaking or writing in a meaningless manner, driving on the wrong side of the road, or driving to a strange place without recalling the episode is noted. These episodes of automatic behavior may result from partial sleep episodes, frequent lapses, or microsleeps.
The clinical presentation of narcolepsy with cataplexy in children may be different from that of adults ( ). Children may present with prolonged nocturnal sleep or paradoxically may present with hyperactive behavior, insomnia, and bizarre hallucinations. Children may present with characteristic cataplectic facies (facial weakness with tongue protrusion) not associated with emotion, or cataplexy may be manifested with perioral dystonic movements ( Fig. 101.40 ) ( ).

In addition to the major manifestations, patients with narcolepsy may also have several important comorbid conditions (see ![]() eTable 101.6 ): sleep apnea, PLMS, and RBD. Sleep apnea is noted in approximately 30% of narcoleptic patients; most commonly central apnea is seen, but obstructive or mixed apnea is also reported. Associated sleep apnea may aggravate sleep attacks. It is important to diagnose obstructive sleep apnea (OSA) in these patients because treatment with continuous positive airway pressure (CPAP) will relieve apnea, with curtailment of EDS. RBD generally occurs in men with narcolepsy—in up to 36% of all patients with the disorder. Mostly commonly, the stimulants and tricyclic antidepressants or selective serotonin reuptake inhibitors (SSRIs) used to treat narcolepsy-cataplexy syndrome may induce or exacerbate RBD. PLMS have been noted in 10%–60% of narcoleptic patients. There is a high prevalence (exact percentage is not known in limited studies) of both eating disorder ( ) and increased BMI ( ) not related to each other in narcoleptic patients, as well as anxiety disorders, depression, and fatigue which is distinct from sleepiness.
eTable 101.6 ): sleep apnea, PLMS, and RBD. Sleep apnea is noted in approximately 30% of narcoleptic patients; most commonly central apnea is seen, but obstructive or mixed apnea is also reported. Associated sleep apnea may aggravate sleep attacks. It is important to diagnose obstructive sleep apnea (OSA) in these patients because treatment with continuous positive airway pressure (CPAP) will relieve apnea, with curtailment of EDS. RBD generally occurs in men with narcolepsy—in up to 36% of all patients with the disorder. Mostly commonly, the stimulants and tricyclic antidepressants or selective serotonin reuptake inhibitors (SSRIs) used to treat narcolepsy-cataplexy syndrome may induce or exacerbate RBD. PLMS have been noted in 10%–60% of narcoleptic patients. There is a high prevalence (exact percentage is not known in limited studies) of both eating disorder ( ) and increased BMI ( ) not related to each other in narcoleptic patients, as well as anxiety disorders, depression, and fatigue which is distinct from sleepiness.
The most common conditions that should be differentiated from narcoleptic sleep attacks are illustrated in Box 101.13 and Table 101.7 . Narcolepsy-related sleep attacks should be differentiated from other causes of EDS. These include sleep deprivation and insufficient sleep syndrome, OSAS, alcohol- and drug-related hypersomnolence, idiopathic hypersomnia ( ), circadian rhythm sleep disorders, and other medical, neurological, and psychiatric disorders causing hypersomnolence. OSAS (which will be reviewed later in the chapter) is the most common cause of EDS in patients referred to a sleep laboratory for evaluation. It is characterized by repeated episodes of obstructive and mixed apneas during NREM and REM sleep in overnight PSG recordings. These patients have prolonged daytime sleep episodes followed by fatigue and drowsiness on awakening, which contrasts with a fresh feeling in narcoleptic patients on awakening from brief sleep attacks. All patients with hypersomnolence can be excluded after careful history and physical examination and overnight PSG recording.
Obstructive sleep apnea syndrome
Sleep deprivation
Insufficient sleep syndrome
Alcohol/drug-related hypersomnolence
Periodic hypersomnolence
Medical, neurological, and psychiatric disorders causing hypersomnolence
Idiopathic hypersomnia
Circadian rhythm sleep disorders
| Narcoleptic Mimics | Confounding Overlapping Features | Clinical Clues | Objective Verifiable Indicators | |
|---|---|---|---|---|
| Laboratory/Neuroimaging | Neurophysiology | |||
| Neuromuscular diseases | Hypotonia Hyporeflexia |
Additional neurological findings (weakness, worsening with exercise, etc.) | Elevated CPK, aldolase, LDH, abnormal muscle biopsy imaging/histology | Altered EMG/NCV pattern |
| Epileptic attacks | Head drops Axial drops Falls Negative/positive myoclonus |
Loss of consciousness, rapid onset, post ictal confusion, tongue biting, incontinence | Increased CPK after generalized episodes Unique metabolic abnormalities related to different etiologies Neuroimaging abnormalities |
Critical epileptic EEG discharges, Inter-ictal EEG epileptic abnormalities. |
| Cerebellar ataxias | Unstable gait, slurred speech, hypotonia and hyporeflexia | Persistent condition (although fluctuating), absence of emotional triggers, additional neurological findings (e.g., nystagmus, appendicular ataxia) frequent involvement of other CNS structures | Different biochemical/genetic abnormalities according to different underlying diseases Neuroimaging abnormalities |
|
| Choreic disorders | Dyskinesia with prominent facial involvement and psychiatric comorbidities, recent streptococcal infection, and/or elevated streptococcal antibodies titer | Presence of piano-playing choreiform movements, systemic involvement | Evidence of recurrent streptococcal infections coupled with clinical worsening Different biochemical and genetic findings may occur in different choreic conditions |
Active bursts on EMG leads (vs. hypotonia in NT1) |
| Encephalitis | Acute onset of sleep disturbance, abnormal movements (orofacial dyskinesias, stereotyped movements), psychiatric/behavioral changes | Impaired consciousness, severe psychosis or catatonia, frequent seizures, autonomic dysfunction, and severe cognitive impairment | Serological autoimmune/paraneoplastic markers Altered CSF findings (e.g., altered brain blood barrier) |
EEG abnormalities (e.g., potential occurrence of epileptic activity) |
| ADHD | Hyperactivity, lack of attention, decreased school performances, fidgeting | Lack of pathological EDS | ||
Idiopathic hypersomnia (see later discussion) closely resembles narcolepsy syndrome. In contrast to narcolepsy, the sleep episodes in idiopathic hypersomnia are prolonged, and the sleep is not refreshing. PSG recordings and MSLT scores do not show sleep-onset REM sleep but do show pathological sleepiness. There is no disturbance of REM-NREM organization on PSG recordings. Some patients with idiopathic hypersomnia may have a positive family history.
Cataplectic attacks may be mistaken for partial complex seizures, absence spells, atonic seizures (as well as gelastic-atonic seizures characterized by laughing followed by loss of muscle tone), drop attacks, basilar migraines, vertebrobasilar insufficiency, syncope, and pseudocataplexy ( Box 101.14 ). Pseudocataplexy is a functional disorder, often seen in patients with cataplexy, in which there are negative thoughts rather than laughter, the typical precipitant of true cataplexy, and characterized by spells extending over minutes to hours rather than the seconds to minute/s characteristic of bona fide cataplexy ( ; ). A partial complex seizure, however, is characterized by an altered state of consciousness, unlike cataplexy. In addition, patients with seizures may have generalized tonic-clonic movements, postictal confusion, and epileptiform discharges on EEG. Absence spells are characterized by staring with vacant expression lasting up to 30 seconds, with an altered state of alertness associated with characteristic 3-Hz spike-and-wave patterns in the EEG. Atonic seizures are accompanied by transient loss of consciousness and EEG evidence of slow spike-and-wave discharges or multiple spike-and-wave discharges.
Partial complex seizure
Absence spell
Atonic seizure
Drop attack
Syncope
Vertebrobasilar insufficiency
Basilar migraines
Pseudocataplexy
Sleep paralysis, in the context of narcolepsy, should be differentiated from isolated physiological and familial sleep paralysis in which other manifestations of narcolepsy are absent. Automatic behavior should be differentiated from the automatisms observed in partial complex seizures and psychogenic fugue. The clinical history, pre/post event triggers/confusion, physical examination, and EEG should be helpful in differentiating these conditions. Differential diagnosis of narcolepsy and cataplexy are summarized in Table 101.7 ( ).
“Symptomatic narcolepsy” or “secondary narcolepsy” is the previously used term to describe narcolepsy associated with underlying structural, genetic, inflammatory, or vascular abnormality impacting the hypothalamus and leading to severe CNS hypersomnolence with or without cataplexy or abnormal CSF hypocretin levels. Depending on the presence of cataplexy/reduced CSF hypocretin levels, narcolepsy type 1 and 2 may be found. Leading CNS causes include diencephalic and midbrain tumors, MS, strokes, cysts, vascular malformations, encephalitis, cerebral trauma, and paraneoplastic syndrome with anti-Ma2 antibodies and it may present with narcoleptic-like sleep attacks and other manifestations ( ; ; ). Cataplexy may develop in children affected with Niemann-Pick disease type C. Fig. 101.41 illustrates a scenario from the authors’ practice of a gentleman with a history of neurosarcoid of the diencephalon and hypocretin deficiency illustrating a case of secondary/symptomatic narcolepsy ( ).
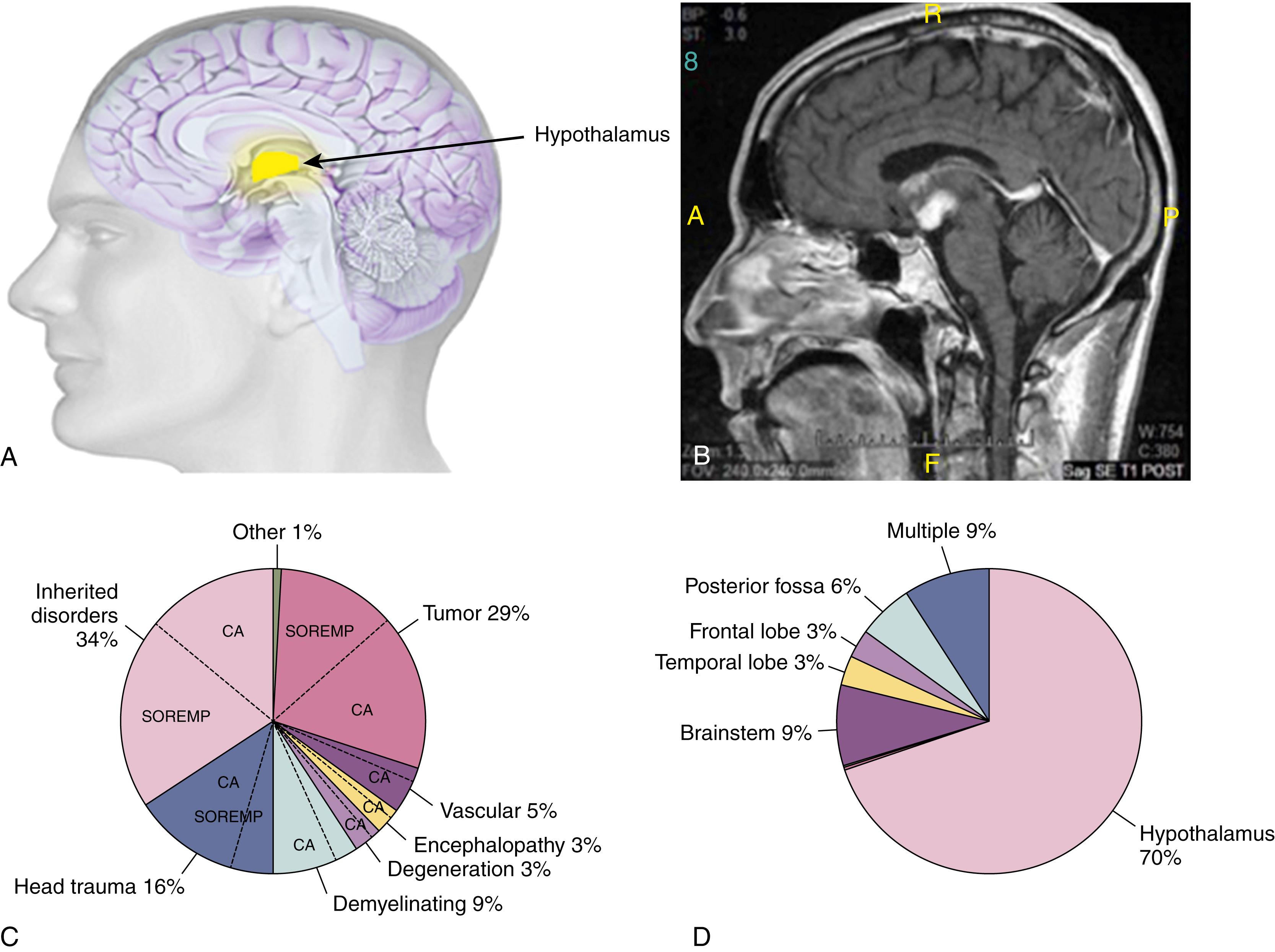
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here