Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
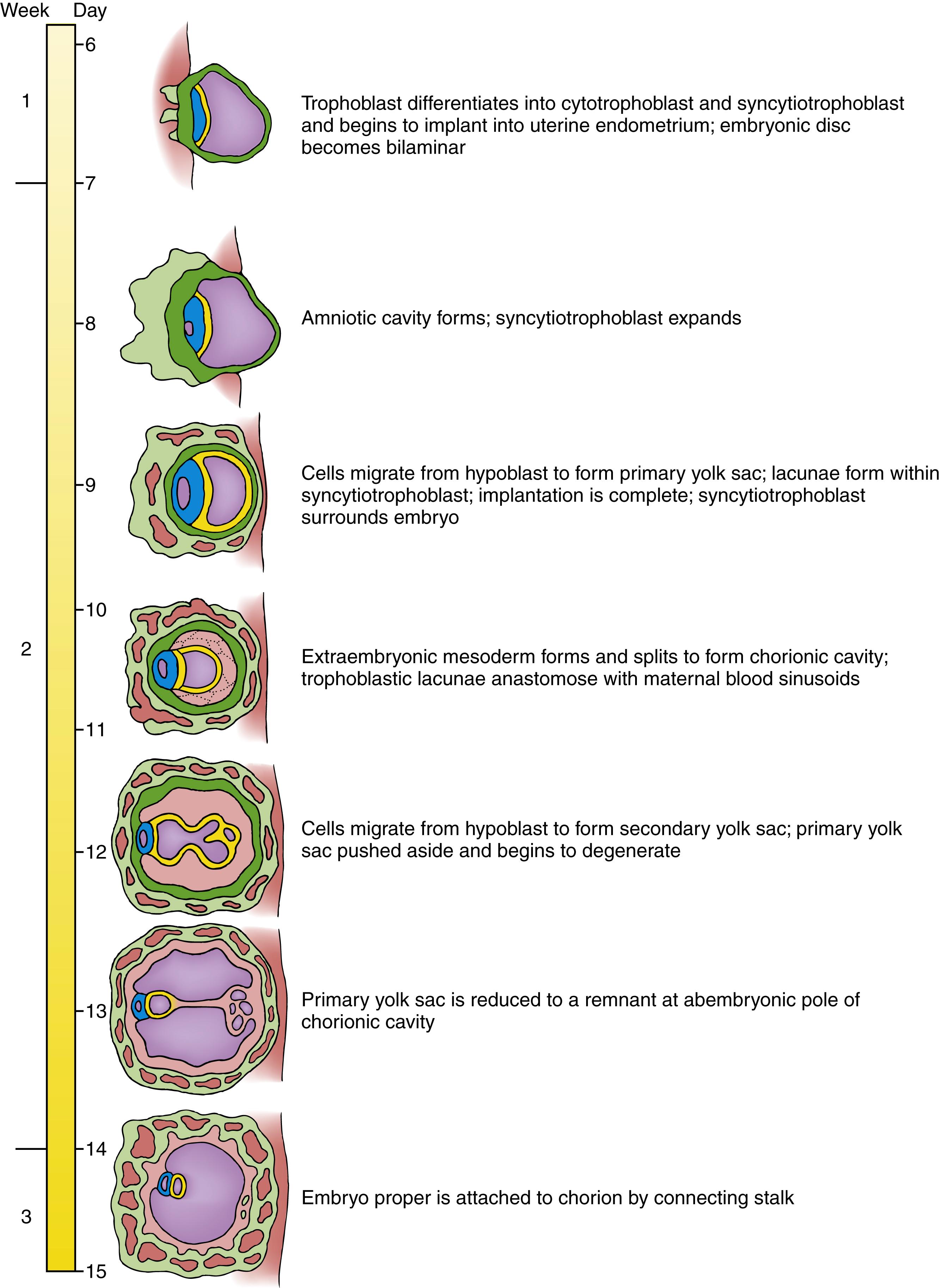
As covered in the preceding chapter, the morula—formed by cleavage of the zygote—transforms during the first week into a blastocyst consisting of an inner cell mass, or embryoblast, and a trophoblast. At the beginning of the second week, the embryoblast splits into two layers: the epiblast and the hypoblast , or primitive endoderm . A cavity, called the amniotic cavity , develops at the embryonic pole of the blastocyst between the epiblast and the overlying trophoblast. It quickly becomes surrounded by a thin layer of cells derived from epiblast. This thin layer constitutes the lining of the amnion , one of the four extraembryonic membranes . The remainder of the epiblast and the hypoblast now constitute a bilaminar embryonic disc , or bilaminar blastoderm , lying between the amniotic cavity (dorsally) and the blastocyst cavity (ventrally). The cells of the embryonic disc develop into the embryo proper and also contribute to extraembryonic membranes . During the second week, the hypoblast apparently sends out two waves of migratory endodermal cells into the blastocyst cavity (blastocoel). The first of these waves forms the primary yolk sac (or the exocoelomic membrane or Heuser’s membrane ), and the second transforms the primary yolk sac into the secondary yolk sac.
In the middle of the second week, the inner surface of the trophoblast and the outer surface of the amnion and yolk sac become lined by a new tissue, the extraembryonic mesoderm . A new cavity—the extraembryonic coelom , or chorionic cavity —develops as the extraembryonic mesoderm splits into two layers. With formation and splitting of the extraembryonic mesoderm, both the amnion and yolk sac (now sometimes called the definitive yolk sac) become double-layered structures: amnion, consisting of ectoderm on the inside and mesoderm on the outside; and yolk sac, consisting of endoderm on the inside and mesoderm on the outside. In addition, the outer wall of the blastocyst is now called the chorion ; like the amnion and yolk sac, it too contains a layer of mesoderm.
Meanwhile, implantation continues. The trophoblast differentiates into two layers: a cellular trophoblast, called the cytotrophoblast , and an expanding peripheral syncytial layer, the syncytiotrophoblast . These trophoblast layers contribute to the extraembryonic membranes, not to the embryo proper. The syncytiotrophoblast, cytotrophoblast, and associated extraembryonic mesoderm, together with the uterus, initiate formation of the placenta . During this process, the fetal tissues establish outgrowths, the chorionic villi , which extend into maternal blood sinusoids .
A 6-month-old boy is referred by his primary care physician to University Hospital for genetic evaluation because of failure to thrive : both his weight-for-height and height-for-age fall below the third centile for age, as assessed using standard growth charts. His mother is 23 and his father is 29, and the boy is their first child. The woman became pregnant 2 months after stopping birth control (contraceptive sponge), and her pregnancy went smoothly with only a couple of weeks of mild morning sickness. She went into labor during the 39th week of gestation, but because labor progressed poorly and abnormal fetal heart rhythms were detected, her child was delivered by cesarean section 23 hours later.
At the child’s 2-month well baby examination, his mother expressed the concern that her baby did not nurse well and seemed to have a weak cry. He also seemed not to move very much. On examination, the boy was somewhat small for his age and was hypotonic (had limp muscles). On a follow-up visit a few weeks later, the infant continued to show poor weight gain, and failure to thrive was diagnosed. To stimulate catch-up growth, the pediatrician recommended supplementing breast feeding with gavage feeding (feeding by tube) of high-calorie formula to achieve 150% of the caloric requirement for the boy’s expected weight if he were at the 50th centile.
Genetic testing occurred at 7 months. It revealed that the boy has a deletion of a portion of the long arm of chromosome 15, and he was diagnosed with Prader-Willi syndrome . The boy’s parents are counseled about their son’s prognosis and are given an information packet, which contains information about a local support group for parents of children with Prader-Willi syndrome. In meetings with the support group, they see other children of various ages with Prader-Willi, as well as their parents, and some children who are said to have the same chromosomal deletion but who act very differently than their son. They are told that these children have a different syndrome called Angelman syndrome . Later, by searching the web, they find that both Prader-Willi syndrome and Angelman syndrome result from abnormalities in a process called imprinting , and that the difference in the two syndromes depends on whether the defect was inherited from the mother or from the father.
At 9 months of age, the boy is started on growth hormone replacement therapy, which has been shown to normalize height and increase lean muscle mass in children with Prader-Willi syndrome.
Many events occur in twos during the second week. Thus, a “rule of twos” constitutes a handy mnemonic for remembering events of the second week. During the second week, the embryoblast splits into two layers: the epiblast and the hypoblast. The trophoblast also gives rise to two tissues the cytotrophoblast and the syncytiotrophoblast. Two yolk sacs form, first the primary and then the secondary. Two new cavities form the amniotic cavity and the chorionic cavity. The extraembryonic mesoderm splits into the two layers that line the chorionic cavity, and the amnion, yolk sac, and chorion all become two-layered membranes.
![]() .
.
Animations are available online at StudentConsult.
As described in Chapter 1 , the blastocyst adheres to the uterine wall at the end of the first week. Contact with the uterine endometrium induces the trophoblast at the embryonic pole to proliferate. Some of these proliferating cells lose their cell membranes and coalesce to form a syncytium (a mass of cytoplasm containing numerous dispersed nuclei) called the syncytiotrophoblast ( Fig. 2.1A ).
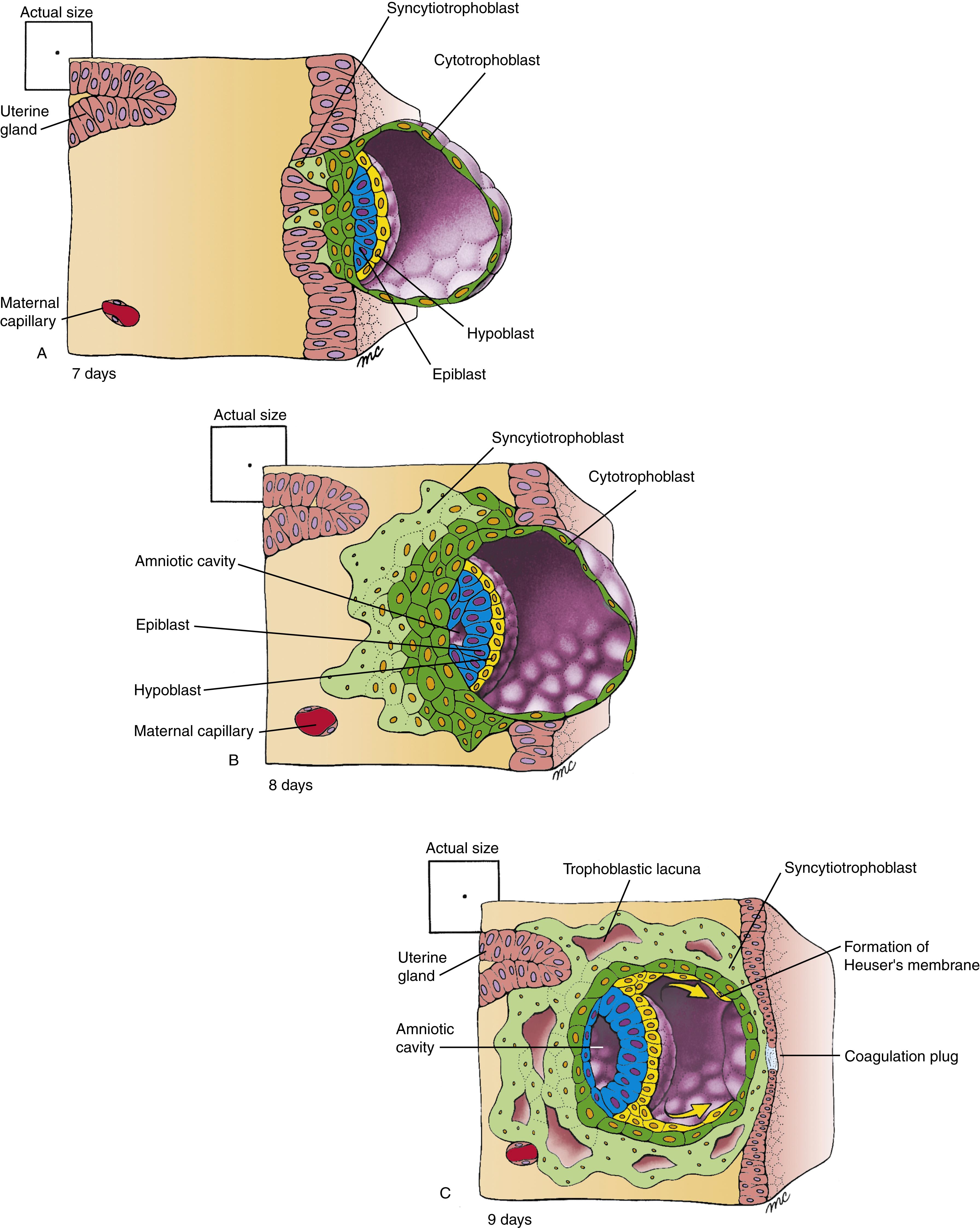
By contrast, the cells of the trophoblast that line the wall of the blastocyst retain their cell membranes and constitute the cytotrophoblast . The syncytiotrophoblast increases in volume throughout the second week as cells detach from the proliferating cytotrophoblast at the embryonic pole and fuse with the syncytium (see Fig. 2.1B,C ).
Between days 6 and 9, the embryo becomes fully implanted in the endometrium. Proteolytic enzymes, including several metalloproteinases, are secreted by the cytotrophoblast to break down the extracellular matrix between the endometrial cells. Active finger-like processes extending from the syncytiotrophoblast then penetrate between the separating endometrial cells and pull the embryo into the endometrium of the uterine wall (see Fig. 2.1A,B ). As implantation progresses, the expanding syncytiotrophoblast gradually envelops the blastocyst. By day 9, the syncytiotrophoblast blankets the entire blastocyst, except for a small region at the abembryonic pole (see Fig. 2.1C ). A plug of acellular material, called the coagulation plug , seals the small hole where the blastocyst implanted, temporarily marking this point in the endometrial epithelium.
Before about 6 or 7 days post-fertilization, both the blastocyst and the apical surface of the uterine epithelium are non-adhesive. Therefore, changes must occur in both the blastocyst and the uterine epithelium to allow blastocyst attachment and the initiation of implantation.
The uterus cycles through receptive and non-receptive stages. As covered in Chapter 1 , entry into the receptive stage, during which implantation is possible, is controlled by estrogen and progesterone. For a relatively short period of time, called the implantation window , the uterus is receptive to implantation. Estrogen , acting through the estrogen receptor , stimulates the uterine endometrium to undergo proliferation by inducing the production of growth factors such as insulin-like growth factor 1 . It also prevents programmed cell death within the uterine epithelium. Progesterone , in turn, acting through the progesterone receptor , induces the transcription factor hand2 , which blocks continued endometrial growth and allows implantation to occur.
As the uterus enters the receptive stage, its apical glycocalyx (a polysaccharide matrix surface coating of epithelial cells including—in the case of the uterine epithelium—abundant high–molecular-weight mucin glycoproteins) decreases in amount and in negative charge. Moreover, apical microvilli , which are normally abundant, retract to establish a flattened surface in many areas of the epithelium, and large apical protrusions called pinopodes form.
The blastocyst undergoes maturation from an attachment-incompetent stage to an attachment-competent stage. Although the presence of the non-adhesive zona pellucida before blastocyst hatching certainly prevents blastocyst attachment, experimental removal of the zona a few days earlier demonstrates that the blastocyst itself is still at the attachment-incompetent stage. As blastocysts mature to the attachment-competent stage, they express perlecan , a heparan sulfate proteoglycan , on their surface. Heparan sulfate proteoglycans are known to have a high degree of specific binding to various extracellular matrix proteins and growth factors/cytokines and thus could serve as attachment factors. A particularly intriguing finding, with respect to the role of perlecan in attachment, is that the uterus at the time of implantation dramatically upregulates expression of heparin-binding epidermal growth factor – like growth factor ( Hb-Egf ) at implantation sites, presumably in response to blastocyst signaling. Studies have shown that binding of Hb-Egf to the blastocyst requires that the blastocyst expresses both the Egf receptor and heparan sulfate proteoglycan. Perlecan-null mice do not exhibit defects in implantation, suggesting that perlecan has functional redundancy with other heparan sulfate proteoglycans that can substitute (or are compensatorily upregulated) in its absence.
In addition to heparan sulfate proteoglycans, other factors possibly involved in adhesion include selectins (a type of lectin—a sugar-binding protein), αvβ3 and αvβ5 integrins (transmembrane glycoproteins involved in adhesion and cell signaling; Chapter 5 provides more details), metalloproteases (enzymes that bind metal such as zinc and degrade proteins) and their inhibitors, cytokines ( Lif and interleukin-11 ), and a cell adhesion complex called trophinin - tastin - bystin . Some of these latter factors (e.g., metalloproteases) play a role in trophoblast invasion of the endometrium, in addition to possibly functioning in attachment.
The conceptus, which expresses both maternal and paternal genes, can be considered to be like an allograft; that is, tissue transplanted from one member of a species to another member of the same species (e.g., from one human to another). Allografts typically elicit an immune response in the host, resulting in rejection of the graft. In such a host-versus-graft reaction, peptides bound to major histocompatibility complex (MHC) molecules generate tissue alloantigens that are recognized by maternal T cells. Medawar (who won the Nobel prize in 1960) proposed over 50 years ago three possibilities for why the developing conceptus is not rejected by its mother: fetal and maternal cells are physically separated from one another; the conceptus is antigenically immature; or the maternal immune system is suppressed or becomes tolerant to the conceptus during pregnancy.
It is likely that a combination of these possibilities prevents rejection of the conceptus. The trophoblast, which separates the actual tissues of the developing fetus from its mother, poorly expresses MHC molecules. Thus, the tissues are only partially separated and the conceptus is antigenically immature. However, there is evidence that maternal T cells are activated during pregnancy. Hence, because there is no completely cell-impermeable barrier between fetus and mother to prevent exposure of fetal alloantigens to maternal T cells (e.g., fetal cells can be found in maternal blood during pregnancy, and maternal cells can be found in the fetus) and because fetal tissues are antigenic, it is likely that tolerogenic mechanisms block maternal T-cell responses and prevent fetal rejection. The unique hormonal conditions of pregnancy that prepare the uterus for implantation and growth of the blastocyst apparently also induce tolerance. Such tolerance is specific for fetal antigens; for example, maternal antiviral immunity is not suppressed during pregnancy, as shown in HIV+ women who do not suffer from AIDS-like disease during pregnancy.
One way in which tolerance to paternal antigens expressed by fetal tissue might occur is through the selective loss of maternal immune cells that respond to these antigens. For example, it has been proposed that maternal-activated T cells are induced to undergo apoptosis through the Fas/Fasl system. Trophoblast cells produce Fasl, a member of the tumor necrosis factor (Tnf) and Cd40 ligand family, which signals through the Fas receptor (also called Cd95, a membrane protein of the Tnf family). In support of this possibility, mice lacking functional Fasl display extensive leukocyte infiltrates at the placental-decidual interface, and deliver small litters.
In addition to a potential host-versus-graft reaction during pregnancy, as just described, a graft-versus-host reaction could occur in which the fetus mounts an immune reaction against its mother. Why a graft-versus-host reaction does not occur is unknown, but it has been suggested that the mother’s immune system interacts with the conceptus either to prevent maturation of the fetus’ immune system or to evoke tolerogenic mechanisms.
Recent evidence provides insight into the suppression of both a graft-versus-host reaction and a host-versus-graft reaction during pregnancy. It is now known that immune cells, called regulatory T cells , are produced by both mother and fetus, and that these cells can cross the placenta, such that fetal cells reside in the mother’s blood and vice versa. Maternal regulatory T cells recognize paternal antigens and suppress the mother’s immune system to prevent rejection of the fetus. These cells persist in the maternal bloodstream long after birth, abrogating the immune response to other fetuses in subsequent pregnancies. Similarly, maternal regulatory T cells that cross the placenta come to reside in fetal lymph nodes, where they induce the development of fetal regulatory T cells that suppress the fetus’ immune response to maternal antigens. Moreover, these maternal cells persist in the child until adulthood is reached, suggesting that they continue to regulate immune responses after birth.
Even before implantation occurs, cells of the embryoblast begin to differentiate into two epithelial layers. By day 8, the embryoblast consists of a distinct external (or upper) layer of columnar cells, called the epiblast , and an internal (or lower) layer of cuboidal cells, called the hypoblast , or primitive endoderm (see Fig. 2.1A,B ). An extracellular basement membrane is laid down between the two layers as they become distinct. The resulting two-layered embryoblast is called the bilaminar embryonic disc , or bilaminar blastoderm . With formation of the bilaminar embryonic disc, the primitive dorsal-ventral axis of the embryo is defined (i.e., epiblast is dorsal, hypoblast is ventral).
The hypoblast, or primitive endoderm, is the first layer to form from the inner cell mass. Studies mainly in Xenopus and zebrafish suggest that a series of factors initiate endoderm formation. These include a T-box–containing transcription factor (VegT), which activates nodal (a member of the Tgfβ family of growth factors), which in turn induces expression of downstream transcriptional regulators (mixer, a paired-homeobox–containing transcription factor; Gata, a zinc finger GATA-binding transcription factor; and Fox, a forkhead box transcription factor). This in turn regulates expression of a relay of HMG-box–containing Sox-family transcription factors that ultimately result in the expression of Sox17, a critical factor in endoderm development.
The role of these genes in endoderm formation in mouse is less clear. Loss-of-function mutants of the mouse homolog of VegT (eomes, also known as eomesodermin) arrest very early in development, precluding analysis of their role in endoderm formation. Nodal loss-of-function mutants fail to form a primitive streak and node (covered in Chapter 3 )—critical events in the genesis of not only endoderm but also mesoderm—so the exact role of nodal in mouse endoderm formation is unclear. However, the use of a hypomorphic nodal allele (i.e., a mutation in which nodal expression is severely downregulated but not completely eliminated), as well as a cripto loss-of-function mutation (cripto is an essential co-factor required for nodal signaling), provides more convincing evidence that nodal signaling is required for endoderm formation. Additional loss-of-function mutations are consistent with a role for both mixer and Sox17 in mouse endoderm formation. Hence, in conclusion, it is likely that the same general cascade of factors initiate endoderm formation in all vertebrates.
Other loss-of-function studies in mouse suggest that at least four other transcription factors are required for endoderm formation and maintenance: Gata6, (a homeobox-containing transcription factor), Hnf4 (a member of the steroid hormone vHnf1 receptor family that functions as a ligand-activated transcriptional regulator), and Foxa2 (a forkhead transcription factor previously known as Hnf3β). A regulatory hierarchy exists among some of these genes, with the first two factors (Gata6 and vHnf1) regulating expression of Hnf4. Foxa2 functions not only in formation of the endoderm but also in the formation of other lineages, such as the notochord and floor plate of the neural tube (covered in Chapter 4 ). Interestingly, orthologs of these genes also function in endoderm formation in other organisms (e.g., the forkhead genes in Drosophila and the Pha4 gene in Caenorhabditis elegans are orthologs of the Hnf3 genes; serpent in Drosophila and End1 and Elt2 in C. elegans are orthologs of Gata genes).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here