Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Skin is divided into the epidermis, dermis, and subcutaneous layers ( Fig. 58.1 ). The epidermis possesses the following appendages , :
Eccrine glands
Apocrine glands
Sebaceous glands
Hair follicles
Nails
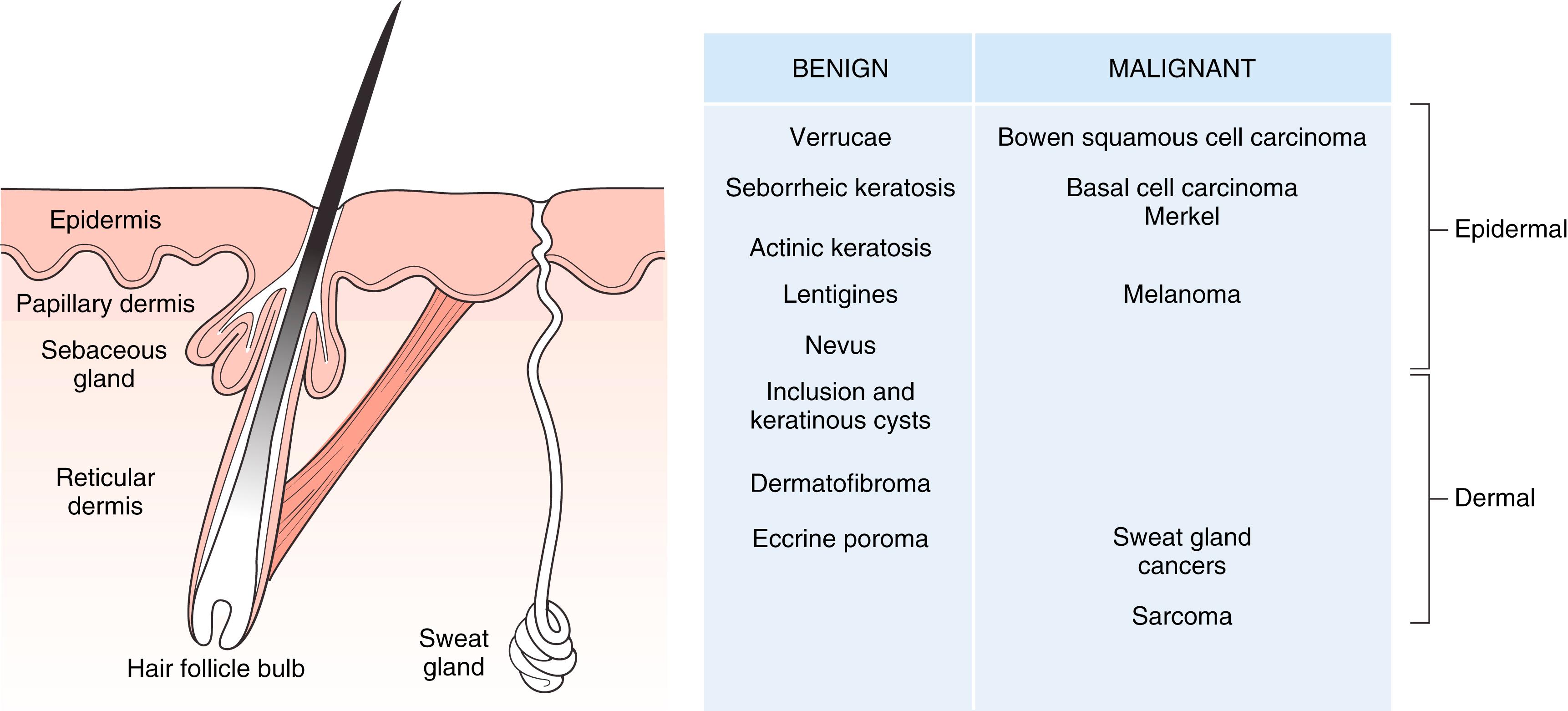
Glands and hair follicles arise as down growths from the epidermis and project into the dermis. The two types of sweat glands are eccrine and apocrine. Eccrine glands exist all over the skin and secrete clear, watery fluid. They begin as a coil in the dermis and open onto the skin surface. Apocrine glands are large sweat glands that open into hair follicles, to which they are attached, and rarely directly to the skin surface. They are located in the axilla, areola, and perianal region, among others, and they do not develop fully until puberty. Secretion is evoked in response to stress, pain, and fright. Sebaceous glands are found all over the body, except palms and soles. They have no lumen and are multilobulated, appearing as a pouch hanging on the side of the hair follicle.
Dermis is divided into two parts: (1) papillary and (2) reticular. Papillary dermis rests against the epidermis, which sends papillary extensions down into it called rete ridges. These ridges resist shear disruption between the dermal and epidermal layers. The reticular dermis is deeper than the papillary dermis. It is composed of coarser bundles of connective tissue fibers and extends to the subcutaneous layer. The dermis contains:
Connective tissue fibers: collagen, elastin, and reticulin
Cellular elements: migratory cells (leukocytes, histiocytes) and fixed cells (fibroblasts, mast cells)
Blood vessels
Nerves and specialized nerve endings
Smooth muscle
Lymphatics
Ground substance
The epidermis is superficial to the basal lamina and is composed histologically of four cell layers (five layers in the palm) ( Fig. 58.2 ):
Basal layer (stratum basale) consists of a single layer of columnar cells that stain deeply basophilic. At haphazard intervals between these basal cells are melanocytes, which are clear cells with small nuclei. Melanin is produced through the conversion of tyrosin to dopa, facilitated by the enzyme tyrosinase. Melanocytes may multiply and form nests in the deep epidermis and even spread into the subjacent dermis, where they lose the ability to synthesize melanin but retain the melanin storing property and remain pigmented. These intradermal clusters are called nevus cells and give the appearance of nevi. Melanocytes are actually dendritic cells and connect to each other over distances. They are derived from neural crest. Merkel cells, like melanocytes, are nonkeratinocyte intraepidermal cells. They also occur in the basal cell layer and are associated with sensory axons for slow-adapting mechanoreceptors and pressure sensors. They are also found in hair follicles.
Malpighian (or prickle cell) layer (stratum spinosum) is made up of cells connected to each other by intracellular bridges and so artifactually (during tissue processing) give the spiny or prickle appearance of this layer.
Granular layer (stratum granulosum) is made of a few layers of diamond-shaped cells filled with basophilic granules (keratohyalin).
Horny layer (stratum corneum) has cells that have no nuclei and are continuously shed from the surface.
Clear layer (stratum lucidum), in the palm only, gives a translucent appearance to the skin. It is composed of up to five layers of “dead” keratinocytes, and this layer is situated between the stratum granulosum and the stratum corneum. Cells are filled with eleidin, an intermediate form of keratin.
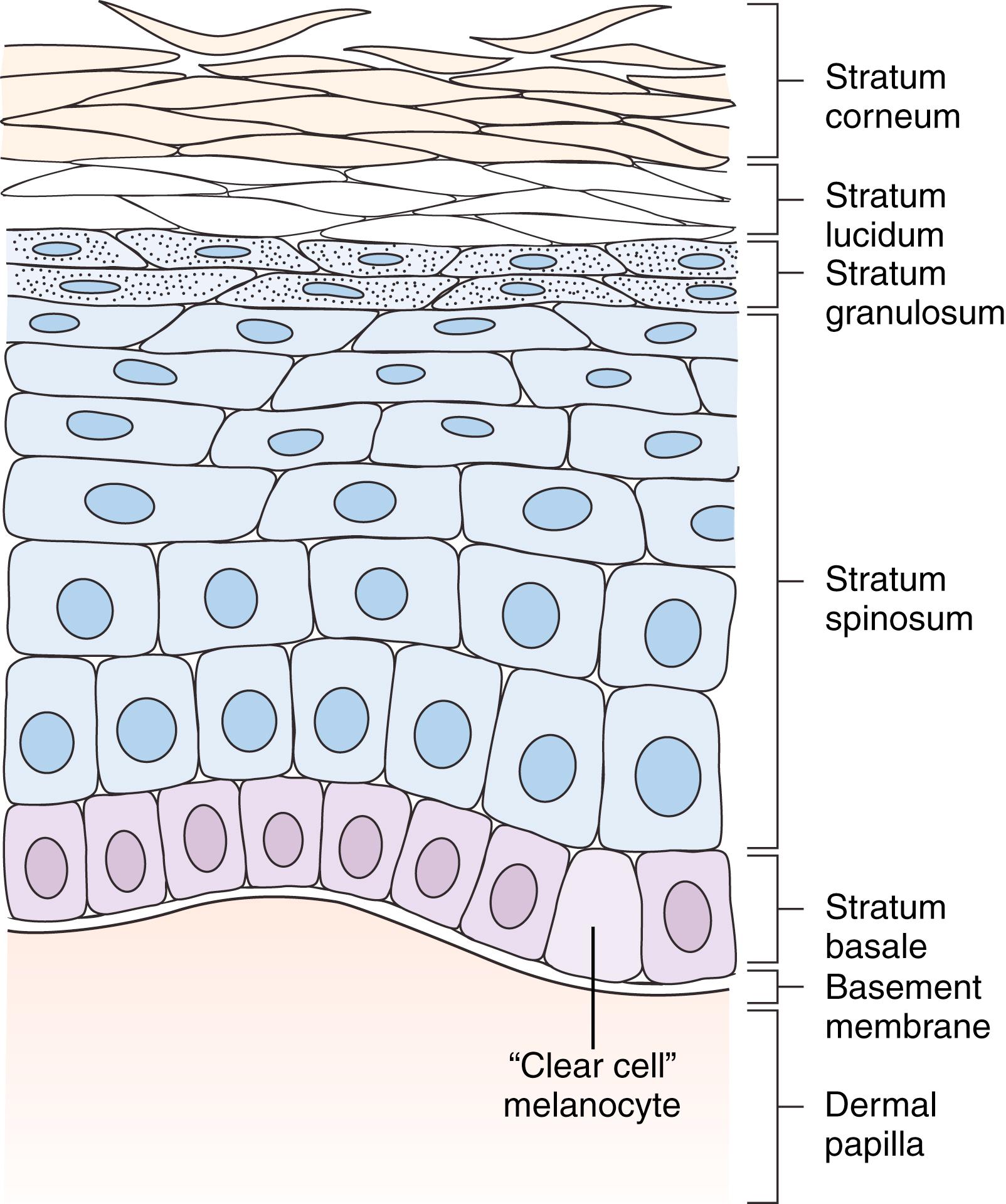
There is a normal progression of maturation from deep to superficial layers of the epidermis. In epidermal cancers, this natural progressive maturation is lost and becomes disorderly and ultimately anaplastic. , Relative invasiveness of these cancers is described first by invasion deep to the basement membrane, and then depth of invasion, next into the papillary dermis and on into the deeper reticular dermis. Thus carcinoma in situ is a true cancer that is disorganized in the progressive epidermal cell maturation but nonetheless has not invaded through the basement membrane of the dermis. Originally, Clark levels were applied descriptively to the depth of melanoma invasion into the papillary dermis, reticular dermis, and on into subcutaneous sites. However, Breslow levels of actual millimeter thickness of invasion are more accurate prognostically. In the subungual regions, rete ridges and papillary dermis are less well defined, and there is also no subcutaneous fat, rendering the distance of tumor penetration between epidermis and periosteum to be very small. In this region, Clark and Breslow measurements cannot be used as an effective treatment guide.
Benign and malignant skin tumors may arise from epithelial elements (in which case malignancies are termed carcinoma) of the epidermis, as well as from the adnexae in the dermis (hair follicles and sweat glands) (see Fig. 58.1 ). Basal cell carcinoma is derived from the basal cell layer of the epidermis and squamous cell carcinomas from the more superficial keratinocytes.
Epithelial cysts within the dermis may result from implantation into the dermis (epidermal inclusion cyst) or from blocked gland ducts (pilosebaceous cysts) and from hair follicles (trichilemmal cysts). Connective tissue tumors (malignant forms are termed sarcomas) may arise from the fibrous tissue of the dermis, such as dermatofibromas and the rarer dermatofibrosarcoma protuberans. Nerve ending tumors and those relative to blood vessels also occur in the dermis, such as neurofibroma, glomus tumor, and Merkel cell tumor. Hemangiomas and capillary stains and vascular malformations are discussed elsewhere.
Having knowledge of skin anatomy and tumor location is helpful in arriving at a clinical diagnosis. Intraepidermal lesions may be plaque-like but are not elevated into papules or nodules (the hallmark of intradermal lesions). Junctional (between dermis and epidermis) lesions are also not elevated, as is the case with a junctional nevus, which is a flat, pigmented lesion. Dermatofibromas and the more vertically invasive nodular melanomas, in contrast, are elevated into a nodule. Melanomas are usually pigmented because their cell of origin is the melanocyte, which produces melanin. However, melanocytes do also pass melanin into adjacent basal and spongy cells and so both basal cell carcinomas (BCCs) and squamous cell carcinomas (SCCs) may be pigmented.
Epidermal lesions often have an “irritated” surface, which may be ulcerative or thickened. Hyperkeratosis is a thickening of the stratum corneum, leading to an exuberant, flaking, and cracked appearance of the skin as in actinic keratosis and verrucae (or warts). In acanthosis, there is a thickening of the stratum spinosum. It gives the skin a leathery folded appearance, and if it is pigmented, it is called acanthosis nigricans. This may occur in simple obesity, but it may be a marker for internal malignancy. Hyperkeratosis may be so pronounced as to become heaped up and form a cutaneous horn. It is most commonly associated with actinic keratosis but may have an underlying SCC or keratoacanthoma. Similarly, greasy scaling lesions that have a “stuck on” appearance are typical of seborrheic keratosis.
In contradistinction, dermal lesions form a papule or nodule. A dermal inclusion cyst retains its attachment to the overlying epidermis (a punctum) and a dermatofibroma arises in the superficial dermal layers, and so the overlying epidermis is adherent to the deeper lesion. In fact, there may be overlying pressure necrosis from a dermatofibroma, leading to an almost pathognomonic feature of central umbilication. The nodule in dermatofibroma is hard, but a nodular nevus is soft and indents with the back end of a cotton-tipped applicator.
The lesions of the deeper dermis will not have an overlying epidermal adherence. Lesions that are deeply attached will be nonmobile. One should always assess clinically for deep fixation and lack of deep mobility of skin cancers since this implies a deeper invasion.
Regional clinical evaluation of lymph node drainage basins for malignant lesions is also important, and one must have an understanding of this anatomy. There are superficial and deep lymphatics. Superficial lymphatics from the ulnar side of the hand may drain first to the supratrochlear nodes just proximal to the medial epicondyle, but most pass directly up to the axilla. Superficial radial lymphatics ascend to the deltopectoral glands and then on to the subclavicular axillary nodes. Deep lymphatic vessels accompany the deep blood vessels (radial, ulnar, anterior and posterior interosseous), along which there may be lymph glands. Most pass up to the lateral axillary nodes. Thus it is necessary to examine the forearm and epitrochlear and supratrochlear and deltopectoral regions, as well as axilla (medial, lateral, and apex) and supraclavicular lymph node drainage basins. When planning a “prophylactic” lymph node biopsy in the absence of palpable glands, one cannot rely on a seemingly logical ascent of lymph drainage up the extremity to the axilla, but one must assess for the “sentinel” node that can be at any one of these potential sites.
Most cutaneous lesions do not require special imaging, unless, for example, there is a large, deeply invasive skin cancer, in which case magnetic resonance imaging (MRI) may be helpful in planning resection.
For small lesions, one may proceed directly to excisional biopsy. However, many small lesions are noncancerous or only minimally invasive cancers, and so various diagnostic and/or treatment options are available. Once again, understanding the depth of cutaneous involvement is an important guide. Many epidermal lesions can be treated by shaving or curettage. Where indicated, these have an advantage over elliptical excision, since the deeper dermal layers are not violated and there is less likelihood of adverse scarring (perhaps not as critical in the hand as on the more cosmetically important areas such as the face). Appropriate nonsurgical treatments may be as follows:
Patient surveillance: Total lesion ablation may not be feasible. Periodic clinical examination and follow-up photographs may detect malignant transformation. This course may be chosen when total excision of a large congenital nevus is impractical, when a patient has multiple dysplastic nevi, or for diffuse syndromal conditions with a high risk of development of cutaneous malignancy (such as xeroderma pigmentosum and basal cell nevus syndrome).
Topical medications: Diffusely spread conditions with malignant potential may be treated by topically applied 5-fluorouracil (5-FU) ( Fig. 58.3 ). Bowen lesions and superficial BCCs or SCCs cannot be treated with 5-FU. Verrucae may be treated by topical application of podophyllin or keratolytic agents, such as salicylic acid. 5% imiquimod cream (Aldara, Graceway Pharmaceuticals, Bristol, TN) is approved for treatment of superficial BCCs, applied five to seven times per week for up to 12 weeks. Incomplete data are available for its use in Bowen disease, SCC, or lentigo maligna melanoma.
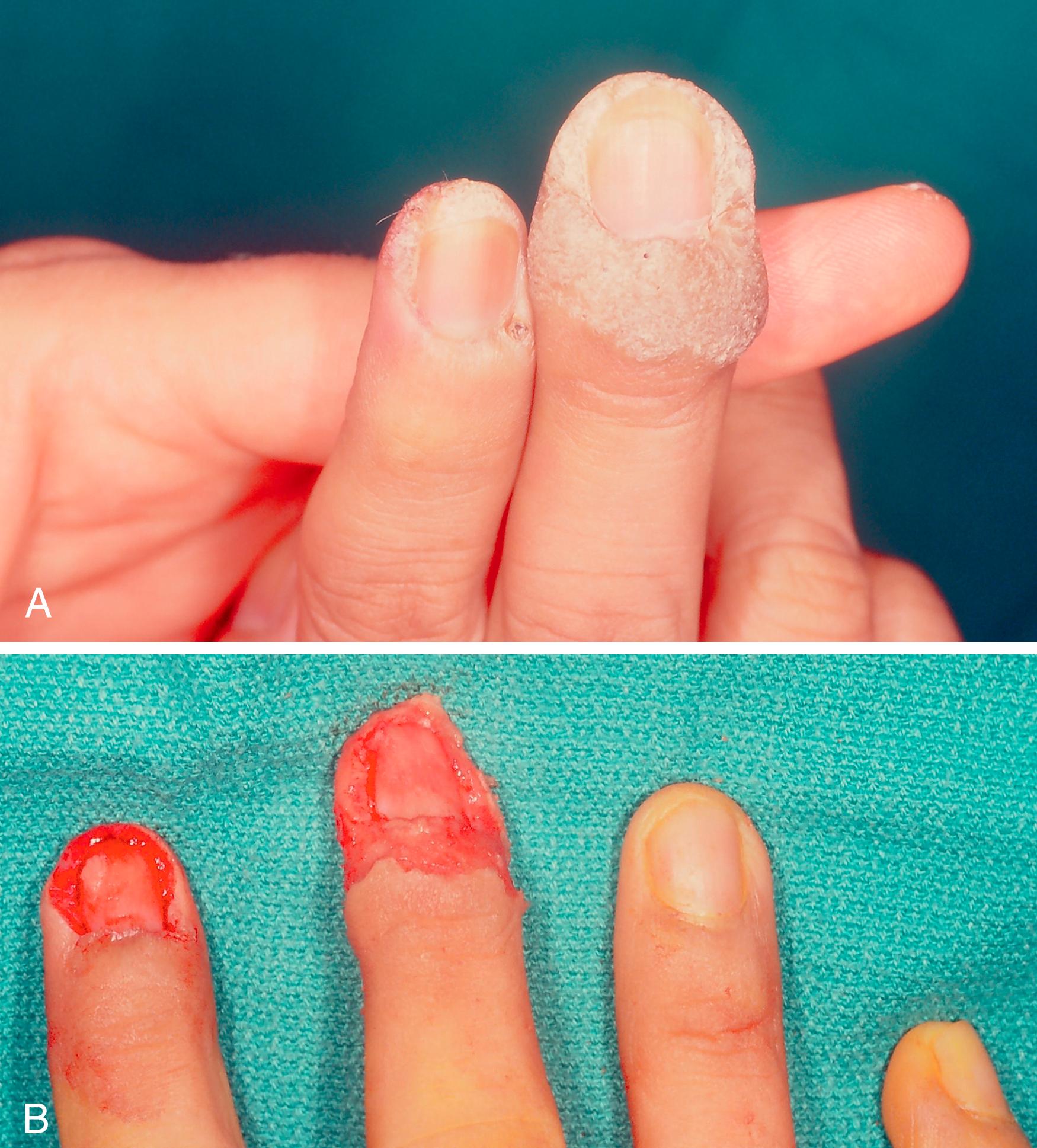
Curettage: A 5-mm curette is most commonly used, and curettage is performed after adequate local anesthesia. It yields material for histologic examination, but it does not allow assessment of tumor margins. It permits safe removal of superficial lesions with minimal scarring and is amenable for actinic and seborrheic keratoses and verruca vulgaris. It is an excellent treatment for subungual verrucae, leaving almost no residual nail deformity. Remove the nail plate, and one can identify a deep curettage cleavage plain using loupe magnification ( Fig. 58.4 ).
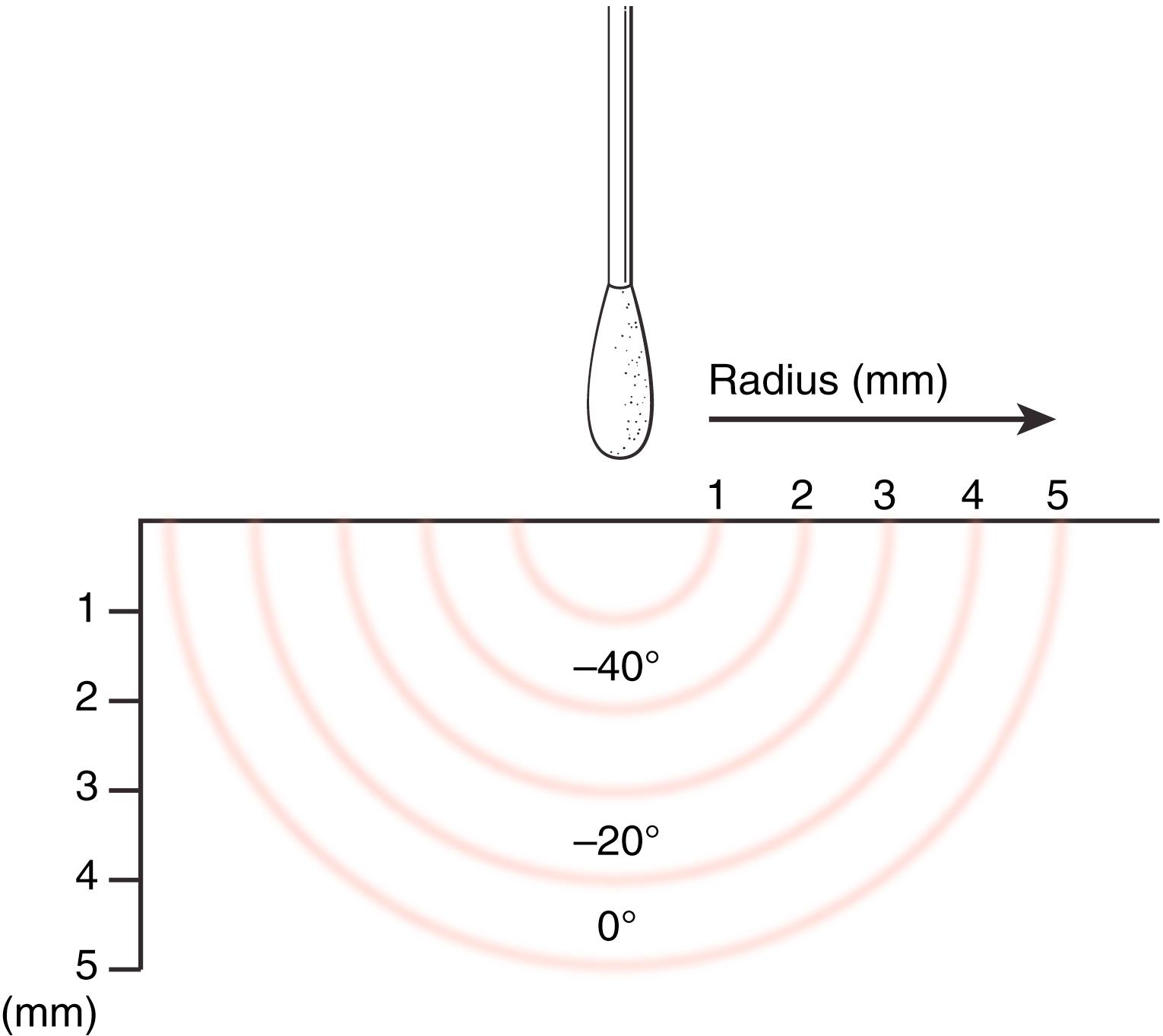
Cryosurgery: Intense cold is applied and all cells within the freezing wave are killed. Liquid nitrogen can remove warts, superficial keratoses, and actinic lentigines. It must be used with caution where there are adjacent peripheral nerves, such as on the volar finger and olecranon, as permanent freeze damage to the nerve may occur. Also, destruction to critical paronychial and nail bed structures can occur at the fingertip. Esthetic results are otherwise usually excellent, but cryotherapy must be used with caution in dark-skinned patients as hypopigmentation may result. It causes little pain and rarely requires anesthesia. A cotton-tipped applicator is used, with cotton teased out and tapered. As liquid nitrogen runs down the applicator, skin at the applicator tip turns white, and the ice front spreads outward ( Fig. 58.5 ). Application ceases when visible freezing extends just beyond the lesion.
Electrosurgery: Electrical energy is converted to heat by tissue resistance. An office hyfrecator is a good electrosurgical instrument and may be used to destroy seborrheic keratoses, skin tags, lentigines, and spider nevi.
Electrodesiccation and curettage: A curette is used to scrape the lesion down to dermis. Scraping is then paused to use a hyfrecator. This causes electrocoagulation (electrodesiccation) of the raw surgical surface. This process is usually repeated three times or until the surgeon is comfortable with the margins. The technique is dependent on clinical experience, but it has the advantage of giving good cosmetic results. Additionally, recurrence is rapidly recognized as it is not obscured by wound closure or flap, and it is a straightforward office procedure that can be used for multiple superficial lesions that would otherwise be difficult or tedious to treat with multiple excisions. It is useful for seborrheic keratoses and viral warts but has also been used for multiple superficial BCCs, keratoacanthoma, and Bowen disease. For larger lesions, sometimes scarring can be obvious.
Before ablative surgery, a histologic diagnosis should always be obtained. The biopsy process may actually be the curative treatment for smaller lesions.
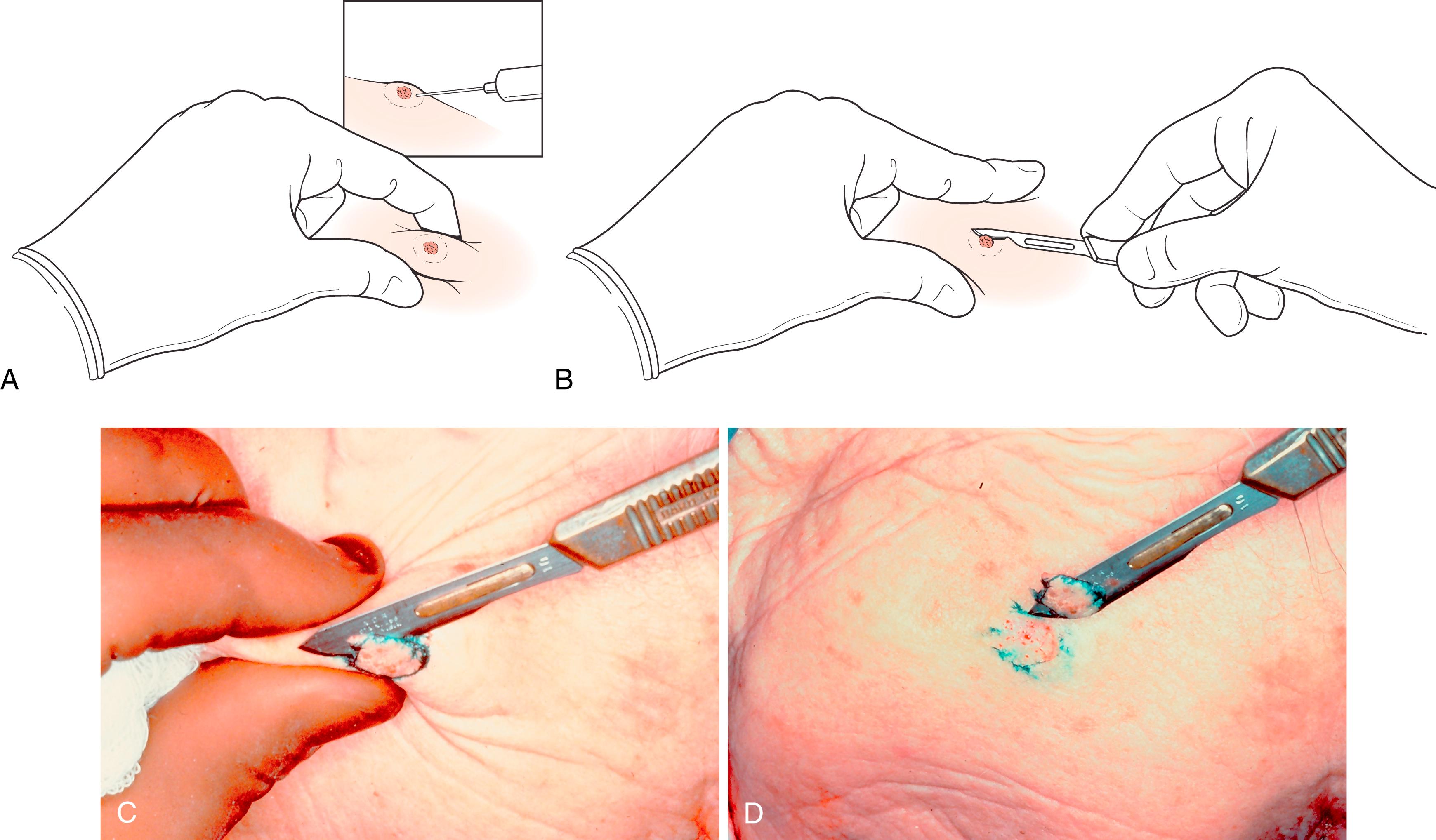
Shave biopsy: This may totally excise the lesion and be curative or may serve as a diagnostic biopsy. Lesion elevation by the mechanical effects of wheal of local anesthetic, and pinching the lesion between thumb and index finger above the plain of the surrounding tissue, enables a full-thickness shave biopsy down to dermis. This can be done with a scalpel blade or a razor blade ( Fig. 58.6 ). Shave biopsies are not suitable for neoplasms that clinically appear to infiltrate dermis and generally not the pigmented lesions with suspicion for melanoma.
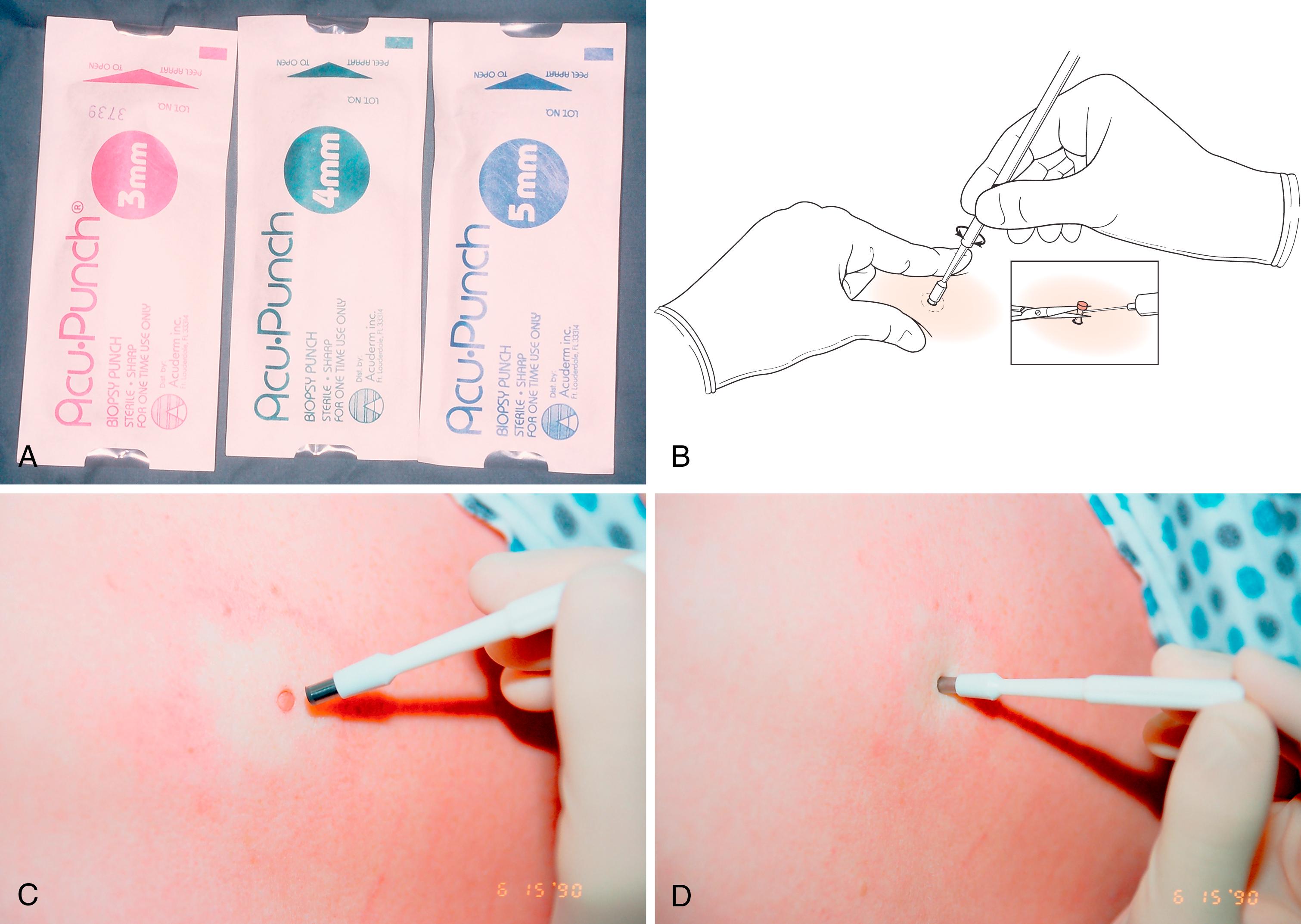
Punch biopsy: This may be incisional or excisional (if the lesion is small enough for the punch to fit around). Variable size cutting punches are available from 1.5 mm to 6 mm—4 mm is most frequently used. Local anesthetic is first injected. When the circular punch incision reaches subcutaneous fat, the punch is removed. The small cylinder of tissue is then lifted and its base transected at the level of the subcutaneous tissue with a small iris scissors ( Fig. 58.7 ). Sutures are generally not used. Sampling error is minimized by careful choice of the site to be biopsied.
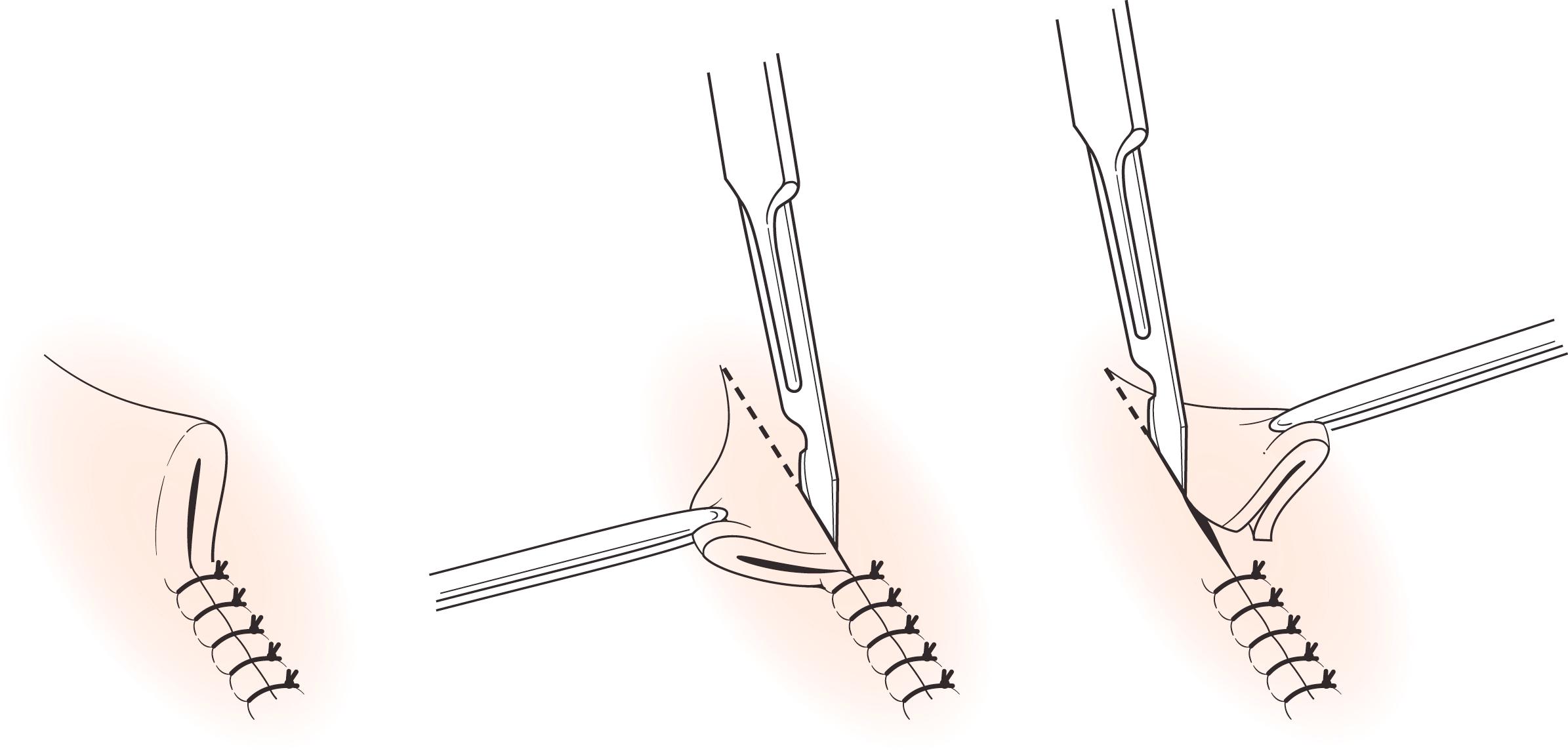
Elliptical incisional/excisional biopsy: If the lesion is too large to make excisional biopsy impractical, incisional biopsy with a margin of adjacent normal skin is performed. Provided that subsequent excision is performed in a timely manner for histologically proven malignant conditions, prior incisional biopsy does not alter the natural history of melanoma and other cutaneous malignancies. Site incisional biopsies longitudinally so that the resulting scar (which may need to be excised later) does not compromise subsequent excision of a potential malignancy. Elliptical excision is usually designed in the line of least skin tension to facilitate linear closure. If the line of least skin tension is difficult to assess, simply excise around the lesion perimeter with appropriate margin and the wound will gape into an ellipse, indicating skin tension, and then simply excise the “dog ears” ( Fig. 58.8 ).
Subungual lesions and longitudinal melanonychia: Remove the nail plate and perform either incisional or excisional longitudinal removal depending on the ability to finely repair the nail bed in a tension-free manner after lateral undermining.
These excisions range from simple elliptical excision and tumor closure, through excision with frozen section assessment of tumor margins, to Mohs micrographic surgery. If the lesion is small, and there is abundant surrounding tissue laxity, then excision and linear closure may be permissible. If permanent sections reveal persistent tumor, then reexcision of the scar is a simple matter. However, if one is planning a complex wound closure by tissue rearrangement or flap coverage, or if the lesion has complex invasion or is difficult to evaluate clinically by inspection and palpation (such as recurrence or certain infiltrative lesions), then intraoperative frozen section analysis of tumor margins is wise or recourse to Mohs micrographic surgery.
When performing a simple tumor excision under local anesthesia, first determine the tumor extent by loupe magnification inspection and by palpation, and mark this tumor margin out, together with the anticipated tumor margin of excision. Do this before local anesthetic infiltration as the infiltration distorts the tissues ( Fig. 58.9 ).
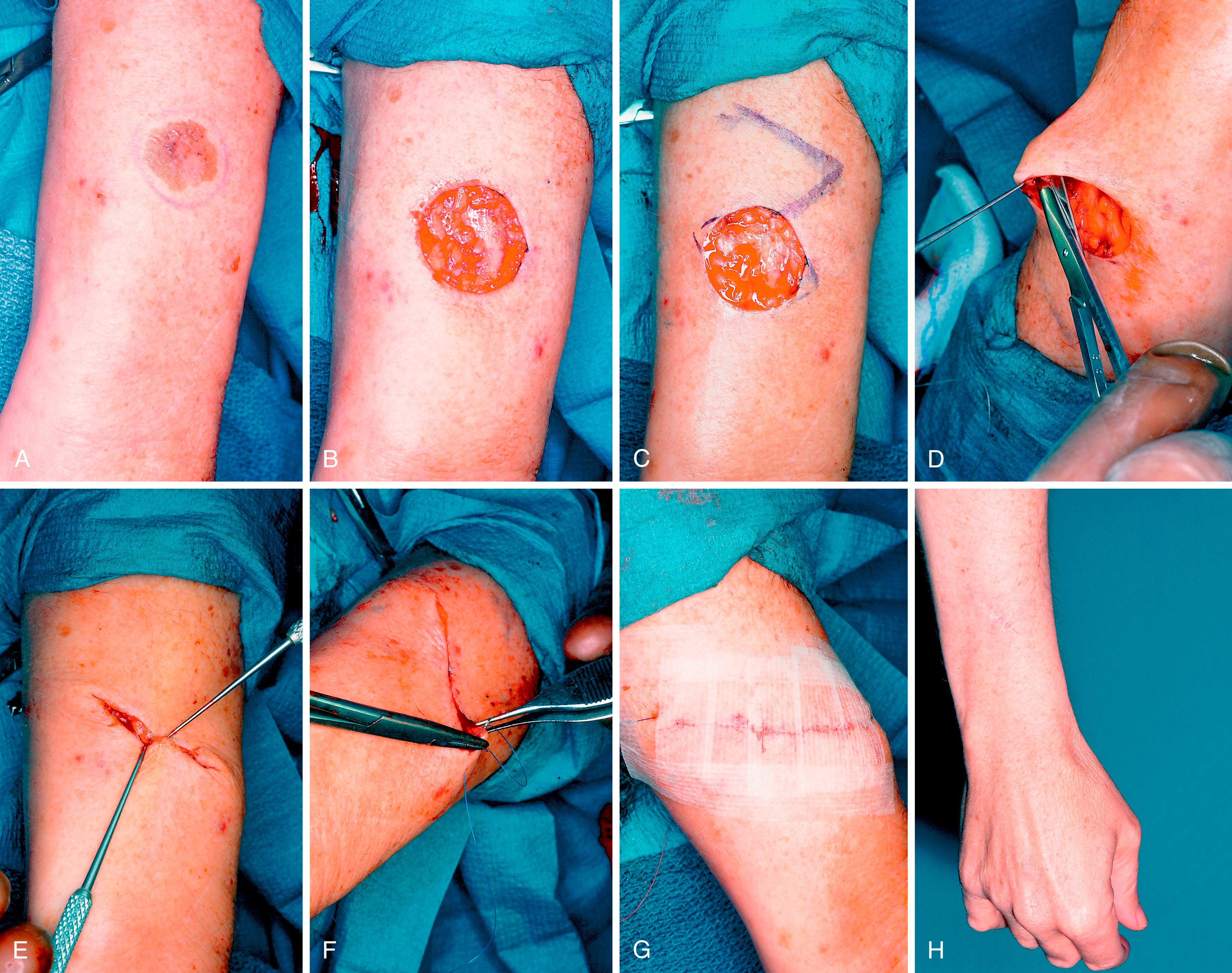
Mohs micrographic surgery is generally reserved for certain widespread lesions with margins that are difficult to interpret clinically and where optimum conservation of uninvolved tissue is necessary, such as with diffuse Bowen lesions ( Fig. 58.10 ). In contrast to the conventional bread-loafed sectioning of the excised specimen, Mohs surgery involves tangential layered excisions and creating maps of residual tumor for further excision, until the whole lesion is excised. It is applicable to recurrent carcinomas, high-risk squamous carcinomas, tumors with ill-defined clinical margins (such as dermatofibrosarcoma protuberans), and areas where maximal conservation of tissues is required to preserve function or cosmesis.
Verruca vulgaris (wart) is caused by human papilloma virus (HPV) and is transmissible by direct contact or autoinoculation. Histologically, one sees hyperkeratosis (thickening of the stratum corneum), acanthosis (thickening of the epidermis), and parakeratosis. The characteristic lesion is a well-demarcated, raised, and a rough cauliflower-like lesion. The less frequent plane wart (verruca plana) is flat. Verrucae are common on the hand and fingers and may occur in periungual and subungual locations. They may be isolated lesions or in groups that combine to form plaques.
Most warts spontaneously regress in 2 years. However, in immunocompromised patients they may reach especially large sizes and may take longer to resolve. A variety of treatment options are available, all of which result in about 70% to 85% cure rate. Most commonly the wart is first pared down and then one may apply a topical keratolytic agent such as salicylic acid solution in collodium, the most commonly used topical agent. Treatment with an emery board has been recommended and various other topical applications (which may need to be repeated) have been used—imiquimod, 5-fluorouracil, and cryotherapy. Silver duct tape (not clear tape) has an unknown mechanism of action. The duct tape is applied for 7 days, and the lesion is then debrided with a pumice stone. Treatments are repeated.
Our preferred treatment is to shave the wart down to the level of the surrounding epidermis. A discrete edge is then seen with magnification loupes and the entire wart is sharply curetted from the surrounding normal epidermis. Since this is an entirely epidermal lesion and the basal epidermal layer is not involved by this technique, reepithelialization occurs rapidly. This technique is an excellent way of treating periungual and subungual warts, resulting in low risk of residual nail or perionychial deformity ( Table 58.1 ).
| Location | Treatment | |
|---|---|---|
| Epidermal | Seborrheic keratosis | Electrodesiccation with curettage, cryotherapy, or tangential shave |
| Actinic keratosis | Cryotherapy, electrodesiccation with curettage, topical treatment | |
| Verruca vulgaris | Cryotherapy, duct tape, tangential shave followed by curettage | |
| Nevus-junctional, compound | Tangential shave | |
| Dermal | Milia | Topical skin care including tretinoin, extraction after lancing each individual milium, cryotherapy, laser therapy |
| Spider angioma | Electrodessication, pulse-dye laser | |
| Pyogenic granuloma | Curettage and electrodessication | |
Seborrheic keratosis is more common in middle-aged and elderly patients. Lesions are more common centrally and thus are more frequently seen on the upper arm and chest than on the hands. The depth of pigmentation is related to the degree of sun exposure. Occasionally, they mimic melanoma and require diagnostic biopsy. They are benign flat lesions that take on a characteristic greasy scale and appear to have been “stuck” on the skin surface ( Fig. 58.11 ). Because they are entirely epidermal lesions, treatment takes advantage of their superficiality and includes curettage, shave biopsy, or application of liquid nitrogen. Since these treatments do not invade the dermis, there is minimal potential scarring.
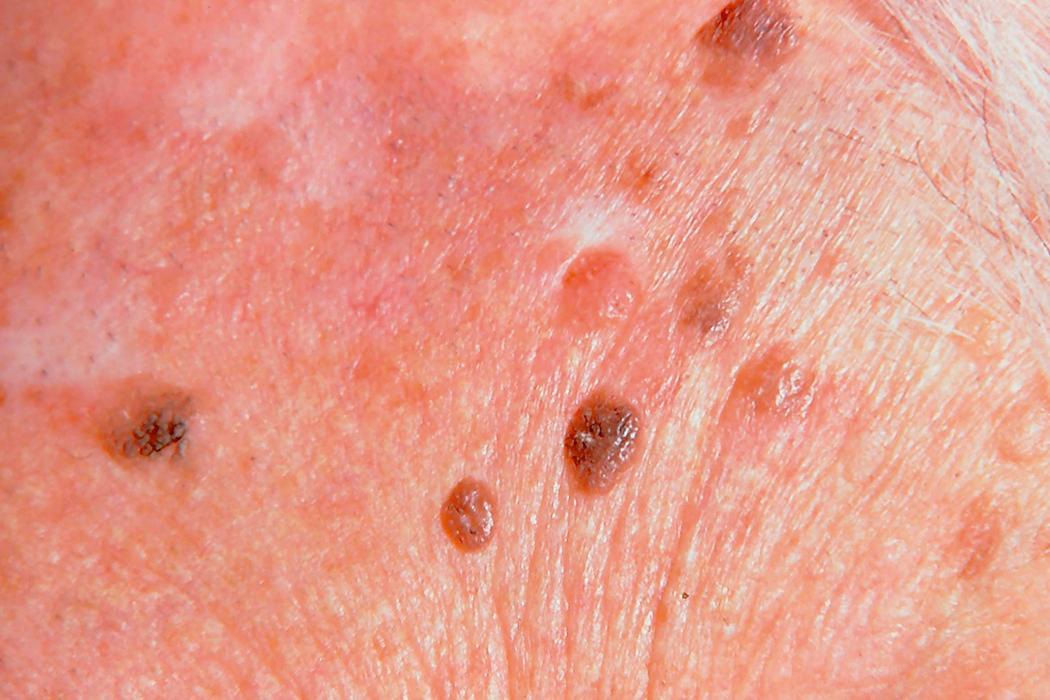
Eccrine poroma is a sweat gland lesion. Because it arises from the eccrine duct within the epidermis and not in the deeper dermal region, it is truly an epidermal lesion. It presents as a moist and red papule with a surrounding collarette and may occasionally be mistaken for a pyogenic granuloma. They are said to be more common on the soles and palms. They may be totally epidermal or may straddle both dermis and epidermis. Malignant change is unusual. Treatment is surgical excision. Multiple palm and sole lesions may occur in the uncommon genetic syndrome of hidrotic ectodermal dysplasia.
Lentigines are benign pigmented macules with increased melanocytic activity. Unlike freckles, they do not fade in the absence of sun exposure. A simple lentigo (lentigo simplex) appears during childhood, is usually less than 5 mm in diameter and may occur on both sun-exposed and non–sun-exposed sites. Solar lentigo (also called senile lentigo) is common on the sun-exposed forearm and dorsum of the hand. There is associated solar damage in the dermis, as indicated by histologic solar elastosis. Their incidence increases with age. They may resemble melanoma, and diagnostic biopsy may be required. Otherwise, specific treatment is unnecessary, though laser therapy may be helpful if the patient is bothered by cosmesis.
Melanocytic nevus is a benign proliferation of melanocytes. Nevi, or simple moles, are the most common tumors in humans. Few are present at birth, and they begin soon after birth, reaching their greatest incidence in young adults.
Junctional nevi have nevus cells confined to the dermoepidermal junction, and intradermal nevi have these nests in the dermis alone. Most nevi in younger patients are junctional and are flat. In adults, most are intradermal and are elevated. Compound nevi have nevus cells at both locations. It is the junctional component of both junctional and compound nevi that may more rarely transform into malignant melanoma. Most melanomas do not arise out of preexisting nevi. Unlike melanocytes, in the basal layer of the epidermis, nevus cells do not have dendritic processes (except in a blue nevus).
Melanocytic nevi may be either acquired or congenital. The latter are classified according to size (small, medium, or giant), and the former are classified according to pathologic findings (common mole, Spitz, blue, and atypical/dysplastic).
Most common moles (nevi) do not require any specific treatment unless malignant transformation is suggested by enlargement, darkening pigmentation, or ulceration—“ABCD”: asymmetry, border irregularity, color variation, diameter greater than 6 mm. Palmar nevi were thought to be prone to malignant transformation, but this view has changed. Palmar nevi occur in 13% of white people and 29% of black people, making routine excision of nevi in these locations both impractical and unnecessary.
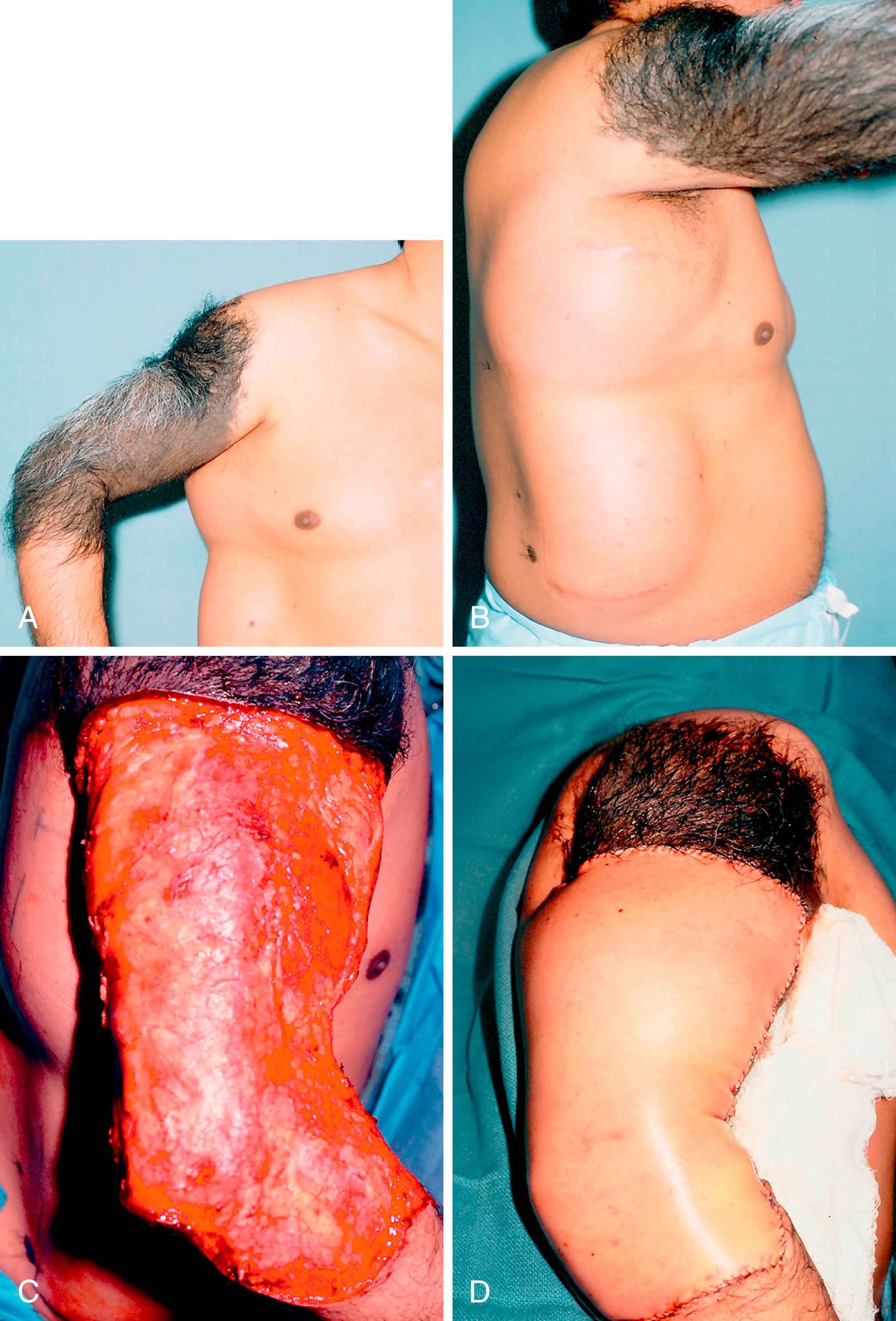
Giant hairy congenital nevi occur most often on the face and bathing trunk area, but they may occur on the upper extremity ( Fig. 58.12 ). It is unclear what the precise risk is for malignancy developing in giant congenital nevi. A systematic review suggests a range of 0% to 9%. The highest risk of malignant transformation with these lesions is actually in childhood and prepuberty, unlike the general incidence of melanoma, which is after puberty. Smaller congenital nevi are generally excised. However, excision of larger nevi may be less practical and may require use of skin expansion, flaps, or skin grafting to close the large wounds after excision. Where excision is not feasible, periodic cancer surveillance and biopsy of suspicious areas must be done.
With spindle (or Spitz ) nevus, which has also been called benign juvenile melanoma, the incidence of malignant transformation is no greater than with other compound nevi. The color ranges from pink to purplish-red, and the minority are darkly pigmented. Treatment is total excision.
Blue nevus contains dermal melanocytes that produce melanin, and unlike the intradermal nevus, these melanocytes also have dendritic processes. Dermal melanin scatters shorter wavelengths of light, giving a blue color. It is more commonly found on the dorsum of the hand as a solitary lesion and arises in adolescence or early adulthood. Malignant degeneration is unusual.
Dysplastic nevus syndrome occurs in both sporadic and familial forms ( Fig. 58.13 ). There are multiple atypical nevi varying from 10 to over 100, occurring mostly on the upper trunk. Lesions are often greater than 1 cm in diameter, with irregular and variegated color. Microscopic appearance is characteristic with large, atypical melanocytes that are spindle or epithelioid in type. The papillary dermis shows fibroplasia. It is impractical to excise all the lesions. Excision of a representative lesion confirms pathologic diagnosis. These patients must be carefully observed, and suspicious lesions excised.
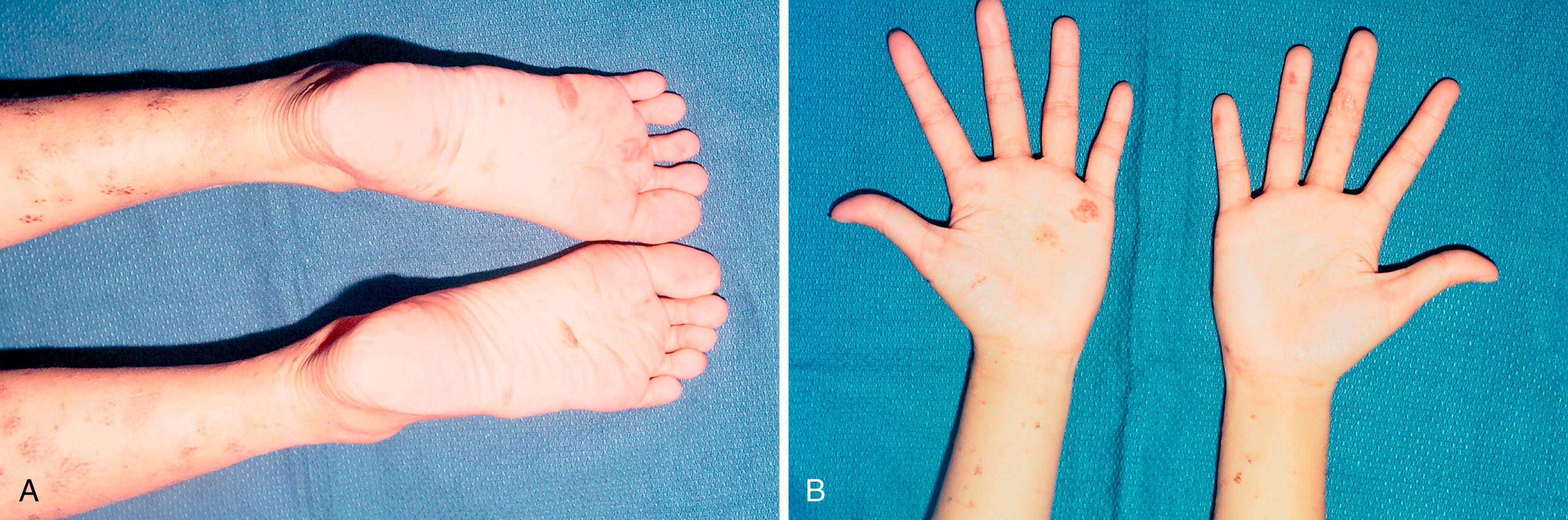
Acral nevi (nevi occurring on the thickened palmar skin) can have histologic findings that may mimic dysplastic nevi.
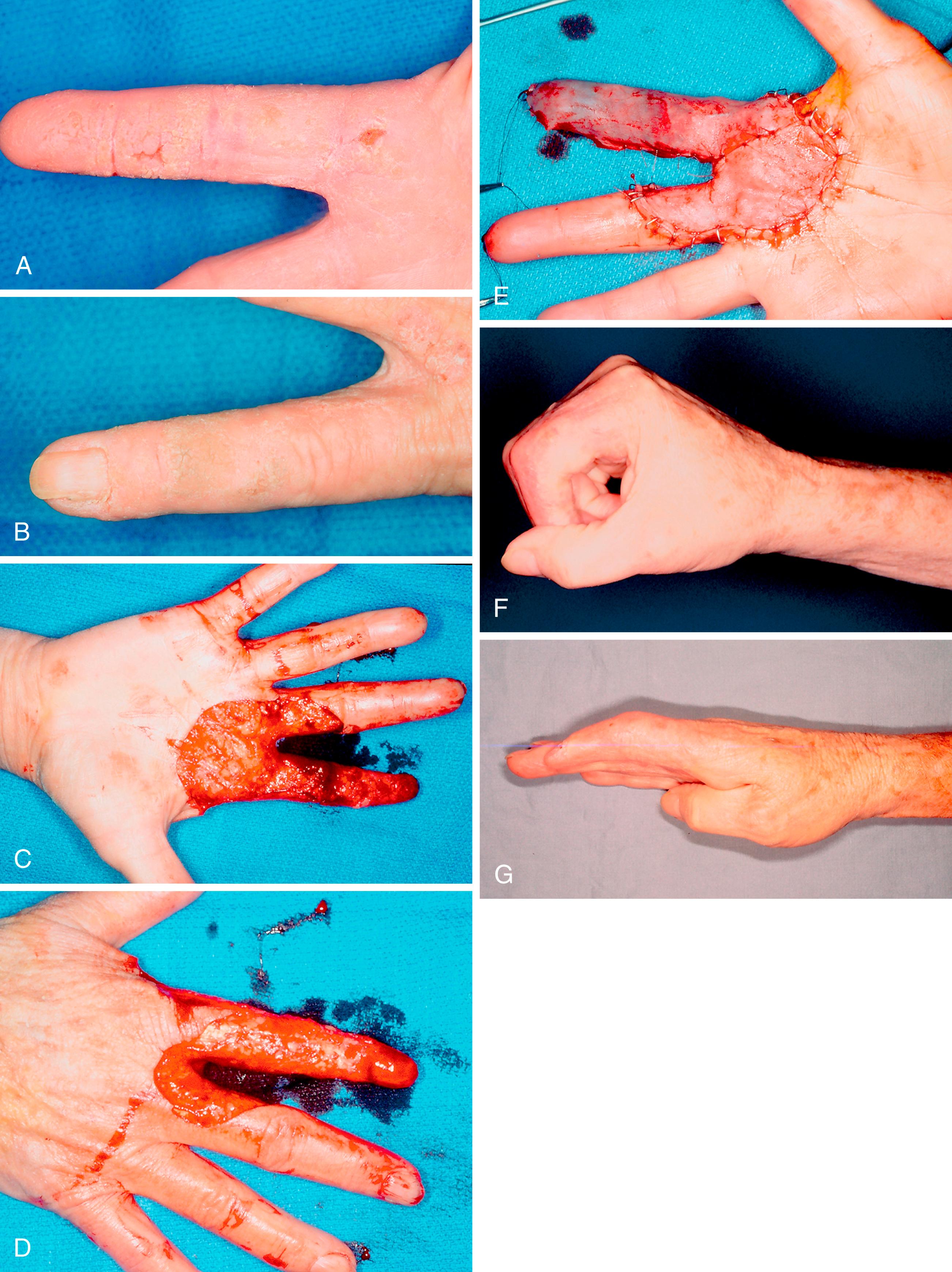
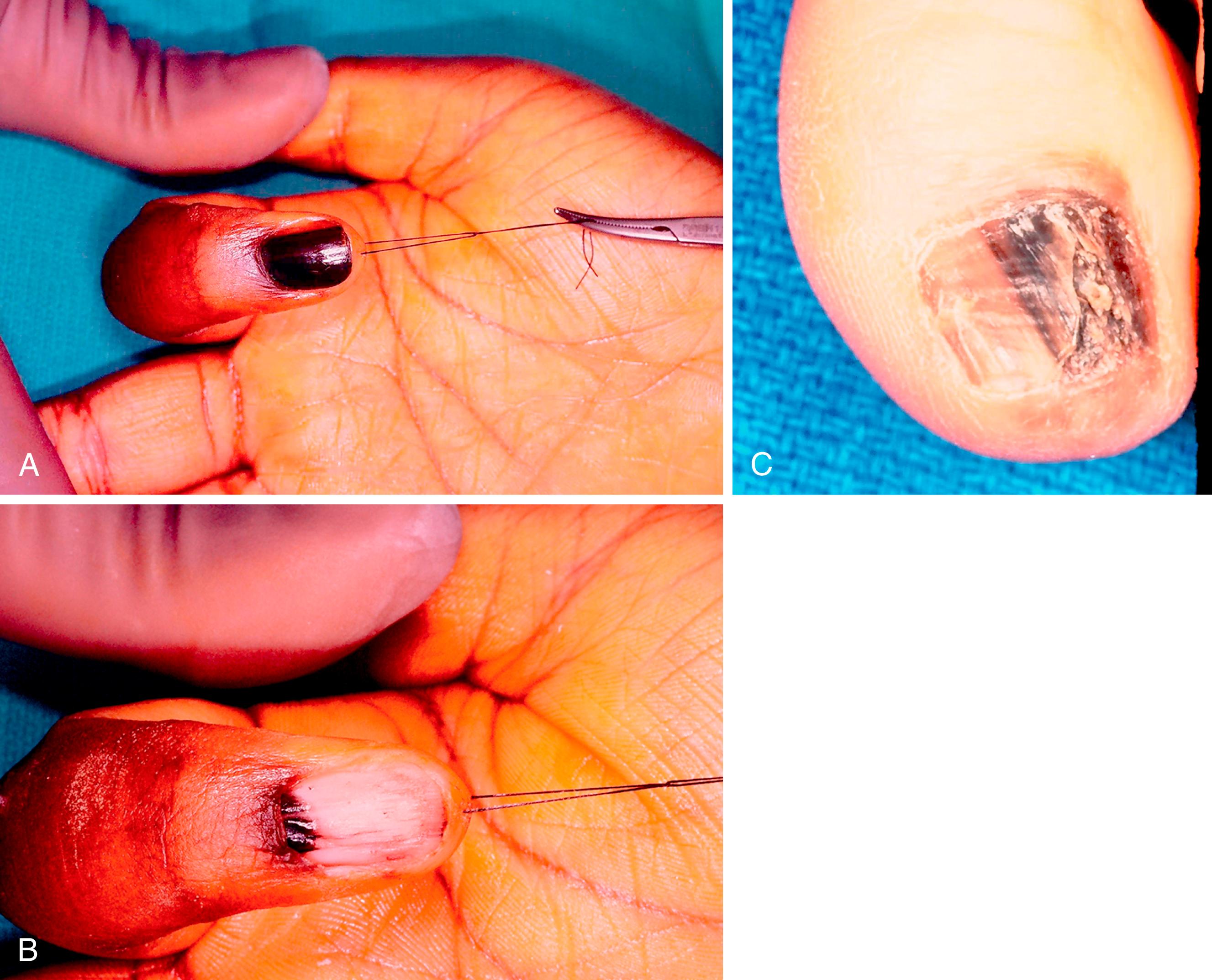
Longitudinal melanonychia refers to the dark streaking of the nail caused by melanin deposition in the nail plate. Acral nevi that involve the nail matrix can present in this way. In dark-skinned patients, longitudinal melanonychia is common and is simply caused by increased melanin production by normal melanocytes. A longitudinal full-thickness nail bed biopsy is required for a suspicious lesion after removal of the nail plate and with repair of the hyponychial nail fold and nail bed. Suspicious new lesions for potential melanoma include those in older patients (50 to 70 years), a longitudinal band over 3 mm in width, change in lesion color and size, involvement of a single digit, nail dystrophy, and extension to the periungual skin (Hutchinson sign) ( Fig. 58.14 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here