Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The dictum “Children are not small adults” is especially true regarding skeletal trauma. Fracture patterns, fracture healing, and fracture complications differ between children and adults. Fortunately, fracture complications are relatively uncommon. In healthy children, the process of fracture healing and remodeling is rapid, particularly in the vascularized metaphysis. Most posttraumatic deformities readily correct with healing and remodeling. The composition of a child's bones and the presence of the growth process both predispose children to types and complications of fractures that are different from those seen in adults.
Falls, injuries at play, and motor vehicle accidents account for a majority of childhood fractures. Unfortunately, children may suffer fractures as a consequence of nonaccidental trauma (child abuse). These injuries may have characteristic patterns and are covered in Chapter 143 . Children are increasingly involved in athletics and competitive sports. Certain types of injuries, including stress injuries, are often associated with sporting activities (see Chapter 144 ). Pathologic fractures may be seen with underlying bone tumors, and insufficiency fractures may be seen with metabolic bone disease (see Chapters 138 and 139 ).
Understanding basic fracture nomenclature is important for effective communication. Fractures are subdivided into two basic categories: (1) incomplete (plastic) and (2) complete. Incomplete fractures include greenstick and buckle fractures. Complete fractures should be described based on orientation: transverse , oblique , longitudinal , and spiral . Spiral fractures are defined by an approximately 180-degree or greater twist in the fracture plane. For any fracture, angulation, displacement, diastasis, comminution, and impaction must be described. On follow-up radiographs, any change, in addition to the presence or absence of fracture healing, should be described. Involvement of an open physis and articular surface also should be described. For physeal fractures, it is acceptable to describe the fracture using the Salter-Harris classification.
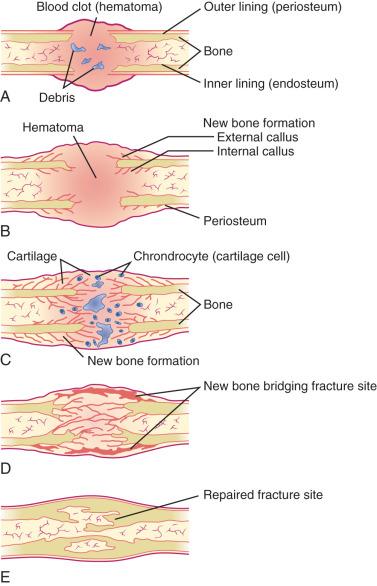
Fracture healing in children has been described in three phases: (1) inflammatory, (2) reparative, and (3) remodeling ( e-Fig. 142.1 ). At the time of fracture, bone and periosteum are disrupted. An inflammatory response is initiated (inflammatory phase), and the organization of a hematoma begins. Bone resorption occurs at areas of necrosis. This peaks 2 to 3 weeks after injury and appears as a poorly defined fracture line. Initial callus is formed during the reparative phase. Callus forming within the hematoma envelops the fracture fragments, joining and stabilizing them. Endosteal callus also forms within the fracture fragments and is seen as increased density on radiographs. Devitalized portions of bone at the margin of the fracture fragments may undergo resorption and appear demineralized on radiographs. An injury that is less than 5 to 7 days old will not show any of these described radiographic features. In time, the woven bone of callus is replaced by organized lamellar bone during the remodeling phase. Remodeling lasts months and occasionally years. The healing process is more rapid and complete in children than in adults. Most childhood fractures heal completely and without residual deformity.
Complications may occur at the time of fracture, during treatment, or as a failure of normal, complete fracture healing. Box 142.1 lists potential complications of pediatric fractures. The incidence of a particular complication varies, depending on many factors, including the site and severity of the fracture, complicating factors (i.e., open fracture), the age and overall health of the patient, associated injuries, and the adequacy of therapy.
Neurovascular injury
Hemorrhage
Fat embolism
Compartment syndrome
Premature growth plate fusion
Delayed union
Nonunion or pseudoarthrosis
Malunion or deformity
Synostosis (cross-union)
Heterotopic ossification or myositis ossificans
Overgrowth
Refracture
Premature degenerative joint disease (osteoarthritis)
Osteomyelitis or septic arthritis
Posttraumatic osteolysis
Avascular necrosis
Posttraumatic cyst
Osteochondroma
Fibrous cortical defect
Aneurysmal bone cyst
Reflex sympathetic dystrophy syndrome (Complex regional pain syndrome)
Soft tissue infection
Hardware misplacement, migration, or infection
Casting complications
Hypocalcemia of immobilization
Superior mesenteric artery syndrome (cast syndrome)
Deep venous thrombosis or pulmonary embolism
In nonunion , healing stops before osseous continuity of the fracture fragments occurs. The fracture fragments may form a pseudoarthrosis. Pseudoarthrosis is most common in the clavicle, humerus, and tibia. Nonunion and pseudoarthroses are uncommon in normal children but may be seen with underlying abnormalities such as neurofibromatosis or congenital insensitivity to pain. Nonunion is suggested when there is lack of progression to union greater than 6 months after injury when there are corresponding clinical signs and symptoms. The incidence of nonunion is increased with greater injury, comminution, and distraction, and with open fractures complicated by substantial soft tissue injury, infection, or both. Mild degrees of malalignment are well tolerated and usually result in no permanent deformity and resolve over time because of remodeling. Surgical interruption of the healing process to correct residual malalignment is occasionally necessary. Posttraumatic synostoses are most common in the paired bones of the forearm and leg. Infection may occur due to an open fracture or as a complication of surgery or percutaneous pinning.
Fracture at certain sites may result in neurovascular injury; however, such injuries are rare and usually only seen with substantial displacement and deformity, as may occur with supracondylar fractures of the distal humerus. Compartment syndrome is an uncommon complication of extremity fractures, most commonly in the leg or with supracondylar elbow fractures, and may lead to Volkmann contracture caused by ischemia.
Premature fusion occurs in approximately 15% of physeal fractures. A bony “bridge” or “bar” forms across the physis. Prognosis depends on the involved bone, the extent and location of the physeal bar (central versus eccentric), and the amount of remaining growth. Morbidity is greater when the remaining growth potential is higher. The phalanges and distal radius are the most common sites of physeal fracture; however, growth arrest is rare in these locations. The distal femur and proximal tibia have a high incidence of posttraumatic physeal fusion but are less common sites for physeal fracture. Premature fusion in the distal femur and tibia is also of greater clinical importance because of possible resultant leg length discrepancy or angular deformity. Indirect physeal insult such as burns, frostbite, and electrical injury, which are discussed later in this chapter, and other processes such as infection, ischemic insult, metabolic bone disease, and iatrogenic causes can also lead to premature physeal fusion ( Box 142.2 ).
Injuries
Fractures involving the physis (Salter-Harris I–IV)
Physeal crush injuries (Salter-Harris V)
Overuse injuries
Electrical injuries
Burns
Frostbite
Infection
Ischemia and osteochondroses
Neuropathic (i.e., myelodysplasia)
Tumor
Congenital/developmental deformities
Metabolic bone diseases
Iatrogenic causes
Radiation therapy
Medications (vitamin A analogues)
Surgical epiphysiodesis
Central fusions cause longitudinal growth disturbances. Central fusions can result in a cupped appearance of the physis, with the epiphysis and physis invaginating into the center of the metaphysis due to tethering. Peripheral fusions result in angular deformities. Premature physeal fusion usually occurs approximately 3 months after injury. Radiographs may show obliteration of the physeal clear space. Comparison views are often helpful, particularly when evaluating an older child in whom the time of normal physeal closure is near. A secondary sign of premature physeal fusion is tethering of growth lines. Growth lines normally form during the healing process. Normal growth lines parallel the physis, whereas with premature fusion, growth lines are angled toward a bony bar.
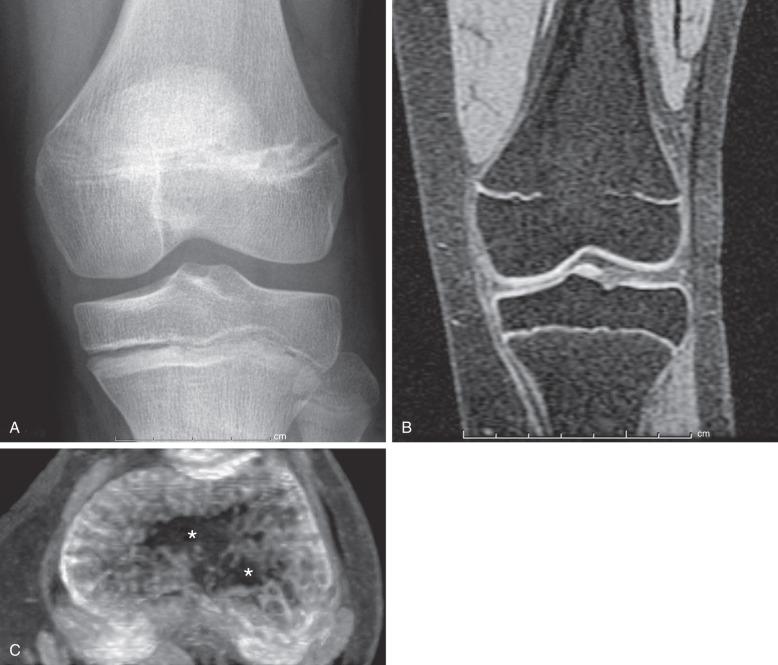
Both computed tomography (CT) and magnetic resonance imaging (MRI) have been used to diagnose premature fusion. A limited CT scan with narrow collimation obtained through the physis can depict physeal bridges, which are best seen on sagittal and coronal reformatted images. Small bars may be seen as a sclerotic band across the physis, whereas with larger areas of fusion, continuity of the marrow space across the fusion is seen. MRI can accurately depict physeal bridges on multiple sequences. The small bridges may appear as low-signal intensity on all sequences, however, if there are stress changes, edema-like signal may be present on T2-weighted sequences. Larger bridges often show signal following fatty marrow on all sequences. A thin-slice sequence with bright physeal cartilage and dark bone (e.g., three-dimensional [3D] spoiled gradient recalled echo with fat saturation) should be performed when evaluating bridges on MRI ( Fig. 142.2 ). Reformatting this sequence into a maximum intensity projection (MIP) image of the physis can be used to create a “physeal map”. On this reformatted image, the physeal cartilage is bright while a bridge across the physis is low-signal intensity. This allows the radiologist to obtain the area of the bridge and the total area of the physis to obtain the percentage of the physis occupied by the bridge (area of bridge total area of physis = percent of physis occupied by bridge). This percentage is important to the orthopedic surgeon considering bar resection, as discussed later. MRI can also detect the potential fracture complication of trapped periosteum within the physis.
Bar resection is considered when a deformity is present or developing from a physeal bridge, which occupies less than 50% of the area of the physis, and the child has at least 2 cm or 2 years of expected remaining growth. Direct and indirect (metaphyseal) resection approaches can be used and an interposition material is placed in the resection site. Bridges occupying greater than 50% of the physis are not resected because of poor outcomes, and very small bridges may resolve with physiologic growth. Depending on the deformity and growth potential of the patient, other orthopedic techniques may be utilized to minimize morbidity from the premature fusion, including osteotomies, lengthening and realignment procedures, and contralateral epiphysiodesis.
Radiographs are the mainstay of imaging of traumatic injuries to the pediatric skeleton. As a general rule, two orthogonal views are obtained to assess for fracture at nonosteoarticular locations and an additional oblique view is added at osteoarticular locations. At some locations, normal anatomy limits the value of orthogonal projections (i.e., the pelvis). At other locations, unique projections are helpful for delineation of anatomy (i.e., an axillary view of the shoulder). When imaging a long bone, both the proximal and distal joints must be included.
Contralateral comparison views are not routinely obtained but frequently aid in differentiating normal developmental variation from pathology. Comparison views are most helpful in areas of complex anatomy such as the elbow. Normal variants are common and may mimic fracture.
At certain sites and with complex patterns of injury, CT is very helpful in diagnosing fractures and delineating the anatomy of fracture planes, resultant deformity, and the extent and type of articular involvement. Occasionally, MRI or ultrasonography can be used to diagnose fractures in children; however, these modalities excel in delineating associated soft tissue injury rather than osseous injury. Ultrasonography, however, may be particularly helpful in infants whose epiphyses are not yet ossified. The cartilaginous epiphysis is well seen with ultrasound and its relationship and continuity with an adjacent metaphysis can be readily assessed. It also can be used to detect lipohemarthrosis as an indirect sign of fracture or to aid in diagnosis of an occult fracture.
The composition of bones in a child is different from that of the bones in an adult. The most apparent anatomic differences in the pediatric skeleton are the presence of the physis and a thick periosteum. The plasticity of a child's bones allows for substantial deformity before fracture ( e-Fig. 142.3 ). Bone may give way and may become permanently deformed before a complete break. The result is an “incomplete fracture” or a “plastic fracture.”
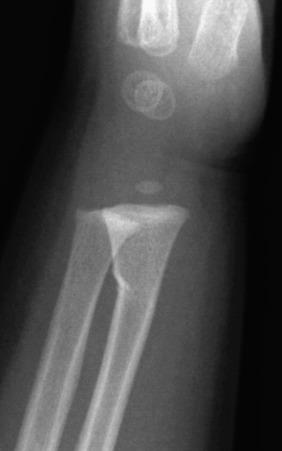
A greenstick fracture occurs when a cortical fracture occurs on the tension side of bone with intact cortex on the loading side of bone. A torus or buckle fracture is a cortical fracture occurring on the loading side of bone with intact cortex on the tension side. A bowing deformity does not have a radiographically identifiable fracture line on either the tension or loading side of bone.
Greenstick fractures most commonly occur in long bones, particularly the radius and ulna. These fractures are most common in the first decade of life, are uncommon in the second decade of life, and are not seen in normally developed and mineralized bones of adults. Buckle fractures most commonly affect the distal radius and ulna, tibia, and proximal first metatarsal bone.
Radiographs are the standard method of evaluation and advanced imaging is usually not indicated. Subtypes of incomplete fractures are buckle or torus fractures ( Fig. 142.4 ), lead pipe fractures (part transverse fracture or part buckle fracture), greenstick fractures ( Fig. 142.5 ), and bowing deformities ( e-Fig. 142.6 ). With incomplete fractures, the periosteum is intact wherever the cortex is intact.
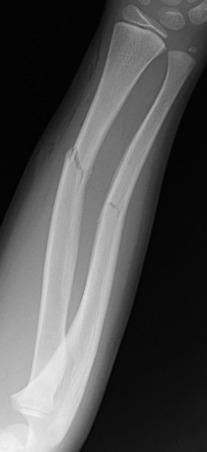
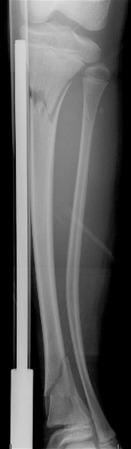
Cast and splinting is usually performed for pain control because most of these fractures are stable. Plastic fractures usually heal completely and have a good prognosis. Rarely, it may be necessary to operatively “complete” the fracture for reduction of severe angular deformity. Mild angular deformities (<15 degrees) usually do not need to be reduced and will remodel nicely without permanent sequelae.
For the musculoskeletal unit of the child, the physis and physeal equivalent regions are points of relative weakness and are thus predisposed to mechanical failure leading to fractures. The physis usually fails before ligamentous or tendinous soft tissue structures fail after biomechanical stress. Once the physis fuses, ligamentous and tendon soft tissue injuries become more frequent, as do metadiaphyseal fractures. Fracture patterns in skeletally mature adolescents are similar to fracture patterns seen in adults.
Approximately 18% of pediatric fractures involve the physes. Physeal fractures are classified by the system of Salter and Harris ( Fig. 142.7 ). This well-accepted classification scheme for describing physeal fractures facilitates efficient communication between clinicians and radiologists.
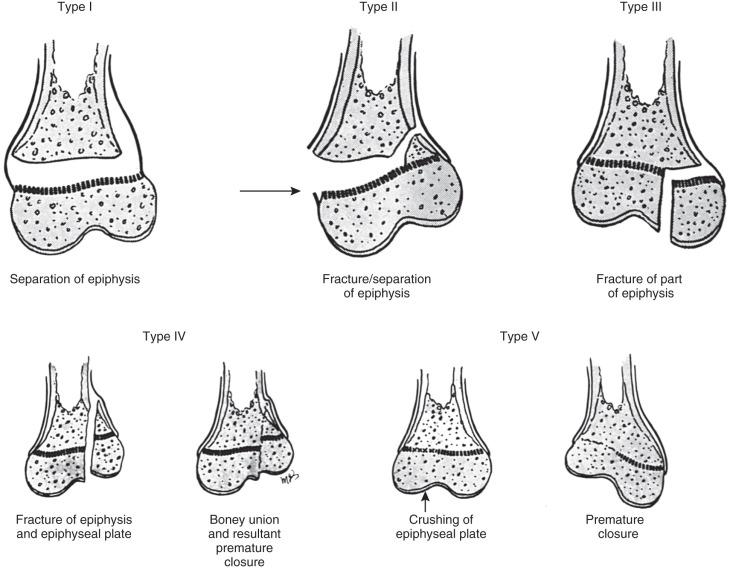
Most physeal fractures are adequately delineated by radiography. The Salter-Harris classification, the degree of displacement and angulation, and intraarticular involvement should be described.
Fractures may pass solely through the physis (Salter-Harris I; e-Fig. 142.8 ), involve the physis and metaphysis (Salter-Harris II; Fig. 142.9 ), involve the physis and epiphysis (Salter-Harris III; e-Fig. 142.10 ), or cross the physis involving both epiphysis and metaphysis (Salter-Harris IV; e-Fig. 142.11 ). Crush injury of the physis (Salter-Harris V) is rare as an isolated injury to the bone and are typically diagnosed retrospectively.
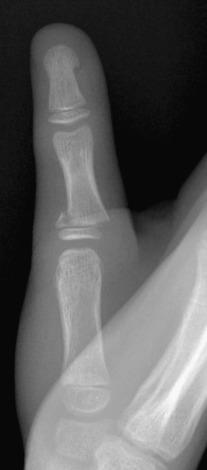
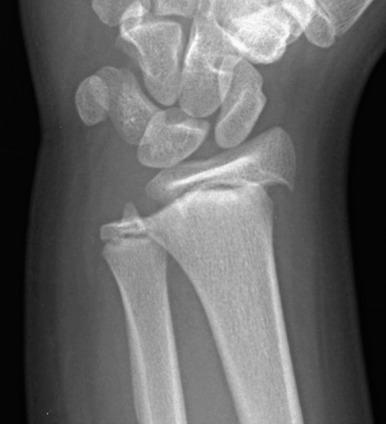
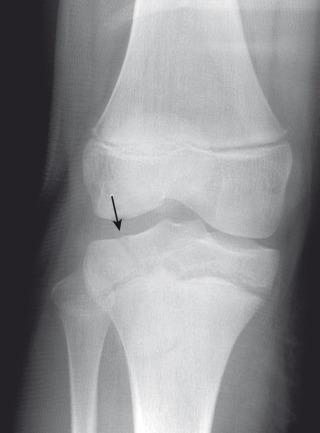
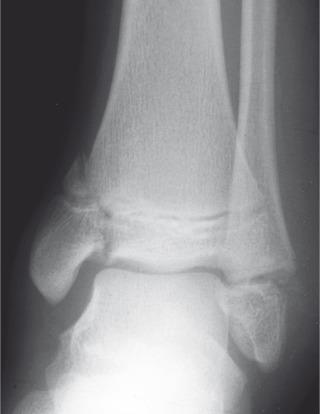
The Salter-Harris classification serves to assign prognosis. Higher grade Salter-Harris fractures have a higher incidence of premature physeal fusion. Most physeal fractures, however, are either Salter-Harris I (approximately 10%) or Salter-Harris II (approximately 75%). Thus although premature physeal fusion is more likely to occur with a higher grade Salter-Harris fracture, this complication is probably more commonly the result of the more common lower grade Salter-Harris fractures. Lesser grades of Salter-Harris fractures that proceed to premature physeal fusion may have a component of crush (Salter-Harris V) injury.
At certain locations, CT or MRI may be used to confirm or further delineate fractures, especially for defining exact measurements of fracture diastasis and whether involvement of an articular surface exists. On MRI, physeal fractures are diagnosed by widening and increased T2-weighted signal within the physis, adjacent bone marrow edema, associated metaphyseal (Salter-Harris II or IV) or epiphyseal (Salter-Harris III or IV) fracture lines, and periosteal disruption. The most significant complication of physeal injuries is growth arrest (see Fig. 142.2 ), which can lead to deformity and longitudinal growth arrest, potentially with limb length discrepancies. A rare complication of physeal fracture diagnosed by MRI is entrapment of periosteum within the fracture ( Fig. 142.12 ). The entrapped periosteum will prevent complete reduction of the fracture.
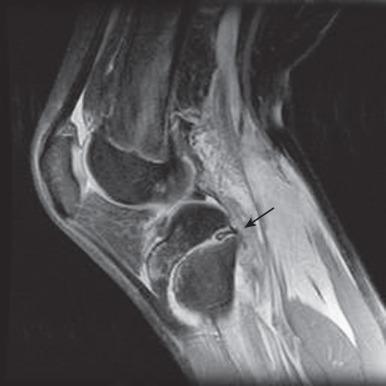
Salter-Harris I and II fractures are usually treated by closed manipulation followed by casting. Salter-Harris type III and IV fractures usually require open reduction and internal fixation, as these types of fractures often are displaced and extend to the joint. To prevent posttraumatic osteoarthritis, it is important to create a smooth articular surface; therefore Salter-Harris fractures with epiphyseal involvement and greater than 2 mm of diastasis or significant angulation usually require surgical fixation.
Infants and toddlers are likely to have a Salter-Harris I fracture of the proximal humeral physis. From 5 to 10 years of age, buckle fractures of the proximal humeral metaphysis are most prevalent ( e-Fig. 142.13 ). In older children, Salter-Harris II fractures predominate ( e-Fig. 142.14 ). Fractures of the humeral diaphysis are also common.
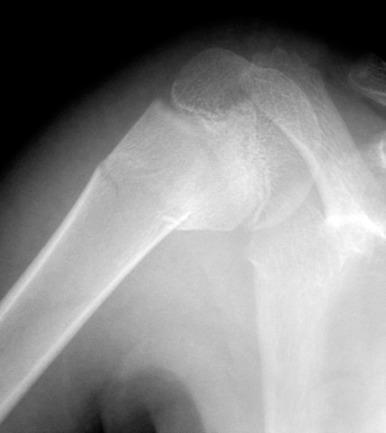
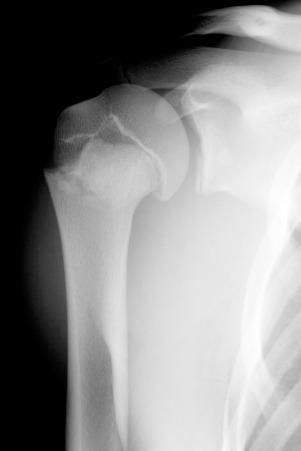
The proximal humeral physis can be a site of birth trauma ( e-Fig. 142.15 ). Similar injuries can also be seen with child abuse.
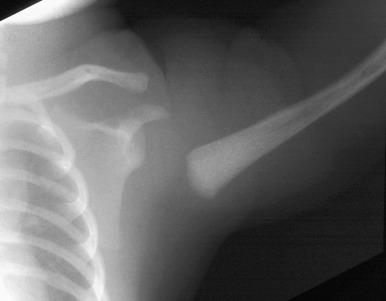
Anteroposterior and lateral radiographs of the humerus should be obtained to initially evaluate a fracture. If the fracture is near or involves the glenohumeral joint, an additional axillary view is recommended. If a Salter-Harris type I fracture is suspected in the neonate, evaluation with ultrasonography is preferable, as the proximal humeral epiphysis is cartilaginous readily seen. For older patients or those with complex proximal humeral fractures, CT or MRI may be useful to evaluate for intraarticular extension and for involvement of the greater and lesser tuberosities.
Radiographically, because the humeral head is usually not ossified or only slightly ossified at birth, fracture through the physis mimics dislocation of the shoulder (see e-Fig. 142.15 ). The humeral metaphysis may appear to align inferior to the glenoid. In the newborn, Salter-Harris I fracture of the proximal humerus is much more common than glenohumeral dislocation. Ultrasound may be used to diagnose the fracture by showing malalignment of the cartilaginous humeral head with the proximal humeral metaphysis and showing motion at the physis ( e-Fig. 142.16 ).
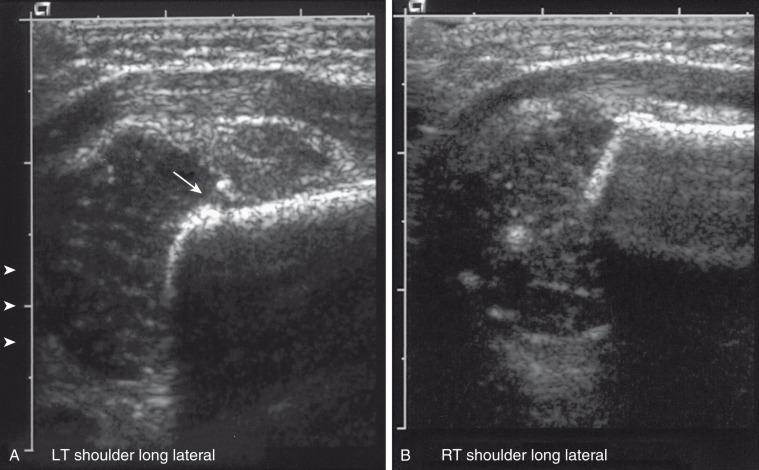
Salter-Harris fractures of the proximal humerus in older children are usually Salter-Harris II fractures, although the metaphyseal fragment is often very small. Occasionally, no metaphyseal fragment exists. Such Salter-Harris I fractures are sometimes called slipped capital humeral epiphysis . Typically, with a Salter-Harris I or II fracture of the proximal humerus, the epiphyseal fragment rotates medially because of unopposed pull by the rotator cuff. Salter-Harris III and IV fractures of the proximal humerus are extraordinarily rare. Avulsion of the greater tuberosity is a Salter-Harris III fracture. Avulsions of the lesser tuberosity caused by hyperextension with avulsion of the subscapularis tendon are rare, often delayed in diagnosis, and are best delineated by an axillary view.
In very young children, fractures of the humeral diaphysis may be incomplete fractures; however, beyond the toddler years, most fractures of the humeral diaphysis are complete fractures. The humerus is one of the more common sites for a pathologic fracture because of its propensity to develop solitary bone cysts. The positioning and alignment of the fragments of a humeral fracture are dependent on the site of fracture and its relationship to the deltoid and pectoral muscular insertions.
Proximal humeral fractures are usually treated nonsurgically, including those with significant angulation and displacement. Fractures will heal and remodel without long-term orthopedic sequelae. However, some advocate operative management in certain situations. Reduction may be necessary in patients near skeletal maturity if the fracture has more than 50 to 70 degrees of angulation in the coronal or sagittal plane. Operative intervention also is indicated in those patients with associated neurovascular injury or who have intraarticular or open fractures.
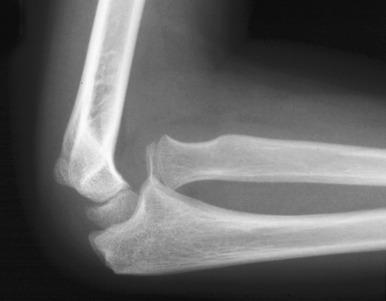
The complex anatomy of the elbow joint and the immaturity of the pediatric skeleton make this joint particularly susceptible to injury. Complications, although uncommon, include neurovascular injury, malunion, and compartment syndrome.
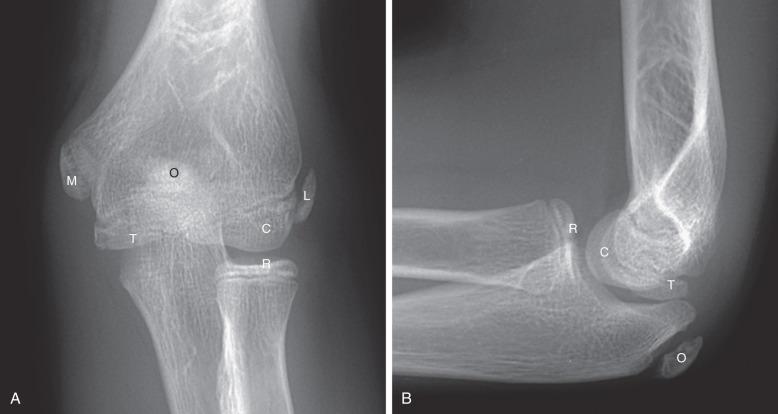
The normal, orderly progression of the appearance of the ossification of the six major ossifications centers of the elbow ( Fig. 142.17 ) is as follows: capitellum (~1–2 years), radial head (~2–4 years), medial (internal) epicondyle (~4–6 years), trochlea (~9–10 years), olecranon (~9–11 years), and lateral (external) epicondyle (~9.5–11.5 years). This can be remembered by the mnemonics CRMTOL or CRITOE. With very rare exceptions, the medial epicondyle ossifies before the trochlea. If the trochlea is ossified, so should be the medial epicondyle. Fusion of the elbow ossification centers is less orderly, occurring after puberty.
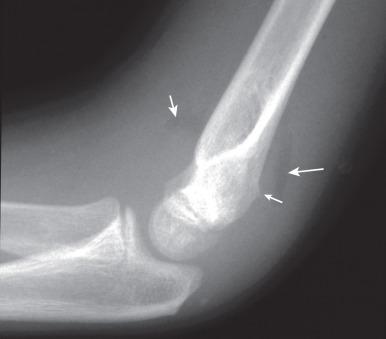
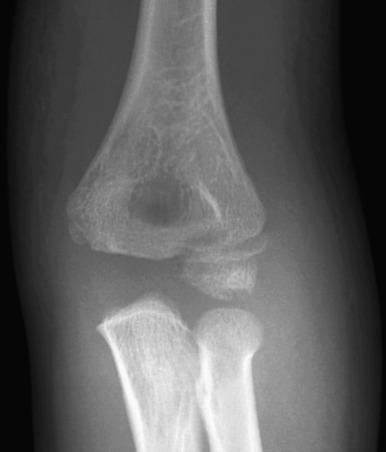
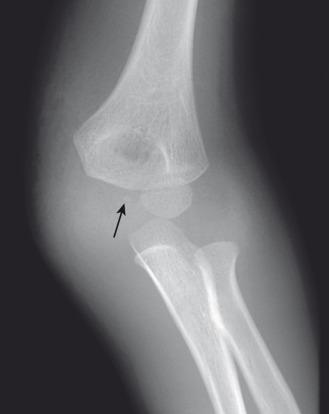
Patients with acute fractures at the elbow that extend into the synovial cavity will usually have an elbow joint effusion. The effusion causes displacement of the anterior and posterior fat pads of the elbow ( Fig. 142.18 ). Although the anterior fat pad is seen normally, it may appear elevated by an effusion (“sail sign”). The posterior fat pad normally resides within the olecranon fossa and is not seen on radiographs unless a joint effusion is present causing posterior displacement. The presence of an elbow joint effusion is strong evidence for the presence of a fracture. Usually, the fracture is obvious. In younger children, a subtle buckle or greenstick fracture of the supracondylar distal humerus may occur. In older children, a subtle fracture of the radial head or radial neck may occur. The presence of an effusion is not unequivocal evidence for a fracture. However, as fractures are often subtle or occult, the presence of an effusion without an identifiable fracture usually prompts splinting of the arm with follow-up radiographs to assess for healing of an occult fracture.
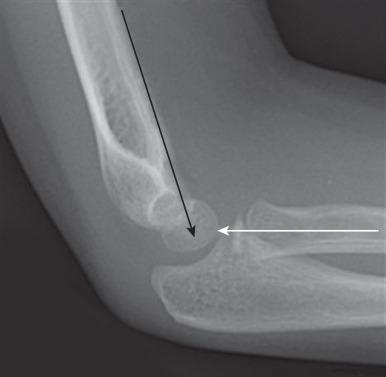
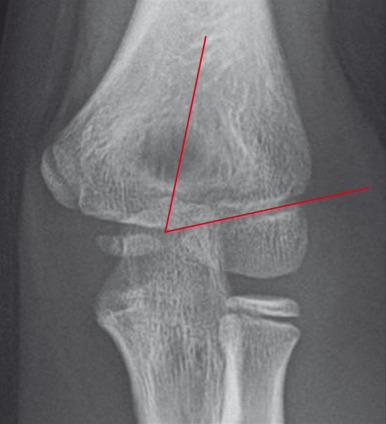
On a properly positioned lateral view, the anterior humeral cortical line is drawn along the anterior cortex of the humerus. This line should pass through the middle third of the capitellum. Disruption of this relationship aids in the detection of supracondylar fractures, which are usually posteriorly angulated and displaced. The radiocapitellar line is drawn along the axis of the radius. Regardless of patient positioning or projection, this line should pass through the capitellum ( Fig. 142.19 ). Disruption of this relationship aids in detection of radiocapitellar dislocation. Other reasons for radiocapitellar line disruption include lateral condylar, radial neck, and Monteggia fractures.
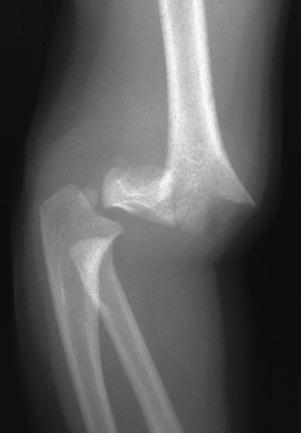
Supracondylar fractures of the distal humerus account for 60% of pediatric elbow fractures. The usual mechanism of injury is hyperextension with impingement of the olecranon on the posterior distal humerus.
The distal fragment of a supracondylar fracture is often displaced or angulated posteriorly. As a result, the anterior humeral cortical line will not bisect the capitellum. If this line passes through the anterior third of the capitellar ossification center or anterior to it, then a supracondylar fracture is likely present.
The modified Gartland classification system of supracondylar fractures is the most commonly used to succinctly describe the fracture and for treatment planning. Type I supracondylar fractures are nondisplaced or minimally displaced less than 2 mm and have an intact anterior humeral line (see Fig. 142.18 ). Type II fractures are displaced greater than 2 mm with angulation and disruption of the anterior humeral line, but with an intact posterior cortex. Type III fractures are displaced with no cortical continuity ( e-Fig. 142.20 ). Most type II and all type III fractures are treated surgically. The risk of developing trochlear epiphyseal osteonecrosis increases with delayed reduction. Neurovascular injury may occur with displaced supracondylar fractures.
Inadequately reduced supracondylar fractures may cause clinically significant limitations in elbow flexion. After reduction, supracondylar fractures often demonstrate a mild degree of posterior displacement or angulation. This will not adversely affect functional outcome; however, fusion with loss of normal cubitus valgus will potentially inhibit the range of motion of the elbow. Normally, slight lateral angulation of the radius exists relative to the humerus (cubitus valgus) and is greater in females. The Baumann angle is created by the intersection of the humeral axis with a line tangent to the physis of the capitellum ( e-Fig. 142.21 ). This angle can be used to reliably predict the final carrying angle of the elbow after supracondylar fractures. The normal angle ranges from 64 to 81 degrees. With posttraumatic cubitus varus, the Baumann angle is increased. An increase in the Bauman angle compared with the contralateral, unaffected elbow is more reliable than using an exact number to predict cubitus varus.
Conservative versus operative management depends on the degree of displacement, age of the patient, location and stability of the fracture, and associated injuries. Most type I or nondisplaced supracondylar fractures can be treated conservatively in a long arm cast. Most type II and III supracondylar fractures are treated operatively with closed reduction and percutaneous pinning.
Lateral condylar fractures are the second most common type of fracture of the pediatric elbow, accounting for 12% to 20% of pediatric elbow fractures. The mechanism is hyperextension with varus stress. Lateral condylar fractures which extend through the distal humeral epiphysis are Salter-Harris IV fractures.
An internal oblique view may better delineate the lateral condylar fracture. The fracture may or may not extend through the unossified portion of the distal humeral epiphysis ( e-Fig. 142.22 ). “Stable” lateral condylar fractures (type I) do not traverse the cartilaginous epiphysis and are incomplete and thus nondisplaced or minimally displaced ( Fig. 142.23 ). Type II lateral condylar fractures are complete and thus “unstable” but with little or no displacement. The lateral condylar fracture line may be quite subtle, often paralleling the adjacent metaphyseal margin. Both MRI and ultrasonography have proven capable of demonstrating the fracture through epiphyseal cartilage indicating a Salter-Harris IV fracture. Type III fractures are complete and “unstable”, and the lateral condylar fragment is displaced and rotated; e-Fig. 142.24 .
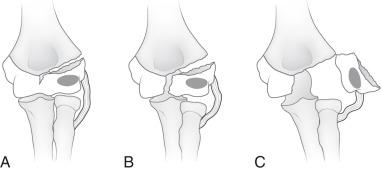
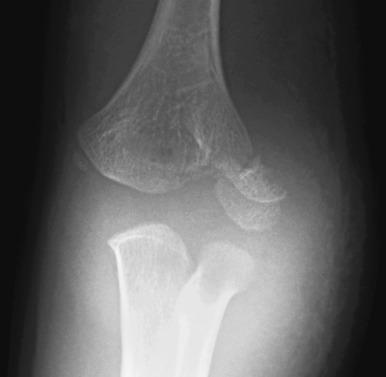
Lateral condylar fractures that are nondisplaced (stable) or are displaced 2 mm or less are managed with cast immobilization. Those that are displaced greater than 2 mm (unstable) are treated with surgical fixation with lateral entry pins. Physeal growth arrest and posttraumatic osteoarthritis are more common complications of lateral condylar fractures compared with supracondylar fractures. The most common long-term deformity is relative lateral overgrowth with subsequent cubitus varus.
The medial epicondyle ossifies by around age 7 years and fuses by around age 16 years. The medial epicondyle is the origin of the forearm flexor mechanism and ulnar collateral ligament. Avulsions thus occur within this age range, although rare avulsions of the unossified medial epicondyle have been reported. Medial epicondyle avulsions may occur secondary to a single acute injury, including elbow dislocations, or from chronic repetitive stress, as is seen in skeletally immature throwing atheletes. The avulsion occurs because of hyperextension, with valgus stress producing traction on the apophysis by the flexor tendons and pronators. In the setting of an acute avulsion, the child will experience sudden-onset medial elbow pain and have point tenderness over the medial epicondyle. Rarely, the avulsed medial epicondyle may displace into the joint mimicking a trochlear ossification center. This occurs because the valgus stress temporarily widens the joint.
Initial assessment should include anteroposterior, oblique, and lateral radiographs of the elbow ( Fig. 142.25 ). With elbow dislocation in the skeletally immature patient, the medial epicondyle is often avulsed from its normal location. When assessing the images of a dislocated elbow, the status of the medial epicondyle should be specifically addressed ( e-Fig. 142.26 ).
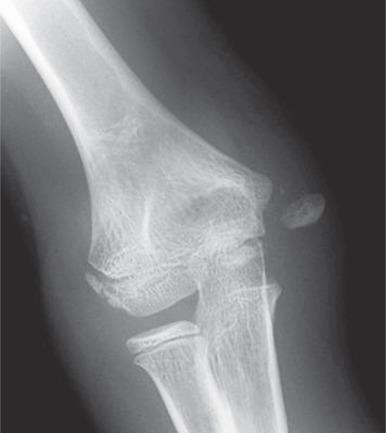
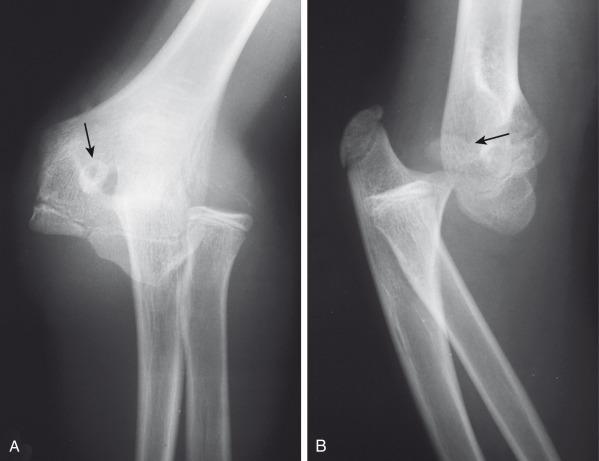
Some cases of medial epicondyle avulsion fracture occur with a transient, unrecognized dislocation. When an elbow dislocation is reduced, the medial epicondyle may be trapped in the elbow joint ( Fig. 142.27 ). A trapped medial epicondyle may superficially mimic a trochlear ossification center in the first decade of life. However, absence of the medial epicondyle at its normal location should prompt a search for it within the joint. If the trochlea is ossified, the medial epicondyle should be as well. This illustrates the importance of the mnemonics CRMTOL or CRITOE for remembering that the medial (internal) epicondyle typically ossifies before the trochlea.
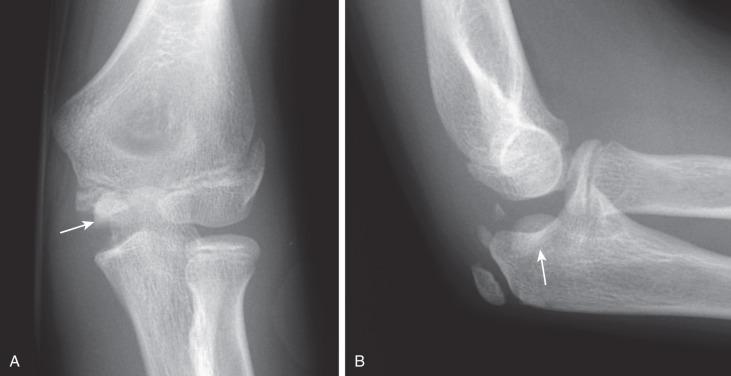
Comparison views may be helpful in confirming mildly displaced medial epicondylar avulsion injuries at the level of the medial epicondyle physeal equivalent region; however, they are usually not necessary. Avulsion of the medial epicondyle before its ossification is distinctly rare but has been reported. Ultrasonography, CT, or MRI can be used for fracture delineation.
Medial epicondyle avulsion fractures can be treated conservatively if the avulsed fragment is not intraarticular, if the child is less than 5 years of age or the degree of displacement is less than 4 mm. Generally, the need for intervention increases with the age of the child, degree of dislocation, and athletic activity.
Distal humeral Salter-Harris I fracture is an uncommon fracture that occurs from birth to approximately age 7 years, peaking at age 2.5 years. It is typically an injury of infants and young toddlers, and is caused by child abuse in half of the cases or by a rare birth injury.
Radiographically, the fracture may be mistaken for a dislocation of the elbow, as the bones do not appear to align. However, the radial axis will be normally aligned with the capitellum, but the capitellum itself will be abnormally related to the distal humeral metaphysis ( e-Figs. 142.28–142.30 ). The radius, ulna, and humeral epiphysis will be medially displaced. Fractures may occur before capitellar ossification.
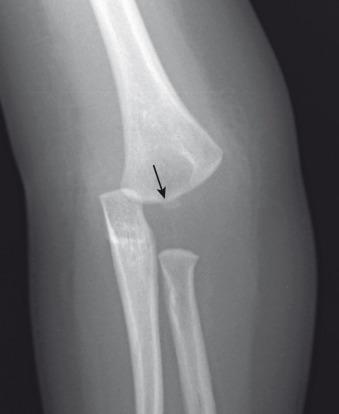
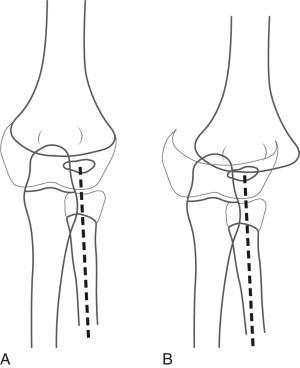
Nondisplaced fractures will be treated with splinting or percutaneous pinning. Displaced fractures usually are treated with open reduction and internal fixation.
Fractures of the radial head and neck account for 5% of pediatric elbow fractures. Mechanism of injury is falling on an outstretched hand. Fractures in children often occur at or just distal to the physis; this is unlike the adult population where fractures typically involve the radial head. Associated injuries include fracture of the olecranon, avulsion of the medial epicondyle, or medial collateral ligament injury.
Radiographs are usually sufficient for diagnosis and follow-up. External oblique views best delineate the radial head and neck morphology. Radial neck fractures are usually buckle fractures or Salter-Harris II fractures. Isolated radial neck fractures as well as fractures with extension to the radial head are unusual. The radiocapitellar joint is often preserved even when radial neck fractures are displaced and angulated.
Most pediatric radial head and neck fractures can be treated nonoperatively with closed reduction and immobilization. If there is greater than 30 degrees of residual angulation, greater than 3 to 4 mm of displacement at the fracture site or less than 45 degrees of pronation or supination, then operative intervention is suggested.
Olecranon fractures are relatively uncommon accounting for approximately 4% to 6% of elbow fractures in children. Common mechanisms include falling on an outstretched hand, twisting injury, or direct trauma. The olecranon is the insertion site of the triceps muscle and prone to avulsion fractures. Olecranon fractures commonly have associated injuries, including radial neck fractures, medial epicondylar fractures, coronoid fractures, and osteochondral injuries.
Radiography is usually sufficient for defining olecranon fractures. Olecranon fractures may be transverse, oblique, or longitudinal. Buckle, bowing, or greenstick fractures are common in younger patients.
The olecranon physis and apophyseal ossification centers vary considerably in size, location, and degree of fragmentation. The juxtaphyseal fragmentary appearance of the olecranon ossification center may be mistaken for fracture, and vice versa. Comparison views may be helpful. Olecranon ossifications are well corticated and appear oval or round. Olecranon fractures have a thin, sliver-like appearance, are usually not corticated, and are located near and parallel the olecranon metaphyseal equivalent zone ( e-Fig. 142.31 ). Other olecranon injuries include stress fractures and sleeve fractures. An olecranon sleeve fracture is an avulsion of the triceps tendon from the olecranon process ( e-Fig. 142.32 ). Stress fractures of the olecranon physis occur in adolescent baseball pitchers. Nondisplaced stress fractures of the olecranon most commonly occur in adolescence and may require MRI for diagnosis.
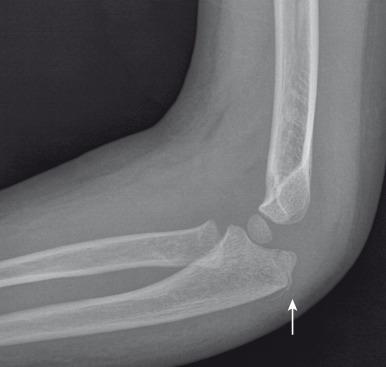
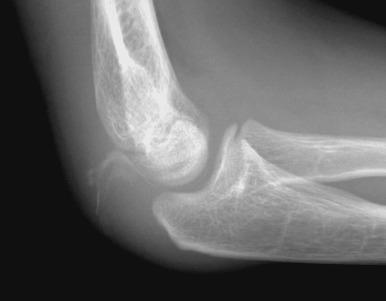
Nondisplaced or minimally displaced olecranon fractures are treated conservatively with immobilization and splinting. Displaced fractures are treated with tension wiring across the apophysis rather than screw fixation.
Nursemaid's elbow occurs due to an upward pull on an extended elbow in pronation. The annular ligament of the proximal radius is disrupted or displaced, allowing the radial head to sublux or dislocate anteriorly relative to the capitellum ( e-Fig. 142.33 ). This injury most commonly occurs in the toddler years, up to age 5 years.
An astute pediatrician will make the diagnosis clinically and reduce the radius without imaging. Often, when imaging is requested, the dislocation is reduced in the course of properly positioning the hand in supination for the anteroposterior view of the elbow. Ultrasonography may also play a role in detecting dislocation of the radial head with respect to the annular ligament. The utility of radiography is not to make the diagnosis of nursemaid's elbow, but to exclude an underlying fracture. After nursemaid's elbow is successfully reduced, the only radiographic sequelae may be a joint effusion without underlying fracture.
When radiographs demonstrate a persistent anterior dislocation despite attempts at reduction, a congenital radial head dislocation should be considered, which has characteristic findings including a convex radial head, small capitellum, and concave shaped posterior margin of the olecranon.
MRI may be indicated when radiographs show persistent radial head dislocation despite attempted reduction to look for obstacles to reduction such as an entrapped annular ligament.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here