Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Recurrent infections, including those involving the skin, raise the possibility that a child has an immune deficiency. The most common cause of immunodeficiency in children is acquired immunodeficiency resulting from human immunodeficiency virus (HIV) infection (see Chapter 15 ). Less commonly, children with evidence of an immunodeficiency have an inherited disorder. Genetic immunodeficiency disorders may show a variety of cutaneous abnormalities, some of which are unique and characteristic of the disorder, and others, such as dermatitis, that are shared by other immunodeficiencies and other disorders.
It is beyond the scope of this chapter to review all immunodeficiencies, or even all that may manifest with mucocutaneous features. In addition, primary immunodeficiency disorders are frequently reclassified, with a major update most recently in 2015. Some of these immunodeficiencies are discussed elsewhere because of their manifestations. Wiskott–Aldrich syndrome and hyperimmunoglobulinemia E (hyper-Ig) syndrome are discussed in Chapter 3 due to the common presence of dermatitis. Chediak–Higashi and Griscelli syndromes, the silvery hair syndromes associated with immunodeficiency, are reviewed in Chapter 11 with the disorders of pigmentation. The telangiectasias that allow ataxia–telangiectasia to be distinguished from other forms of ataxia are described in Chapter 12 . Complement deficiencies are mentioned in Chapter 14 because individuals with a deficiency of a late complement component have an increased risk of neisserial infection, in Chapter 20 because hereditary angioedema (HAE) can be confused with angioedema, and in Chapter 22 because deficiency of the early components of complement may lead to a lupus-like disorder. Given its characteristic recurrent and recalcitrant candidal infections, chronic mucocutaneous candidiasis is reviewed in Chapter 17 .
Signs that should raise the suspicion of acquired or hereditary immunodeficiency are listed in Box 25.1 . Screening laboratory tests for a patient with recurrent cutaneous infections suspected of having an immunodeficiency are suggested in Table 25.1 . Although flow cytometry is increasingly used to identify immune cell deficiencies and diagnose specific subtypes of immunodeficiency, multicolor flow cytometry has recently been shown to reveal distinct populations among patients with a wide variety of immunodeficiencies, allowing early diagnostic screening beyond seeking cellular deficiencies.
History of infections
Increased frequency, severity, and duration
Unusual manifestations
Unusual infecting agents
Chronic infections, incomplete clearing
Poor response to appropriate agents
Severe viral infections
Recurrent osteomyelitis
Failure to thrive
Diarrhea, vomiting, malabsorption
Clues to specific types of immunodeficiency
Hematologic abnormalities (e.g., Wiskott–Aldrich syndrome)
Arthritis (e.g., Wiskott–Aldrich syndrome or early complement deficiency)
Paucity of lymph nodes (e.g., SCID) or lymphadenopathy (e.g., CGD)
Follicular-based pustules in a neonate (e.g., hyper-IgE syndrome)
Hepatosplenomegaly (e.g., CGD, Omenn syndrome, or Wiskott–Aldrich syndrome)
Oral ulcers (e.g., chronic granulomatous disease or hyper-IgM syndrome)
Poor wound healing (e.g., leukocyte adhesion deficiency)
Silvery hair (e.g., Chediak–Higashi or Griscelli syndromes)
Telangiectasia (e.g., ataxia-telangiectasia)
| Test | Primary Immunodeficiency |
| Complete blood count with differential, platelet count, examination of smear | Giant leukocyte granules (Chédiak–Higashi syndrome) Thrombocytopenia (Wiskott–Aldrich syndrome) Leukocytosis (chronic granulomatous disease and leukocyte adhesion deficiency) |
| Quantitative immunoglobulins | X-linked hypogammaglobulinemia (all Igs ↓) Transient hypogammaglobulinemia (all Igs ↓) PLAID * and APLAID syndromes (all Igs ↓) Common variable immunodeficiency (IgG↓, IgA↓ ±IgM↓) Selective IgA deficiency (IgA↓) Selective IgM deficiency (↓gM↓) Hypohidrotic ectodermal dysplasia with immunodeficiency (HED-ID) (±IgG↓ ±IgA↓ IgM↓) Hypogammaglobulinemia with hyper-IgM (Igs↓ IgM↑) Ataxia–telangiectasia (IgG2↓ IgG4↓ ↓IgA IgE↓) Wiskott–Aldrich syndrome (IgM↓, ±IgG↓ IgA↑ IgE↑) Hyperimmunoglobulin E (↑↑IgE) |
| Flow cytometry (T and B cells) | Severe combined immunodeficiency (lack of T cells ± B cells); specific subtypes have markers, such as γc chain in X-linked SCID Leukocyte adhesion defect (CD18 deficiency) Chronic granulomatous disease (deficient gp91 phox , p22 phox , p40 phox , p47 phox , p67 phox ) |
| Total hemolytic complement (CH50) | Complement deficiencies (marked reduction) |
| Quantitative dihydrorhodamine flow cytometry | Usually <10% (but can be up to 30%) of normal reduction in chronic granulomatous disease |
| Hair shaft examination | Small, regular melanin aggregations in Chediak–Higashi syndrome Large, irregular aggregations in Griscelli syndrome |
* PLAID: Phospholipase Cc2-associated antibody deficiency and immune dysregulation; APLAID: Autoinflammation and PLAID.
Children with deficiencies of immunoglobulins (Igs) primarily manifest with bacterial infections beginning at 3 to 6 months of age, at a time when transplacentally derived maternal Igs wane. , In general, the treatment of hypogammaglobulinemia is antibody replacement by intravenous infusions of immune serum globulin and vigorous antibiotic therapy.
The most common Ig deficiency is selective IgA deficiency, found in 1 in 500 individuals, of whom 10% to 15% show clinical manifestations. About 5% of patients with IgA deficiency have mutations in TACI , which encodes a tumor necrosis factor (TNF) receptor family member. B cells in individuals with TACI mutations have impaired isotype switching and do not produce IgA, and often IgG, in response to TACI ligand. The most common features are sinopulmonary bacterial infections and Giardia gastroenteritis. Approximately one-third of patients with clinical manifestations develop immune-mediated disorders, some of which involve the skin. These include an atopic-like dermatitis, lupus erythematosus with selective IgA deficiency linked to mutations in IFIH1 , vitiligo, recurrent candidal infections, lipodystrophy, and idiopathic thrombocytopenic purpura. Two percent of individuals with celiac disease have full or partial selective IgA deficiency; this subgroup of patients has more concomitant autoimmune disorders and may have persistent elevation of IgG serologies, even with disease improvement. The risk of allergy is also increased with selective IgA deficiency, including asthma, cow’s milk allergy, and allergic rhinoconjunctivitis.
In addition to their decreased levels of IgA, 30% to 50% of affected individuals have serum anti-IgA IgG antibodies, leading to a risk of fatal anaphylactic reactions upon administration of blood products with IgA-bearing lymphocytes; if intravenous immunoglobulin (IVIG) is necessary, only IVIG with low IgA content should be administered.
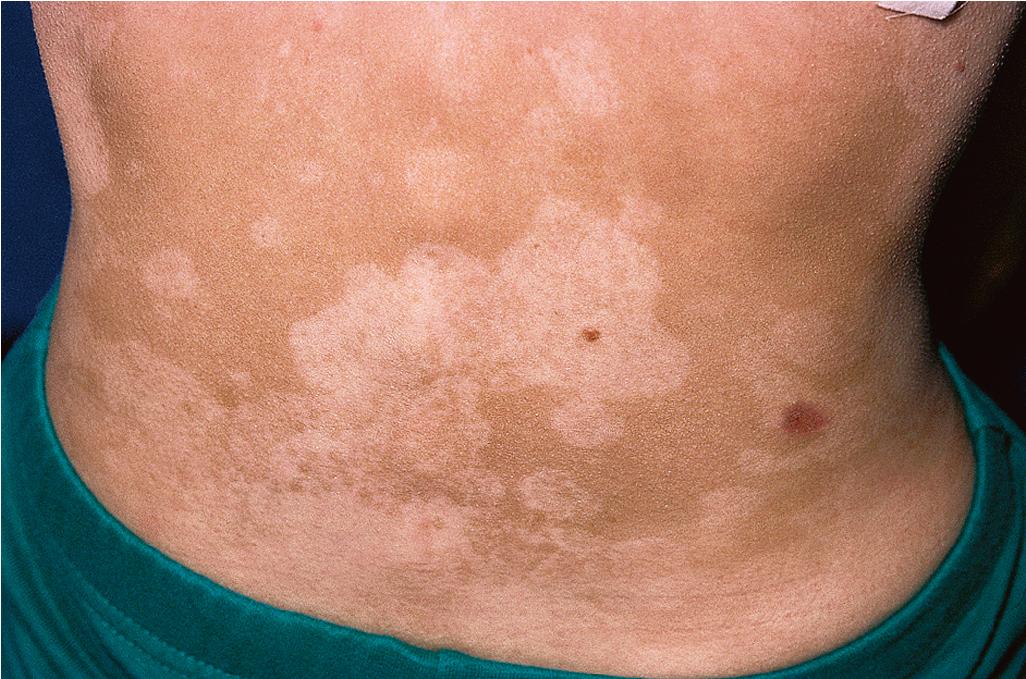
IgA deficiency may transition into (and is seen with increased incidence in families with) combined variable immunodeficiency (CVID), a heterogeneous group of disorders with decreased Ig levels (IgG, IgA, and sometimes IgM) and variable functional T-cell abnormalities. , Patients with CVID most commonly show pyodermas, extensive warts, widespread dermatophyte infections, and dermatitis. They are predisposed to pyogenic infections of the upper and lower respiratory tract, as well as gastrointestinal infections, particularly those caused by Giardia . Noncaseating granulomas of the lungs, liver, spleen, and/or skin have been described and are not associated with microorganisms. These granulomas have recently been shown to result from emergence of immunodeficiency-related viruses with altered immune, replication, and persistence properties from the live attenuated rubella vaccine, often persistent for decades subclinically. , Individuals with CVID also have an increased risk of autoimmune diseases, including vitiligo ( Fig. 25.1 ), alopecia areata, and vasculitis. The incidence of lymphoma is increased 400-fold and that of cancer overall 8- to 13-fold. CVID is primarily a disorder of adults, with the mean age of onset 23 to 33 years ; however, 25% of cases are diagnosed before 21 years of age, with a peak incidence in children aged 5 to 10 years and a minimum age of 4 years used to exclude patients with other primary immunodeficiency disorders. Death in patients with CVID usually results from lymphoma, hepatitis, respiratory insufficiency, or gastrointestinal disease.
Most cases of panhypogammaglobulinemia in children represent X-linked hypogammaglobulinemia (also called X-linked agammaglobulinemia ), a disorder caused by mutations in BTK , which encodes a tyrosine kinase that regulates the conversion of pre-B cells to B cells able to differentiate and produce Igs. Less commonly, pediatric patients may have a transient form during infancy with early failure to thrive, protracted diarrhea, sinopulmonary infections, pyodermas, and cutaneous abscesses, but with reversal when Ig is produced at 18 to 30 months of age. Approximately 10% have an autosomal recessive form of panhypogammaglobulinemia.
Boys with X-linked hypogammaglobulinemia develop recurrent bacterial infections in the first year of life and have an increased susceptibility to hepatitis B and enteroviral infections. Furuncles and cellulitis are the most common infections. An atopic-like dermatitis is common, and noninfectious cutaneous granulomas have been described. There is an increased predisposition to the development of pyoderma gangrenosum, which has been linked to Warthin–Starry-positive Helicobacter bilis infection; although difficult to grow, the Helicobacter organisms are detectable by polymerase chain reaction (PCR) and electrospray ionization time-of-flight mass spectrometry. A small percentage of patients develop a dermatomyositis-like disorder with slowly progressive neurologic involvement, usually related to echoviral meningoencephalitis. Up to 6% of patients develop lymphomas.
Patients with X-linked hypogammaglobulinemia with hyper-IgM (HIM) tend to have neutropenia and deficiencies of IgA, IgE, and IgG, but increased levels of IgM and isohemagglutinins. Rather than a primary B-cell defect, affected individuals have a primary T-cell defect. Cross-linking of CD40 on B cells induces switching of Ig classes from IgM to IgG, IgA, or IgE. Mutations in X-linked HIM lead to a dysfunction of the ligand for CD40 (CD40L). B cells from patients with HIM express functional CD40, but the T cells express the defective CD40 ligand and cannot bind CD40. , Three other forms of HIM are autosomal recessive; these result from deficiency of CD40 itself, activation-induced cytidine deaminase (AICD), or uracil-N-glycosylase (UNG), the latter two being signaling components downstream of the CD40 receptor that are critical to B-cell differentiation and class switching. A rare form of X-linked immunodeficiency sometimes associated with HIM is linked to hypohidrotic ectodermal dysplasia (see Chapter 7 ) and has been linked to mutations in the NEMO (IKBKG) gene, the same gene that is mutated in incontinentia pigmenti (see Chapter 11 ).
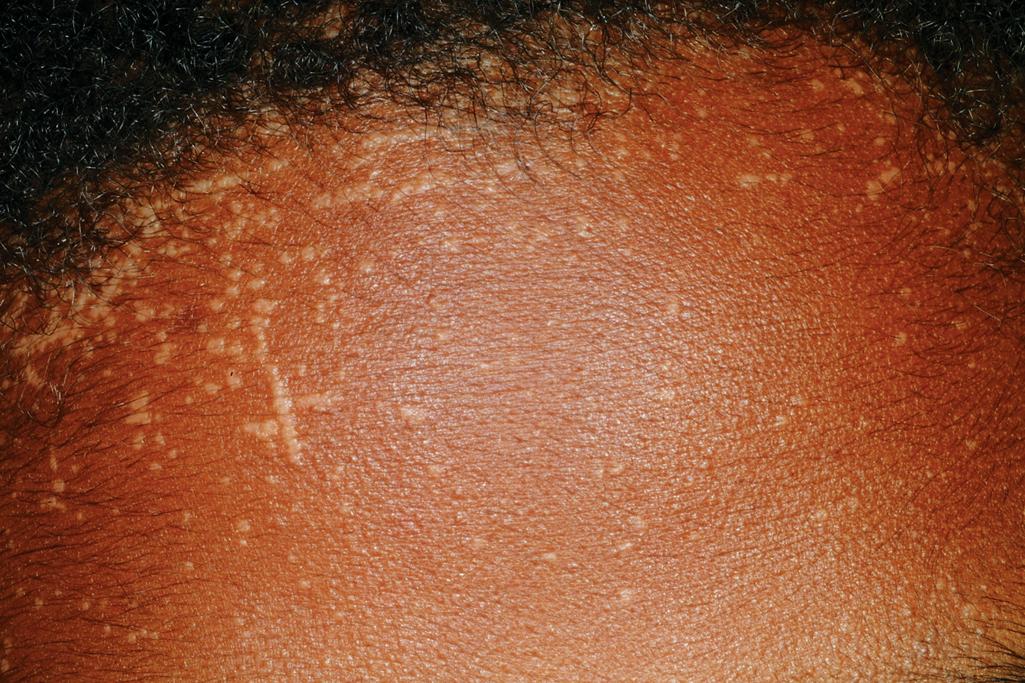
Patients with HIM often seek treatment during infancy with bacterial and sometimes fungal or opportunistic sinopulmonary infections (most common feature), pyodermas, and gastrointestinal infections (especially with opportunistic infections such as Cryptosporidium ), hepatosplenomegaly, cervical adenitis, autoimmune disorders (especially thyroiditis and hemolytic anemia), and an increased risk of lymphoma. , Numerous widespread warts ( Fig. 25.2 ), oral and perianal area ulcerations, and sclerosing cholangitis are additional features. Extensive warts are also a feature of w arts, h ypogamma g lobulinemia, i nfections, and m yelokathexis (WHIM) syndrome, which results from gain-of-function mutations in the chemokine receptor CXCR4 and has been treated with plerixafor, a selective antagonist of CXCR4. Additional disorders with T-cell impairment, including EVER1 and EVER2 deficiency, DOC8 deficiency (see Chapter 3 ), Netherton syndrome ( Chapter 5 ), GATA2 deficiency, TAOK2 deficiency, and STK4 deficiency, , also feature an increased risk of warts.
X-linked lymphoproliferative disease (XLP) is characterized by an abnormal response to Epstein–Barr virus infection because of mutations in either SH2DIA , which encodes signaling lymphocytic activation molecule (SLAM)-associated protein (SAP) (XLP-1) or XIAP (XLP-2), critical proteins for cytotoxic T-cell function. Affected boys are healthy until they first develop infectious mononucleosis during childhood or adolescence. Fever, pharyngitis, maculopapular rash, lymphadenopathy, hepatosplenomegaly, purpura, jaundice, and hemorrhagic colitis, and often hypogammaglobulinemia as well, are typical features. The virus stimulates a rapidly progressive B-cell lymphoma, often with superimposed bacterial sepsis, which leads to death in 70% of affected boys, especially with hemophagocytic lymphohistiocytosis and without transplantation.
Chronic granulomatous disease (CGD) is a group of disorders characterized by severe recurrent infections. These infections result from an inability of phagocytic leukocytes to generate oxidative metabolites and activate neutrophil granule elastase and cathepsin G, with the resultant inability to kill certain intracellular bacteria and fungi. , The estimated incidence is 1 in 200,000 births, approximately half of that of SCID. In all forms of CGD, the function of the nicotinamide dinucleotide phosphate (NADPH) oxidase complex is reduced. The disorder usually presents with acute bacterial infections, particularly suppurative lymphadenitis (classically from Serratia marcescens ) and recurrent pneumonias. Hepatosplenomegaly and lymphadenopathy may be associated. Some affected children present with inflammation, particularly with very early-onset inflammatory bowel disease. Patients develop granulomas, primarily of the lungs, liver, skin, and genitourinary and gastrointestinal tracts, as an abnormal immune response.
The most common form of CGD is X-linked recessive (two-thirds of cases) and results from mutations in CYBB , which encodes the membrane-bound component of NADPH oxidase cytochrome b-245 beta-chain or gp91 phox ( phox is short for phagocyte oxidase ); 90% of affected individuals are male. Autosomal recessive forms (25% to 30% of patients) are due to mutations in other components of phagocyte NADPH oxidase. The most common cause is mutations in NCF4 , encoding the cytoplasmic component p47 phox , but mutations in CYBA (encoding p22 phox , also called neutrophil cytochrome b light chain ) and NCF2 (also called NOXA2 ; encoding p67 phox ) have also been described. Patients with CGD commonly show leukocytosis, anemia, elevated erythrocyte sedimentation rate (ESR), and hypergammaglobulinemia. Skin tests for delayed hypersensitivity, phagocytosis, and chemotaxis are normal. Carrier and affected patients are often detected by quantitative dihydrorhodamine flow cytometry to measure dihydrorhodamine 123 (DHR) oxidase activity. The nitroblue tetrazolium (NBT) screening assay is now rarely performed (in which the oxidized yellow form of NBT is reduced to a blue formazan precipitate). Flow cytometry , and immunoblots can detect the selective loss of membrane phagocyte oxidase components. However, mutations that lead to deficiency of gp91 phox or p22 phox cannot be differentiated by these techniques because both are components of cytochrome b 558 and the entire cytochrome is absent if one component is deficient; thus gene sequencing is required.
The skin, lungs, and perianal area are most often the sites of infection. Early lesions are usually cutaneous staphylococcal pyodermas and abscesses of the face and perianal area, sometimes associated with purulent dermatitis and regional lymphadenopathy. Cutaneous lesions can resemble seborrheic dermatitis, Sweet syndrome, and scalp folliculitis; perioral and intraoral ulcerations may resemble aphthous stomatitis.
The organisms associated with CGD are usually catalase-positive and include Staphylococcus aureus and opportunistic gram-negative bacteria (especially Enterobacteriaceae Serratia and Klebsiella , as well as Pseudomonas ). These organisms all require oxidative metabolism (generation of superoxide anion and other reactive oxygen species) for intracellular killing by neutrophils, monocytes, macrophages, and eosinophils. Other organisms that may cause infection in patients with CGD with increased incidence are Aspergillus , Candida , Cryptococcus , Fusarium , and Nocardia . Bronchopneumonia and suppurative lymphadenitis are the most prevalent noncutaneous infections. These infections respond to appropriate antibacterial therapy and, in some cases, surgical drainage. The extracutaneous organs most commonly involved in CGD are the lymph nodes, lungs, liver, spleen, and gastrointestinal tract ( Box 25.2 ). Female carriers are at increased risk of infection if DHR activity is <20% and particularly <10%, leading to the suggestion that antimicrobial prophylaxis be used in these individuals as well.
Seen in more than 50% of patients
Lymphadenopathy, lymphadenitis
Hepatosplenomegaly
Bronchopneumonia
Failure to thrive
Seen in 25% of patients or less
Persistent diarrhea
Pleuritis or empyema
Septicemia or meningitis
Hepatic or perihepatic abscess
Osteomyelitis
Seen in less than 25% of patients
Perianal abscess
Periorificial dermatitis, folliculitis
Aphthous stomatitis
Cutaneous signs of systemic and discoid lupus
Ocular inflammation
Lung abscess
Peritonitis
Obstructive granulomas
Noninfectious inflammation is a common complication, especially in those with CYBB mutations. Approximately 40% of patients with X-linked CGD develop some form of inflammatory bowel disease. CGD has been misdiagnosed as Crohn’s disease because of the overlap in features (failure to thrive, diarrhea, abdominal pain, weight loss, perianal abscesses, ulcers and fistulas, bowel obstruction, anemia, hypoalbuminemia, and granulomatous colitis). , At least 50% of patients have noninfectious lung issues, especially during adulthood. Although lung infections are most common, inflammatory complications are also described (interstitial lung disease, chronic obstructive pulmonary diseases, lung nodules, and pleural effusions) and may be difficult to diagnosis without tissue evaluation. Granulomas are a classic noninfectious finding, particularly of the colon, stomach, and bladder. Chorioretinitis, uveitis, and ocular granulomas occasionally occur. In a recent study of patients who reached adulthood, 13% developed skin issues. These included granulomatous acne, inflammatory nodules, photosensitivity, cutaneous lymphocytic infiltration, and aphthous stomatitis. In fact, development of systemic or discoid lupus erythematosus is the most commonly seen autoimmune complication in affected individuals, including in those with a recessive form. Female carriers of CYBB mutations even more commonly show aphthous stomatitis, lupus-like eruptions, arthralgias, and photosensitivity. , In contrast to infection, these features have not been correlated with baseline oxidative burst function in carriers. Photosensitivity reactions have been described in patients administered voriconazole, and poor wound healing, including the development of pyoderma gangrenosum, has occurred postoperatively.
Antibiotic treatment of infection with surgical intervention as needed, as well as prophylactic trimethoprim–sulfamethoxazole and itraconazole therapy, have been used in most affected individuals. Short courses of systemic corticosteroids have been helpful for patients with obstructive visceral granulomas. Restoration of normal oxidase activity in ≥20% of circulating neutrophils markedly reduces the risk of infection. Stem cell therapy with replacement of defective neutrophils is better than conventional antimicrobial therapy with respect to fewer serious infections and surgeries, better growth, and improved quality of life , in patients with X-linked CGD. Transplantation should be performed as early as possible, given better outcomes, especially before the occurrence of CGD-related organ damage. The estimated survival of nontransplanted patients has been 88% at 10 years of age and 55% at 30 years old. In a recent study, the survival with unrelated donors was equivalent in outcome to parental (carrier) donors, suggesting that X-linked carrier donors should be avoided, given their increased risk of autoimmune and inflammatory issues. Targeted gene-editing technologies are under investigation preclinically, especially for patients with autosomal recessive CGD.
The leukocyte adhesion deficiencies (LADs) are a group of three autosomal recessive disorders that affect the ability of neutrophils, cytolytic T lymphocytes, and monocytes to be mobilized into extravascular sites of inflammation. LAD1 is most common and results from mutations in CD18, leading to deficiency or dysfunction of the β 2 subunit of integrins. The principal ligand for these integrins is ICAM1, which participates actively in neutrophil and monocyte chemotaxis and phagocytosis.
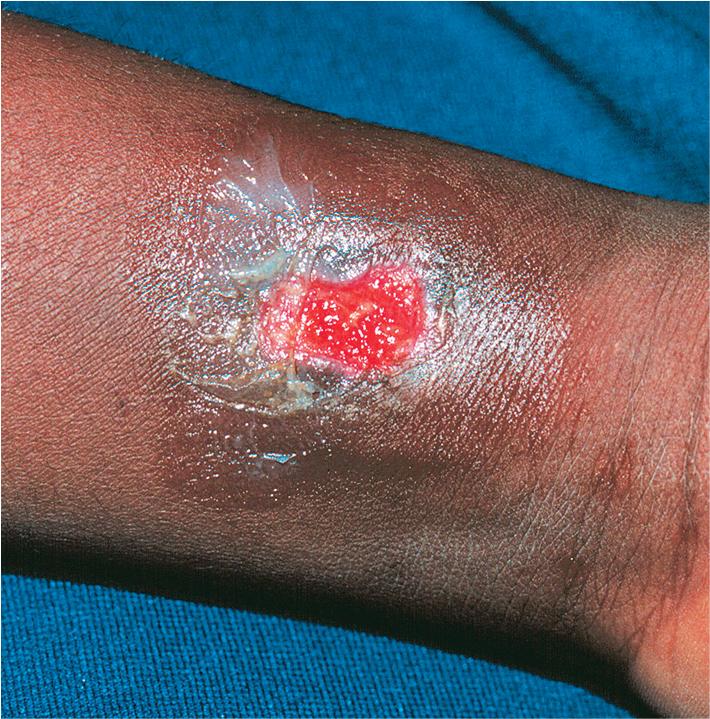
Patients with LAD have frequent skin infections (especially facial cellulitis and perianal infection), mucositis, and otitis, with a 5- to 20-fold increase in peripheral blood leukocytes. Poor wound healing is characteristic and leads to paper-thin or dysplastic cutaneous scars. Minor skin wounds may become large ulcerations that resemble pyoderma gangrenosum ( Fig. 25.3 ), especially if secondarily infected. Periodontitis is a typical feature and if, severe, may lead to loss of teeth. Bacterial and fungal infections may be life threatening. Delayed separation of the umbilical cord is a common historic clue to the diagnosis. Psoriasis has been described in affected individuals with mild disease. More than 75% of patients with severe disease die by 5 years of age; more than half of the patients with moderate deficiency die between the ages of 10 and 30 years.
A second form of LAD (LAD2) is an autosomal recessive disorder caused by mutations in FUCT1. FUCT1 encodes a GFP-fucose transporter that is required for formation of sialyl-Lewis X, a ligand for selectins on the surface of neutrophils. In addition to their elevated leukocyte counts and recurrent bacterial infections, patients have short stature, a distinctive facies, and developmental delay. A third type of LAD (LAD3), which features a bleeding tendency, is caused by a mutation in FERMT3, which encodes kindlin-3 and prevents activation of β1, β2, and β3 integrins. Dermal hematopoiesis, leading to the “blueberry muffin” presentation, has been described in affected infants and children. Petechiae and mucosal hemorrhage are common, and mucosal bleeding is the presenting sign in more than 70% of patients.
Therapy of the soft-tissue infections includes antimicrobial agents and, as appropriate, debridement of wounds. Scrupulous dental hygiene reduces the severity of the periodontitis. Death usually occurs by 2 years of age in patients with severe LAD unless successful bone marrow transplantation or cord blood transplantation is performed. Oral fucose has been helpful for some patients with LAD2.
SCID is a group of disorders with similar clinical manifestations and immune dysfunction but different biochemical, cellular, and molecular features ( Table 25.2 ). , Newborn screening for SCID using the T-cell receptor excision circle (TREC) assay to detect neonatal T-cell lymphopenia has allowed much earlier detection and is now the most common means of detecting SCID in the United States. TREC assays also allow detection of “leaky” SCID in which some T cells are present with limited function.
| Disorder | Gene | Diagnostic Tests | Cells |
| ADA deficiency | ADA | Red cell ADA levels and accumulation of toxic metabolites | T − /B − /NK − |
| AK2 deficiency | AK2 | Defective survival of hematopoietic precursors | T + /B + /NK − |
| Artemis deficiency | Artemis | Defects in V(D)J recombination; increased sensitivity to radiation | T − /B − /NK + |
| CD8 deficiency | CD8 | CD8 expression deficiency | T + /B + /NK + (CD8 − ) |
| CARD11 deficiency | CARD11 | CARD11 expression; abnormal IL-12 production, Treg-cell deficiency | T + /B + /NK + |
| CD45 deficiency | CD45 | CD45 expression | T − /B + /NK + |
| IL-7 receptor deficiency | IL-7 receptor α | IL-7 receptor α expression | T − /B + /NK + |
| JAK3 deficiency | JAK3 | JAK3 expression/signaling | T − /B + /N −− |
| MHC class I deficiency | TAP1 and TAP2; TAPBP; B2M | MHC class I expression | T + /B + /NK + (CD8 − ) |
| MHC class II deficiency | CIITA RFX5 RFXAP |
HLA-DR expression | T + /B + /NK + (fewer CD4 + ) |
| Moesin deficiency | MSN | Deficient moesin expression | T − /B − /NK − |
| PNP deficiency | PNP | Red cell PNP levels and metabolites | T − /B − /NK − |
| RAG deficiency * Omenn syndrome | RAG1 and RAG2 | Defects in V(D)J recombination; T- and B-cell clonal analysis | T − /B − /NK + |
| T-cell receptor deficiency | CD3g | CD3 expression | T − /B + /NK + |
| X-linked SCID † | Common γ chain | γ c expression by FACS | T − /B + /NK − |
| ZAP70 deficiency | ZAP-70 | ZAP-70 expression | T − /B + /NK − |
^ For current classification (2015), see Picard C, Al-Herz W, Bousfiha A, et al. Primary immunodeficiency diseases: An update on the classification from the International Union of Immunological Societies Expert Committee for Primary Immunodeficiency 2015. J Clin Immunol 2015;35:696–726.
* Most common autosomal recessive form (15% of autosomal recessive types have biallelic RAG1 mutations).
Based on newborn screening, the prevalence of SCID is now estimated to be 1 in 50,000 births and the percentage with the X-linked recessive form has decreased from approximately 50% to only 19%, although it still the most common form. This X-linked recessive form results from mutations in the gene that encodes the γ c chain, a component of several interleukin receptors, which is critical for T-cell and natural killer (NK)-cell function. Recombination activating gene (RAG) proteins RAG1 or RAG2 mediate deoxyribonucleic acid (DNA) double-strand breaks that allow variable (diversity) joining (V[D]J) recombination and Ig diversity, and their deficiency results in Omenn syndrome (15% of SCID). Mutations in adenosine deaminase (ADA1) occur in 10% of neonates and lead to accumulation of adenosine, which is toxic to lymphocytes.
Infants may present with a generalized seborrheic-like dermatosis, a morbilliform eruption, or exfoliative erythroderma with alopecia. Extensive cutaneous inflammation is a characteristic of 98% of infants with Omenn syndrome, a subset of SCID with reticuloendothelial cell proliferation. Patients with Omenn syndrome typically show hepatosplenomegaly (88%), lymphadenopathy (80%), alopecia (57%), eosinophilia, and a high serum IgE level. Oral and genital ulcers are characteristic of defects in patients with an Artemis mutation, especially in Athabaskan-speaking American Indian children. Multicentric dermatofibrosarcoma protuberans (DFSP) and pulmonary alveolar proteinosis have been described in association with ADA deficiency. Recurrent varicella and bacterial infections are seen in the recently described form related to deficiency of moesin. Profound T-cell deficiency has also more recently been related to biallelic loss-of-function mutations in CARD11 (or its signaling partners BCL10 and MALT1), leading to recurrent and severe infections; monoallelic missense mutations in CARD11 lead to abnormal T-cell responses with susceptibility to infection, multiorgan atopy (including atopic dermatitis and urticaria), and autoimmunity (lichen sclerosis, cutaneous necrotizing granulomatous inflammation). Major histocompatibility complex (MCH) class I deficiency may manifest with vasculitis and/or pyoderma gangrenosum.
Acute graft-versus-host disease (GVHD) from maternal cell engraftment or nonirradiated transfusions should also be considered in an infant with an extensive cutaneous eruption. Biopsy will allow differentiation. A more chronic form of GVHD (often with the acute form in utero ) may also present as lesions that resemble lichen planus, lamellar ichthyosis, or scleroderma (see Graft-Versus-Host Disease section).
Recurrent infections, diarrhea, and failure to thrive are apparent by 3 to 6 months of age. The most common early infections are candidal infections and pneumonia caused by bacteria, viruses, or Pneumocystis jiroveci . Patients with SCID usually lack tonsillar buds, a thymus, and palpable lymphoid tissue despite recurrent infections. Nearly all patients with SCID have a profound deficiency of T lymphocytes and a low absolute lymphocyte count. Patients are further classified by the results of fluorescent activated cell sorter (FACS) analysis into those with B lymphocytes (T−/B+ SCID) and those without B lymphocytes (T−/B− SCID). Further subclassification can be made according to the presence or absence of NK cells (see Table 25.2 ). However, it is now clear that many immunodeficiency disorders have cells that are differentiated into these subtypes but do not function normally, leading to immune dysregulation, infection, and sometimes cancer. The specific diagnosis is confirmed primarily by direct genetic analysis and flow cytometric analysis of peripheral blood mononuclear cells with antibodies directed against specific proteins missing from the cell surface, such as JAK3 or γ c.
Early diagnosis of SCID is critical, preferably before the administration of live vaccines, nonirradiated blood products, and, in countries where applicable, bacille Calmette–Guérin (BCG). Patients with SCID have a high risk of BCG-associated complications (34% disseminated and 17% localized) and subsequent death, especially if they are given the vaccination at 1 month of age or younger and with T-cell numbers of 250/mcL or less at diagnosis. The natural outcome for SCID is poor, and most patients die by 2 years of age without intervention.
Hematopoietic stem cell transplantation in infants is the treatment of choice and leads to 5-year survival of 95% of infants if performed in the first 3 months of life, regardless of donor or conditioning regimen. Most deaths after transplantation occur during the first 2 to 5 years after transplantation and result from infections. Bone marrow–derived CD34+ cells transduced with the SIN-γ c modified γ-retrovirus vector without preparative conditioning has led to T-cell recovery without evidence of leukemia after up to 3 years of follow-up in boys with X-linked SCID. Enzyme replacement therapy (ERT) is available for ADA SCID, both as a bridge to definitive treatment and in addition to stem cell transplantation; however, it is costly, requires weekly to biweekly intramuscular injections, and has a decreasing effect on lymphocyte counts with time, likely due to the development of anti-ADA antibodies. Even definitive therapy with transplantation does not prevent late complications or comorbidities, including the continued need for immunoglobulin injections (up to 58%), chronic human papillomavirus infections (up to 25%), chronic gastrointestinal issues (especially poor feeding and diarrhea, 20%), sinopulmonary infections (20%), growth retardation ("15%), and neurocognitive issues, especially attention-deficit/hyperactivity disorder. There is a high risk of autoimmunity (especially hemolytic anemia) and GVHD.
Children who undergo transplantation are at risk for several cutaneous complications. Most common among these are infections, drug reactions (given the large number of prescribed medications), GVHD, and the increased risk after transplantation of skin cancer.
GVHD should be distinguished from host-versus-graft reaction , which occurs when a graft or transplant (e.g., skin, heart, or kidney) is placed in a normal individual and the host’s circulating immune cells react to the foreign tissue, causing graft or transplanted tissue destruction. In GVHD, the reverse happens. , GVHD most commonly occurs in children with malignancy who receive hematopoietic stem cell transplantation after radiation and chemotherapy or in immunodeficient children who receive nonirradiated blood products. Reactions to transfusions often occur 7 to 10 days after the transfusion. Nevertheless, GVHD may be advantageous in patients with hematologic malignancies, helping to clear host neoplastic cells (e.g., graft-vs.-leukemia). In utero GVHD may occur in immunodeficient babies exposed to maternal antigens. GVHD also uncommonly occurs in recipients of solid organ transplants (most often small bowel and liver transplants) and can be fatal. In these patients, 87% have skin involvement, typically described as a “maculopapular rash.” Although poorly described morphologically, the eruption occurs an average of 48 days after transplantation, is rarely pruritic, and is associated with a mortality rate of 72% from sepsis and multiorgan failure. Patients who receive autologous transplants may have a mild cutaneous form of aGVHD that occurs 1 to 3 weeks after transplantation and resolves spontaneously.
GVHD was previously classified as acute (aGVHD) or chronic (cGVHD) based on the threshold of before or after 100 days posttransplant. However, specific clinical features that define acute vs. chronic disease do not necessarily agree with the timing after transplantation. In addition, changing practices that affect recipient immune status, among them the introduction of reduced-intensity conditioning, donor lymphocyte infusions, and second allogeneic stem cell transplants, have contributed to the changes in GVHD presentation. Classification now includes (1) classic aGVHD; (2) persistent, recurrent, or late-onset aGVHD (beyond 100 days after transplantation); (3) classic cGVHD; and (4) an overlap syndrome with features of both aGVHD and cGVHD. The latter two types can present any time, including before 100 days after transplantation ( Table 25.3 ). It is recognized that the pathomechanism of aGVHD vs. cGVHD is distinct. In aGVHD, the immune dysregulation results from stimulation of the host’s antigen-presenting cells by activated donor mature T cells (especially Th1 lymphocytes) in host target organs, amplifying T-cell activation and expansion, and culminating in target organ apoptosis and dysfunction. cGVHD, however, occurs by a complex mechanism that includes activation initially of innate immune responses, followed by more chronic inflammation mediated by adaptive immune activation (T and B cells) and ultimately the production of profibrotic cytokines by activated T cells. The risk of GVHD is greater in patients given a transplant from a partially matched family donor or an unrelated volunteer than an allogeneic transplant from a human leukocyte antigen (HLA)-identical donor.
| Type of GVHD | Time after Transplantation | Features |
| Acute GVHD (aGVHD) | ||
| Classic | ≤100 days | aGVHD only |
| Persistent, recurrent, or late onset | >100 days | aGVHD only |
| Chronic GVHD (cGVHD) | ||
| Classic | Any | cGVHD only |
| Overlap syndrome | Any | aGVHD + cGVHD |
The most common cutaneous manifestation of aGVHD is an erythematous to violaceous macular or papular (morbilliform) eruption, which often begins on the ears, face, neck, palms, and soles and then becomes generalized ( Figs. 25.4 to 25.6 ). The cutaneous eruption may become confluent and desquamate. Variants are ichthyotic, eczematous, psoriatic, pityriasis rubra pilaris–like, or diffuse and perifollicular. Distribution along Blaschko lines has been reported. , Mild to moderate aGVHD most often presents with a nonspecific cutaneous eruption and thus can be confused with infection (especially viral), drug reaction, and reactions related to the transplant (e.g., the self-limited eruption of lymphocyte recovery; engraftment syndrome, characterized by associated fever and vascular leak with weight gain, edema, ascites, pulmonary infiltrates, and hypotension ; and toxic erythema of chemotherapy, which is painful and usually localized to the palms, soles, and intertriginous areas). Severe aGVHD may resemble exfoliative erythroderma or toxic epidermal necrolysis (TEN). The TEN associated with aGVHD must be differentiated from TEN associated with a drug eruption based on time course and drug exposure. Eosinophilic pustular folliculitis has recently been described as another posttransplantation eruption, which can be confused with aGVHD but occurs 2 to 3 months after transplantation and responds well to topical corticosteroids.
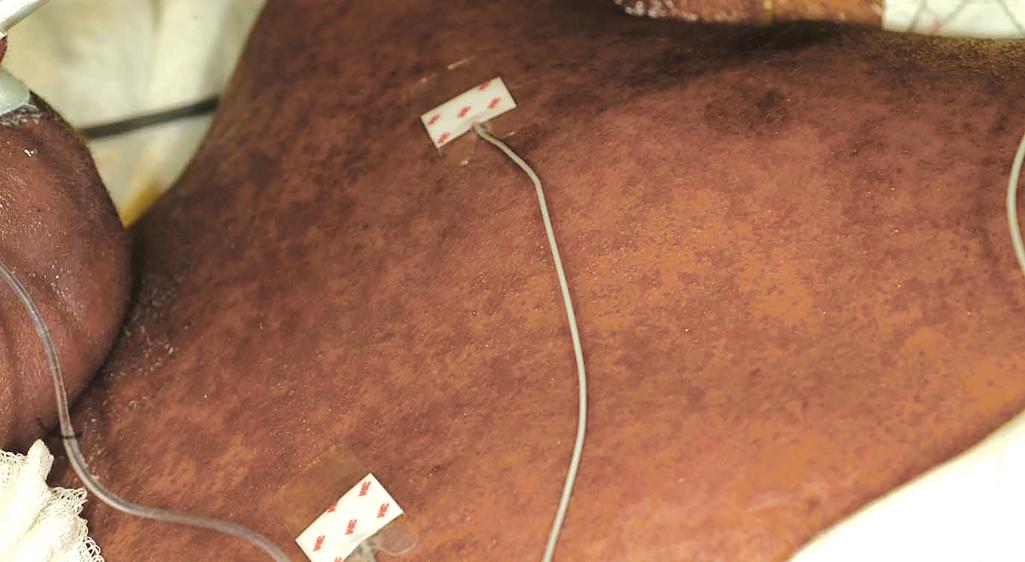
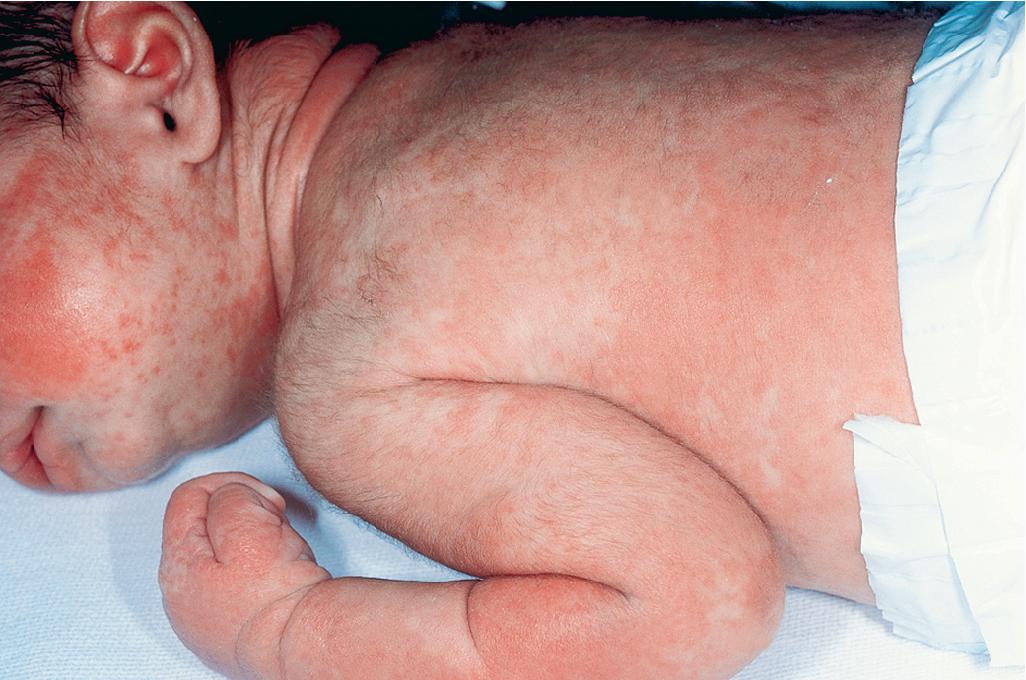
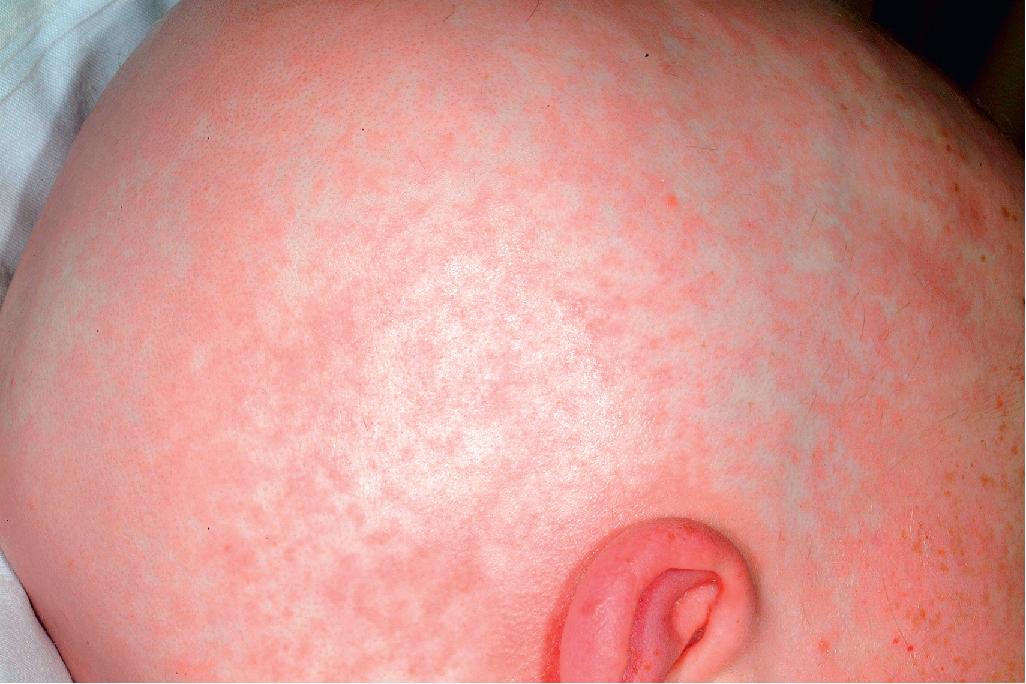
aGVHD usually involves the skin, gastrointestinal tract, and liver (in that order of incidence). Patients with aGVHD may complain of nausea or crampy abdominal pain and have watery or bloody diarrhea, hepatomegaly, and hepatic function abnormalities. Many patients are anorexic and have fever. Staging depends on the extent of specific organ involvement (of skin, liver, and gut), and grading reflects the staging of each organ collectively. Staging of skin in aGVHD is based on extent (stage 1: <25%; 2: 25% to 50%; 3: >50%; and 4: generalized erythroderma, usually with bulla formation). Grade 1 aGVHD involves stage 1 or 2 skin involvement and no organ involvement, whereas grade 4 aGVHD requires stage 4 skin and liver involvement.
Abnormalities seen in biopsy specimens reflect the clinical severity of the cutaneous manifestations and range from nonspecific changes, to basal keratinocyte vacuolization with scattered necrotic keratinocytes surrounded by lymphocytes (the characteristic “satellite cell necrosis”), to severe epidermal necrosis. As such, biopsies are not helpful for patients with milder disease and do not tend to affect treatment decisions. Laboratory tests may show eosinophilia and elevation of bilirubin and hepatic transaminase levels. aGVHD grading predicts prognosis and is based on extent and degree of organ system involvement, as well as percentage of body surface area involved by the aGVHD.
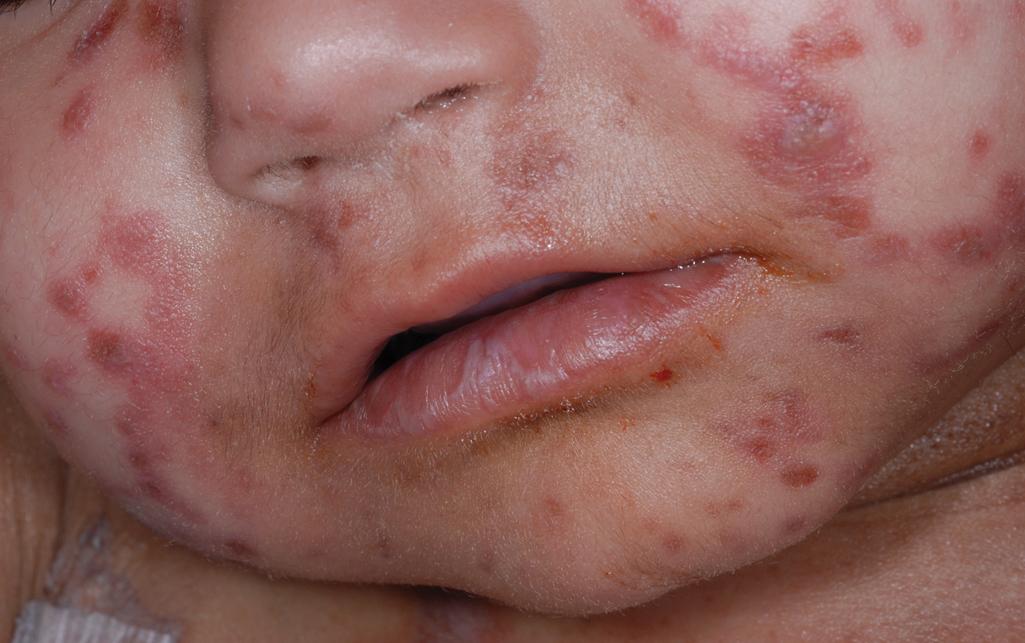
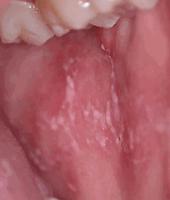
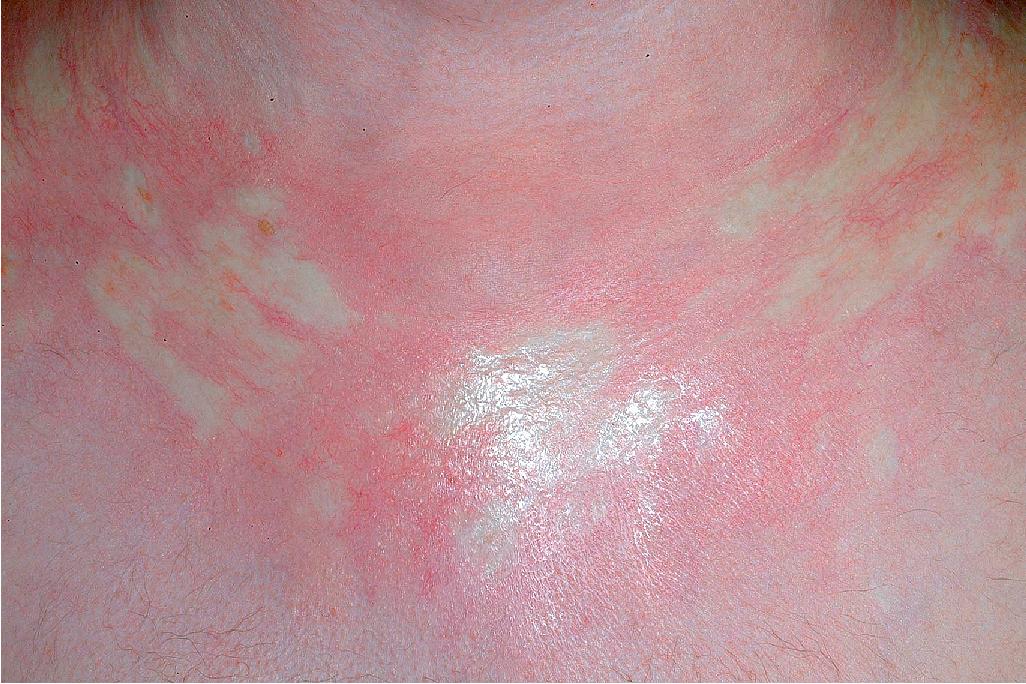
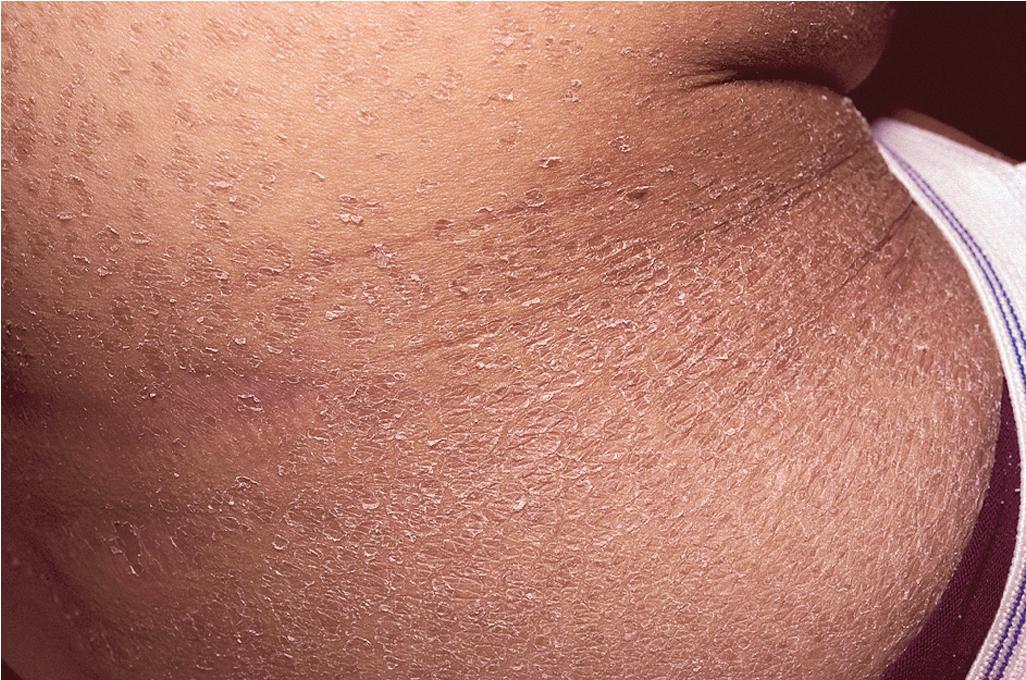
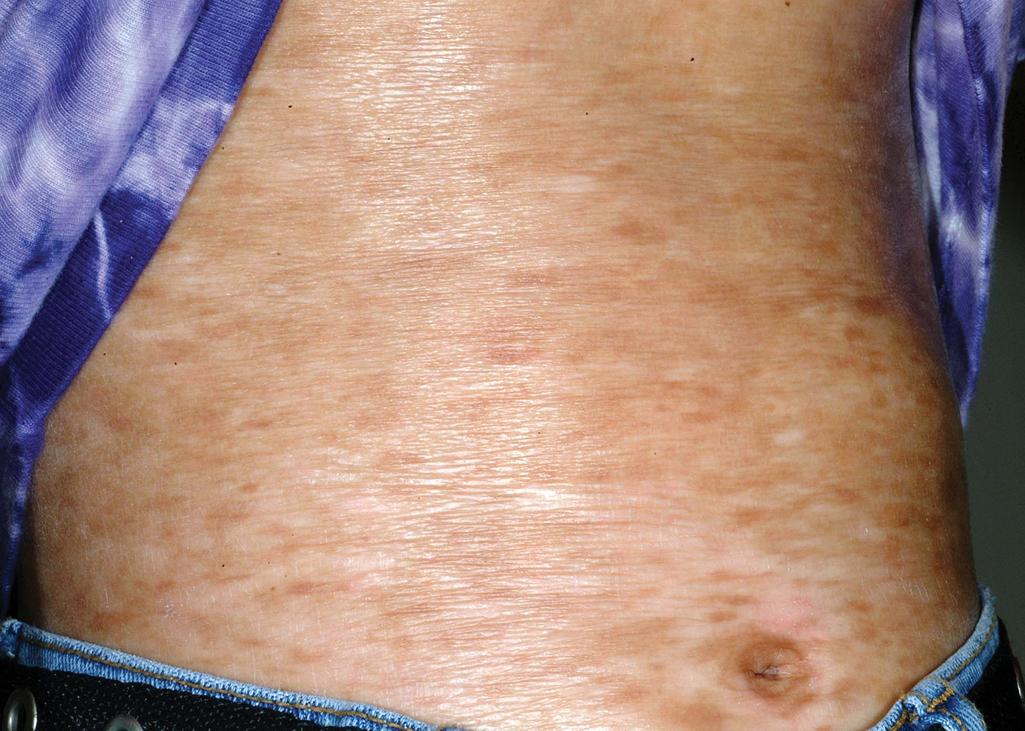
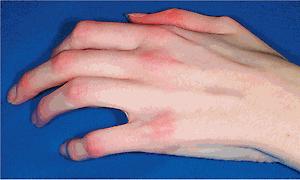
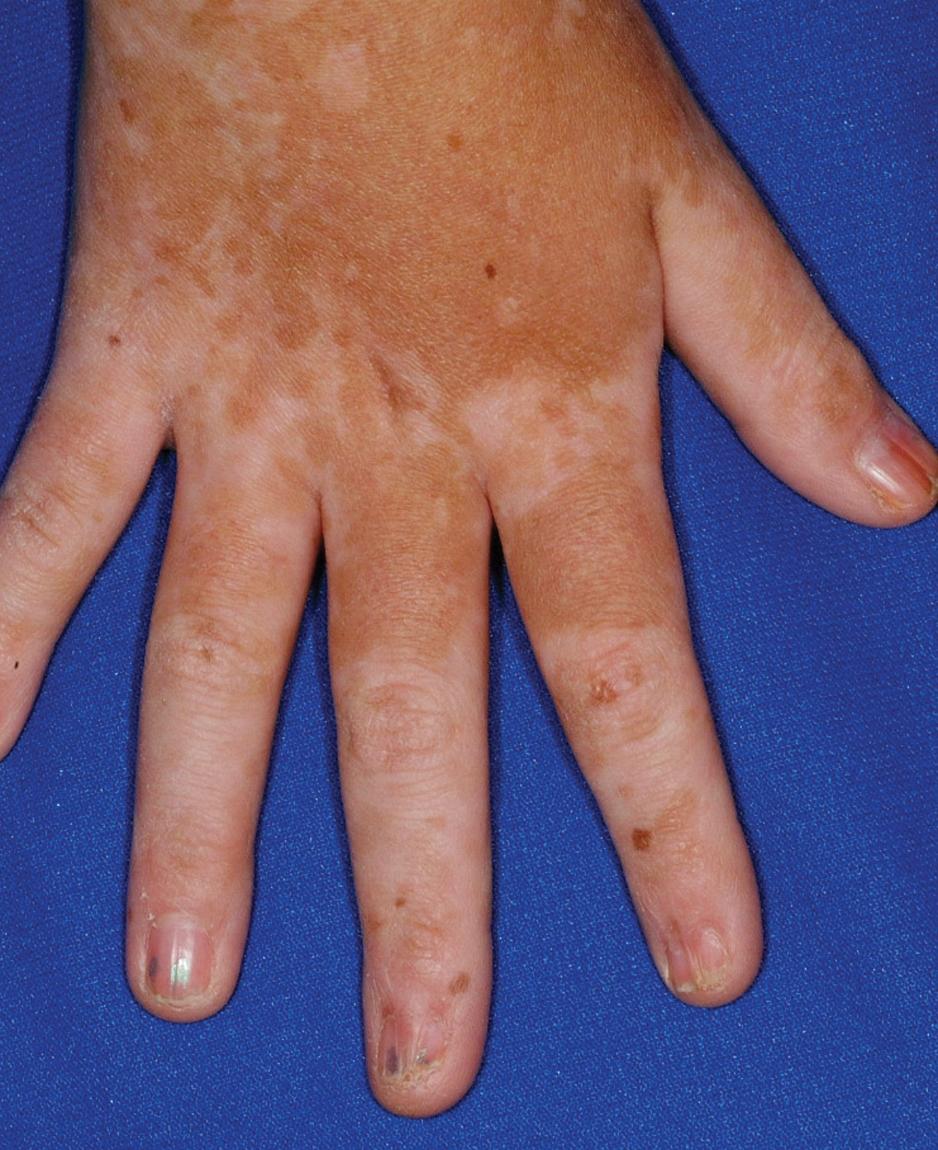
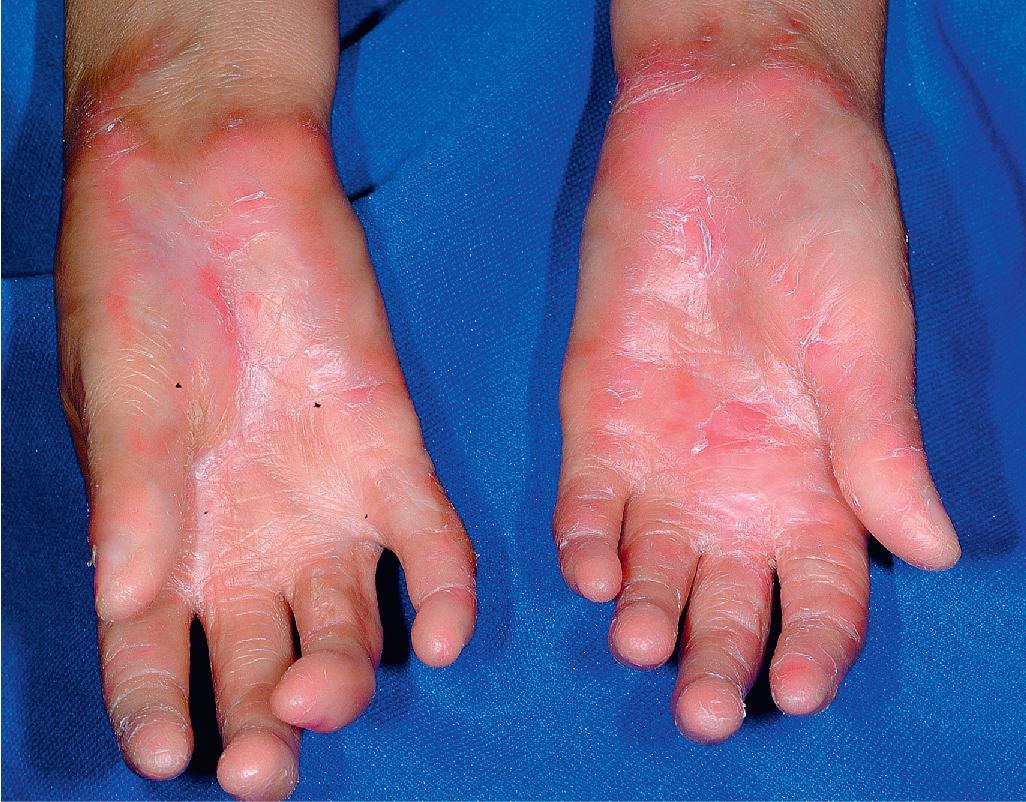
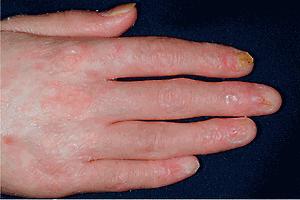
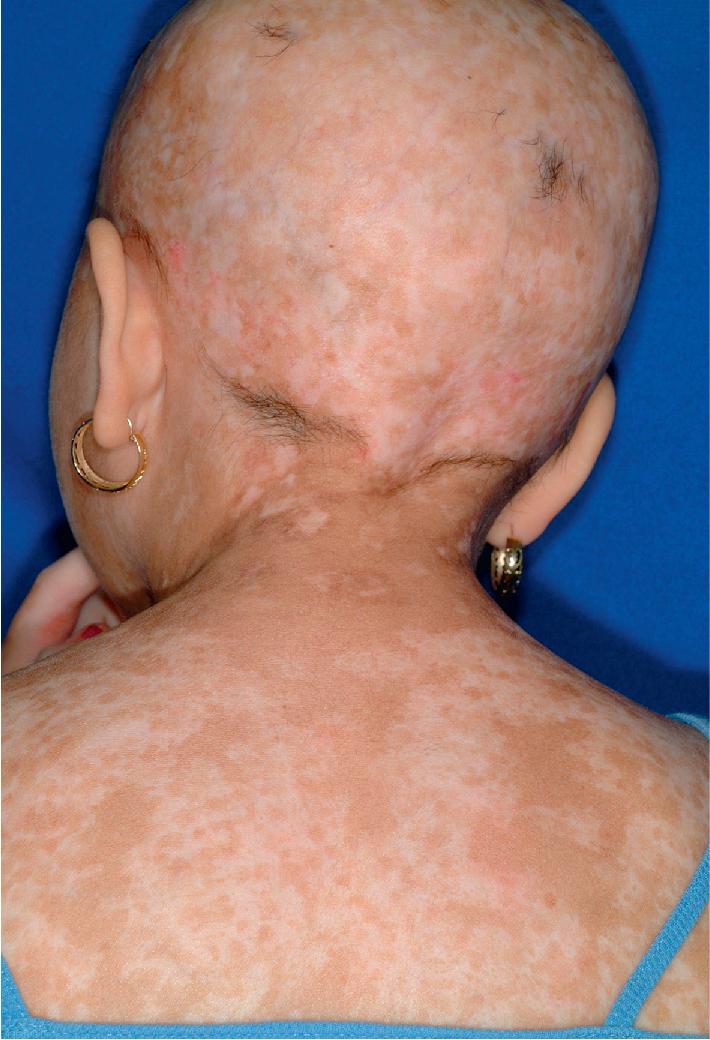
cGVHD often resembles autoimmune disorders and, in contrast to aGVHD, can affect virtually any organ, leading to significant morbidity with decreased quality of life and overall survival ( Table 25.4 ). Early lesions of cGVHD most commonly resemble lichen planus with flat-topped violaceous papules and plaques (including mucosal changes of lichen planus) ( Figs. 25.7 and 25.8 ), which is the most common manifestation (see Chapter 4 ). Lichenoid lesions may be localized to Blaschko lines or to the site of previous herpes zoster infection. Some patients with cGVHD manifest lichen sclerosus ( Fig. 25.9 ). Early features may also be nonspecific, manifesting as xerosis with or without follicular prominence or ichthyotic ( Fig. 25.10 ). Eczematous and psoriatic variants of cGVHD are rarely seen. , Later changes of cGVHD include generalized xerosis, patchy dyspigmentation, and sclerodermatous changes , , ( Fig. 25.11 ); joint contracture ( Figs. 25.12 and 25.13 ); vitiligo ( Fig. 25.14 ); progressive poikiloderma (characterized by reticulated hypopigmentation and hyperpigmentation, atrophy, and telangiectasia); ulcerations ; keratoderma (see Fig. 25.13 ); and nail dystrophy, sometimes with pterygium formation ( Fig. 25.15 ; see Fig. 25.14 ) resembling that of dyskeratosis congenita (see Chapter 7 , Fig. 7.69 ). Alopecia that may be diffuse (and sometimes permanent), localized (areata), androgenetic, or cicatricial ( Fig. 25.16 ) has been noted, and permanent alopecia often relates to the use of a conditioning regimen with busulphan. Disseminated porokeratosis has also been described after stem cell transplantation and may respond to acitretin. The many autoimmune changes associated with cGVHD likely reflect reduced Treg cells.
| Location | Characteristic Features | Other Features |
| Skin | Lichen planus–like Poikilodermatous Lichen sclerosis–like, including vaginal scarring, stenosis Sclerotic (or lichen sclerosus–like) |
Depigmentation Hypopigmentation and hyperpigmentation Ichthyotic changes Genital erosions, ulcers, fissures Erythema, maculopapular rash Pruritus Hypohidrosis Keratosis pilaris |
| Hair | Alopecia, nonscarring or cicatricial; can be diffuse Premature graying |
|
| Nails | Ridging, onycholysis, loss, pterygium | |
| Oral | Lichen planus–like | Mucosal atrophy Xerostomia Ulceration Mucocele Keratotic plaques Sclerosis with restricted opening |
| Eyes | Dry eyes Cicatricial conjunctivitis Keratoconjunctivitis sicca Punctate keratopathy |
|
| Joints/muscle | Contracture from sclerosis Fasciitis, arthritis |
Myositis |
| Gastrointestinal | Chronic diarrhea Upper esophageal strictures, stenosis, webbing Hepatomegaly |
|
| Lungs | Bronchiolitis obliterans Interstitial fibrosis |
Affected individuals may have dry eyes (as in Sjögren syndrome; see Chapter 22 ), conjunctivitis/keratitis, and desquamative esophagitis with stricture formation. Weight loss can result from dysphagia and mucositis. Other manifestations are chronic diarrhea, hepatomegaly, lymphadenopathy, myositis, arthritis, pleural and pericardial effusions, and pulmonary fibrosis. cGVHD is subclassified as mild, moderate, or severe based on the number of organs involved, the presence of pulmonary involvement, and resultant disability. ,
Patients with cGVHD show evidence of immune dysfunction and have recurrent infections. The majority of patients with cGVHD have eosinophilia and hypergammaglobulinemia. Many have thrombocytopenia and increased titers of a wide variety of autoimmune antibodies, especially antinuclear antibodies (ANAs). Liver function testing may show evidence of cholestasis. Pulmonary function tests are abnormal in approximately 50% of patients, and chest radiographs may reveal interstitial fibrosis. Biopsies of skin lesions show changes consistent with the clinical picture. For example, patients with lichenoid lesions have the histologic changes of lichen planus (see Chapter 4 ), often with satellite cell necrosis. Patients with sclerosis tend to show band-like inflammation at the dermal–epidermal junction and dermal sclerosis with obliteration of adnexae. Direct immunofluorescence analysis of skin specimens often shows the linear deposition of immunoreactants at the dermal–epidermal junction.
cGVHD occurs in 6% (matched sibling cord blood) to 65% (matched unrelated donor peripheral blood stem cell transplants) of pediatric patients; in one recent study, cGVHD occurred in 18% of all transplanted children, with 90% showing lichenoid lesions and 30% developing sclerosis. Although patients may develop cGVHD without preceding aGVHD, having aGVHD that resolves or progresses directly is a major risk factor for development of cGVHD (11-fold increased risk), emphasizing the importance of preventing aGVHD. Other risk factors are an unrelated or mismatched donor, use of peripheral blood stem cells, older age of donor or recipient, use of total body irradiation, male recipient with a female donor, and malignant disease. For patients with myeloablation (full-intensity conditioning, which eliminates all malignant and marrow hematopoietic cells), prophylaxis usually involves 2 to 12 months of tacrolimus (or cyclosporine), with or without a short course (about 1 month) of methotrexate. With reduced-intensity conditioning before transplant, which does not fully eradicate malignant and hematopoietic cells and instead relies on immunologic antihost and antitumor effects, prophylaxis often involves administration of oral tacrolimus and mycophenolate mofetil (MMF) (or corticosteroid). In both situations, anti–T-cell serotherapy with either antithymocyte globulin or alemtuzumab (Campath) is usually added for unrelated donor transplants and also reduces graft rejection; for haploidentical donors, T-cell depletion or CD34 selection is often performed. Environmental isolation, bowel rest through hyperalimentation, irradiation of blood products, and prevention of infection are other protective measures.
The mortality rate of aGVHD ranges from 12% for mild disease to 55% for severe aGVHD, usually because of infection. The mortality of cGVHD is 25%, usually from infection, hepatic failure, and malnutrition. Decreased survival of patients with cGVHD has been associated with thrombocytopenia, progressive onset, extensive cutaneous involvement, gastrointestinal involvement, and a low Karnofsky performance status at diagnosis.
Topical corticosteroids, with or without topical calcineurin inhibitors, are used for grade I disease. Ultraviolet (UV) light (preferably, nbUVB and UVA1) may also be helpful for skin-predominant aGVHD. If grade II through IV aGVHD develops, the first-line therapy is continuing prophylactic immunosuppression (optimizing calcineurin inhibitor levels) and adding intravenous methylprednisolone at 2 mg/kg per day. Steroids are tapered when there is a clinical response, given the high risk of adverse events with continued use of high doses. For skin GVHD, topical steroid and/or tacrolimus may be added, with oral administration of budesonide for gastrointestinal GVHD. Patients who fail to respond to corticosteroid alone after 5 to 7 days (60% to 70% of patients) can be treated with salvage therapy, such as MMF, anti-TNF antibodies (e.g., infliximab), mammalian target of rapamycin (mTOR) inhibitors (e.g., sirolimus/rapamycin), anti-interleukin (IL)-2 antibodies (e.g., daclizumab or basiliximab), , antithymoglobulin, the combination of an anti-IL2 and an anti-TNF antibody, or extracorporeal photopheresis (ECP). Responses in general are poor if initial steroid treatment is not successful and the risk of infection is high. If at least two second-line treatments fail, alternatives are alemtuzumab, methotrexate, pentostatin, , and mesenchymal stem cells. ,
Similar regimens have been used to treat cGVHD, although there is no proven standard. Prednisone and calcineurin inhibitors are the most commonly used first-line therapies. The mean duration of therapy for patients with cGVHD is 3 years, with about 50% of patients able to discontinue therapy 5 years after transplantation. Second-line treatment (steroid resistant or steroid sparing) includes one of more of the following: ECP, rituximab, imatinib mesylate (may increase range of motion with sclerotic cGVHD), MMF, mTOR inhibitors, and low-dose methotrexate. ECP has been associated with cutaneous response rates of 60% to 80% and the ability to reduce the dose of systemic corticosteroid. , ECP works by collecting white blood cells, exposing them to 8-methoxypsoralen, and treating with UV light before reinfusing in an effort to apoptose cells but activate regulatory T cells. Disadvantages of ECP are the time required for each treatment, the frequency of required therapy, and the potential for large volume shifts in small children. Overall, the 5-year survival rate is 50% for those who respond to therapy for GVHD and 30% for those with an incomplete or no response.
Anecdotal reports of responses in pediatric patients with cGVHD to systemic ruxolitinib have also been described. However, responses are variable (31% failure rate) and toxicity is high. Targeting concurrently the IFNγ and IL-6 receptors by JAK inhibition (baricitinib more than ruxolitinib) has been shown to both prevent and reverse established GVHD in a mouse model while enhancing graft-versus-leukemia, a therapeutic benefit of GVHD. Ibrutinib, an inhibitor of Bruton tyrosine kinase that prevents and treats cGVHD in mouse models, , was recently shown to treat cGVHD in an early phase human trial.
In addition to use of topical antiinflammatory agents, mucocutaneous manifestations of GVHD may improve from administration of thalidomide or acitretin. Although nbUVB has been used for cutaneous sclerotic GVHD, UVA1 phototherapy is a favored intervention, because its longer wavelength penetrates more deeply. Phototherapy is not immunosuppressive, but its long-term effects are unknown. Supportive intervention for cGVHD includes supplemental nutrition (usually parenteral), scrupulous care of wounds, physical therapy to prevent joint contractures and disability, artificial tears, cutaneous emollients and sunscreens, and prophylaxis against Pneumocystis infection with trimethoprim–sulfamethoxazole. Routine skin surveillance is important for transplanted patients, not only to monitor for GVHD but also for the potential later development of mucocutaneous squamous cell carcinomas, which occurs more frequently in individuals who have had cGVHD. , Education about the risk of skin cancer and self-examination is also of importance. Overall, 13% to 55% of cancers after organ transplantation involve the skin. Squamous cell carcinomas and basal cell carcinomas occur more often than Merkel cell carcinoma and melanoma. In one study, 20% of transplanted pediatric patients developed nonmelanoma skin cancer at an average of 17.2 years after renal transplantation.
Anorexia nervosa and bulimia nervosa are severe disorders associated with potentially serious medical complications ( Box 25.3 ). Anorexia nervosa occurs in up to 3% of pubertal females and less commonly in prepubertal females or males. In both disorders, patients have an intense preoccupation with food and an irrational concern about gaining weight. Bulimic patients tend to binge eat and attempt to lose weight by vomiting and abusing laxatives, diuretics, diet pills, or emetics. The cutaneous features, which reflect endocrinologic changes and malnutrition, usually occur when the body mass index drops to less than 16 kg/m 2 . , Affected individuals often show self-induced lesions as well, particularly the characteristic superficial ulcerations and hyperpigmented calluses or scars on the dorsal aspect of the second and third fingers (Russell sign), the result of repeated abrasion of the skin against the maxillary incisors during forced emesis. , Other emesis-associated complications include recurrent sore throat, hoarse voice, and dental erosions. Nutritional deficiency, such as zinc deficiency, scurvy, and pellagra, have occurred, as has erythema ab igne from chronic use of a heating pad for warmth. , Because of the potential severity of these disorders, it is important that physicians recognize the early signs and symptoms, particularly in view of the fact that patients tend to deny their illness or minimize the severity of their symptoms. The cutaneous manifestations are reversible when weight is gained. Many patients respond well to the combination of antidepressant and cognitive behavioral therapy.
Xerosis
Generalized pruritus
Loss of subcutaneous fat
Carotenemia
Acne
Petechiae and purpura
Edema, especially pretibial and pedal
Calluses on the dorsum of the hand (Russell sign)
Other self-inflicted injuries
Poor wound healing
Pellagra
Scurvy
Acrodermatitis enteropathica
Seborrheic dermatitis
Acral changes
Periungual erythema
Acrocyanosis
Acral coldness
Perniosis
Oral changes
Cheilitis/perlèche
Aphthous stomatitis
Gum recession
Enamel erosion and dental caries
Hair changes
Telogen effluvium
Increased lanugo-like body hair
Dry hair
Brittle, dystrophic nails
Enlarged parotid and salivary glands (full cheeks despite malnutrition)
Heating pad–induced erythema ab igne
Innate immunity is activated in response to infection or tissue injury, including in skin. Neutrophils, mast cells, macrophages, and NK cells are key effectors of the innate immune system, although T and B lymphocytes, which are more typically associated with adaptive immunity, may contribute. Autoinflammatory disorders are characterized by recurrent episodes of inflammation in affected organs because of excessive antigen-independent innate immune activation. These bouts of inflammation are not associated with infection, high-titer autoantibodies or antigen-specific T cells, or allergy. Many of these disorders have recurrent fevers, and the originally characterized autoinflammatory disorders were called periodic fever syndromes.
Monogenic autoinflammatory disorders are the best characterized forms and have been classified as inflammasomopathies, interferonopathies, ubiquitinopathies/relopathies, or others ( Table 25.5 ). In general, these disorders first manifest during childhood, although the onset may be in adults with milder or atypical forms. Cutaneous features may include urticaria, pustules, papulosquamous lesions (psoriatic- or pityriasis rubra pilaris–like), neutrophilic dermatosis, acne, hidradenitis suppurativa/sterile abscesses, pyoderma gangrenosum, eczematous lesions, granulomatous lesions, purpura with vasculitis, livedo, lipodystrophy, nonspecific cutaneous inflammatory eruptions, and aphthous ulcers ( Table 25.6 ). Systemic inflammatory manifestations include fever, arthritis, uveitis, serositis (peritonitis, pleuritic), and/or meningitis. Some patients may have organ enlargement (lymphadenopathy, splenomegaly) and amyloidosis with chronic, long-term disease.
| Disorder | Inheritance | Genetic Defect | Gene Product | Associated Features |
|---|---|---|---|---|
| Inflammasomopathies | ||||
| Familial Mediterranean fever | AR | MEFV | Pyrin (marenostrin) | Recurrent fever of short duration (2–3 days) Serositis with abdominal pain, pleuritis, and chest pain Pericarditis, scrotal swelling, splenomegaly, erysipelas-like eruption in minority of patients High risk of renal amyloidosis if untreated Good response to colchicine, IL-1 blockade |
| TNF receptor–associated periodic syndrome (TRAPS) | AD | TNFRSF1A | P55 TNF receptor | Recurrent fevers that are prolonged (1–3 weeks) Serositis, rash, conjunctivitis, periorbital edema, arthritis 10%–25% incidence of renal amyloidosis Response to etanercept and IL-1 blockade |
| Mevalonate kinase deficiency (MVK)/hyper-IgD syndrome (HIDS) | AR | MVK | Mevalonate kinase | Early onset (usually first year) Periodic fever lasting 4–5 days Urticarial eruption (>90%), abdominal pain with vomiting and diarrhea, arthritis Headache, hepatosplenomegaly, painful cervical adenopathy, aphthous stomatitis, leukocytosis, high levels of IgD Response to steroids, NSAIDs, simvastatin, IL-1 blockade Often improves by adulthood Amyloidosis rare + |
| Familial cold autoinflammatory syndrome (familial cold urticaria) * | AD | NLRP3/ClAS1 | Cryopyrin | Cold-induced nonpruritic urticaria, arthritis, fever and chills, leukocytosis Responds to IL-1 blockade |
| Muckle–Wells syndrome * | AD | NLRP3/CIAS1 | Cryopyrin | Recurrent urticaria, sensorineural hearing loss, amyloidosis Responds to IL-1 blockade |
| Neonatal onset multisystem disease (NOMID) * † | AD | NLRP3/ClAS1 | Cryopyrin | Neonatal onset urticaria, chronic aseptic meningitis, arthropathy and bone deformities, fever, ocular changes and hearing loss Responds to IL-1 blockade |
| NLRC4-activating mutations | AD | NLRC4 | NLRC4 | Fevers, macrophage activating syndrome or erythematous plaques, and arthritis |
| NLRP1-activating mutations | AD | NLRP1 | NLRP1 | Fevers, spiky hyperkeratoses or keratosis lichenoides chronica–like lesions, arthritis, autoimmune disorders |
| NLRP12-activating mutations | AD | NLRP12 | NLRP12 | Cold urticaria (FCAS2), aphthous stomatitis, abdominal discomfort, and diarrhea |
| Periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis (PFAPA) syndrome | Likely polygenic inheritance | Unknown | Unknown | Periodic fevers, aphthous stomatitis, pharyngitis, and cervical adenitis, malaise, headache |
| Pyogenic sterile arthritis, pyoderma gangrenosum, and acne (PAPA) syndrome | AD | PSTPIP1/C2BP1 | PSTPIP1/C2BP1 | Pyogenic (sterile, destructive) arthritis, pyoderma gangrenosum, acne, myositis |
| PASH, PAPASH, PsAPASH (see column at right for definitions) | AD | PSTPIP1 or NCSTN in some cases of PASH | PSTPIP1/C2BP1 or nicastrin | PASH combines PG, acne, hidradenitis suppurativa/HS; PAPASH either combines PAPA and PASH or adds psoriasis; PsAPASH combines psoriatic arthritis |
| Interferonopathies | ||||
| STING-associated vasculopathy with onset in infancy (SAVI) syndrome | AD | TMEM173 | STING | Acral telangiectatic vasculopathy with soft-tissue loss, sometimes large pustules Fevers, adenopathy, interstitial lung fibrosis May respond to JAK inhibitor |
| Chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE) syndrome; also called proteasome-related autoinflammatory syndrome (PRAAS) (see Chapter 20 ) | AR | PSMB8 Less often PSMB4, PSMB9, PSMA3 |
Proteasome subunit β type | Chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (fever), lymphadenopathy, purpuric patches and nodules, arthralgias Same as Nakajo–Nishimura syndrome May respond to JAK inhibitor |
| Aicardi–Goutieres syndrome | AD, AR | 7 genes; see Chapter 20 | 7 gene products; see Chapter 20 | Pernio, arthralgias, positive antinuclear antibodies, fevers, neurologic issues vary in severity but often result in profound intellectual disability and/or encephalopathy, developmental regression, dystonia, and spasticity |
| Ubiquitinopathies/relopathies | ||||
| LUBAC component deficiency | AR | HOIL-1L, SHARPIN, HOIP | HOIL-1L, SHARPIN, HOIP | Autoinflammation, immunodeficiency, myopathy; lymphangiectasia in HOIP deficiency |
| Otulipenia (otulin-related auto-inflammatory syndrome [ORAS]) | AR | OTULIN | Otulin | Fever, diarrhea, panniculitis, arthritis, failure to thrive |
| Haploinsufficiency A20 | AD | TNFAIP3 | A20 | Familial Behçet disease; mucosal ulcers, arthritis, vasculitis |
| Others | ||||
| Blau syndrome | AD | NOD2/CARD15 | CARD15 | Onset usually <5 years old Granulomatous dermatitis, uveitis, synovitis Good response to TNF inhibitors; skin eruption responds to macrolides, especially erythromycin |
| Majeed syndrome | AR | LPIN2 | Lipin-2 | Multifocal osteomyelitis, congenital dyserythropoietic anemia, inflammatory dermatosis Responds to IL-1 blockade |
| Deficiency of the IL-1 receptor antagonist (DIRA) | AR | IL1RN | IL-1 receptor antagonist | Fetal distress, joint swelling with periosteal inflammation, severe osteopenia, lytic bone lesions, respiratory involvement, thromboses, aphthae, pyoderma gangrenosum, pustulosis Responds to IL-1 blockade |
| Deficiency of the IL-36 receptor antagonist (DITRA) | AR | IL36RN | IL-36 receptor antagonist | Usually generalized pustules, fever and malaise May have palmoplantar pustulosis or AGEP-like disorder Nail dystrophy, arthritis, cholangitis |
| CARD14-mediated pustular psoriasis (see Chapter 4 ) | AD | CARD14 | CARD14 | Pityriasis rubra pilaris, plaque psoriasis, erythroderma with pustular psoriasis; AGEP Usually responds to ustekinumab or Th17 inhibition May respond partially to methotrexate TNF inhibition |
| PLAID syndrome (phospholipase Cγ2-associated antibody deficiency and immune dysregulation) | AD | PLCG2 | Phospholipase Cγ2 | Evaporative cold urticaria, acral ulcerations, granulomatous plaques, hypogammaglobulinemia, infections, sometimes allergic and autoimmune disorders |
| APLAID syndrome (Autoimmunity + PLAID) | AD | PLCG2 | Phospholipase Cγ2 | Neonatal-onset vesiculopustules, cutaneous granulomas, acral hemorrhagic blisters; immunodeficiency with recurrent infections; enterocolitis; arthralgias; deafness |
| Deficiency of adenosine deaminase 2 (DADA2) | AR | ADA2 | Adenosine deaminase 2 | Livedo reticularis to PAN-like systemic vasculopathy, early-onset fevers; vasculitis of CNS (strokes), intestine, liver, kidneys; hypogammaglobulinemia; blood cell deficiencies |
* One of the cryopyrin-associated periodic syndromes (CAPS).
+ Amyloidosis is unusual in Blau, HIDS, PAPA, and PFAPA syndromes.
† Also called chronic infantile neurologic cutaneous and articular (CINCA) syndrome.
| Mucocutaneous Feature | Autoinflammatory Disorder/Gene |
| Urticaria | FMF/ MEFV NOMID/CINCA (CAPS)/ NLRP3 Muckle-Wells (CAPS)/ NLRP3 Mevalonate kinase disorders/ MVK TRAPS/ TNFRSF1A |
| Cold urticaria | FCAS/ NLRP3 FCAS2 (NLRP12-AD)/ NLRP12 PLAID and APLAID/ PLCG2 |
| Pustulosis, papulosquamous features (psoriasis- or PRP-like) | DIRA/ IL1RN DITRA/ IL36RN CAMPS/ CARD14 PAPASH syndrome/ PSTPIP1 |
| Neutrophilic dermatosis or vasculitis | FMF/ MEFV NOMID/CINCA (CAPS) NLRP3 Familial Behçet syndrome/ TNFAIP3 PAPA syndrome/ PSTPIP1 SAVI syndrome/ TMEM173 CANDLE (PRAAS)/ PSMB8 ORAS syndrome/ OTULIN Majeed syndrome/ LPIN2 ADA2 deficiency/ ADA2 Blau syndrome/ NOD2 |
| Macrophage activation syndrome | NLRC4-MAS/ NLRC4 |
| Panniculitis | CANDLE (PRAAS)/ PSMB8 ORAS syndrome/ OTULIN Blau syndrome/ NOD2 |
| Acne, hidradenitis suppurativa, sterile abscesses, and/or pyoderma gangrenosum | PAPA, PASH, PAPASH, PsAPASH syndromes/ PSTPIP1 |
| Eczematous | PLAID and APLAID/ PLCG2 |
| Granulomatous | Blau syndrome/ NOD2 PLAID and APLAID/ PLCG2 ADA2 deficiency/ ADA2 |
| Erysipelas-like eruption | FMF/ MEFV |
| Livedo | Aicardi–Goutieres/many genes ( Chapter 20 ) SAVI syndrome/ TMEM173 ADA2 deficiency/ ADA2 |
| Lipodystrophy | CANDLE/PRAAS/ PSMB8 ORAS syndrome/OTULIN |
| Aphthous stomatitis | Familial Behçet/ TNFAIP3 FCAS2 (NLRP12-AD)/ NLRP12 Majeed syndrome/ LPIN2 PAPA-like/ PSTPIP1 PFAPA/polygenic |
Autoinflammatory disorders reflect abnormalities in innate immunity, often with increases in proinflammatory cytokines (e.g., IL-1β, IL-17, IL-18, IL-36, TNF). These cytokines are also increased and involved in the pathogenesis of several polygenic autoinflammatory disorders, such as psoriasis, neutrophilic disorders, and granulomatous disorders, suggesting common pathogenic mechanisms. In addition, features typical of polygenic disorders (e.g., pyoderma gangrenosum, pustular psoriasis, Behçet disease, and sarcoidosis) are seen in monogenic autoinflammatory disorders, which have led to classification of these polygenic disorders as autoinflammatory. As such, many of these disorders are described in this chapter, before the presentation of the monogenic disorders. Some autoinflammatory disorders are described in other chapters (e.g., see Chapter 4 for psoriasis and chronic recurrent multifocal osteomyelitis [CRMO]; Chapters 4 and 8 for synovitis, acne, palmoplantar pustulosis and psoriasis, hyperostosis, and osteitis syndrome [SAPHO]; Chapter 20 for Sweet syndrome; and Chapter 22 for systemic juvenile idiopathic arthritis [JIA]).
Pyoderma gangrenosum (PG) is a severe, chronic, ulcerative autoinflammatory disorder of the skin. Although much more commonly described in adults, 4% of cases have occurred in infants and children. , PG has an incidence of 0.26 per 100,000 per year in children 10 to 19 years of age and 0.06 per 100,000 per year in children under 10 years. PG has been described in siblings. It is a feature of some rare forms of inflammasomopathies.
Although PG commonly occurs in children without associated disease, it often develops in pediatric patients before, concurrently, or after onset of a systemic disorder. Most common associations in children are inflammatory bowel disease (more in Crohn’s disease—see Crohn’s Disease section—than in ulcerative colitis), but immunodeficiency (especially HIV infection, leukocyte adhesion defect, , SCID, CVID, chronic granulomatous disease, hyper-IgE syndrome, and selective IgA deficiency), hematologic disorders (especially leukemia), , Takayasu disease with or without airway or pulmonary disease, and rheumatic disorders (especially JIA, systemic lupus erythematosus, Henoch–Schönlein purpura, and in association with antiphospholipid antibodies) , have all been reported. PG can also be seen in patients with PAPA (see Fig. 25.33 , B ), PASH, PAPASH, and PsAPASH syndromes (see The Inflammasomopathies section); chronic recurrent multifocal osteomyelitis ; Majeed syndrome ; and SAPHO syndrome. PG tends to occur during adolescence in most pediatric patients with inflammatory bowel disease and inflammasomopathies. In children with onset during infancy, Takayasu disease is the most common association and may occur years after the onset of PG. PG can occur as a drug reaction, particularly with use of TNF inhibitors, , despite their use as therapy (see Chapter 4 for the paradoxical occurrence of psoriasis-like lesions with the use of TNF inhibitors); in adults, PG has been associated with pegfilgrastim (granulocyte-stimulating factor) and gefitinib (epidermal growth factor [EGF] receptor inhibitor). PG-like ulcerations have been described as well in granulomatosis with polyangiitis, a systemic, necrotizing, small vessel vasculitis associated with circulating antineutrophil cytoplasmic antibodies (ANCAs).
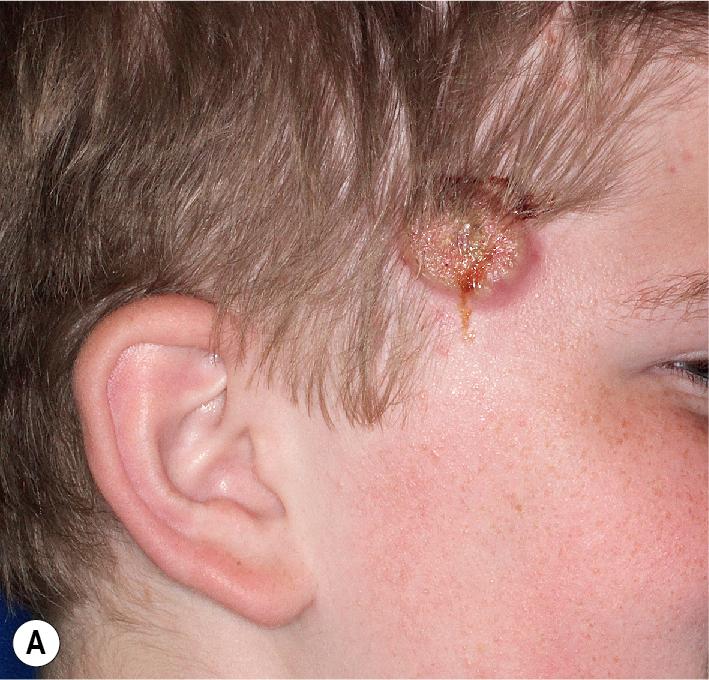
The diagnosis is considered based on clinical features. PG is characterized by a painful sloughing ulceration with purulent or vegetative base and an elevated dusky blue or reddish purple undermined border surrounded by a rim of inflammation ( Figs. 25.17 and 25.18 ). The lesion usually begins as a tender papulopustule or erythematous nodule that undergoes necrosis and ulcerates. Ulcer extension is variable but can be rapid, with rates of up to 1 cm in a day. Cultures yield no organisms. During healing, the border collapses and epithelial projections extend into the ulcer ( Gulliver sign ). The ulceration heals with cribriform scarring ( Fig. 25.19 ). Pathergy, the development of lesions at sites of minor trauma due to release of proinflammatory cytokines and chemokines that recruit and activate neutrophils, occurs in 20% of patients with PG and may be more common in childhood cases. PG developed at multiple sites of physical abuse in one child. Pathergy is also thought to contribute to the tendency for PG to occur around peristomal sites , in children with associated inflammatory bowel disease, as well as in postoperative PG. , Consensus-based diagnostic criteria have recently been described that require the major criterion of biopsy of the ulcer edge (ideally when not on immunosuppressive medication or when flared) showing neutrophilic infiltration, as well as having at least four of eight minor criteria. These include exclusion of infection; pathergy; history of inflammatory bowel disease or inflammatory arthritis; history of papule, pustule, or vesicle ulcerating within 4 days of appearing; peripheral erythema, undermined border, and tenderness at ulceration site; multiple ulcerations, at least one on an anterior lower leg; cribriform or “wrinkled paper” scar(s) at healed ulcer sites; and decreased ulcer size within 1 month of initiating immunosuppressive medication(s). Having many of these minor criteria may allow diagnosis without biopsy in children. If a biopsy is performed, however, an elliptic specimen of a well-developed edge (not a fresh area of extension) that includes the margin and ulcer floor is recommended; sections show massive infiltration with neutrophils, as well as hemorrhage and vessel necrosis.
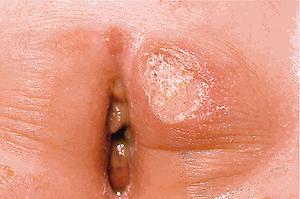
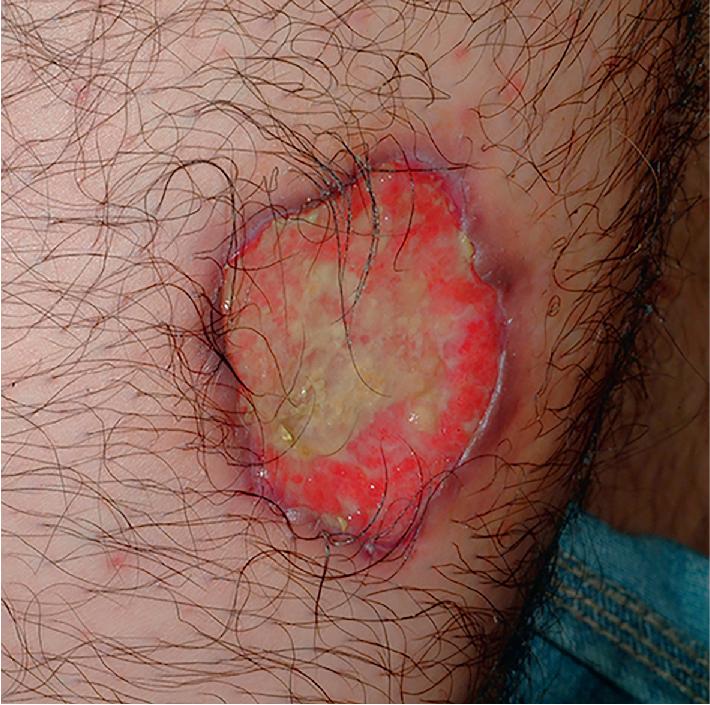
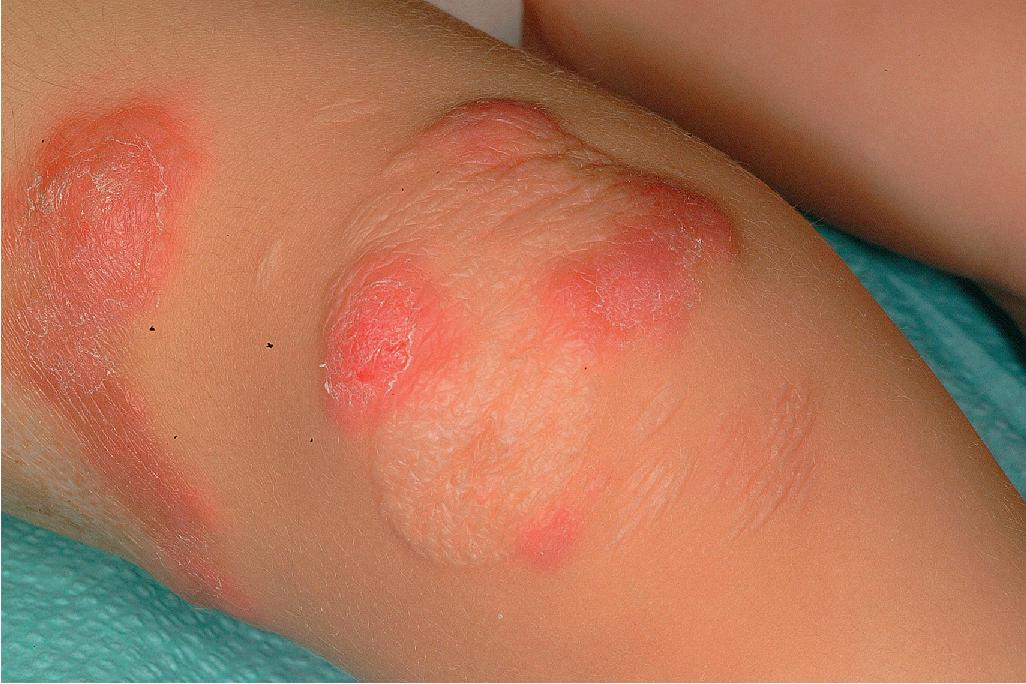
Lesions in older children and adolescents (as in adults) are most often found on the lower extremities (especially the anterior tibial surface) but can be seen anywhere on the body, including mucosal and peristomal sites. In one pediatric study, lesions were found as commonly on the trunk as on the lower extremities (both 77%). The head, airway, and anogenital areas are more common sites of involvement in neonates, infants, and younger children. Pustular, bullous, vegetative, and superficial , variants have been described. The bullous form may be difficult to distinguish from bullous Sweet syndrome (see Chapter 20 ). Biopsy findings are nonspecific, but special stains and cultures of biopsy specimens may help distinguish PG from other disorders, including vasculitis and infectious causes of ulceration.
Affected individuals may experience fever, malaise, myalgias, and arthralgias that range from monoarthritis to destructive polyarthritis. Pulmonary and splenic lesions have also been described.
Treatment of PG involves use of systemic and topical antiinflammatory medications to promote wound healing and decrease pain, as well as a vigorous attempt to control the underlying disease. Debridement and other mechanical trauma may worsen the ulcerations by increasing pathergy and should be avoided. Systemic corticosteroids are usually the initial drug of choice. Cyclosporine or systemic tacrolimus, TNF-α inhibitors (etanercept, adalimumab, infliximab), ustekinumab, IL-1β/IL-1 receptor inhibitors (canakinumab and anakinra), dapsone, clofazimine, MMF, and IVIG , have been used successfully as steroid-sparing agents. Although IL-17 inhibitors may be useful for treating PG, some patients have developed PG during IL-17 inhibitor therapy. , Topical tacrolimus ointment applied a few times daily has improved ulcer healing ; intralesional steroid injections may be appropriate for adolescents. Stoma closure and resection of affected bowel in patients with inflammatory bowel disease have been advocated for peristomal involvement.
Although occurring primarily in adults, amicrobial pustulosis of the folds (APF) has been described in children, especially in the second decade of life. Three major criteria are required to make this diagnosis: (1) pustulosis involving one or more major folds, one or more minor folds (e.g., perinasal, retroauricular, and external auditory canals), and the anogenital area; (2) histologic pattern consisting of intraepidermal spongiform pustules and a mainly neutrophilic dermal infiltrate; and (3) negative culture from an unopened pustule. One additional minor criterion is required for diagnosis from among the following: (1) association with one or more autoimmune or autoinflammatory disorders, (2) positive ANAs at a titer of 1/160 or higher, or (3) presence of one or more serum autoantibodies. Pustules tend to be symmetrically in the folds and often involve the scalp and nails. APF has a chronic, relapsing course and typically responds to corticosteroids. However, dapsone, cyclosporine, and TNF inhibition has been used effectively as well. The condition has been associated with several autoimmune disorders and inflammatory bowel disease, including in patients treated with anti-TNF agents, as a paradoxical reaction.
Behçet disease is a chronic multisystemic autoinflammatory disorder characterized by recurrent oral and genital ulcerations and inflammatory disease of the eye. Overall, the prevalence globally is 10.3 per 100,000 persons, with the highest prevalence rates in Turkey. Although even neonatal onset without maternal Behçet disease has been noted, Behçet disease usually begins in patients between 10 and 45 years of age, with approximately 17% of cases beginning before 17 years of age. Neonatal Behçet disease usually occurs in infants born to mothers with the disease and generally disappears spontaneously by the time the infants reach the age of 6 months. Characterized by aphthous stomatitis and skin lesions, affected neonates rarely have severe, life-threatening complications. ,
Although most studies have suggested that Behçet disease has a predilection for males, including in pediatric patients, an international registry enrolled equal numbers of boys and girls. Only 2% to 5% of cases are in children. In general, the disease is less severe and more delayed in its full manifestation in pediatric patients.
Behçet disease is thought to be an autoinflammatory disorder influenced by genetics and environment, with Th1/Th17 skewing and decreased Treg cells. Most patients carry HLA-B*5 and particularly its subset Bw51 alleles, the strongest-known risk factor. , Some 15% to 45% of children have an affected family member, in contrast to 8% of adults. , Dominantly inherited Behçet disease is now recognized to result from haploinsufficiency of A20, encoded by TNFAIP3 (see the A20 Haploinsufficiency section). Loss-of-function mutations lead to activation of the NF-κB pathway.
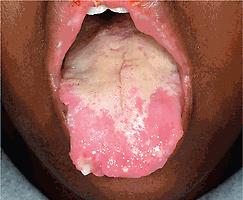
Cutaneous and oral ulcerations are the most common clinical features of pediatric Behçet disease. Oral lesions, noted at some point in 95% of affected children, are the initial manifestation in 83% of pediatric patients and occur approximately 8 years before other signs. The ulcerations begin as erythema and within 1 to 2 days become superficial erosions that range from smaller ulcerations, indistinguishable from aphthous stomatitis, to deeply punched-out necrotic ulcers on the lips, buccal mucosa ( Fig. 25.20 ), tongue ( Fig. 25.21 ), and, less commonly, the gingivae. They have regular sharp edges and vary from a few millimeters to a centimeter in diameter. Minor aphthae have a diameter of less than 1 cm, resolve in 7 to 10 days, do not scar, and are most common, whereas major aphthae are larger, deep, more painful, and tend to scar. Sometimes aphthae are grouped (herpetiform aphthae). The ulcer base is covered with a yellowish-gray exudate, and the margin is surrounded by a red halo. Oral lesions recur at intervals varying from weeks to months; by definition, aphthae must recur at least three times yearly. Features of Behçet disease and relapsing polychondritis have also been described as mouth and genital ulcers with inflamed cartilage (MAGIC) syndrome.
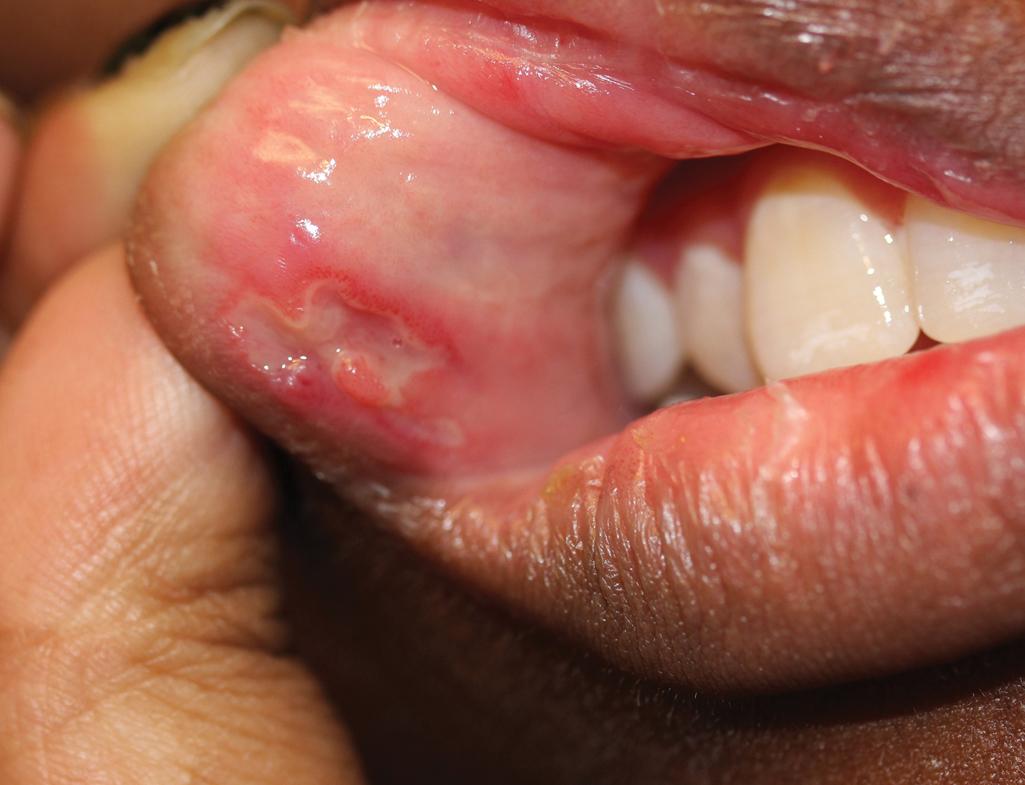
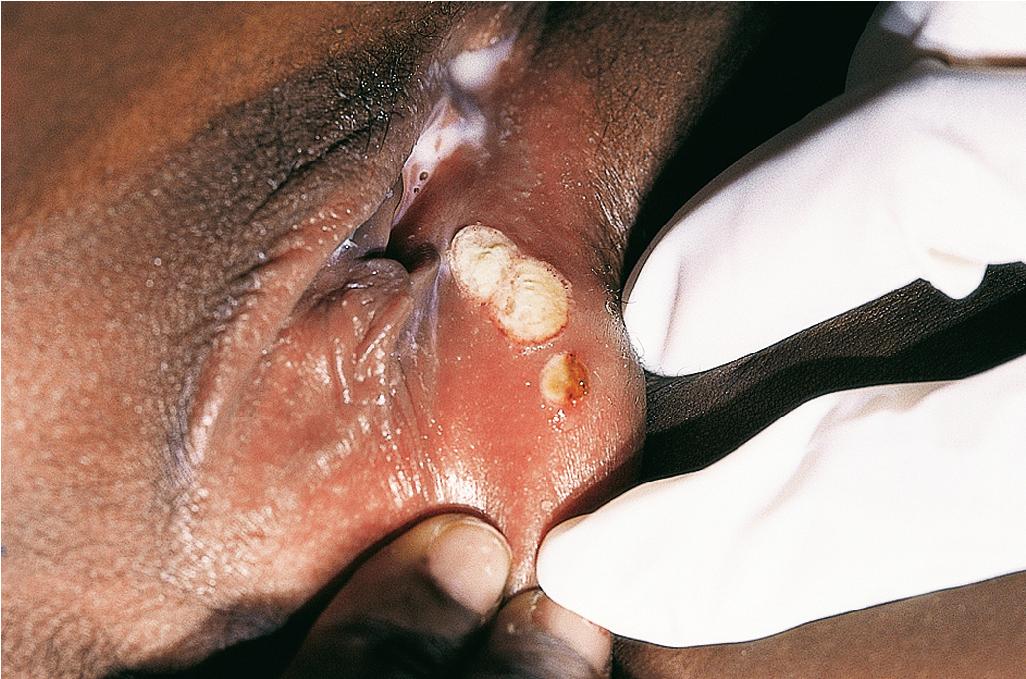
Genital ulcerations are seen in 34% to 60% of children with Behçet disease and occur more often in girls than in boys. They are found on the vulva and vagina of females ( Fig. 25.22 ) and the scrotum or base of the penis in males. They are similar in appearance to the oral ulcerations but less painful and may be overlooked. Perianal ulcerations are less common but have been noted more often in children than in adults (up to 30%). Genital ulcerations are usually deeper than oral ulcerations ( Fig. 25.23 ) and may scar. As with oral ulcerations, they remit in 1 to 2 weeks and tend to recur. Epididymo-orchitis has also been described, characterized as scrotal pain and swelling.
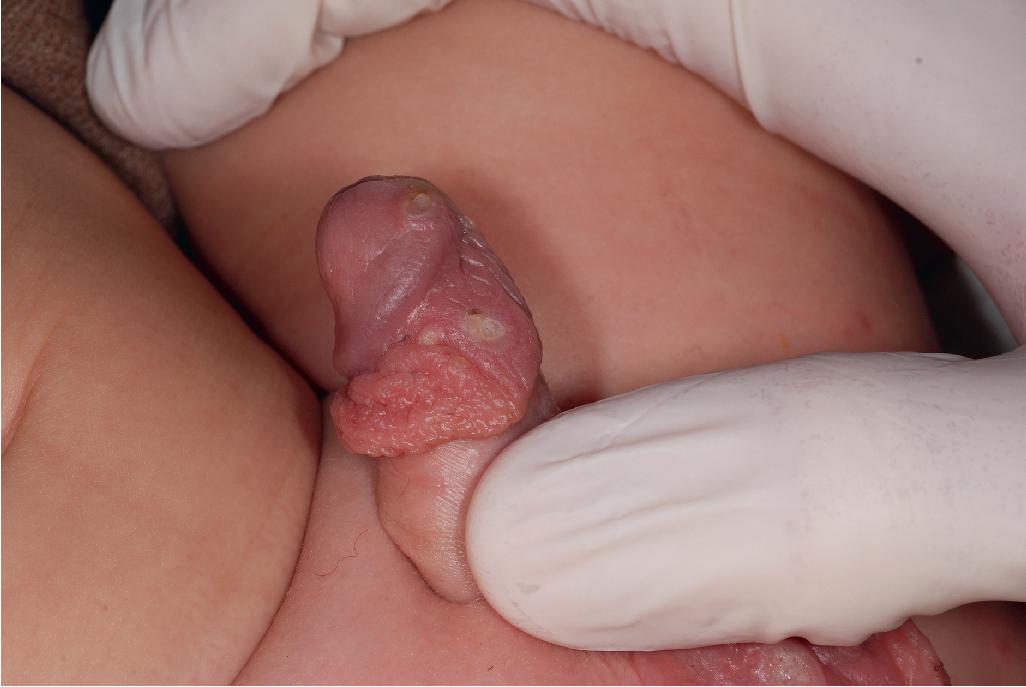
Other cutaneous lesions of Behçet disease present a varied picture. Seen in approximately 80% of affected children, they consist of folliculitis, pustules, pyoderma, acneiform lesions, furuncles, abscesses, ulcerations, erythema nodosum–like lesions, and palpable purpura ( Fig. 25.24 ). Pathergy (the formation of an ulceration at an area of trauma, skin prick, or venipuncture) is a common feature.
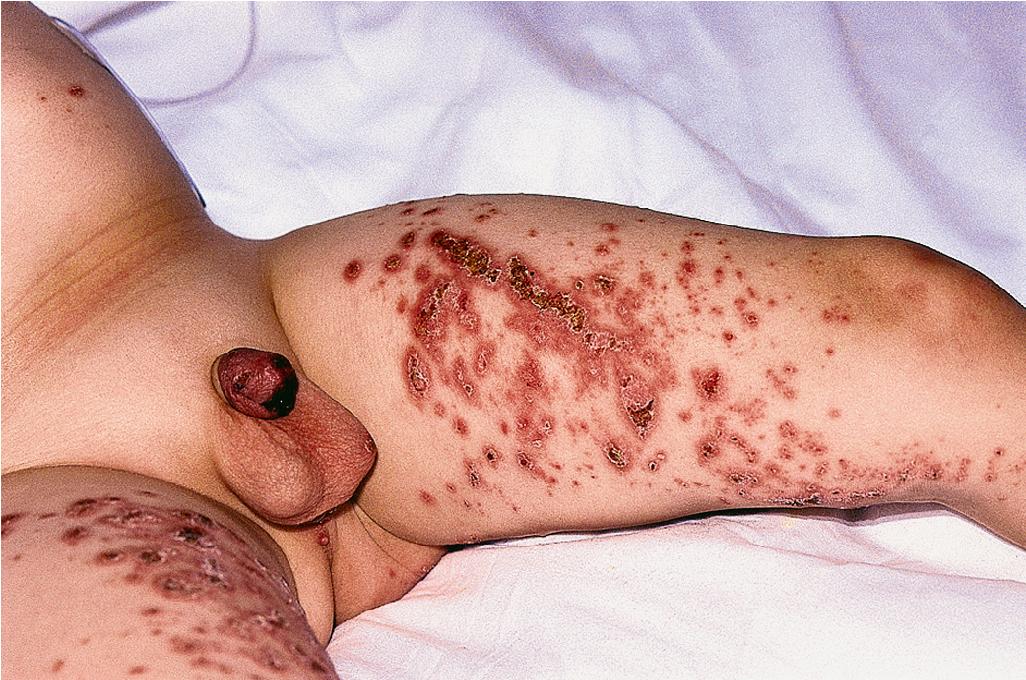
Ocular lesions are seen in approximately 50% of boys but only 15% to 20% of affected girls. They usually occur after 10 years of age, are less common than in adults, and are associated with a worse prognosis. Conjunctivitis and photophobia may be early ocular findings, but bilateral panuveitis is the most common manifestation (more than 80% of children with eye findings) ; posterior uveitis is found more often than anterior uveitis. Retinal vasculitis and retinitis occur in the majority of children with ocular features, and cataracts, maculopathy, and optic atrophy have been described.
Fever and constitutional symptoms are variable. Musculoskeletal complaints, especially arthralgia and arthritis, are found in up to 75% of affected children. Gastrointestinal complaints are more common in children than in adults and may warrant endoscopic evaluation, especially in children under 10 years of age. They range from vague abdominal pain (up to 50%) and anorexia to diarrhea and hemorrhagic colitis. Just as in inflammatory bowel disease, fecal calprotectin has been found to be a noninvasive marker for intestinal involvement. Central nervous system (CNS) involvement is often the most severe prognostic feature of this disorder. Seen in 20% to 50% of patients, it often manifests as headache and most commonly reflects cerebral venous sinus thrombosis. , Arterial or venous thrombosis can also affect the lower extremities and can be recurrent. Renal involvement, an uncommon complication, may be seen, with a spectrum ranging from asymptomatic abnormalities detected on urinalysis to a rapidly progressive glomerulonephritis and nephrosis. Other unusual findings in children include pericarditis, orchitis, and epididymitis.
The diagnosis of Behçet disease is based on the constellation of typical clinical manifestations. Diagnosis in a patient with the full triad of oral and genital ulcers and ocular inflammation is not difficult, but many show partial manifestations. Criteria to diagnose Behçet disease include the International Study Group (ISG), the International Criteria for Behçet Disease (ICBD), and the Paediatric Behçet’s Disease (pediatric specific; PEDBD) criteria. ISG criteria, established in 1990, had low sensitivity and required the presence of oral aphthae at least three times yearly, despite the fact that not all patients met this criterion. ISG criteria diagnosed only 29% to 53% of the pediatric Behçet disease patients, , whereas the ICBD criteria have been met in 80%. The ICBD were proposed in 2014 for adults, and the PEDBD represent a 2015 adaptation for pediatric patients ( Box 25.4 ). In a cohort of Turkish and Israeli children, PEDBD criteria were 73.5% sensitive and 98.9% specific. However, the PEDBD criteria only classified 46% and 26% of affected children from Italy and the UK, respectively. This modification for pediatric patients , eliminated potential points for pathergy and gave equal weight to all clinical signs, including giving the cutaneous (particularly folliculitis, acneiform lesions, and erythema nodosum), neurologic, and vascular signs vs. the oral and genital ulcerations. Although pathergy is common, a negative pathergy test does not rule out the diagnosis. Pathergy can also be a feature of pediatric PG, leukemia, and bowel bypass syndrome (see Chapter 20 ). Biopsy is usually not helpful, but having HLA-B51 positivity can be useful supporting evidence of the diagnosis.
| CRITERION | ADULT † | PEDIATRIC * |
|---|---|---|
| Recurrent oral aphthous stomatitis (≥3 attacks/year) | 2 points | 1 point |
| Genital ulcers (typically with residual scarring) | 2 points | 1 point |
| Other skin involvement (acneiform lesions, necrotic folliculitis, erythema nodosum) | 1 point | 1 point |
| Ocular manifestations (anterior or posterior uveitis, retinal vasculitis) | 2 points | 1 point |
| Neurologic signs (beyond isolated headaches) | 1 point | 1 point |
| Vascular signs (venous thrombosis, arterial thrombosis, arterial aneurysm) | 1 point | 1 point |
| Positive pathergy test | 1 point | 0 points |
† ≥4 points are required for diagnosis of adult Behçet disease. Pathergy testing is optional, but if positive adds 1 point toward the score.
* ≥3 points are required to classify as pediatric Behçet disease.
Treatment of the aphthous ulcers with potent topical or intralesional corticosteroids, topical application of tacrolimus, oral rinses with elixir of diphenhydramine, or application of 2% viscous lidocaine can give symptomatic relief. For mucocutaneous ulcerations, colchicine is often helpful. Therapy is begun with a dosage of 0.6 mg twice daily for the first week. If the patient has no nausea, vomiting, or diarrhea, the dosage can be increased to three times a day, but colchicine is poorly tolerated by pediatric patients. Dapsone can also be helpful, especially in children with recurrent oral ulcerations. In severe cases, corticosteroids, alone or in combination with immunosuppressive agents such as methotrexate and azathioprine, are usually successful and are still the recommendation for management of thromboses. However, the demonstrated activation of inflammatory cytokines TNF-α, IL-1β, and IL-6 has led to new therapeutic approaches. Inhibitors of TNF, IL-1 (anakinra, canakinumab), IL-6 (tocilizumab), and IL-12/IL-23 (ustekinumab), as well as rituximab, have been used successfully. Thalidomide 50 to 100 mg once weekly to once daily has also led to improvement, but its use is limited by constipation, fatigue, and peripheral neuropathy, which may be irreversible, and it is now rarely used. The overall mortality rate of Behçet disease in children is 3%; death usually results from large-vessel vasculopathy (aneurysm, thrombosis), intestinal perforation, CNS involvement, or as a complication of therapy.
Orofacial granulomatosis (OFG; also called cheilitis granulomatosa or granulomatous cheilitis ) ( Fig. 25.25 ) is characterized by face and lip swelling (macrocheilitis). It is the most common manifestation (93% with facial swelling and 66% with lip swelling) of Melkersson–Rosenthal syndrome, which also includes recurrent facial paralysis (30%) and a furrowed or “scrotal” tongue (lingua plicata, 30%) ( Fig. 25.26 ). , No prodrome warns of attacks, and patients experience no associated erythema, pain, or pruritus. The disorder is more prevalent in males and has a mean age of onset of 11 years. The swelling can increase the size of the lip by twofold to threefold, leading to chapping from exposure of the mucosa. Both lips may be involved, and the swelling may be bilateral or unilateral. In addition to the swelling of the lips and other oral mucosal structures, the eyelids may be swollen. The attacks usually disappear within days or weeks but commonly tend to persist after several recurrences. Facial palsy usually occurs on the side of the facial swelling and tends to resolve spontaneously.
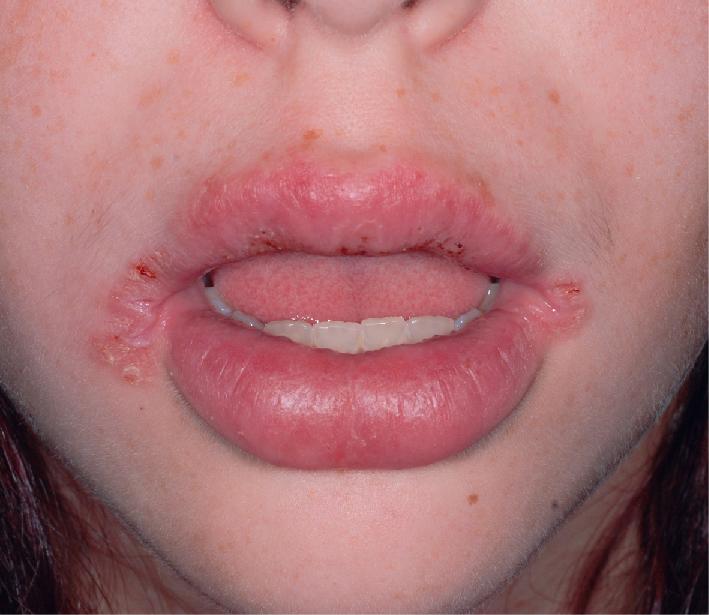
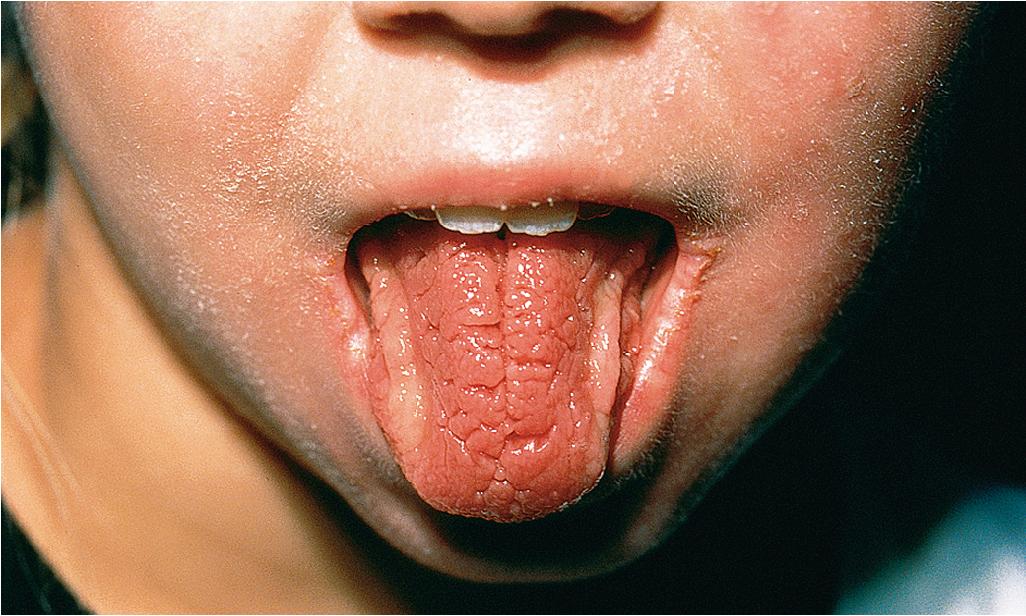
The cause is unknown. Attacks usually start during adolescence with paralysis of a facial nerve; repeated severe headaches; edema of the circumoral tissue of the upper lip or cheeks; and occasionally edema of the gingivae, sublingual area, and lower lip. The edema is usually asymmetric, but the whole face may be involved. Associated signs include hyperhidrosis, loss of taste, and visual impairment. Biopsy specimens show noncaseating granulomas in an edematous stroma that may be indistinguishable from the granulomas of Crohn’s disease, and the relationship between Crohn’s disease and OFG remains controversial. In one systematic review, gastrointestinal signs were present in 26% at the time of OFG diagnosis, 40% of children were diagnosed with Crohn’s disease, and OFG preceded signs of Crohn’s disease in more than 50%. Concomitant perianal disease and a family history of Crohn’s disease are associated with a higher risk of Crohn’s disease.
Intralesional injections of triamcinolone or systemic administration of corticosteroids can control the facial and mucosal swelling; intermittent application of topical clobetasol gel may also lead to improvement. Minocycline (or macrolides in younger children), with or without corticosteroids, has been helpful. , Other therapies include thalidomide, dapsone, azathioprine, clofazimine, and TNF-α inhibitors, particularly infliximab.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here