Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Unmyelinated (UNM) and small myelinated (M) axons that convey nociception and temperature sensation terminate in laminae I and V (origin of the spinothalamic tract). Other UNM axons terminate in the dorsal horn, from which neurons for polysynaptic reflexes and for the spinoreticular system originate. M axons for touch and pressure terminate in the dorsal horn, from which additional reflex connections, spinothalamic projections, and supplementary epicritic projections to the dorsal column (DC) nuclei originate. M axons also project directly into fasciculi gracilis and cuneatus, destined for nuclei gracilis and cuneatus; these lemniscal pathways process epicritic information for conscious interpretation. M proprioceptive axons (Ia afferents) terminate directly on lower motor neurons (LMNs) and on the Ia interneuronal pool. Additional M axons terminate in the dorsal horn on neurons of origin for the spinocerebellar tracts.
Primary afferents include both epicritic afferents (mainly larger diameter M axons that convey fine, discriminative touch; vibratory sensation; and joint position sense) and protopathic afferents (mainly small M or UNM axons that convey mainly nociceptive information and temperature sensation). These axons can be affected differentially in neuropathies. Some peripheral neuropathies can affect all modalities, leading to a total loss of sensation; other peripheral neuropathies affect selected populations of axons and their related modalities. Selective loss of protopathic modalities may occur in leprosy, in amyloid neuropathy, and in some cases of diabetic neuropathy, leading to insensitivity to pain and temperature. Selective loss of epicritic sensation may occur in some distal symmetrical polyneuropathies, neuropathy with vitamin B 12 deficiency, Guillain-Barré syndrome, and others, accompanied by paresthesias (numbness and tingling, “pins and needles,” abnormal sensations), dysesthesias (disagreeable or abnormal sensations in the absence of stimulation), hyperesthesia (increased sensation with stimulation), or hypesthesia (diminished sensation with stimulation). Some neuropathic conditions also are accompanied by allodynia (pain evoked by normally nonpainful stimuli) and burning, stabbing, radiating pain. Peripheral neuropathies that affect larger diameter M axons often can also affect the motor axons, leading to weakness and hyporeflexia or areflexia. Some small fiber neuropathies, especially diabetic neuropathies, may affect small autonomic axons to bowel, bladder, reproductive organs, and peripheral blood vessels, leading to orthostatic hypotension, bladder dysfunction, chronic gastrointestinal problems, or erectile dysfunction.
The monosynaptic reflex (the muscle stretch reflex ) is tested in a clinical neurological examination. Specific muscle tendons are tapped, with the expected result of contraction of the homonymous muscle (e.g., tapping of the patellar tendon resulting in contraction of the ipsilateral quadriceps muscle). The muscle stretch reflexes routinely tested in a neurological examination include the biceps reflex, triceps reflex, brachioradialis reflex, patellar (knee-jerk) reflex, and ankle-jerk reflex on both sides. The reflexes are graded on a numerical scale ranging from hyporeflexic to normoreflexic to hyperreflexic; normal physiological reflexes may vary in responsiveness, so the result of reflex testing must be considered in conjunction with other clinical signs and symptoms. For example, hyperreflexia in a pathological state such as stroke or spinal cord injury may be accompanied by hypertonia of the affected muscle, spasticity, abnormal reflexes (extensor plantar response), and repetitive alternating hyperreflexic responses (clonus). In contrast, hyporeflexia or areflexia accompanying peripheral neuropathy may be accompanied by muscle weakness and flaccidity and diminished sensation of epicritic modalities, protopathic modalities, or both. More formal testing of reflexic responses can be done with electromyography and conduction velocity studies.
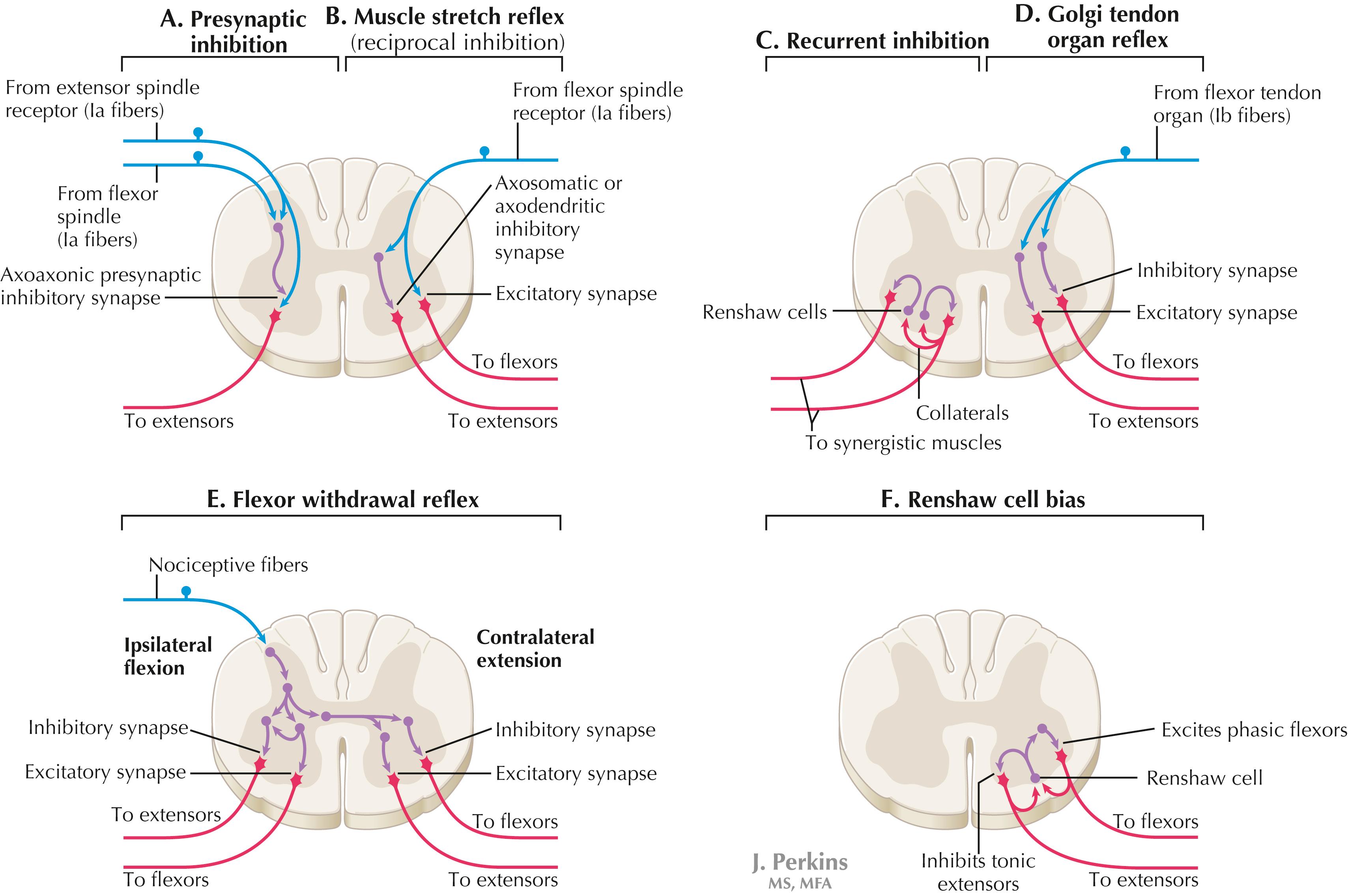
A, Presynaptic inhibition. Some interneurons synapse on the terminal arborizations of other axons, as in the case of some afferent pools associated with muscle stretch reflexes. These axoaxonic contacts permit the modulation of neurotransmitter release from the second (target) axon terminal by depolarization of the terminal membrane, altering the influx of Ca ++ . B, Muscle stretch reflex. In the muscle stretch reflex, Ia afferents excite the homonymous LMN pool directly and inhibit the antagonist LMN pool reciprocally via Ia inhibitory interneurons. C, Recurrent inhibition. Some interneurons receive recurrent collaterals from axons (e.g., LMN axons) and project back onto the dendrites or cell body of origin of that axon, usually inhibiting that neuron. This process can help to regulate the excitability and timing of excitation of the target neurons. Collaterals of LMN axons excite Renshaw cells (large interneurons), which inhibit the LMN of origin as well as LMNs projecting to synergistic muscles. Renshaw inhibition permits wiping the slate clean, after original excitation, of pools of LMNs, requiring additional incoming stimulation in order to excite these LMNs again. D, Golgi tendon organ reflex. Ib axons from Golgi tendon organs in muscle tendons terminate on pools of interneurons that inhibit LMNs to the homonymous muscle disynaptically and excite LMNs to the antagonist muscle reciprocally. The action of this reflex as a protective mechanism to prevent damage to a muscle during generation of maximal tension on the tendon is seen in attempted passive stretch of a spastic muscle; the resultant inhibition of the homonymous LMN pool is called a clasp-knife reflex. E, Flexor withdrawal reflex. A flexor reflex (also called a withdrawal reflex or a nociceptive reflex) occurs when afferents derived from a noxious stimulus terminate on pools of interneurons that excite appropriate pools of LMNs (often flexor LMNs) to bring about a protective withdrawal from the source of the noxious stimulus. These interneurons also inhibit the antagonist LMNs through reciprocal inhibition. Flexor reflexes can extend throughout the spinal cord, as happens when one touches a hot stove with a finger; the result is the removal of the entire arm, or even the entire body, away from the source of heat. These flexor reflexes may involve both sides of spinal cord. F, Renshaw cell bias. Some reflex responses such as Renshaw reflexes (see part C) may result in the distribution of influence (bias) in a manner that favors a particular type of action. Renshaw cells receive inputs from axon collaterals of both flexor and extensor LMNs, but their projections are directed mainly toward the inhibition of tonic extensor LMNs (and through reciprocal inhibition with the excitation of phasic flexor LMNs). Thus, the Renshaw cell response favors flexor movements and helps to inhibit extensor movements.
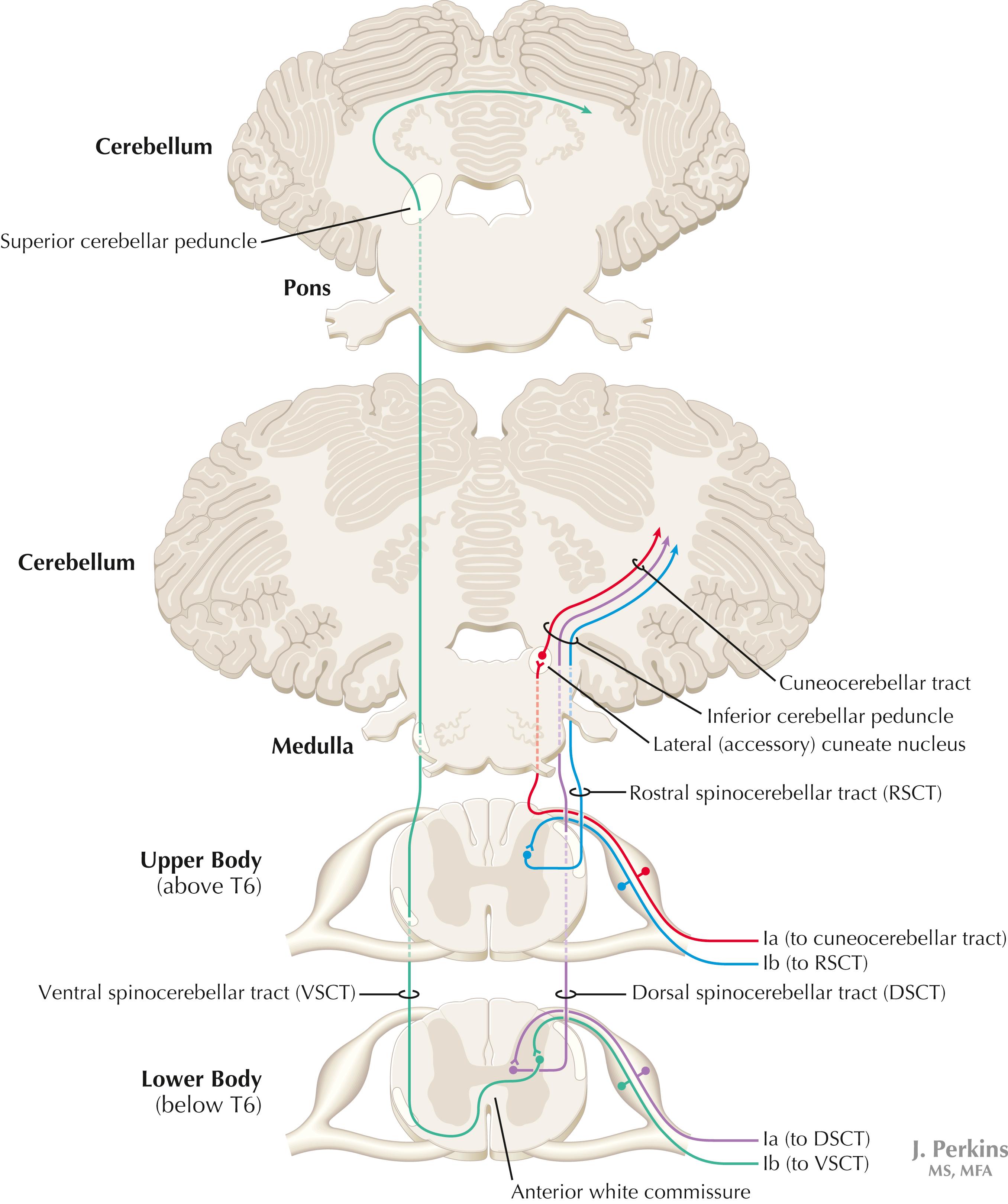
Proprioceptive primary somatosensory axons from joints, tendons, and ligaments (represented in this figure by Ib afferents from Golgi tendon organs) terminate on neurons of origin (border cells, dorsal horn) of the ventral spinocerebellar tract (VSCT) and the rostral spinocerebellar tract (RSCT) from the lower and upper body, respectively (level T6 is the cut-off point). Proprioceptive primary somatosensory axons from muscle spindles (represented in this figure by Ia afferents) terminate on neurons of origin (Clarke’s nucleus, lateral [external] cuneate nucleus of the medulla) of the dorsal spinocerebellar tract (DSCT) and the cuneocerebellar tract from the lower and upper body, respectively (level T6 is the cut-off point). The DSCT, RSCT, and cuneocerebellar tracts remain ipsilateral. The VSCT crosses twice, once in the anterior white commissure of the spinal cord and again in the cerebellum.
The dorsal and ventral spinocerebellar pathways travel in a conspicuous site at the lateral edge of the lateral funiculus throughout most of its length; these pathways are vulnerable to lesions that impinge on this zone of the spinal cord. They include tumors, radiculopathies with accompanying myelopathies, combined-system degeneration, demyelinating diseases, vascular infarcts in the anterior circulation of the cord, Brown-Séquard lesions, and other pathologies. Such a lesion, if superficial in the lateral funiculus, results in ipsilateral ataxia, dysmetria, clumsiness, and mild hypotonia, with impaired ability to perform heel-to-shin testing and tandem walking. However, lesions of the lateral funiculus often also involve the descending upper motor axons of the lateral corticospinal tract and the rubrospinal tract. Lesions that involve these tracts cause ipsilateral spastic hemiparesis or monoparesis below the level of the lesion, depending on the level of the lesion. The resulting spastic weakness, hyperreflexia, and hypertonus predominate in the clinical picture, thus masking the spinocerebellar symptomatology. Thus, an initial picture of spinocerebellar damage may give way to a progressive picture of spastic paresis on the same side.
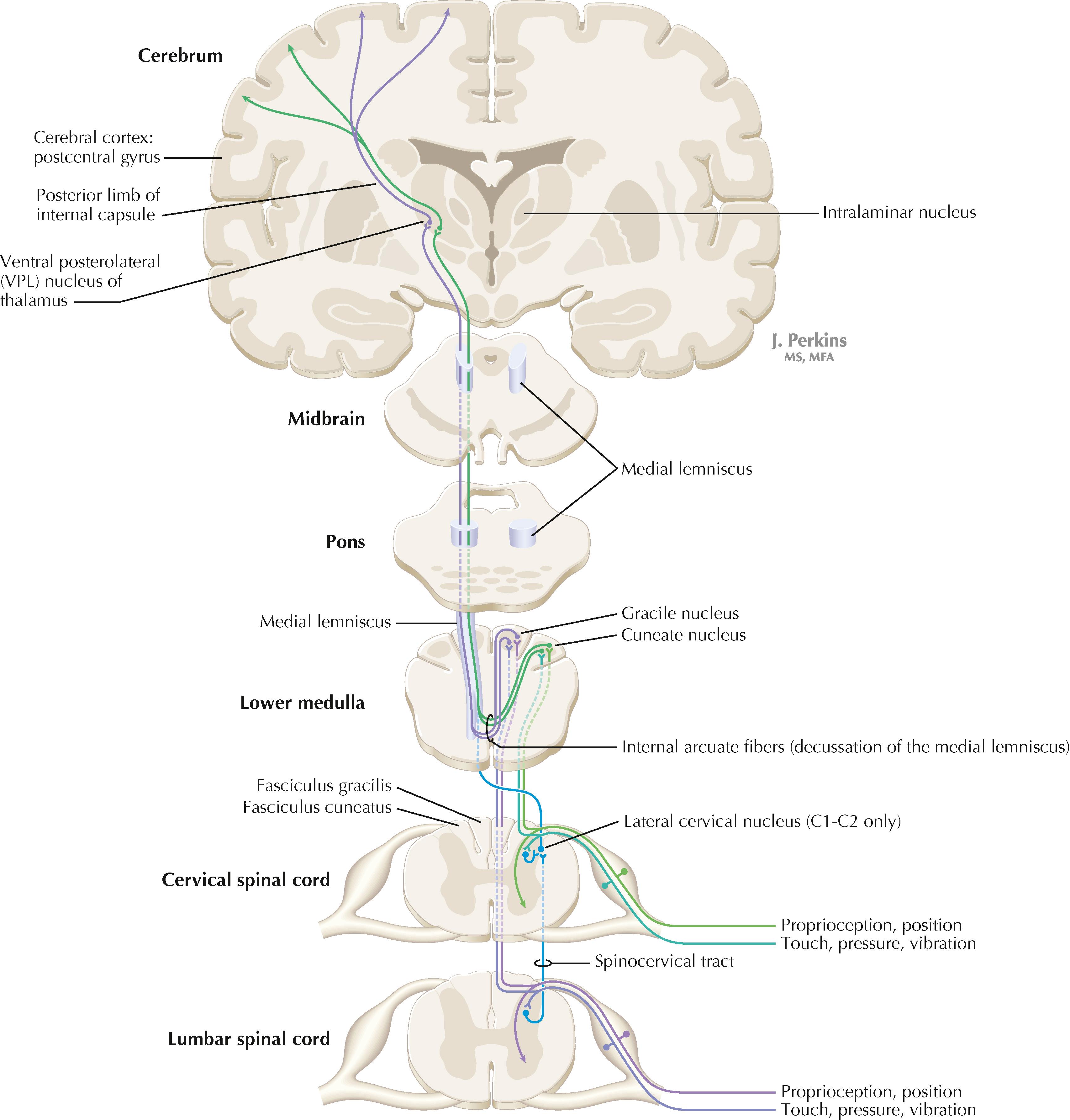
Primary somatosensory myelinated axons conveying fine, discriminative touch, pressure, vibratory sensation, and consciousness of joint position project directly into the DC system (fasciculus gracilis for lower body, below T6, and fasciculus cuneatus for upper body, T6 and above), where they are topographically organized. They terminate in nuclei gracilis and cuneatus, respectively, from which the medial lemniscus originates. This tract crosses (decussates) in the medulla, rostral to the decussation of the pyramids, and projects to the ventroposterolateral (VPL) nucleus of the thalamus. Axons of neurons in the VPL nucleus terminate in the primary sensory cortex topographically. The entire DC/medial lemniscal system is topographically organized; the lower body is represented medially in the primary somatosensory cortex, and the upper body (and face from trigeminal projections) is represented laterally. This representation is sometimes drawn proportionally (the resultant figure is called a homunculus); information from the fingers and hands has far greater representation in the cerebral cortex than information from the back. The spinocervical system is a small supplement to the DC system. Primary afferent projections terminate in the medial part of the dorsal horn; these neurons project to the lateral cervical nucleus (in C1 and C2 only). This nucleus contributes additional crossed axons with polysynaptic mechanoreceptive information. The supplemental epicritic information contributing to the dorsal column/medial lemniscal system ascends in the dorsal portion of the lateral funiculus.
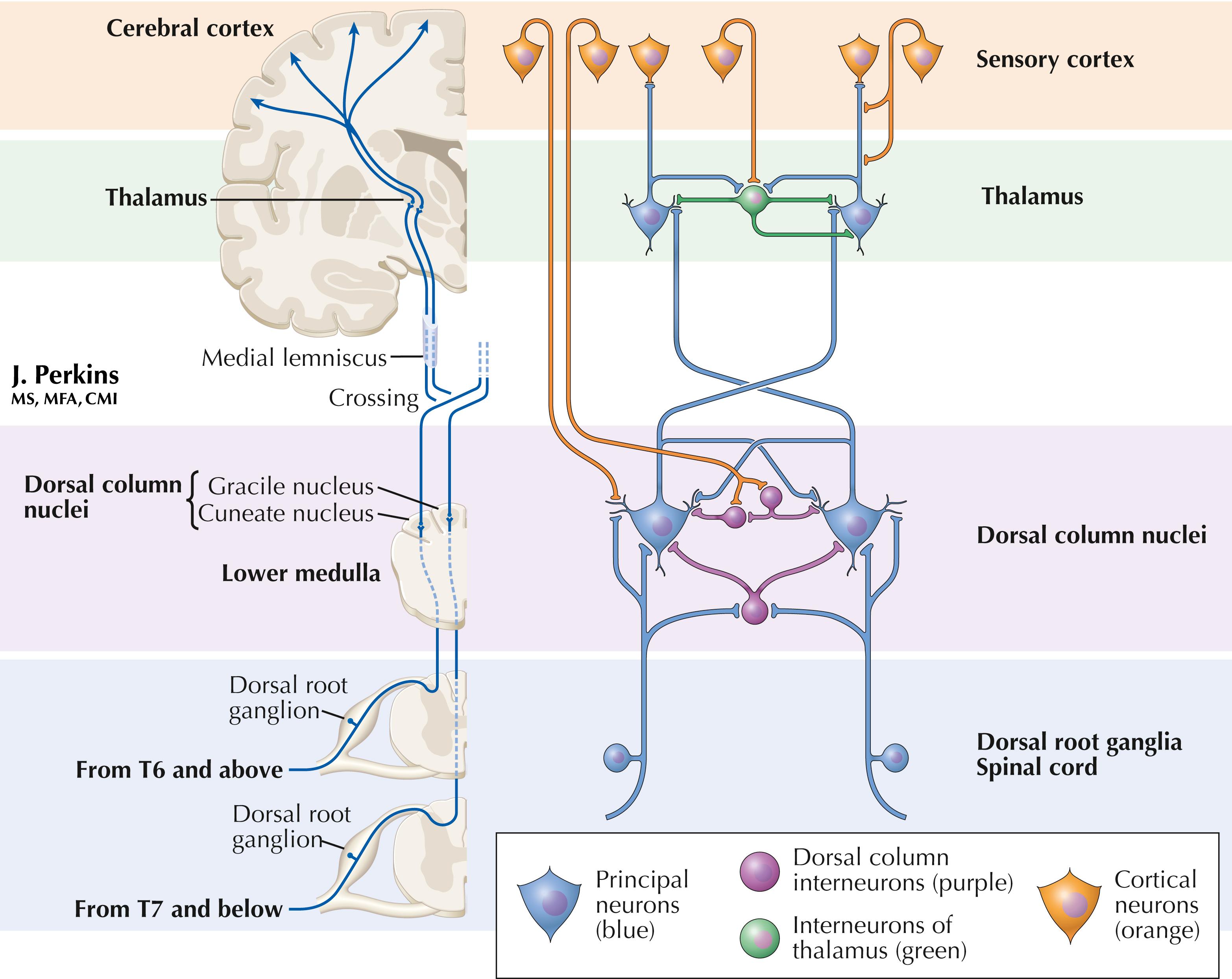
The classic organization of the epicritic somatosensory system is illustrated on the left part of the plate. The more complex interactions in the dorsal column and thalamic nuclei are illustrated on the right. The dorsal column nuclei (gracilis and cuneatus) project to the thalamus (ventral posterolateral nucleus, VPL) but also to the posterior thalamus, inferior colliculus, pontine nuclei, cerebellum, several brainstem nuclei, and spinal cord. Collaterals from the principal neurons can inhibit adjacent dorsal column neurons, sharpening the somatosensory message. The dorsal column nuclei (DCN) contain inhibitory intrinsic neurons, using glycine and GABA as neurotransmitters. The cerebral cortex sends corticonuclear fibers to the DCN and can disinhibit transmission through these neurons. The cortex also can select the frequency and intensity of inputs to these neurons. Corticonuclear axons end on small and medium dendrites of DCN principal neurons and also end on the interneurons. The DCN interneurons themselves postsynaptically inhibit the DCN principal neurons.
Slow-conducting corticonuclear fibers tonically inhibit the DCN principal neurons as well as some inhibitory interneurons. Fast-conducting corticonuclear fibers can activate principal neurons during movement, particularly during limb manipulation and exploratory activities, overriding underlying inhibition.
Interneurons also are present in the thalamic VPL nucleus. The interneurons are fewer in number than the principal neurons in both the DCN and VPL. In VPL, the inhibitory interneurons form dendrodendritic connections with principal neurons and inhibit flow of information to the sensory cortex. Input to principal neurons in VPL from axons of the medial lemniscus produces a brief depolarization for flow of information to the sensory cortex, followed by hyperpolarization from the interneurons and a cessation of information flow to the sensory cortex. The sensory cortex controls principal neurons in VPL through connections with distal dendrites, not with inputs to the synaptic glomeruli formed by medial lemniscus axon connectivity to VPL principal neurons.
Thus, the interneurons fine-tune somatic sensation and responses to it through a small, but powerful, set of inhibitory interneurons in the DCN and nucleus VPL of the thalamus.
See next page.
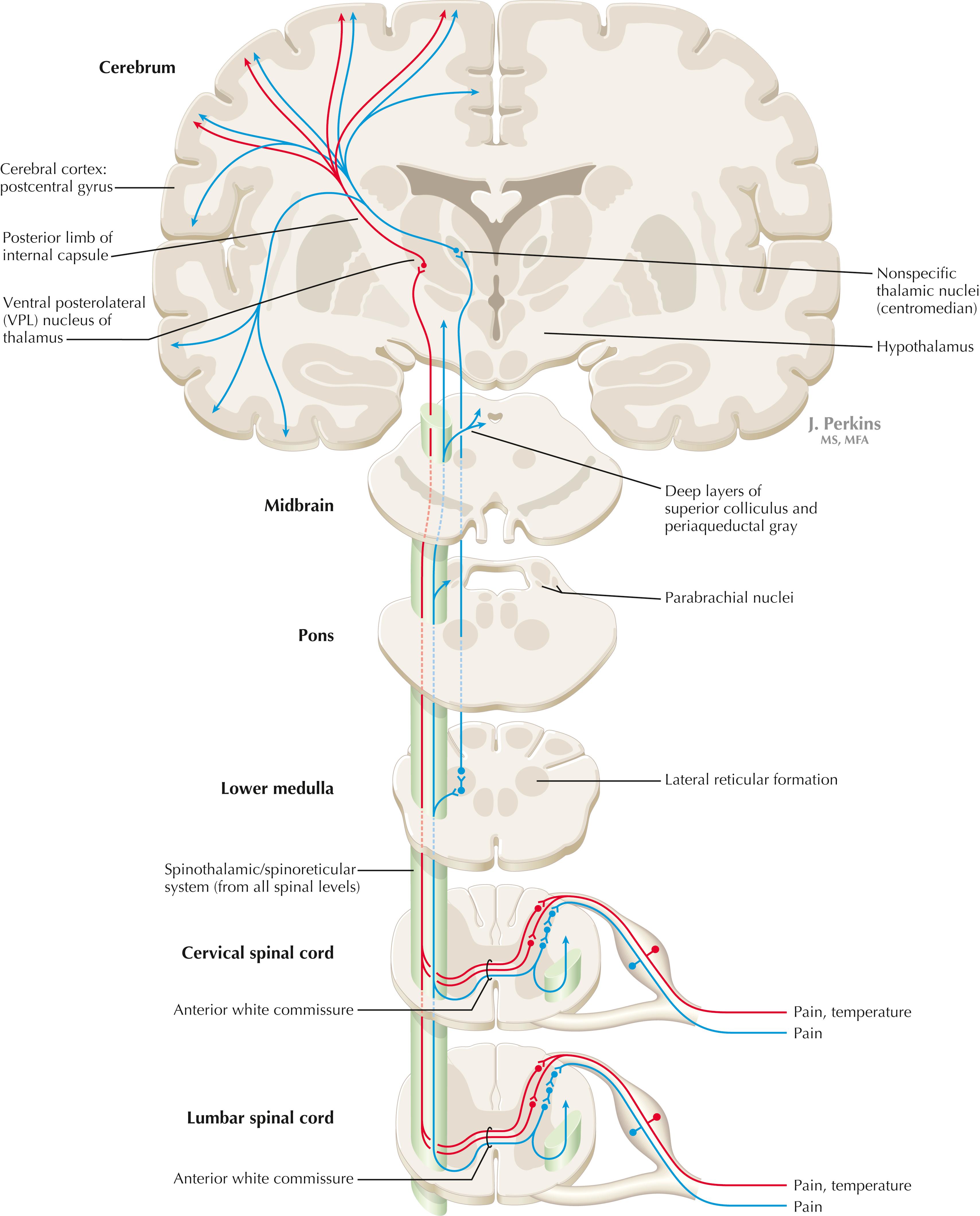
Primary somatosensory unmyelinated (C) fibers and small myelinated (A delta) fibers that convey nociceptive information (fast, localizing pain), temperature sensation, and light, moving touch terminate on neurons in laminae I and V. These dorsal horn neurons send crossed axons into the spinothalamic tract, projecting to neurons in the VPL nucleus of the thalamus (red). This pool of neurons in the VPL nucleus is different from the pool receiving input from nuclei gracilis and cuneatus from the DC system. These thalamic neurons in the VPL nucleus project to the second somatosensory cortex (SII) as well as to the primary sensory cortex. Primary sensory C fibers also terminate in the dorsal horn and contribute to a large, cascading network for bilateral projections into the spinoreticular tract (blue) . This system ends mainly in the reticular formation, from which polysynaptic projections lead to nonspecific medial dorsal and anterior thalamic nuclei. Some spinoreticular fibers also terminate in the deeper layers of the superior colliculus (spinotectal pathway), in the parabrachial nuclei of the pons, and in the periaqueductal gray. Cortical regions such as the cingulate, insular, and prefrontal regions then process and interpret nociceptive information related to slow, agonizing, excruciating pain. In addition, axonal projections from neurons in the dorsal horn of the spinal cord, descending nucleus of V, and parabrachial nuclei of the pons terminate directly in the hypothalamus. These nociceptive axons to the hypothalamus help to coordinate visceral responses (e.g., fight-or-flight, autonomic reactions to pain such as blood pressure and cardiovascular responses, stress hormone secretion of cortisol and epinephrine, and emotional responses). Direct somatosensory inputs also help to mediate sexual responses and oxytocin release for milk letdown from suckling.
The spinothalamic tract conveys lemniscal information from primary afferents for nociception and temperature sensation to secondary sensory neurons in laminae I and V of the dorsal horn of the spinal cord. These dorsal horn neurons then project contralateral spinothalamic tract axons to the VPL nucleus of the thalamus, which in turn sends some information about “fast pain” (not outlasting the duration of the stimulus) to sensory cortices I and II in the parietal lobe. This is the principal protopathic system tested in the neurological examination, using light pin prick and touching the body with test tubes containing water of various temperatures. This spinothalamic tract system does not convey chronic, agonizing, deep pain that characterizes many chronic diseases; such chronic “slow” pain is conveyed through a vast polysynaptic network through the dorsal horn of the spinal cord and then the lateral reticular formation of the brain. This spinoreticular processed information eventually reaches the nonspecific thalamic nuclei (such as the centromedian) and is conveyed to limbic structures for more subjective, interpretative aspects of pain and to the hypothalamus for appropriate visceral autonomic and hormonal responses to pain. This latter spinoreticular network can be influenced by a host of other inputs, including the cortex, the limbic system, the descending forebrain and diencephalic systems, and collaterals of the DC system. Collaterals of the DC system can gate nociceptive processing through the dorsal horn by activating neurons that dampen transmission of information through the cascading dorsal horn network. This process is evoked in a simple fashion by light rubbing on or adjacent to an injured part of the body. In a more chronic fashion, DC stimulation (by a transcutaneous electrical nerve stimulation [TENS] unit) can electrically activate large-diameter axons that then gate the painful stimuli bombarding the dorsal horn nociceptive axons.
The DC system consists of fasciculus gracilis (lower half of the body, with T6 cutoff) and fasciculus cuneatus (upper half of the body). These pathways consist of primary sensory axons conveying fine, discriminative touch sensation, vibratory sensation, and joint position sense (the epicritic sensations) toward the first synapse in the secondary sensory nuclei gracilis and cuneatus in the caudal medulla. These epicritic sensations are called primary DC modalities, the basic information coded mainly by large-diameter myelinated axons. Additional DC modalities are sometimes tested if the primary modalities are intact, including two-point discrimination, stereognosis (knowing what an object is just by touch), and graphesthesia (interpreting a number drawn into the palm of the hand). These are considered cortical modalities of the DC system; they require that the primary DC modalities be intact and also require the ability of the sensory cortices to interpret the information conveyed and to draw conclusions about that information. If the primary modalities are impaired, there is no reason to attempt to test the cortical modalities that depend on unimpaired primary modalities. Pure lesions of the DC system do not entirely eliminate the primary epicritic modalities; they just remove some interpretive capabilities. Such a patient may realize that a vibratory stimulus is being applied to the upper extremity but may be unable to distinguish vibratory stimuli of different frequencies. The dorsal portion of the lateral funiculus carries additional epicritic information to the DC nuclei from the spinal cord dorsal horn. A lesion of both the DC and the dorsal portion of the lateral funiculus results in total loss of epicritic sensation on the affected side.
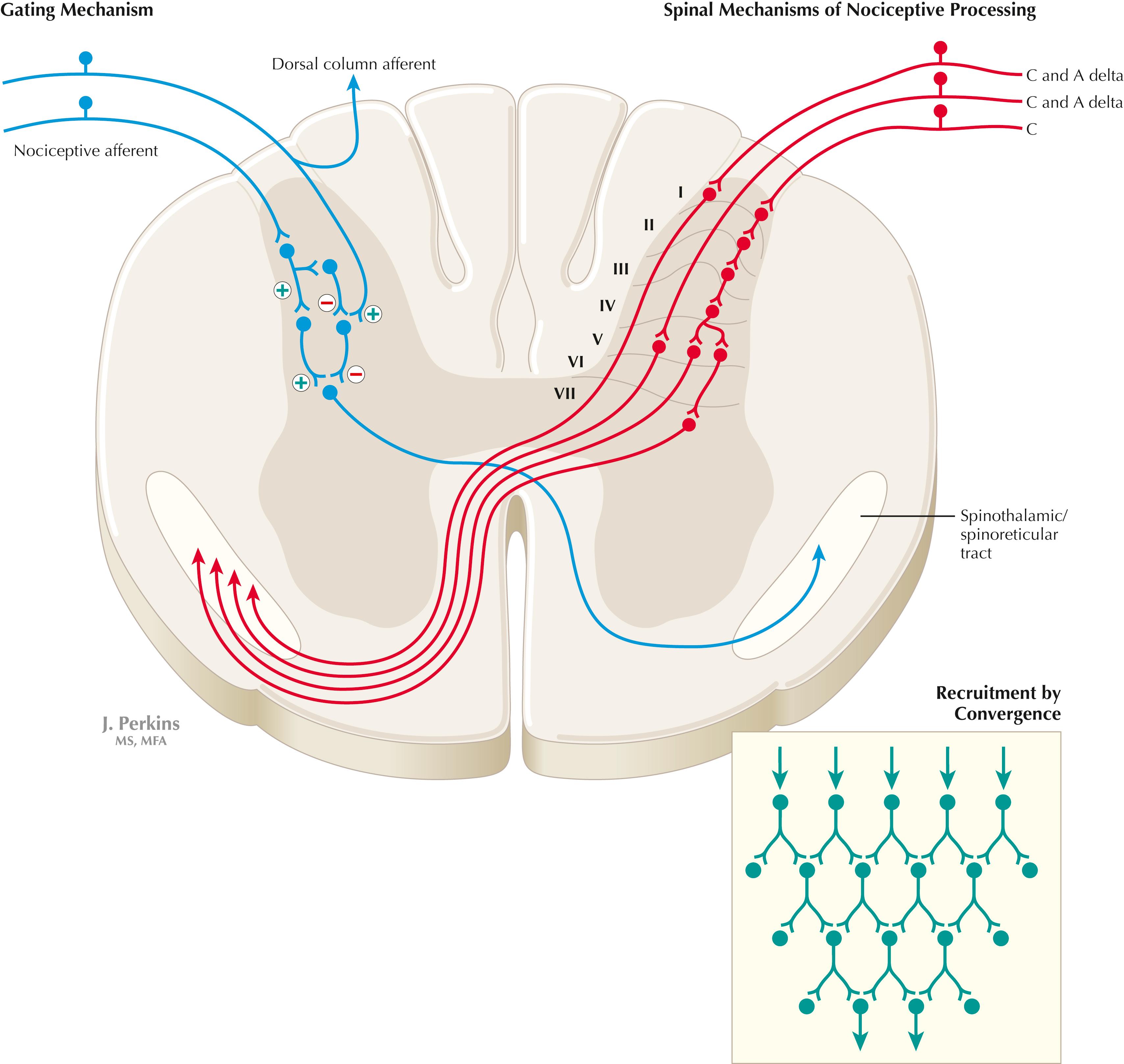
Primary afferents (C and A delta fibers) conveying fast, localized pain and temperature sensation terminate in laminae I and V of the dorsal horn of the spinal cord, from which the crossed spinothalamic axons originate. Unmyelinated primary afferents (C fibers) also terminate on neurons in the dorsal horn, from which a cascading system involving recruitment, convergence, and polysynaptic interconnections originates. This system (shown in red) contributes to the spinoreticular tract (mainly crossed, but some are uncrossed), which projects into the RF and continues polysynaptically to nonspecific medial dorsal and anterior thalamic nuclei. This system contributes to perception of excruciating pain and its emotional connotation via cortical regions such as the cingulate, insular, and prefrontal cortices. The gating mechanism, shown in blue on the left, allows primary DC axon collaterals to dampen pain processing in the dorsal horn via inhibitory interneuronal connections that inhibit the flow of information through the cascading dorsal horn system that contributes to the spinoreticular pathway.
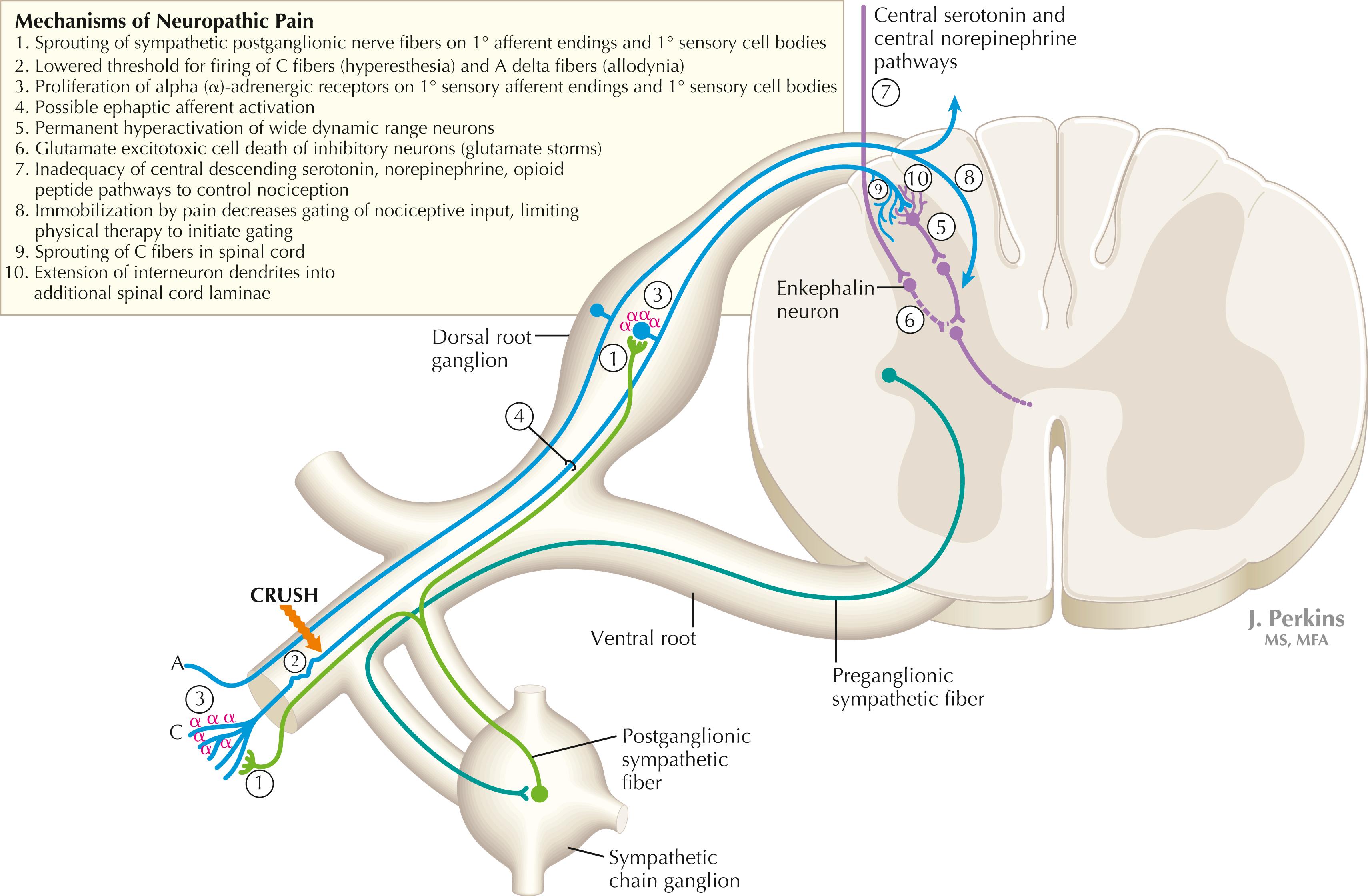
The cascading dorsal horn system receives primary afferent C fibers of nociceptive origin and projects into the spinoreticular system for the conscious interpretation of excruciating pain and neuropathic pain, shown in this illustration. Connections from the sympathetic nervous system can innervate terminals and cell bodies of primary nociceptive neurons directly. In neuropathic pain syndromes such as complex regional pain syndrome (CRPS), formerly called reflex sympathetic dystrophy (RSD), sympathetic postganglionic neurons may activate receptors on greatly sensitized primary afferent nerve terminals and cell bodies, either directly (via synapses) or indirectly (through secretion of norepinephrine into the blood); such activation may exacerbate the perception of the neuropathic pain. Multiple mechanisms are thought to contribute to sensitization of pain-related neurons and presence of chronic, agonizing neuropathic pain in CRPS and related syndromes. These mechanisms are noted in this illustration as numbered sites. Descending central noradrenergic and serotonergic projections are thought to play an important modulatory role in the processing of neuropathic and nonneuropathic pain.
In some cases of nerve damage or compression, particularly that associated with a sprain, a crush injury, a direct injection into a nerve, or even relatively minor trauma, a pathological reaction of primary afferents can result in a chronic neuropathic pain syndrome called reflex sympathetic dystrophy , more recently renamed CRPS . It is related to the type of chronic, agonizing central pain experienced in phantom limb syndrome. CRPS affects the hand, arm, and shoulder to a greater extent than the lower extremity. Intense burning or stabbing pain is felt, with allodynia and hyperesthesia (extreme sensitivity to touch and painful stimuli, respectively). When this phenomenon affects one nerve (perhaps following a bullet wound) it is sometimes called causalgia. The primary afferents involved in CRPS appear to proliferate alpha-adrenergic receptors on their sensory receptor endings and on the dorsal root ganglion cell body and often show extraordinary sensitivity to catecholamines, which provoke a lower threshold for response to nociceptive stimuli. In syndromes such as CRPS, permanent destruction of dorsal horn inhibitory interneurons (by glutamate excitotoxicity) and permanently altered thresholds for wide dynamic range spinoreticular neurons also have been observed. Sympathetic-related characteristics may be noted in CRPS, such as changes in skin appearance due to vascular flow changes (vasomotor), atrophic skin and nails (trophic changes), altered sweating and skin temperature (sudomotor), and altered bone density on a triphasic bone scan. Treatment must occur quickly after detection and must employ simultaneous vigorous therapeutic approaches. Treatment choices normally include analgesics, tricyclic or other antidepressants to alter pain threshold in the spinal cord, membrane-stabilizing agents (e.g., Neurontin), physical therapy, and nerve stimulation of large-diameter myelinated “gating” axons.
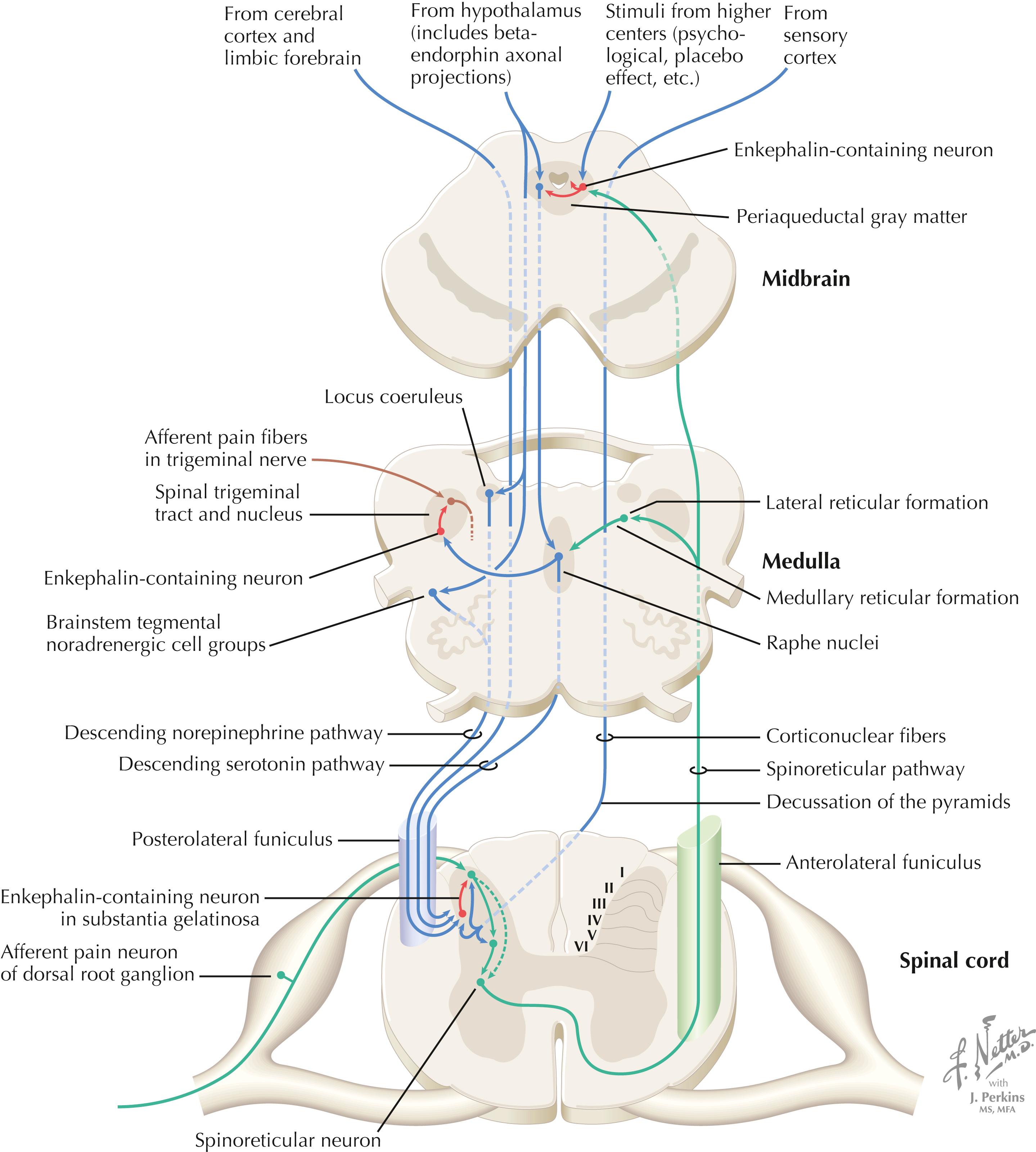
The processing of nociceptive information in the dorsal horn of the spinal cord can be modulated by descending connections from the cerebral cortex, limbic forebrain structures, hypothalamus (paraventricular nucleus), periarcuate beta-endorphin neurons, periaqueductal gray, RF of the brainstem, central noradrenergic neurons (of locus coeruleus and other brainstem tegmental groups), and serotonergic (5HT) neurons (nucleus raphe magnus). The central descending noradrenergic and 5HT pathways, modulated by the periaqueductal gray and other higher centers, are particularly important for endogenous and exogenous (i.e., opioid) modulation of pain.
Several regions of the central nervous system (CNS) send projections, direct and indirect, to regulate nociceptive processing through the dorsal horn of the spinal cord for the body and the descending nucleus of V for the face. These areas include regions of cerebral cortex, limbic forebrain areas, hypothalamic regions including endorphin nuclei, and sensory cortical centrifugal connections. Some of these projections use endogenous opiates . Enkephalin and dynorphin interneurons are found in pain-processing regions, particularly in the dorsal horn of the spinal cord and the descending nucleus of V, and in many hypothalamic and limbic sites that may be involved in the subjective interpretation of pain. The beta-endorphin neurons of the periarcuate region (sometimes called just the arcuate nucleus ) of the hypothalamus send connections to the periaqueductal gray, locus coeruleus and brainstem noradrenergic nuclei, raphe nuclei, and many limbic regions. The periaqueductal gray is particularly important for opioid activation of the nucleus raphe magnus and other descending monoamine pathways that activate enkephalins and assist in opiate analgesia. The periaqueductal gray–raphe connection is essential for full functionality of opioid analgesia. Systemic administration of synthetic opiates activates neurons of the periarcuate region of the hypothalamus and periaqueductal gray, resulting in analgesia.
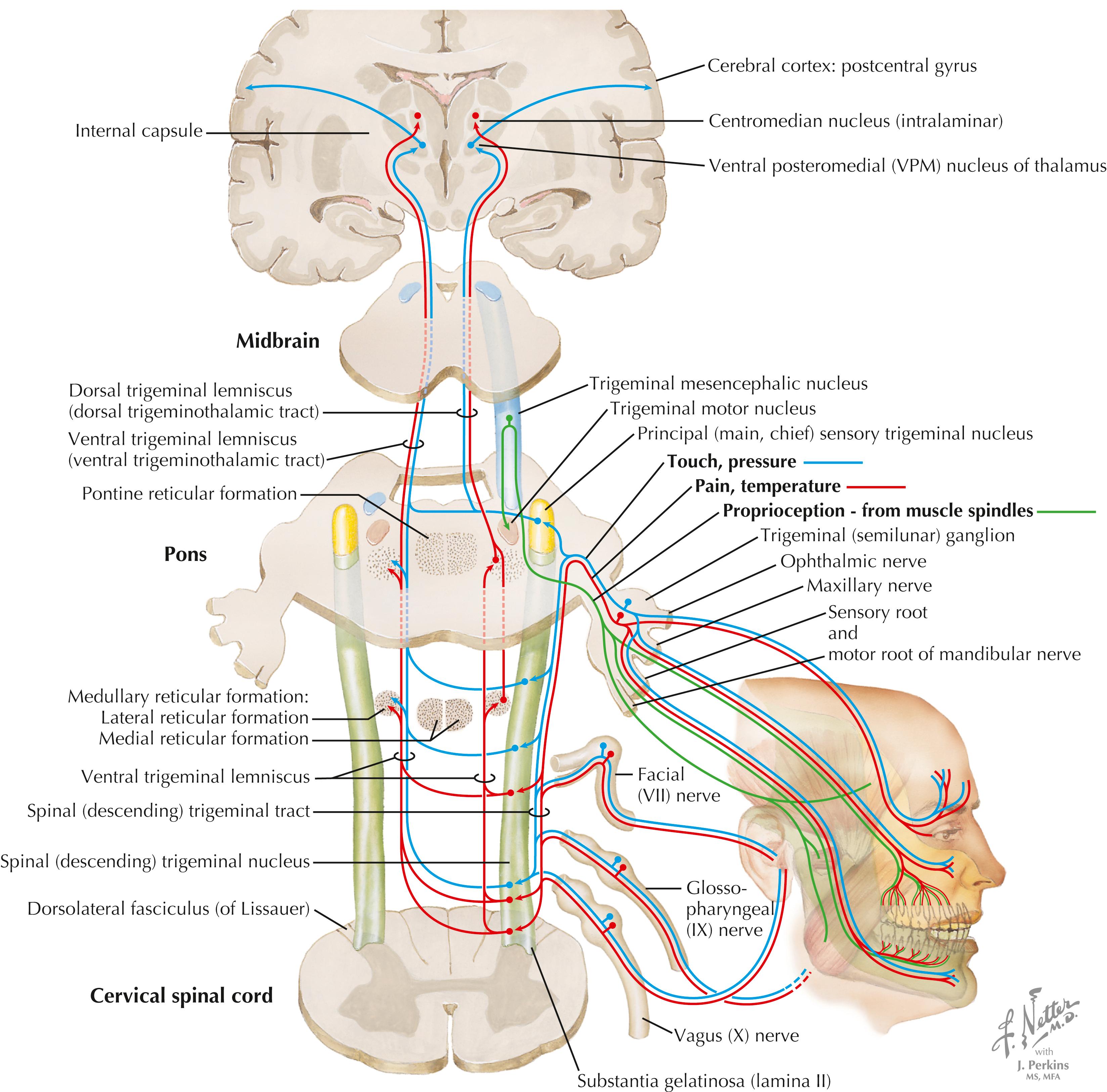
Axons of primary sensory trigeminal neurons enter the brainstem, travel in the descending (spinal) tract of V, and terminate in the descending (spinal) nucleus of V. Axons of the trigeminal ganglion (V) supply the face, anterior oral cavity, and teeth and gums; axons of the geniculate ganglion (VII) and jugular ganglion (X) supply a small zone of the external ear. Axons of the petrosal ganglion (IX) supply general sensation to the posterior oral cavity and pharynx. Axons of the descending nucleus of V project into the ventral trigeminal lemniscus (ventral trigeminothalamic tract; mainly crossed axons) and terminate in the ventral posteromedial (VPM) nucleus of the thalamus. The VPM nucleus projects to the lateral primary sensory cortex and to intralaminar thalamic nuclei, which are associated with nociceptive processing. The caudal descending nucleus also sends contralateral projections to the RF for processing of excruciating pain (similar to the spinoreticular system). Primary sensory axons carrying fine, discriminative modalities from V (similar to the DC system) terminate in the rostral portion of the descending nucleus of V and in the main (chief) sensory nucleus of V, which contribute to the ventral trigeminothalamic tract. A portion of the main sensory nucleus also projects ipsilaterally to the VPM nucleus via the dorsal trigeminothalamic tract. Although most of the trigeminal system is represented on the lateral portion of the contralateral primary sensory cortex (postcentral gyrus), part of the epicritic trigeminal projections as well as taste are represented in the ipsilateral sensory cortex. The mesencephalic nucleus of V is the only primary sensory nucleus found inside the CNS; these neurons supply muscle spindles for masticatory and extraocular muscles and mediate associated muscle spindle reflexes. See page 433 for a Clinical Point.
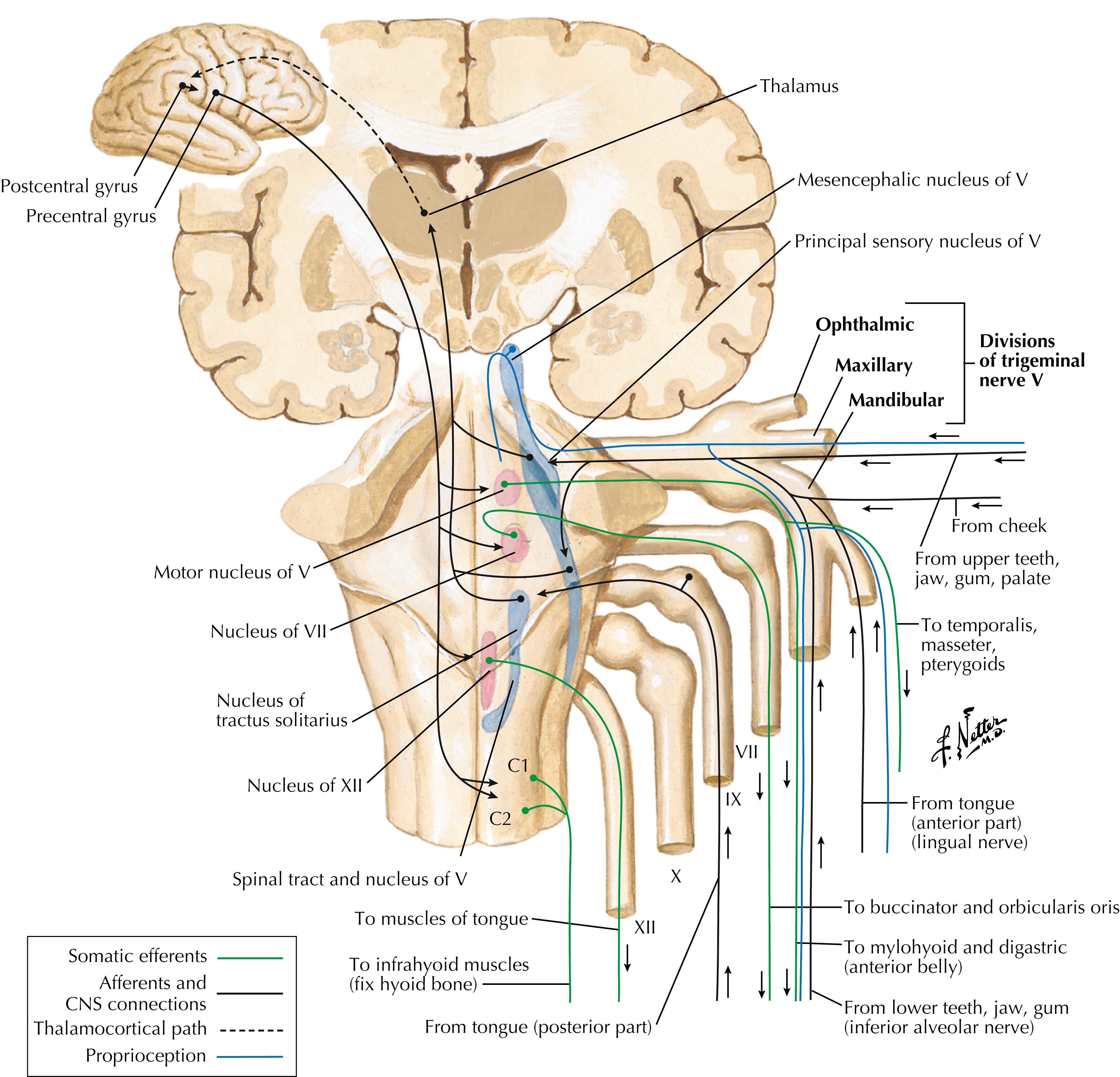
This illustration is a summary of trigeminal-related pathways and connections, including cortical and reflex connections.
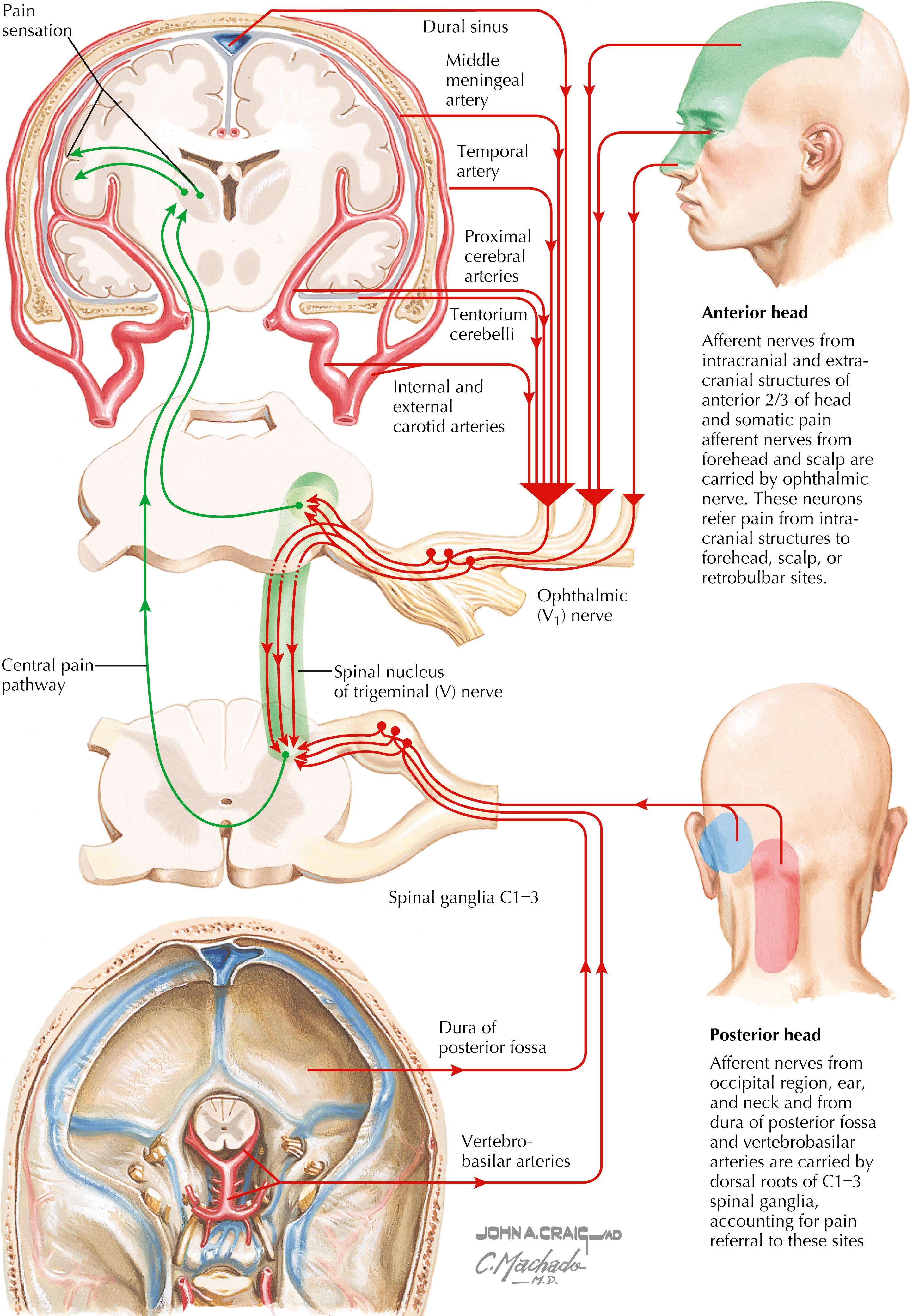
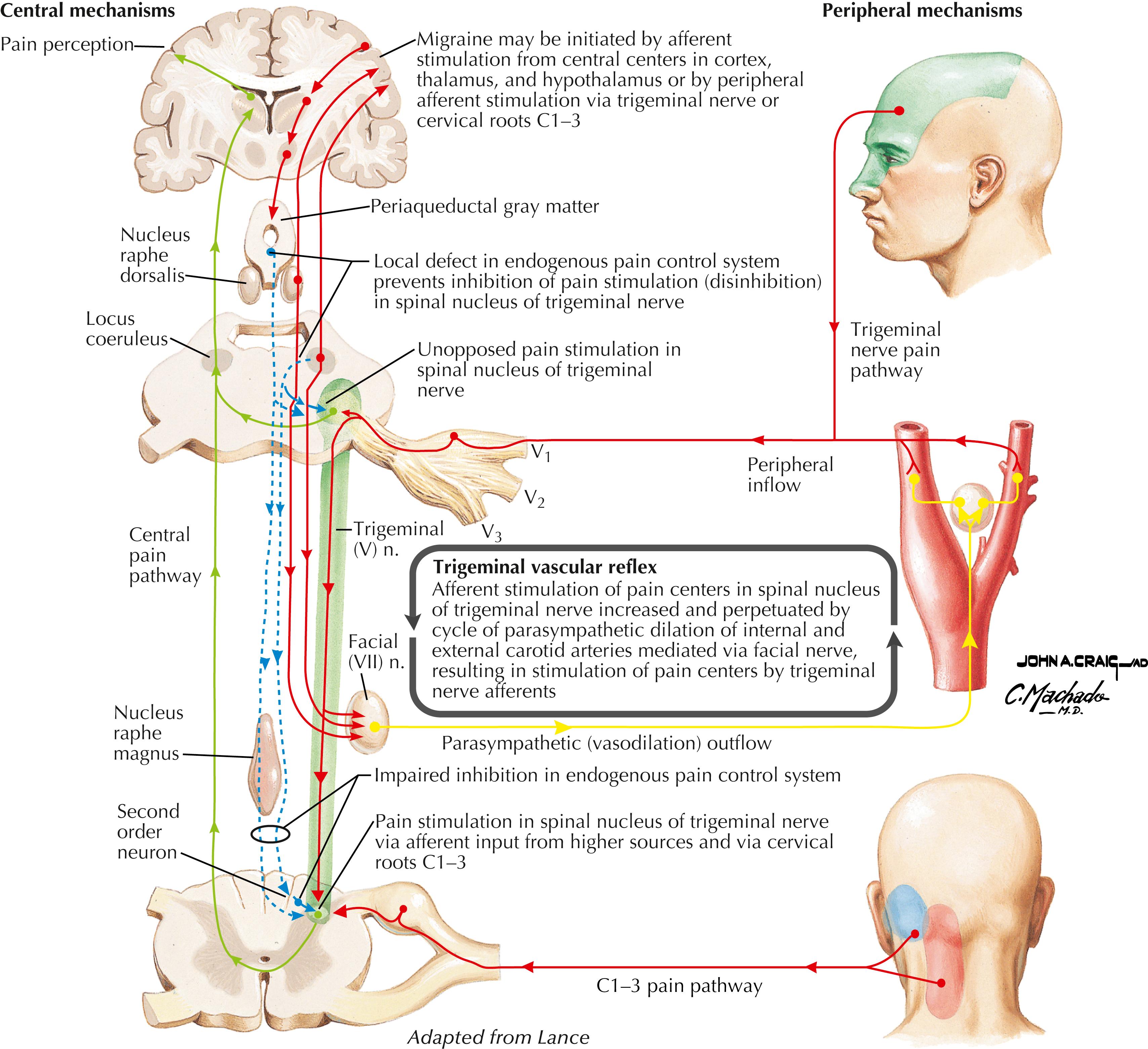
Pain-sensitive structures of the head include dural structures (e.g., sinuses, tentorium cerebelli), arteries, and muscles. Primary headaches can arise as migraine headaches, tension headaches, and neuralgias. Secondary headaches can arise from tumors, abscesses, hematomas, bleeding (e.g., ruptured berry aneurysm), and meningitis or meningeal irritation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here