Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This category includes a diverse group of neoplasms believed to derive from the hormone-producing ovarian cortical stroma. Based on their predominant cell population and appearance, the World Health Organization (WHO) classification divides them into two main categories: (1) pure lesions, which are in turn divided into pure stromal tumors (with a mesenchymal appearance suggestive of derivation from gonadal stroma; e.g., fibroma, thecoma, and Leydig cell tumor) and pure sex cord (with an epithelioid appearance suggestive of derivation from gonadal sex cords; e.g., granulosa cell tumor and Sertoli cell tumor [SCT]); and (2) mixed lesions ( Box 15.1 ). By far, the most frequently encountered are in the pure stromal subcategory (fibromas and thecomas) followed by tumors in the pure sex cord subcategory (adult granulosa cell tumor [AGCT] being the most common).
Pure stromal tumors
Fibroma
Cellular fibroma
Thecoma
Luteinized thecoma associated with sclerosing peritonitis
Fibrosarcoma
Sclerosing stromal tumor
Signet-ring stromal tumor
Microcystic stromal tumor
Leydig cell tumor
Steroid cell tumor
Pure sex cord tumors
Adult granulosa cell tumor
Juvenile granulosa cell tumor
Sertoli cell tumor
Sex cord tumor with annular tubules
Mixed sex cord-stromal cell tumors
Sertoli–Leydig cell tumor
Sex cord stromal tumors, NOS
This group of tumors is composed predominantly of stromal elements, including those with thecal and fibromatous phenotype. If present, sex cord elements (granulosa cells) must be minor (<10% of the tumor).
This is the most common sex cord-stromal tumor of the ovary, accounting for approximately 70% of tumors in this category, although they only represent ∼4% of all ovarian tumors.
Fibromas may occur at any age but are seen most commonly in middle-aged women, with an average age at presentation in the fifth decade. They are uncommon in women <30 years, except for patients with Gorlin syndrome (nevoid basal cell carcinoma syndrome), an autosomal-dominant disorder characterized by congenital abnormalities (e.g., developmental defects of the skeletal system) and predisposition to multiple basal cell carcinomas, ovarian fibromas (which are frequently bilateral, multinodular, and calcified), as well as other tumors. Most fibromas are incidental findings removed at the time of surgery for an unrelated condition; however, abdominal pain is the presenting symptom in approximately 40% of cases. Fibromas are rarely associated with evidence of steroid hormone production, but they have been reported to be associated with elevated levels of Cancer antigen (CA)125, which not surprisingly raises clinical suspicion for ovarian carcinoma. Fibromas >10 cm may also be associated with ascites in 10%–15% of cases or with Meigs syndrome (ascites and pleural effusion) in a small percentage of patients, both typically disappearing after removal of the tumor.
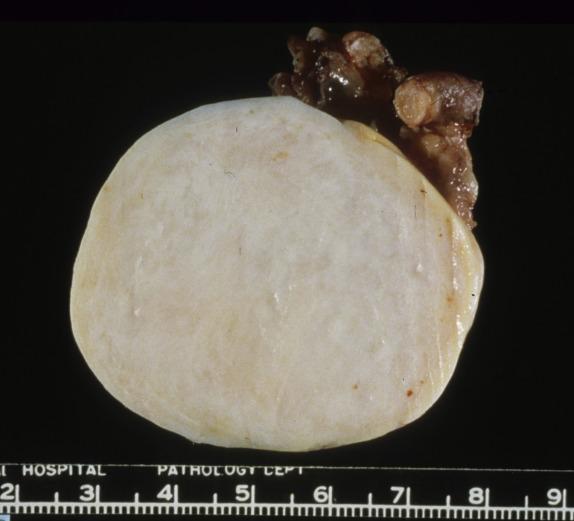
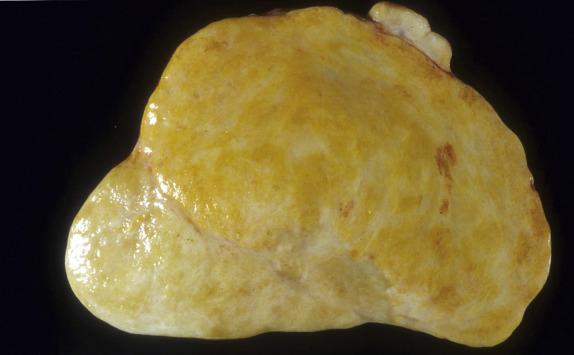
Conventional fibromas are typically unilateral (90%) and may range from microscopic to >20 cm (average 6 cm). They have a smooth or lobulated surface and a homogeneous, firm, white cut section ( Fig. 15.1 ). Edema and pseudocyst formation are frequent; calcification is seen in up to 10% of tumors. Hemorrhage and necrosis are rare. Fibromas associated with Gorlin syndrome, however, are bilateral in approximately 75% and typically show multinodular growth and calcifications in almost all cases. Fibromas that are histologically cellular tend to be larger, and may have a softer, white to yellow cut surface in comparison to conventional fibromas ( Fig. 15.2 ). It is not infrequent for cellular fibromas to be associated with ovarian surface adhesions, adhesions to surrounding structures, or rupture.
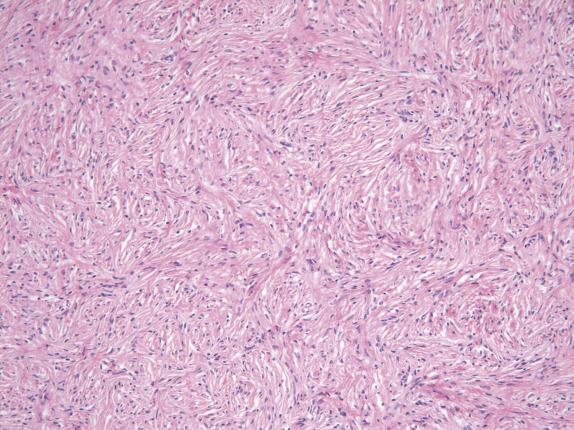
Fibromas are composed of fascicles of bland spindle cells that often display a striking storiform arrangement ( Fig. 15.3 ). The cells have scant cytoplasm and uniform oval to elongated wavy nuclei with pointy ends. The cell population is uniform and monotonous, with minimal cytologic atypia. These tumors are variably cellular depending on the amounts of edema and collagenous stroma. Hyaline bands and calcified plaques are common. Small foci of hemorrhage may be seen, but necrosis is typically absent (except with infarction).
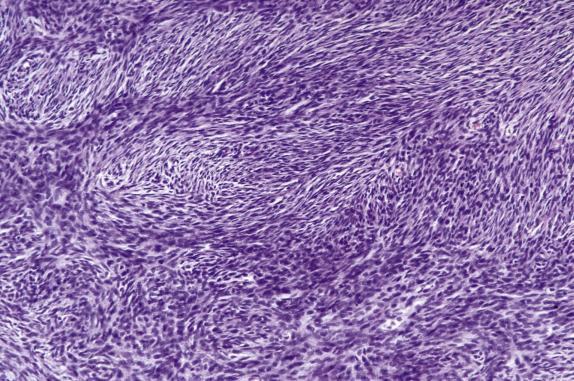
Fibromas with increased cellularity, similar to that seen in diffuse AGCTs, are termed cellular fibromas . Tumors with this degree of cellularity are uncommon and represent approximately 10% of all ovarian fibromas. Cellular fibroma frequently has areas morphologically similar to conventional fibroma; however, in contrast to its typical counterpart, it is much less frequently associated with edema and hyaline bands. Importantly, these tumors show only minimal cytologic atypia, which is the most important diagnostic clue ( Fig. 15.4 ). Another unusual feature is the presence of an increased mitotic rate. Conventional fibromas have on average 1–2 mitoses/10 high-power fields (HPFs); mitotically active fibromas have a mean mitotic rate of 6.7/10 HPFs (ranging from 4 to 19). Importantly, 15% of these tumors have 10–19 mitoses/10 HPFs ( Fig. 15.5 ). Mitotic activity by itself is not indicative of malignancy, as it has been shown that fibromas with mitoses exceeding 3 in 10 HPFs have a similar indolent outcome to conventional tumors ( Fig. 15.5 ).
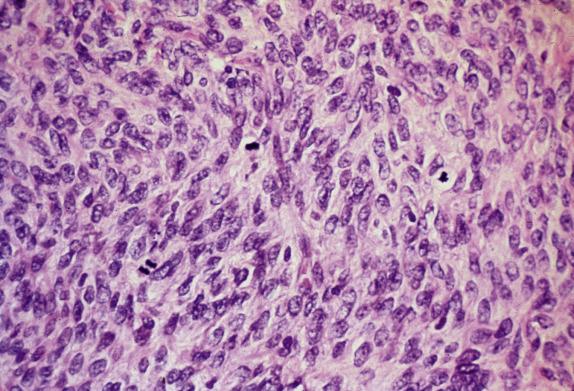
In contrast to conventional fibromas, both cellular and mitotically active cellular fibromas may show necrosis that appears sharply demarcated from the surrounding viable tumor, the latter commonly present in a perivascular location, a morphologic appearance that overlaps with tumor cell necrosis seen in malignant smooth-muscle tumors.
Other unusual findings in fibroma include sex cord-like differentiation comprising <10% of the tumor (termed “fibroma with minor sex cord elements”), intracytoplasmic hyaline droplets, bizarre nuclei and Verocay-like areas. More than one of these unusual features may be present in the same tumor.
Reticulin stain reveals a dense fibrillar network with a pericellular distribution (fibers wrap individual cells). Fibromas typically are positive for FOXL2 (but lack the corresponding mutation), SF1, vimentin, CD56, WT1, estrogen receptor (ER), and progesterone receptor (PR) but are usually negative or weakly positive for CD99 and CD10. Calretinin, inhibin, and smooth muscle actin are also frequently positive, although inhibin is usually patchy in extent. Fibromas are rarely positive for S100, CD34, and desmin.
FOXL2 and DICER1 mutations have not been identified in ovarian fibroma. Patients with Gorlin syndrome have mutations in the human homologue of Drosophila patched gene (PTCH) . Some sporadic typical and cellular fibromas have shown loss of heterozygosity (LOH) at 19p13.3, with the highest frequency at microsatellite marker D9S15 which localizes proximal to the PTCH gene. In one study, LOH at 19p13.3 was seen in 50% of cellular fibromas and 25% of conventional fibromas, but not in fibrosarcomas. These results suggest that sporadic cellular fibromas and fibromas associated with Gorlin syndrome may arise through similar genetic pathways.
Conventional fibroma has morphologic overlap with stromal hyperplasia, massive ovarian edema, and thecoma. In contrast to fibroma, stromal hyperplasia is typically bilateral and does not present as a mass-forming lesion; it is characterized by a diffuse and cellular proliferation of ovarian stromal cells within the cortex and medulla of the ovary, which may exhibit variable nodularity and lacks collagenous stroma and hyaline plaques. Massive ovarian edema and fibroma are usually unilateral and may be histologically quite similar; however, massive ovarian edema does not displace or peripherally compress normal ovarian structures (e.g., follicles, corpora lutea, and corpora albicantia). Fibroma and thecoma may show significant morphologic overlap; indeed, a combination of fibroma and thecoma morphologies within the same tumor is a frequent event (hence use of the term “fibrothecoma”). Unlike thecomas, pure fibromas lack nodular growth and cells with abundant pale or vacuolated cytoplasm; they only express inhibin focally and are negative for CD10. Tumors with an appreciable number of lutein cells in an otherwise conventional fibroma background are best classified as thecoma. Occasionally, fibromas have striking pseudocystic degeneration and may be confused with a serous cystadenofibroma (particularly on gross examination); however, the spaces lack an epithelial lining.
Cellular fibroma and mitotically active fibroma should be distinguished from fibrosarcoma, diffuse adult-type granulosa cell tumor (AGCT), and luteinized thecoma. On gross examination, fibrosarcoma tends to be larger and softer and more commonly shows hemorrhage and necrosis. Furthermore, fibrosarcoma is rare. Morphologic criteria for the distinction between cellular fibroma and fibrosarcoma have evolved over time, and now it is recognized that cytologic atypia is the only reliable feature that distinguishes between these two entities. Fibromas have absent to at most mild nuclear atypia; conversely, moderate to severe nuclear atypia warrants the diagnosis of fibrosarcoma. As mentioned earlier, the mitotic rate can be elevated in indolent fibromas. The border of cellular and mitotically active fibromas is usually smooth; however, if extraovarian extension is present, the tumor interface with involved pelvic tissue may appear infiltrative; by itself, this finding is not diagnostic of malignancy. Like cellular fibroma, luteinized thecoma with sclerosing peritonitis (LTSP) may be densely cellular, lack cytologic atypia, and have a brisk mitotic rate. However, cellular fibromas are more homogeneously cellular; they lack luteinized cells, are typically unilateral, and are not associated with sclerosing peritonitis. Furthermore, hormonal manifestations are common in LTSP but extremely unusual in cellular fibroma. Diffuse AGCTs , which may sometimes have a striking fibromatous background, can be distinguished from cellular fibromas at higher magnification as the cells in the former often show grooved nuclei although this feature alone is not entirely specific. A reticulin stain will characteristically show a pericellular reticulin fiber distribution in fibroma, whereas AGCT has, at least focally, nests of tumor cells devoid of pericellular reticulin. Finding other areas with patterns characteristic of AGCT is also helpful.
Finally, neoplasms that rarely enter the differential diagnosis but should be considered include primary or metastatic endometrioid/endometrial stromal sarcoma (evenly spaced thin arteriolar vasculature, lack of storiform pattern, strong and diffuse CD10, negative calretinin), gastrointestinal stromal tumor (CKIT and DOG1 positivity), and Brenner tumor (solid or glandular nests of squamoid epithelial cells resembling urothelial elements).
Benign fibromatous tumor of varying cellularity composed of spindle to oval collagen-producing cells
70% of all sex cord-stromal tumors (∼4% of primary ovarian tumors)
Typically unilateral
Commonly bilateral if associated with Gorlin syndrome
Secondary torsion
If cellular, may be associated with extraovarian adhesions
Average fifth decade
Younger in patients with Gorlin syndrome
Pelvic mass, abdominal/pelvic pain, ascites, urinary frequency
Increased CA125 serum levels
Meigs syndrome in 1% (ascites and pleural effusion)
Association with Gorlin syndrome (hereditary basal cell nevus syndrome)
Excellent prognosis
Oophorectomy; complete surgical resection if adherent to other structures (e.g., pelvic wall, omentum)
Adhesions and rupture may be associated with recurrence in cellular and/or mitotically active fibroma
Long-term follow-up recommended if cellular and/or mitotically active
Conventional fibromas are benign tumors, and oophorectomy is adequate treatment. Cellular and mitotically active fibromas also behave in a benign fashion even when associated with ovarian adhesions or extraovarian extension. Thus, these tumors should be treated conservatively with surgery. Long-term follow-up is advised, particularly for those patients with tumor rupture or adhesions as they may recur locally.
1–21.5 (average 6) cm
Solid with firm, white cut surface; may be lobulated
Cellular lesions have soft, white to yellow cut surface
Pedunculated or polypoid growth (∼20%)
Edema and pseudocystic change (∼25%)
Hemorrhage and necrosis if complicated with torsion
Bilaterality, multinodularity, and calcification if associated with Gorlin syndrome
Uniform and monotonous spindle cells arranged in storiform pattern
Variable degrees of collagen production with hyaline plaques
Variants:
Cellular (cellularity similar to diffuse AGCT)
Mitotically active (4–19 [mean 6.7] mitoses/10 HPFs)
Other features:
Sex cord–like differentiation (<10% of tumor)
Intracytoplasmic hyaline droplets
Bizarre nuclei
Verocay-like areas
Prominent edema
Reticulin shows a dense pericellular pattern
FOXL2, SF1, vimentin, CD56, WT1, ER, and PR positive
Smooth muscle actin, inhibin, calretinin can be positive (patchy)
desmin, S100, and CD10 typically negative
Negative for FOXL2 and DICER1 mutations
Conventional fibroma
Stromal hyperplasia
Massive ovarian edema/fibromatosis
Thecoma
Cellular mitotically active fibroma
Fibrosarcoma
LTSP
Diffuse AGCT
Thecomas are uncommon, representing less than 1% of all ovarian neoplasms. They typically occur in postmenopausal women, most commonly in the sixth decade, but they may occur at any age, with some presenting in women ≤30 years (more often luteinized thecomas); however, they are rare before puberty. These tumors are commonly estrogenic, and most women present with abnormal vaginal bleeding. Approximately 20% of patients will also develop concurrent endometrial carcinoma. Of note, luteinized thecomas are androgenic in approximately 10% of cases.
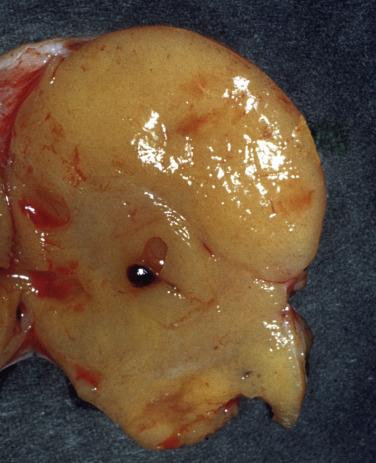
The vast majority of thecomas are unilateral (∼95%) and can range in size from microscopic tumors to masses between 5 and 10 cm. These tumors are typically solid (but may occasionally be cystic) and have a tan-yellow, sometimes lobulated sectioned surface ( Fig. 15.6 ). On occasion, they may show areas of necrosis, hemorrhage, or calcification, the latter more common in tumors occurring in younger women.
These tumors are composed of sheets or nodular aggregates of plump round to spindle cells with pale (lipid rich) vacuolated to eosinophilic cytoplasm with indistinct cell borders, arranged in a vaguely fascicular or storiform pattern ( Fig. 15.7A ). Their nuclei are round to oval with fine, dispersed chromatin and infrequent mitotic figures (<2/10 HPFs). This population resembles the theca interna of a developing follicle. This characteristic cell component can be seen exclusively, or variably admixed with a spindle cell component identical to ovarian fibroma, with scant cytoplasm and lack of vacuolization. The term “luteinized thecoma” has been applied when single or sharply demarcated clusters of lutein cells are present within the tumor. Like thecomatous cells, the lutein cells have abundant cytoplasm but tend to be more eosinophilic than vacuolated and their nuclei typically contain vesicular chromatin and a prominent central nucleolus. Hyaline plaques are often present and more common in between nodules ( Fig. 15.7B ); keloid-like sclerosis can occasionally occur ( Fig. 15.7C ). Stromal calcification may be seen and is sometimes prominent, particularly in tumors occurring in younger women. Minor (<10%) sex cord differentiation can be observed. Rarely, fatty metaplasia and bizarre nuclei may be seen.
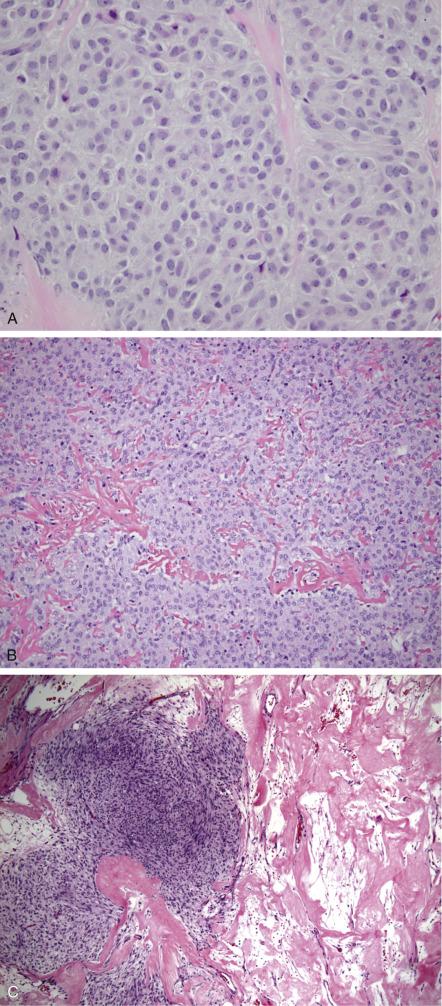
Reticulin stain reveals a dense fibrillar network that surrounds individual tumor cells. Oil red O stain demonstrates intracytoplasmic lipid. Thecomas are typically positive for SF1, vimentin, inhibin, and calretinin and may be CD10 positive, but are negative for CD99, cytokeratin and EMA.
Thecomas are often FOXL2 positive by immunohistochemistry; nonetheless, only 7/45 (15%) of tumors analyzed to date have shown a corresponding FOLX2 mutation; however, it is unclear as to whether these positive cases in fact represent adult granulosa cell tumors with a prominent thecomatous component.
The principal differential diagnostic considerations include fibroma, AGCT (diffuse pattern or with extensive luteinization), and sclerosing stromal tumor.
The distinction between fibroma and thecoma is not only frequently challenging but also largely academic. The presence of a significant thecomatous (lipid-rich) component warrants a diagnosis of thecoma. The diagnosis of fibroma is reserved for those lesions purely or predominantly spindled. The term “fibrothecoma” can be applied to lesions with relatively even admixtures of thecomatous and fibromatous components, although in practice, one component usually predominates, leading to easy categorization as either fibroma or thecoma. The diffuse pattern of adult-type granulosa cell tumor (AGCT) may mimic a thecoma, particularly in cases with prominent luteinization or those with a prominent thecomatous stromal component. AGCT can be distinguished from thecoma by the presence of more typical growth patterns and characteristic nuclear grooves (although this may require careful scrutiny of nuclear morphology) but the most helpful feature is the lack of investment of individual cells by a dense fibrillar network in AGCT, as highlighted by a reticulin stain. Although thecomas are often positive for FOXL2, by immunohistochemistry, they usually lack the corresponding mutation. Thecoma may mimic a sclerosing stromal tumor as it may show a prominent nodular growth and cells with spindle and epithelioid appearance. However, sclerosing stromal tumor more commonly occurs in the first three decades of life and is less commonly associated with clinical manifestations. In addition, sclerosing stromal tumors are more vascular and contain numerous vessels with a hemangiopericytoma-like appearance. In pregnant women, thecomas may undergo extensive luteinization and may mimic pregnancy luteoma . However, the latter lacks a fibromatous or thecomatous background, and the cells contain little or no lipid. Steroid cell tumors typically have a diffuse growth of cells showing well-defined borders and containing abundant lipid vacuoles.
Thecoma (including luteinized thecoma) is a benign tumor, and unilateral salpingo-oophorectomy is considered adequate treatment. Because of their hormone production and association with endometrial proliferation, endometrial sampling should be performed to exclude endometrial neoplasia.
Stromal tumor composed of lipid-containing cells resembling theca cells with a variable fibromatous component
Uncommon
95% unilateral
Abnormal endometrial proliferation due to estrogen production
Conventional thecoma: mostly postmenopausal women (mean age 59 years)
Luteinized thecoma: 30% in patients ≤30 years
Pelvic mass or swelling
Vaginal bleeding (both conventional and luteinized thecoma)
Endometrial adenocarcinoma in one-fifth of patients (secondary to estrogen production)
Virilization (more common in luteinized thecoma)
Benign
Unilateral oophorectomy
Unilateral
<1 to 23 cm; most between 5 and 10 cm
Solid and yellow to gray-white and sometimes lobulated cut surface
Occasional pseudocystic change, focal calcification, hemorrhage, and necrosis
Extensive calcification in young women
Vaguely arranged fascicular or storiform aggregates of round to spindle cells with abundant pale to vacuolated cytoplasm and round to oval nuclei (theca cell component)
Theca cell component predominant or admixed with nonvacuolated spindled cells (fibromatous component)
Luteinized thecoma: single or sharply demarcated groups or lutein cells (abundant eosinophilic cytoplasm, round nuclei with vesicular chromatin, and visible nucleoli)
Minimal cytologic atypia and rare mitotic figures
Hyaline plaques and, less frequently, calcification may be seen.
Unusual features:
Minor sex cord elements
Bizarre nuclei
Fatty metaplasia
Reticulin shows dense pericellular pattern
SF1, inhibin, calretinin, FOXL2, and vimentin typically positive; CD10 can be focal
CD99, EMA, and cytokeratin negative
Typically lacks FOXL2 mutations
Fibroma
Granulosa cell tumor
Sclerosing stromal tumor
Pregnancy luteoma (vs. luteinized thecoma)
Steroid cell tumor (vs. luteinized thecoma)
This unusual process, part of the luteinized thecoma spectrum, is categorized as a neoplasm in the WHO classification; however, some investigators consider it to be nonneoplastic and the term “luteinized thecomatosis” has been proposed.
LTSP is a variant of thecoma that typically affects women in their third and fourth decades. This is an enigmatic entity in which patients present with abdominal swelling and pain, ascites and, in some cases, pleural effusion or symptoms of bowel obstruction. On pelvic examination, they are found to have unilateral or (more frequently) bilateral pelvic masses.
The ovarian lesions may form bilateral large masses (up to 31 cm) or result in only slightly enlarged ovaries ( Fig. 15.8A ), replacing the entire ovary or less commonly involving the cortex. When they form a mass, the tumors typically have a prominent nodular or cerebriform surface and a solid, homogeneous, white to tan-pink and edematous cut section ( Fig. 15.8B ).
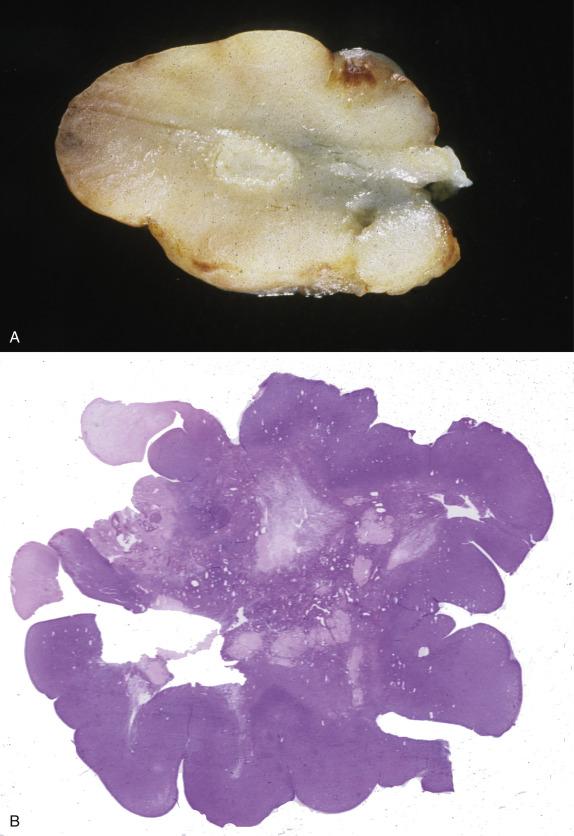
Sclerosing peritonitis, which can involve omentum, peritoneum, and intestinal serosa, appears as areas of thickening up to 2–3 mm, well demarcated from the underlying normal tissue.
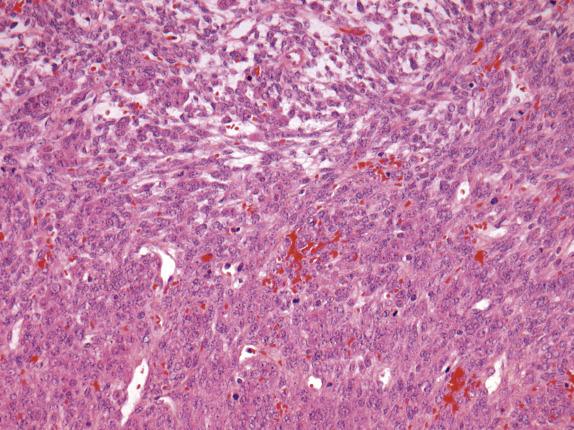
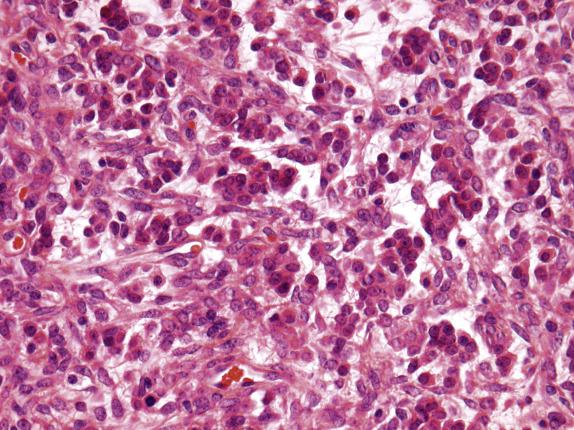
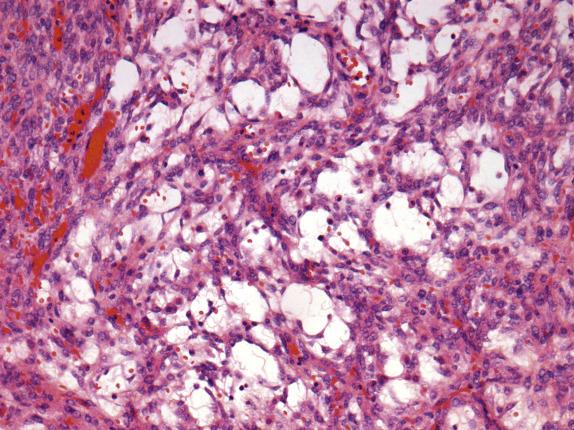
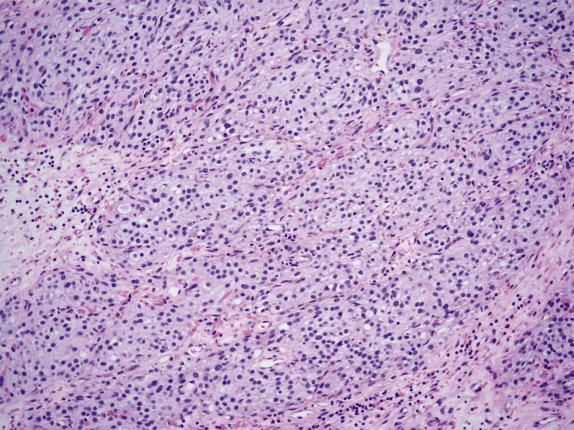
The ovarian lesion is poorly demarcated and is composed of sheets or intersecting fascicles of cells with a fibromatous to thecomatous appearance ( Fig. 15.9 ). In some cases, discrete masses are not present, but rather there is diffuse cortical spindle cell expansion mimicking stromal hyperplasia. Scattered throughout the fibrothecomatous background are distinct small nests and clusters of luteinized stromal cells (hence designation as luteinized thecoma) ( Fig. 15.10 ). These lutein cells have visible eosinophilic cytoplasm and round nuclei with inconspicuous nucleoli. The mitotic rate can be striking (up to 80 mitoses/10 HPFs), sometimes with markedly varied counts between the ovarian lesions on both sides ( Fig. 15.10 ). Significant edema may be seen with secondary cavitation (pseudocyst formation) ( Fig. 15.11 ). Residual entrapped normal ovarian elements are often present within the lesion.
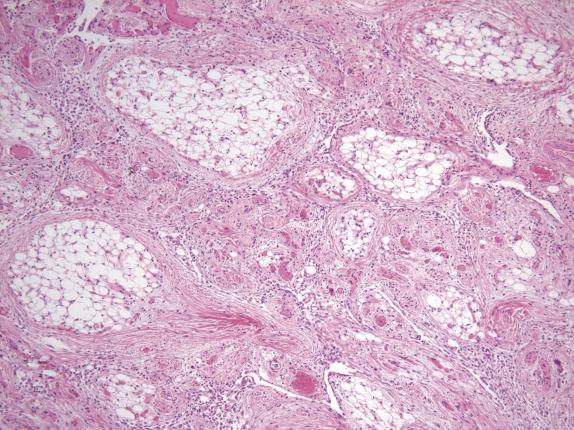
The associated sclerosing peritonitis is composed of a noninfiltrative, superficial proliferation of bland-appearing fibroblasts, which are arranged in fascicular and less commonly storiform patterns with variable cellularity, collagen deposition, and inflammation ( Fig. 15.12 ). Surface fibrin and focal mesothelial hyperplasia may also be present. Extensive involvement of the omentum results in envelopment of individual lobules of adipose tissue by the fibroblastic proliferation, resulting in a dramatic accentuation of the normal lobular architecture.
The luteinized cells are strongly and diffusely positive for inhibin, calretinin, and CD56. The spindle cell component is typically negative for inhibin but positive for SF1 and FOXL2 (but lacks mutation); it also shows variable ER and PR expression and can be focally positive for smooth muscle actin, desmin, cytokeratin AE1/AE3, calretinin, and CD56.
LTSP is part of the morphologic spectrum of luteinized thecoma (and fibrothecoma). It is different from conventional luteinized thecomas in its association with sclerosing peritonitis; at the morphologic level, the lutein cells are smaller than in conventional luteinized thecomas.
Stromal hyperthecosis falls into the differential diagnosis of LTSP because it is almost always bilateral and characterized by the presence of nests of luteinized cells within hyperplastic stroma, and, similarly to LTSP, it can also entrap normal ovarian follicles; however, it is not accompanied by ascites, prominent ovarian enlargement, or obliteration of the ovarian architecture. In addition, the stromal cells in stromal hyperthecosis are not mitotically active, which is in stark contrast to most LTSPs. Although massive ovarian edema may cause significant ovarian enlargement, occasionally may be bilateral, and contain rare foci of luteinized cells, they are typically hypocellular and associated with either diffuse marked edema or collagen deposition. Cellular fibroma can be quite edematous but is distinguished from LTSP because it is typically unilateral, and although it may be associated with ascites, it is not associated with sclerosing peritonitis. Sclerosing stromal tumor is also typically unilateral and differs morphologically from LTSP in that it is composed of a biphasic population of mitotically inactive spindle-shaped and luteinized cells arranged in a characteristic pseudolobular arrangement; in addition, the prominent hemangiopericytoma-like vascular pattern is characteristic. Even though fibrosarcoma can exhibit brisk mitotic activity, it is associated with tight fascicles of highly atypical spindle cells, features not seen in LTSP.
Treatment consists of removal of the ovarian lesion(s), as well as omentectomy, lysis of adhesions, and bowel resection when necessary, to relieve symptoms of obstruction. Rare patients have benefited from hormonal treatment (gonadotropin-releasing hormone agonist) and high-dose corticosteroids. Although it appears that the ovarian lesions are benign with no evidence of recurrence or metastases, a small number of patients have died of complications related to the sclerosing peritonitis.
Densely cellular tumor composed predominantly of fibrothecomatous elements admixed with a less prominent lutein cell component and sclerosing peritonitis
Rare
Typically bilateral but can be unilateral
Bowel obstruction and enterocutaneous fistulae secondary to sclerosing peritonitis
No recurrence or metastases
Death secondary to abdominal and pelvic complications
Reproductive age (mean 25 years; frequently <40 years)
Abdominal pain or swelling
Symptoms secondary to bowel obstruction
Prognosis related to intestinal/peritoneal disease
Bilateral oophorectomy and resection of adhesions
Bowel obstruction managed by surgical intervention, conservative measures, or hormonal therapy
Adhesions increase the risk of bowel obstruction, requiring extensive and multiple surgeries
Typically bilateral
Range from slightly enlarged to nodular ovaries or large masses (up to 31 cm)
Cerebriform surface
Solid and edematous cut surface
Frequent diffuse ovarian involvement
Densely cellular with a predominant diffuse or loose fascicular fibrothecomatous appearance
Minor component composed of groups of small lutein cells
Absent to mild cytologic atypia
Brisk mitotic rate in fibrothecomatous cells (up to 80/10 HPFs)
Not infrequently, edema with pseudocyst formation
Entrapped normal ovarian structures can be observed.
SF1 and FOXL2 positive (but lacks mutation)
Inhibin, calretinin, and CD56 positive in luteinized cells
Smooth muscle actin, desmin, calretinin, CD56, and AE1/3 focally positive
Inhibin negative in spindle cells
Thecoma
Stromal hyperthecosis
Massive edema
Cellular fibroma
Sclerosing stromal tumor
Fibrosarcoma
Sclerosing stromal tumor is a rare benign neoplasm that accounts for 2%–6% of ovarian sex cord-stromal tumors.
This lesion is generally found in patients who are younger than those with either thecomas or fibromas, as 80% are < 30 years of age. Patients often present with menstrual irregularities or symptoms related to the presence of a pelvic mass. Evidence of hormone production, either estrogenic or androgenic, is documented only rarely; androgenic manifestations have been reported more frequently in pregnant patients. Ascites, Meigs syndrome, or elevated serum levels of CA125 are infrequent.
Sclerosing stromal tumor is almost always unilateral and ranges from 1.5 cm to 21 cm in largest dimension. It has a well-defined border that, in some cases, has allowed enucleation of the tumor at surgery. They are white to yellow and typically solid; occasional tumors may have a large central cavity ( Fig. 15.13 ).
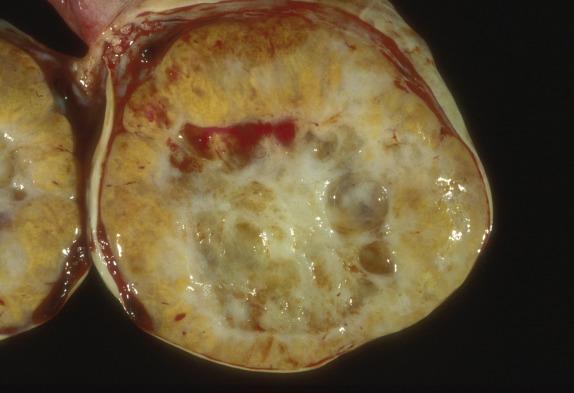
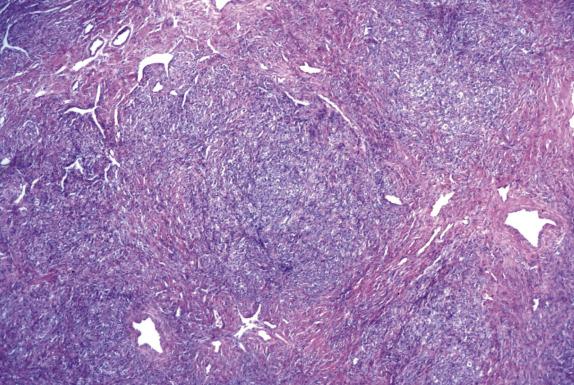
Sclerosing stromal tumor is typically well circumscribed, with a sharply demarcated thin rim of normal ovarian tissue at the periphery. At low magnification, the tumor has a pseudolobular appearance, given by the alternation of cellular nodules with hypocellular edematous to markedly collagenized areas ( Fig. 15.14 ). The cellular nodular areas contain a random and heterogeneous mixture of spindled (fibromatous) cells and clusters of round luteinized cells. The lutein cells have eosinophilic to clear vacuolated cytoplasm and round nuclei with vesicular chromatin and prominent nucleoli ( Fig. 15.15 ). These cells may have a signet-ring appearance. The tumor vasculature is characteristic, composed of numerous thin-walled vessels, some of which can be dilated and branched resembling those seen in hemangiopericytoma ( Fig. 15.14 ). Cytologic atypia and mitoses are rare, with exceptions (more often in pregnant women). Furthermore, in pregnant women, the tumors may contain a larger number of more robustly luteinized cells, which may obscure the typical lobular architecture and dual cell population characteristic of this tumor.
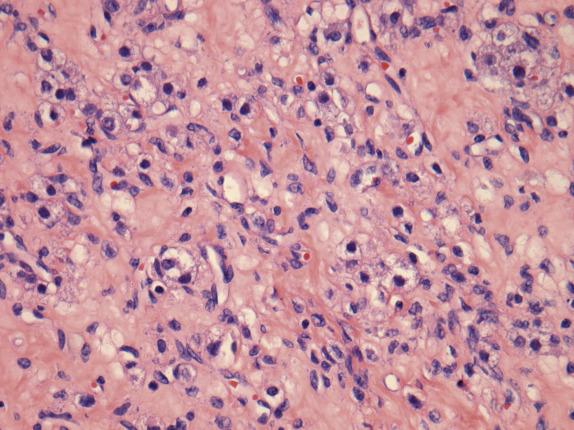
Sclerosing stromal tumors are positive for calretinin and less frequently for inhibin, particularly in the lutein cells. ER, PR, vimentin, and smooth muscle actin are also positive. Keratin and EMA are negative, whereas there are conflicting reports regarding desmin expression.
Depending upon which component is most prominent in the tumor, the entities to be considered in the differential diagnosis can vary. If the spindle cells predominate, a sclerosing stromal tumor may be confused with a fibroma or thecoma. Fibroma tends to occur in older patients (average age is approximately 50 years; <10% are under 30 years of age), and histologically, they lack the pseudolobulation, alternating cellularity, prominent vascularity, and cellular heterogeneity characteristic of sclerosing stromal tumors. Thecomas may be confused with sclerosing stromal tumor as the former may have a lobulated growth; however, thecomas are composed of a uniform population of cells with pale grey cytoplasm and lack the characteristic vascularity of sclerosing stromal tumors. Luteinized thecoma and sclerosing stromal tumor share several clinical and pathologic features. Both occur in young patients in higher frequencies than fibromas and thecomas (30% of patients with luteinized thecoma are 30 years of age or younger); both contain spindled and lutein cells and express inhibin and calretinin. However, the variegated appearance of sclerosing stromal tumor and its prominent thin-walled vessels are absent in luteinized thecoma. Sclerosing stromal tumor can mimic metastatic signet-ring cell carcinoma (Krukenberg tumor) since both lesions have variegated/alternating cellularity of hyper- and hypocellularity, and lutein cells of sclerosing stromal tumors may have a signet-ring cell resemblance. Krukenberg tumors, however, contain mucin and are positive for keratin and EMA, whereas the vacuoles in the signet-ring-like cells of the sclerosing stromal tumor contain lipid. If the lutein cells are arranged in cords, they can mimic metastatic breast carcinoma or carcinoid tumor ; however, both lack the prominent hemangiopericytoma-like vascular network seen in sclerosing stromal tumors. Negative keratin and EMA and positive inhibin and calretinin staining should distinguish sclerosing stromal tumor from these two entities. It should be kept in mind that both sclerosing stromal tumor and metastatic breast carcinoma can be ER and PR positive. Steroid cell tumor may enter into the differential diagnosis when sclerosing stromal tumors are cellular and extensively luteinized; however, the former typically lacks the characteristic admixture of spindled and luteinized cells as well as the striking vascularity. Moreover, the vast majority of sclerosing stromal tumors are not associated with hormonal manifestations. Lastly, sclerosing stromal tumors presenting during pregnancy can mimic pregnancy luteoma, as both lesions usually display prominent luteinization. Again, attention to the variegated appearance and distinct vasculature of sclerosing stromal tumor is most helpful in this differential.
As these tumors are typically benign, simple oophorectomy is adequate therapy.
Benign stromal tumor with cellular areas composed of fibroblasts and lutein cells separated by hypocellular edematous or collagenized areas imparting a pseudolobular appearance
2%–6% of ovarian stromal tumors
<1% of all primary ovarian tumors
Unilateral
Rare torsion
80% of women <30 years of age
Pain, pelvic mass
Very rarely, estrogenic or androgenic manifestations
Benign, excellent prognosis
Unilateral oophorectomy
This is a rare tumor within the category of pure stromal tumors.
1.5 cm–21 cm
Well-defined demarcation from normal ovarian tissue
White to yellow and solid, or solid with pseudocystic cavitation
Occasionally large central cyst
Pseudolobular architecture with alternating zones of cellularity
Cellular nodules composed of spindle (fibromatous) cells and clusters of lutein cells
Numerous thin-walled vessels, some branched and dilated (hemangiopericytoma-like vascular network)
Minimal cytologic atypia and low mitotic rate
Calretinin, ER, PR, vimentin, and smooth muscle actin positive
Variable inhibin and desmin expression
Keratin and EMA negative
Fibroma
Luteinized thecoma
Metastatic signet-ring cell carcinoma (Krukenberg tumor)
Carcinoid tumor
Steroid cell tumor
Pregnancy luteoma
Patients are typically premenopausal, although age range is wide. They usually present with symptoms related to a pelvic mass or it may be an incidental finding. Endocrine manifestations are rare.
The lesion is unilateral and usually <10 cm in maximum dimension (mean 9 cm). It is well circumscribed and typically has a solid appearance; however, small cysts can be grossly apparent. The solid areas often have a lobulated, tan to white cut surface. Foci of hemorrhage and/or necrosis may be seen.
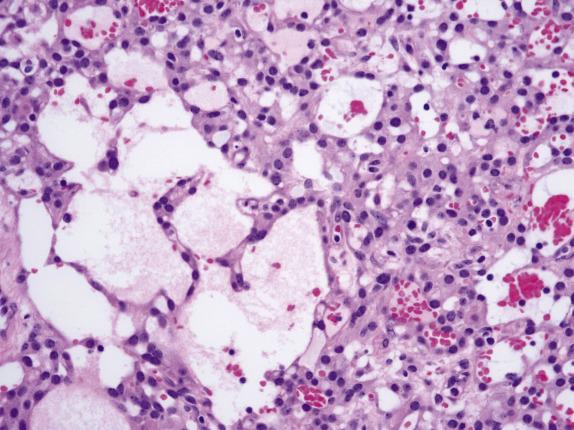
On low-power examination, this neoplasm is composed of densely cellular areas separated by variably sized hyaline bands. The tumor cells are arranged in solid sheets or cords and characteristically form empty cavities (“microcysts”) of various sizes and shapes ( Fig. 15.16 ), which is usually the dominant feature. The microcysts may coalesce and often contain lightly basophilic or clear secretions ( Fig. 15.17 ). The solid areas may vaguely resemble thecoma ( Fig. 15.18 ). Tumor cells have modest amounts of finely granular and lightly eosinophilic cytoplasm and bland round to oval nuclei containing tiny nucleoli ( Fig. 15.19 ). Intracytoplasmic vacuoles are often noted. Bizarre nuclei are not infrequent (present in ∼60% of tumors), and they usually have a patchy distribution within the tumor. Mitotic rate is typically very low, with at most 1 per 10 HPFs. Rarely, psammomatous calcifications can be seen.
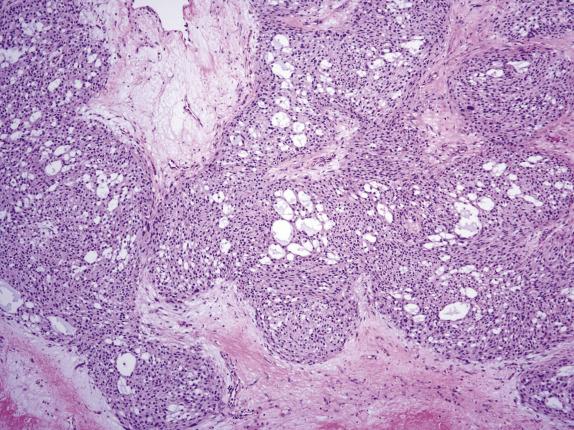
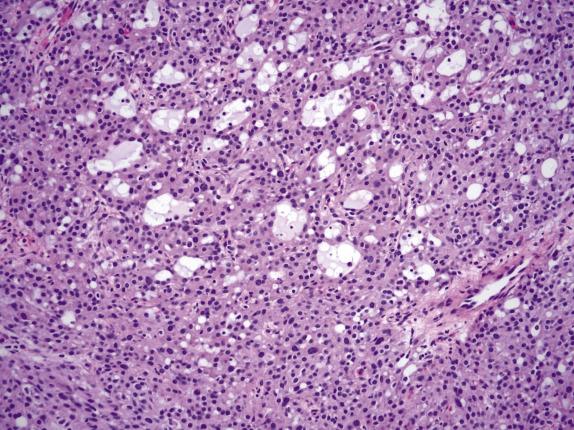
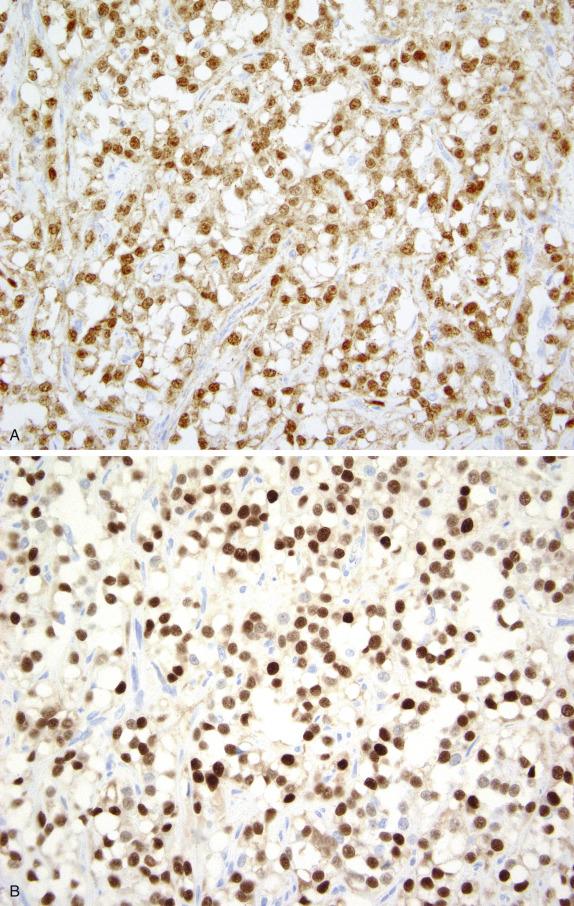
This tumor consistently shows diffuse and strong nuclear staining for beta-catenin ( Fig. 15.20A ). Other positive markers include vimentin, CD10, FOXL2, WT1, and cyclin D1 ( Fig. 15.20B ); most are also positive for SF1. They may show weak positivity for cytokeratin and rarely for inhibin and calretinin, whereas EMA is negative.
Somatic missense point mutations in exon 3 of CTNNB1 have been detected in approximately 50% of microcystic stromal tumors. Although these tumors are strongly positive by immunohistochemistry for FOXL2, they lack the associated mutation. They also lack DICER 1 mutations.
Thecoma is the most common tumor in the differential diagnosis of microcystic stromal tumor as both often occur in patients of similar age and have a vague lobulated architecture with hyaline bands. Furthermore, thecomas can also have bizarre nuclei (albeit rarely); however, they often are associated with estrogenic manifestations, and the tumor cells have more abundant cytoplasm; furthermore, thecomas lack the microcystic component and the intracytoplasmic vacuoles characteristic of microcystic stromal tumors. Thecomas are strongly positive for inhibin and calretinin and negative for nuclear beta-catenin. A steroid cell tumor may also be considered in this differential diagnosis as rarely they may contain microcysts; however, they are associated with hormonal manifestations (more often androgenic) and have a striking yellow to orange and occasionally brown cut surface. In contrast to microcystic stromal tumors, they typically show a diffuse or nested growth and may contain cells with pale, microvacuolated cytoplasm. Although both tumors stain with CD10, calretinin and inhibin positivity establish a diagnosis of steroid cell tumor. Sclerosing stromal tumors and signet-ring stromal tumors may enter into the differential diagnosis as they may have cells with intracytoplasmic vacuoles; however, both contain a second component of spindle cells and sclerosing stromal tumor also has typical luteinized cells and a staghorn vascular pattern as well as positivity for inhibin and calretinin. Nuclear beta-catenin is negative.
Benign stromal tumor with distinctive microcystic appearance
Rare
Unilateral
Wide range, often premenopausal
Symptoms related to pelvic mass
Incidental
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here