Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Conventional gamma cameras use one to three detectors, based on a NaI scintillation crystal and a photomultiplier tube array, that rotate around the patient.
Cameras commonly use parallel-hole collimators for which sensitivity is constant, but spatial resolution degrades as the distance from the collimator increases.
New cardiac SPECT designs use a variety of techniques, including CZT semiconductor detectors, novel collimators, and large numbers of detectors to increase sensitivity.
Compared with conventional cameras, new cardiac SPECT systems have four to eight times the sensitivity and similar or improved spatial resolution.
3D SPECT images are reconstructed from a set of 2D projection data using the FBP algorithm or iterative reconstruction.
Important factors that degrade image quality are gamma ray attenuation and scatter; spatial-resolution loss, which increases with increasing distance from the collimator; patient motion; and image noise.
Iterative reconstruction provides a mechanism to correct for the effects of attenuation, scatter, and collimator resolution losses.
Attenuation correction requires a spatially registered transmission map of the patient tissues, which is most commonly acquired with a CT scan.
Noise in the acquired projections is Poisson distributed, which means that the variance (σ 2 ) in the number of gamma rays detected in a pixel is equal to the number of detected gamma rays (N): σ 2 = N.
Using ECG gating divides the detected gamma rays into separate projection data sets (8 to16 data sets for SPECT and up to 32 data sets for planar imaging) based on the time that has passed since the most recent R-wave of the ECG signal.
ECG gating decreases image blurring caused by cardiac contractile motion (but increases image noise) and provides information on cardiac function (e.g., ejection fraction and wall motion).
Cardiac SPECT instrumentation continues to evolve with ongoing research into the development of dynamic SPECT imaging and respiratory motion correction.
The modern gamma camera traces its origins back to the design introduced by Hal Anger in 1958. , Since then, camera instrumentation has undergone a slow evolution that has continuously improved both its performance and capabilities. Rotating gantry systems have allowed for three-dimensional (3D) single photon emission computed tomography (SPECT) in addition to two-dimensional (2D) planar imaging. The use of multiple detector heads has improved the sensitivity (i.e., detection efficiency) of cameras and reduced scan times. Gating based on the electrocardiogram (ECG) has provided information on cardiac function. Advanced iterative reconstruction algorithms have improved image quality and provided a means to compensate for degrading factors, such as photon attenuation and scatter. More recently, new detector technology has led to the development of novel camera configurations that are further increasing sensitivity and temporal resolution. This chapter provides a brief overview of the hardware and software used to create cardiac SPECT images.
SPECT imaging provides a picture of how radiotracers (tracers labeled with a radioactive isotope) are distributed in a patient’s body. The radioisotopes produce high-energy gamma rays that are invisible to the naked eye and so special radiation detectors are required to detect them. Each detector provides information about the energy and position of a detected gamma ray. Important detector characteristics that influence image quality are the detector efficiency, which is the number of incident gamma rays that are detected; the energy resolution to discriminate against scattered and background radiation; and the intrinsic spatial resolution to locate the position of the detected event on the detector surface. Detectors in cardiac SPECT are based on either scintillation or semiconductor materials.
The most commonly used detector material is the scintillation crystal that converts energy from each gamma ray (high-energy photon) into many low-energy photons, which are subsequently converted to an electronic signal using a light sensor ( Fig. 1.1 ).
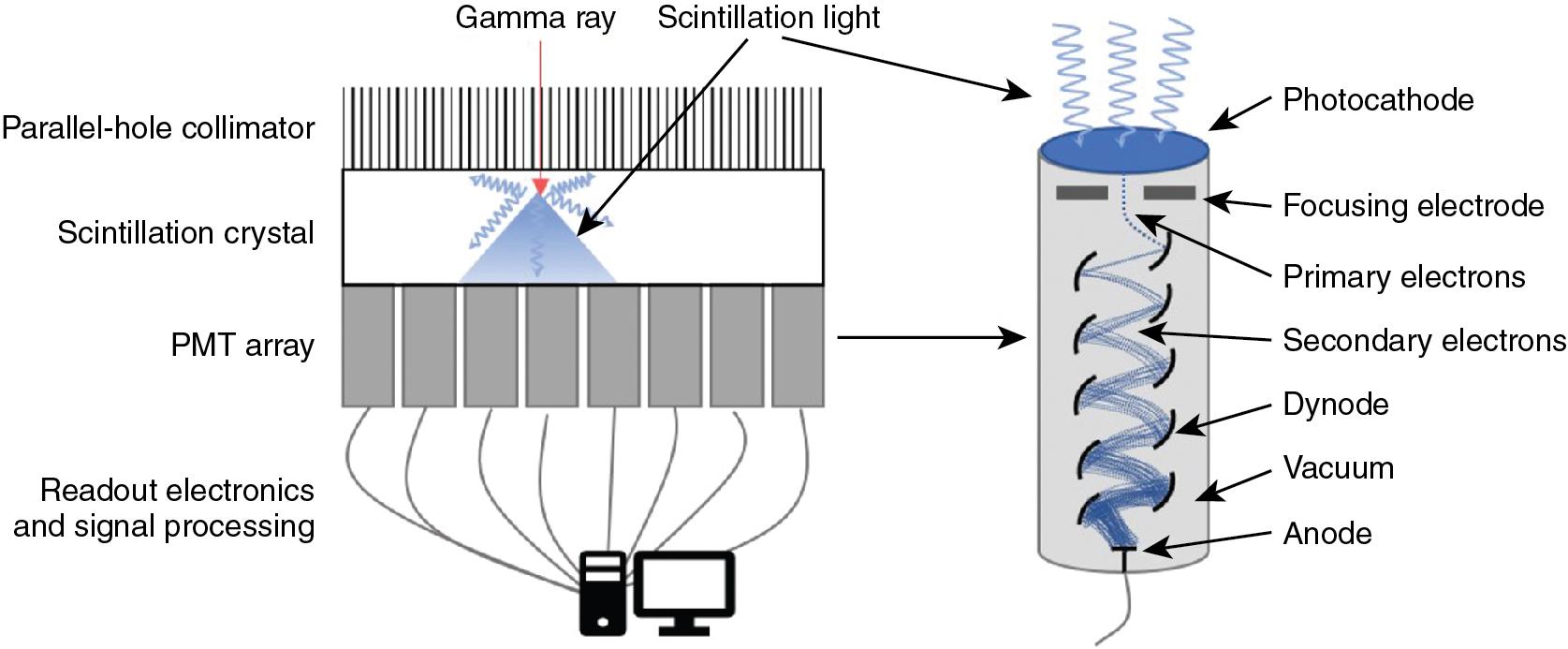
Scintillation materials emit light (low-energy photons) when they interact with gamma rays. Desirable features in a scintillator are a high density to ensure a high efficiency for interacting with gamma rays, a high light yield (number of information carriers), good transparency to those photons to ensure a high energy resolution, and a fast response to process each event quickly to be ready for the next interaction (low dead time). Most SPECT scintillation detector–based systems use sodium iodide (NaI) inorganic ionic crystals or, less commonly, cesium iodide (CsI) crystals. NaI crystals yield 41,000 photons per gamma ray MeV, whereas CsI crystals yield 64,000 photons per MeV. High numbers (N) of scintillation photons are desirable because the gamma ray measurement uncertainty σ is governed by Poisson counting statistics for which σ 2 is proportional to N.
Scintillation detectors produce an electronic signal proportional to the energy of each gamma ray by coupling a light sensor to the scintillation crystal. A photomultiplier tube (PMT) is a light sensor that contains a photocathode and series of dynodes (see Fig. 1.1 ). The photocathode absorbs scintillation photons and relays their energy to ionized electrons. These primary electrons are focused onto the first dynode in the PMT where their kinetic energy ionizes secondary electrons. Electric fields within the PMT accelerate the resulting electrons through a series of dynodes under a vacuum. The number of electrons is increased approximately five-fold after each interaction with a dynode. With 8 to 12 dynodes in a typical PMT, the total signal amplification is approximately 10 6 or 10 7 . The electrical signal read from the back of the PMT is proportional to the amount of incident scintillation light, which is, in turn, proportional to the energy of the detected gamma ray. The PMT signal is, therefore, calibrated to provide a measurement of the gamma ray energy.
For some applications, solid-state light sensors are desired. Avalanche photodiodes (APDs) are silicon-based semiconductors across which a high electric field (>10 7 V/m) is used. Inbound photons liberate an electron in the material to which the electric field provides enough energy to produce an additional electron-hole pair. Subsequent electrons are also accelerated to create more electron-hole pairs. This signal amplification is known as the avalanche effect . Increasing the electric field increases the amount of amplification. The electronic signal obtained from an APD, whose electric field is set to generate an avalanche, is proportional to the number of scintillation light photons detected. APDs are typically around 2 mm thick and have an area up to 30 mm × 30 mm. Higher electric fields lead to an uncontrolled avalanche, allowing APDs to be used like a Geiger-counter such that the signal is independent of the number of photons that interact within the time it takes the detector to reset. Silicon photomultipliers (SiPMs) use arrays of a lot of very small area APDs (side length of 20 to 100 μm) in Geiger-mode to count the number of interacting light photons. The electron signal obtained from a SiPM is proportional to the number of APD cells activated, which is proportional to the number of scintillation light photons, which is, in turn, proportional to the energy of the detected gamma ray. The detectors must be calibrated to the specific expected gamma ray energy. This is important because, for higher gamma energies, there is an increased potential for event pile-up, which is when more than one scintillation photon interacts with an APD cell that can only count one photon at a time. Event pile-ups produce less APD cell activations than there are scintillation photons which can lead to the underestimation of gamma ray energy.
Most clinical SPECT systems use PMTs; however, some small animal systems or evolving research cameras may employ APDs or SiPMs. Solid-state light sensors are much smaller than PMTs, allowing for compact camera designs. When used with appropriate electronics, they can also be used in magnetic fields to enable the development of hybrid SPECT–magnetic resonance imaging (MRI) cameras, which is something that is not possible with PMTs.
A scintillator paired with a PMT produces around 10 information carriers per keV of gamma ray energy. With a scintillator and solid state light sensor, around 29 carriers are produced per keV, allowing for improved energy resolution.
The scintillation light from the detector crystal spreads from the point where the gamma ray interacts with the crystal. The spreading light shower illuminates more than one light sensor and the amount of light seen by a light sensor depends on its distance from the point of interaction. Using the known positions of the light sensors and a weighted combination of the signals measured by each, the location of the point of interaction of the gamma ray with the scintillation crystal can be calculated. The energy and location of the detected gamma ray are recorded and used to build up a 2D picture, also known as a “projection,” of the distribution of the radioisotope in the patient.
Cadmium-zinc-telluride (CdZnTe or CZT) semiconductor detectors directly convert gamma rays into electronic signals. CZT material is sandwiched between a front cathode and an array of pixelated anodes at the back surface. Incoming gamma rays ionize the CZT material to create e-h pairs within the detector. A high voltage is applied across the detector to collect electrons at the anodes. The voltage is set high enough to minimize recombination of electrons with holes, which could result in lost signal and a perceived reduction in the energy of the detected gamma ray. Nevertheless, it is not chosen to be high enough to induce Geiger breakdown like SiPM light sensors do. Thus, the charge collected at an anode is assumed to be proportional to the energy of the detected gamma ray. The single step conversion of gamma ray energy produces around 333 information carriers per keV. Even with some lost signal from charge recombination or lateral drift of charges to spread the signal between anodes, the energy resolution of CZT detectors (6% at 140 keV) is much better than that of scintillation detectors (10% at 140 keV for NaI-PMT). ,
Gamma rays from radiotracers in the patient spread out in all directions such that a 2D image formed on a bare detector would be irrevocably blurred. To provide a clear 2D view, we need information about the trajectory of the detected gamma rays. Collimators provide this context by restricting the angle of the gamma rays that are allowed through to the detector. With a collimator mounted to the surface of a detector, the gamma rays that are detected are known to have traveled a path within a narrow range of angles.
Parallel-hole collimators allow for the detection of gamma rays traveling perpendicular to the detector surface. The collimator has a densely packed array of parallel holes in a high-density material. The diameter of the holes, spacing between holes, and collimator thickness (or hole depth) dictate the resulting spatial resolution and the sensitivity for detecting gamma rays. Fine detail (better spatial resolution) is provided by thick collimators with small-diameter holes. This arrangement, however, drastically limits the number of gamma rays detected from a source. In cardiac imaging, low-sensitivity collimators can mean needing higher patient doses or longer imaging times to acquire sufficient counts. Conversely, when using large holes or thinner collimators, the sensitivity is improved but at the cost of a blurrier image ( Fig. 1.2 A). Collimators are described based on the energy of the isotopes they are designed to detect (isotopes used in cardiac SPECT are typically low energy) and their sensitivity/resolution. A common collimator for cardiac imaging is the low-energy high-resolution (LEHR) collimator.
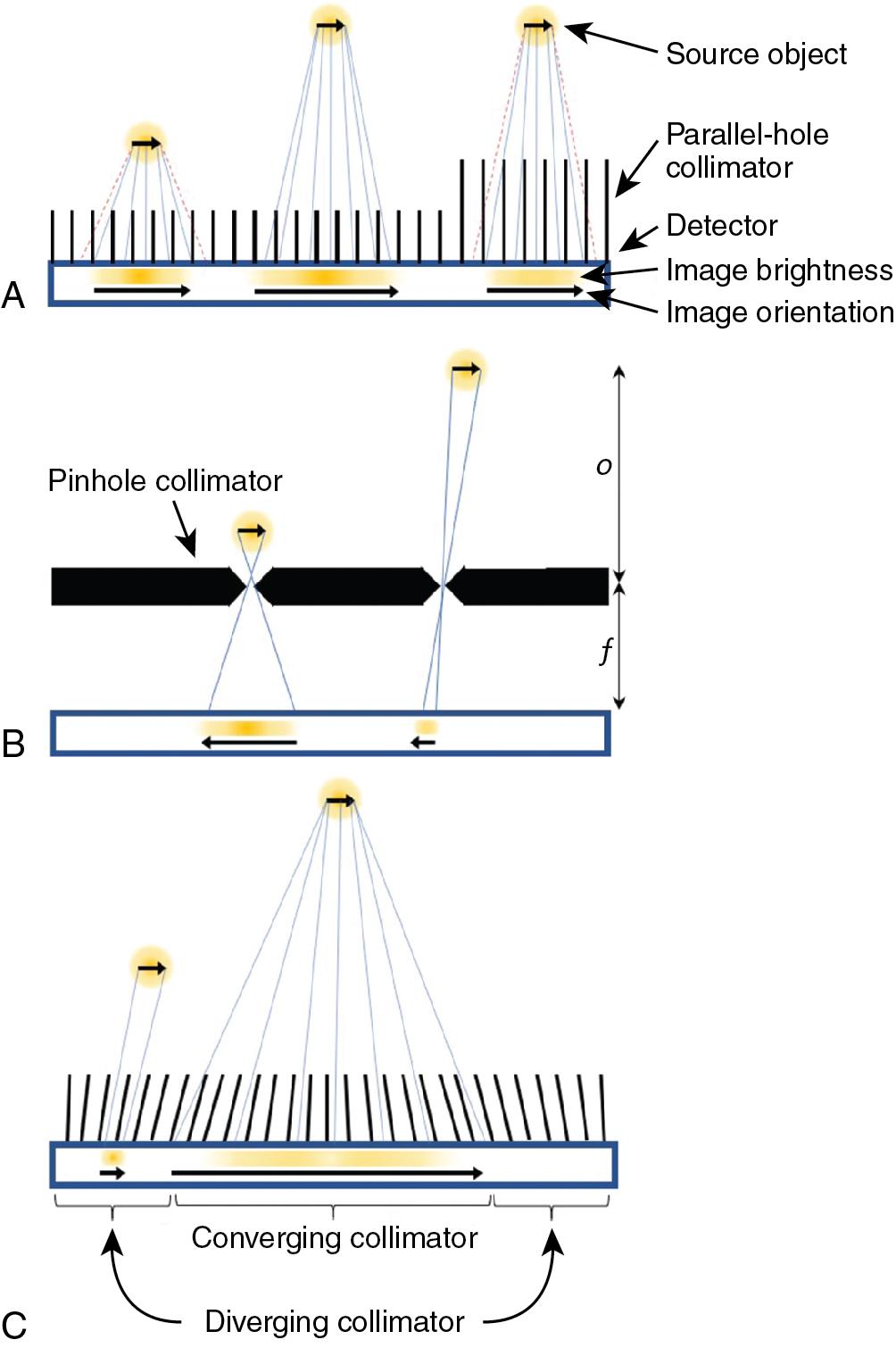
The sensitivity for detecting gamma rays is approximately uniform for varying distances of sources from a parallel collimator. The spatial resolution degrades linearly with distance of the source from the plane of the detector so that an object close to the detector-collimator will be resolved more clearly than an object farther away (see Fig. 1.2 A).
A pinhole collimator has a single hole. Detected gamma rays that have passed through the aperture produce an inverted image of their source (see Fig. 1.2 B). Depending on the ratio of the pinhole-to-detector and detector-to-source distances, the image can either be magnified or minified. Magnification is particularly helpful for small animal imaging systems, whereas minification can allow small-detector-area cameras to avoid truncation of the heart in dedicated cardiac imaging. The spatial resolution of a pinhole collimator-detector depends in part on the aperture diameter and the amount of magnification. The sensitivity for detecting gamma rays depends on the diameter of the pinhole aperture but also on the distance and angle of the source with respect to the pinhole. The sensitivity can be very high for sources close to the pinhole but decreases for gamma rays incident from wider angles and for sources at greater distances. Like the parallel-hole collimator, spatial resolution degrades linearly with distance of the source from the pinhole.
Multifocal collimators are used for specialized applications to improve both sensitivity and resolution compared with traditional parallel-hole collimators using a combination of converging and diverging holes with various focal lengths in a single collimator (see Fig. 1.2 C). The design most relevant to cardiac imaging has holes at the center of the collimator that converge toward the heart and therein sample the heart location more for improved sensitivity and magnify the heart onto the detector for improved resolution compared with parallel hole collimators. Holes closer to the edges of the collimator diverge more the closer they are to the edge until they are nearly parallel, which provides information about surrounding structures and avoids truncation artifacts.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here