Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The overall incidence of salivary gland malignancies in the general population is about 0.9 to 4.0 new cases per 100,000 population per year.
Prognostic factors relate to histology, grade, primary tumor size and extent, lymph node involvement, gender, and age.
Staging includes a thorough history and physical examination, chest radiography, contrast-enhanced computed tomography (CT) scan or magnetic resonance imaging (MRI) of the head and neck region, positron emission tomography (PET) scan, and liver function tests.
Compete surgical resection is the primary therapy for major and minor salivary gland cancers. Small cancers, typically arising within minor salivary glands in cosmetically or functionally critical areas, may be treated with primary radiation therapy with moderate success in the patient who refuses surgery. For minor salivary gland malignancies, transoral robotic surgery (TORS) may offer improved cosmetic and functional results.
Despite an absence of randomized trials, adjuvant radiotherapy is routinely used to improve locoregional control. Indications for adjuvant treatment include positive or close surgical margins, extraglandular extension, bone invasion, high-grade histology, perineural invasion, and the presence of lymph node metastasis.
The 10-year locoregional disease control and overall survival (OS) rates for stages I to III disease treated with surgery and postoperative radiotherapy are ≥80% and ~60%, respectively. When treated with radiotherapy alone, these are ~70% and ~65%, respectively.
The 10-year locoregional disease control and OS rates for stage IV disease treated with surgery and postoperative radiotherapy are ~65% and ~30%, respectively. When treated with radiotherapy alone, these are ~25% and ~20%, respectively. When treated with neutron beam alone, the 6-year actuarial locoregional and OS rates are ~60% and ~60%, respectively. Neutrons can produce exaggerated late effects including central nervous system (CNS) injury at the skull base. The role of concurrent chemoradiation is being investigated in a randomized NRG Oncology trial.
Systemic therapy has been, at best, palliative. Combination chemotherapy regimens have poor response rates, typically less than 25%. Multiple targeted agents have been investigated, demonstrating less toxicity and the suggestion of disease stabilization, but even poorer objective response rates.
The salivary glands are exocrine glands that produce saliva. The salivary gland unit is composed of acini of serous or mucinous cells that drain into a branching ductal system composed of cells forming the intercalated duct, striated duct, and excretory duct. Myoepithelial cells surround the acinar cells and the intercalated ducts and function by contracting and forcing saliva through the ductal system. Stem cells are present in the basal layer of the excretory and intercalated ducts that can differentiate into the different elements of the salivary glands.
The three major paired salivary glands (parotid, submandibular, and sublingual) produce the majority of the saliva. The remaining saliva is produced from more than 600 unencapsulated tubuloalveolar glands, located throughout the lamina propria of the upper aerodigestive tract mucosa. Approximately 50% of these minor salivary glands are located within the mucosa of the hard palate. These glands are predominately mucous secreting. Minor salivary glands may also be seen in the nasal cavity and paranasal sinuses.
The causes of salivary gland tumors (SGTs), both benign and malignant, have not been established. Reports consistently suggest etiologic associations with nutritional deficiencies, exposure to ionizing radiation, ultraviolet exposure, genetic predisposition, history of previous cancer of facial skin, occupational exposure, viral (Epstein-Barr virus) infection, alcohol use, hair dye use, and higher educational attainment.
Exposure to ionizing radiation has been associated with a statistically significant, although small risk for both benign and malignant SGTs. Radiation-induced malignant SGTs (MSGTs) may occur with higher frequency in the minor salivary glands. Studies have demonstrated not only a strong association, but also that the risk has a temporal latency (10 years to 25 years) and a dose-response relationship with the exposure (especially for mucoepidermoid carcinomas) suggesting a cause-and-effect relationship. The relative risk was greater for MSGTs than benign SGTs. These observations were based on several independent long-term cohort studies of victims of atomic bomb exposure and patients receiving therapeutic radiation for benign and malignant indications. The atomic bomb data do demonstrate that second malignancies are increased with single exposures up to approximately 3 Gy and that this relationship is at least linear up to 2 Gy. Unlike radiation-induced sarcomas, which appear to be primarily limited to the high doses seen within radiotherapy prescription volumes, SGTs can also be seen to occur in areas receiving low therapeutic doses of radiation with the risk increasing with higher doses. Although investigators have concluded that higher doses and repeated exposure to radiotherapy increases the develop of radiation-induced SGTs, the nature of the dose-response curve for radiation-induced SGTs is unclear beyond doses 4 Gy or greater.
Salivary gland tumors are uncommon; in Denmark the incidence of SGTs was 1.1/100,000 per year, the mean age 62, and the male to female ratio was 0.97. The top sites of primary tumors are parotid (52.5%), minor salivary glands (33.4%), and submandibular gland (12.2%) ( Table 43.1 ).
| Location | Numbers (%) |
|---|---|
| Parotid gland | 500 (52.5) |
| Submandibular gland | 116 (12.2) |
| Sublingual gland | 15 (1.6) |
| Minor salivary gland | 318 (33.4) |
| Lip | 22 |
| Buccal mucosa | 44 |
| Tongue | 28 |
| Floor of mouth | 27 |
| Palate | 130 |
| Paranasal sinus | 20 |
| Nose | 18 |
| Pharynx | 23 |
| Larynx | 5 |
| Other a | 1 |
| Unknown primary tumor site | 3 (0.3) |
Histologies according to gender, age, and primary site are shown in Tables 43.2 and 43.3 . More men than women had adenocarcinoma not otherwise specified (NOS) and squamous cell histology. Notably, squamous cell carcinomas found in the parotid region usually represent lymph node metastases from cutaneous squamous cell carcinomas rather than primary parotid gland carcinomas. Women had an increased incidence of acinic cell cancers and polymorphous adenocarcinoma (formerly polymorphous low-grade adenocarcinoma).
| Histological Type | N (%) | Males N (%) | Females N (%) | p -Value | Median Age |
|---|---|---|---|---|---|
| Adenoid cystic carcinoma | 240 (25.2) | 107 (11.2) | 133 (14.0) | n.s. | 59 |
| Mucoepidermoid carcinoma | 161 (16.9) | 84 (8.8) | 77 (8.1) | n.s. | 51 |
| Adenocarcinoma NOS | 116 (12.2) | 72 (7.6) | 44 (4.6) | p = 0.004 | 70 |
| Acinic cell carcinoma | 97 (10.2) | 34 (3.6) | 63 (6.6) | p = 0.004 | 55 |
| Carcinoma ex pleomorphic adenoma | 79 (8.3) | 41 (4.3) | 38 (4.0) | n.s. | 63 |
| Polymorphous low-grade adenocarcinoma | 73 (7.7) | 26 (2.7) | 47 (4.9) | p = 0.02 | 58 |
| Squamous cell carcinoma | 52 (5.5) | 35 (3.7) | 17 (1.8) | p = 0.01 | 74 |
| Salivary duct carcinoma | 34 (3.6) | 21 (2.2) | 13 (1.4) | n.s. | 63 |
| Epithelial-myoepithelial carcinoma | 27 (2.8) | 16 (1.7) | 11 (1.2) | n.s. | 67 |
| Lymphoepithelial carcinoma | 25 (2.6) | 9 (0.9) | 16 (1.7) | n.s. | 59 |
| Basal cell adenocarcinoma | 14 (1.5) | 7 (0.7) | 7 (0.7) | n.s. | 65 |
| Oncocytic carcinoma | 15 (1.6) | 7 (0.7) | 8 (0.8) | n.s. | 70 |
| Cystadenocarcinoma | 4 (0.4) | 2 (0.2) | 2 (0.2) | ||
| Mucinous adenocarcinoma | 3 (0.3) | 2 (0.2) | 1 (0.1) | ||
| Myoepithelial carcinoma | 2 (0.2) | 1 (0.1) | 1 (0.1) | ||
| Carcinosarcoma | 1 (0.1) | 0 | 1 (0.1) | ||
| Clear cell adenocarcinoma NOS | 1 (0.1) | 0 | 1 (0.1) | ||
| Not possible to classify—besides carcinoma | 8 (0.8) | 5 (0.5) | 3 (0.3) | ||
| 1990–2005: a national study of incidence, site and histology. Results of the Danish Head and Neck Cancer Group (DAHANCA). Oral Oncol . 2011;47:677–82.TOTAL | 952 (22) | 469 (11) | 483 (51) |
| Histological Type | Parotid Gland N (%) | Submandibular Gland N (%) | Oral Cavity a N (%) | Other b N (%) |
|---|---|---|---|---|
| Adenoid cystic carcinoma | 73 (7.7) | 51 (5.4) | 76 (8.0) | 40 (4.2) |
| Mucoepidermoid carcinoma | 64 (6.7) | 10 (1.1) | 72 (7.6) | 15 (1.6) |
| Adenocarcinoma NOS | 67 (7.0) | 17 (1.8) | 28 (2.9) | 4 (0.4) |
| Acinic cell carcinoma | 86 (9.0) | 2 (0.2) | 7 (0.7) | 2 (0.2) |
| Carcinoma ex pleomorphic adenoma | 64 (6.7) | 10 (1.1) | 4 (0.4) | 1 (0.1) |
| Polymorphous low-grade adenocarcinoma | 3 (0.3) | 0 | 66 (6.9) | 4 (0.4) |
| Squamous cell carcinoma | 43 (4.5) | 9 (0.9) | 0 | 0 |
| Salivary duct carcinoma | 30 (3.1) | 4 (0.4) | 0 | 0 |
| Epithelial-myoepithelial carcinoma | 21 (2.2) | 3 (0.3) | 3 (0.3) | 0 |
| Lymphoepithelial carcinoma | 20 (2.1) | 5 (0.5) | 0 | 0 |
| Basal cell adenocarcinoma | 7 (0.7) | 0 | 4 (0.4) | 3 (0.3) |
| Oncocytic carcinoma | 13 (1.4) | 2 (0.2) | 0 | 0 |
| Cystadenocarcinoma | 1 (0.1) | 1 (0.1) | 2 (0.2) | 0 |
| Mucinous adenocarcinoma | 0 | 0 | 3 (0.3) | 0 |
| Myoepithelial carcinoma | 1 (0.1) | 1 (0.1) | 0 | 0 |
| Carcinosarcoma | 1 (0.1) | 0 | 0 | 0 |
| Clear cell adenocarcinoma NOS | 0 | 0 | 1 (0.1) | 0 |
| Not possible to classify—besides carcinoma | 6 (0.6) | 1 (0.1) | 0 | 1 (0.1) |
| Total | 500 (52.5) | 116 (12.2) | 266 (27.9) | 70 (7.4) |
a Lip, buccal mucosa, tongue, floor of mouth, palate, and the sublingual gland.
b Paranasal sinuses, nose, pharynx, larynx, and the three carcinomas with unknown primary site.
The recognition that radiation exposure is a risk factor for benign SGTs and MSGTs (especially mucoepidermoid carcinomas) argues that long-term surveillance is likely a prudent recommendation for patients with a known history of accidental, diagnostic, or therapeutic head and neck radiation exposure.
Judicious use of diagnostic and therapeutic radiation and avoidance of any unnecessary radiation exposure are currently the most effective preventive strategies that can be recommended.
Significant progress has been made in the understanding of the genetics and molecular biology of salivary gland malignancies, but the clinical applicability has been limited. The incidence of Epstein-Barr virus (EBV) infection in a meta-analysis of malignant salivary gland tumors was 45.1%, but varied geographically. The incidence was 44.2% in American patients, 70% in Asian patients, and only 11.8% in European patients, which does not even look at the incidence by the many strains of EBV and casts doubt on EBV as an etiologic agent.
Ki-67 levels as well has age (and stage) have been found to be independently associated with survival.
An analysis of the national Dutch study found that once performance status was included in the model, age had no significant impact on prognosis, although this seemed to conflict with earlier work. In their more recent analysis, Stage III + IV, performance status greater than 0, and histologic high grade were the statistically significant prognostic factors.
Although relatively common, the behavior of adenoid cystic carcinoma (ACC) is particularly unpredictable. Deletions or translocations of the terminal regions of the long arm on chromosome 6 appear to be a consistent and unique event in the development of salivary gland ACCs. Of these, reciprocal translocations involving 6q and the short arm of chromosome 9 have been frequently reported. Persson et al. have also demonstrated a recurrent and hallmark t(6:9) translocation in ACCs, resulting in the MYB–NFIB fusion oncoprotein. Common mutations include MYB, MYBL1, and NFIB. Mutational burden was low. Looking at the differences between primary lesions and their metastases, the MYB and NFIB mutations are consistently preserved. Next generation sequencing demonstrated new mutations in 3 of 11 patients who developed metastatic disease.
Whole exome sequencing has identified 15 tumors in 102 patients with adenoid cystic cancer who had 18 NOTCH1 mutations. Patients with NOTCH1 mutations had a median survival of 29.6 months compared with 121.9 months for the patients with NOTCH1 wild-type. NOTCH1 mutations have been targeted for therapeutic intervention.
The KIT protein is a membrane tyrosine kinase receptor, which when activated through binding to the ligands’ stem cell factor or mast cell growth factor, provide signals for cell survival, proliferation, and differentiation. Its overexpression has been particularly noted in ACCs and has been suggested as one way in which ACCs may be distinguished from polymorphous adenocarcinoma (PAC, formerly polymorphous low-grade adenocarcinoma), although contradictory findings have also been reported.
Although the independent prognostic significance for KIT overexpression has not been evaluated, it has remained an attractive therapeutic target because of the clinical success of imatinib, a small molecule tyrosine kinase inhibitor (TKI) that competitively inhibits the activation of the KIT receptor and several other structurally similar receptor tyrosine kinases. However, it is clear that it is the nature of any point mutations in the protein that has an impact on its response to inhibition with imatinib. Although several investigators have not demonstrated the presence of mutations, recent studies using more sensitive polymerase chain reaction techniques confirm the presence of multiple mutations in the c-kit gene in ACCs. Although both gain of function (activating function of the receptor) and loss of function c-kit mutations have been described for other malignancies, the therapeutic implications of these recently described mutations in the therapeutic management of ACCs remains to be determined. Although the independent prognostic significance for KIT overexpression has not been evaluated, it has remained an attractive therapeutic target because of the clinical success of imatinib.
The study of perineural invasion (PNI) is an important biologic consideration for many cancers, especially for MSGTs such as ACCs. The molecular determinants of this mechanism of spread, which have only begun to receive attention in recent years, have yielded insights to view this spread pattern as an active and reciprocal interaction between malignant cells and peripheral nerves. Observations in other cancer sites currently suggest that PNI is the result of a mutual neurotropic interaction with cancer cells characterized by the release of various paracrine growth factors. As with neoangiogenesis, it is clear that the in-growth of nerve endings into a tumor may be stimulated by the tumor release of various neurotrophins, such as brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), and its receptor tyrosine kinase A (TrkA). Neurotrophin staining has been demonstrated to be present in ACCs. NGF and TrkA have both been correlated with the presence of perineural invasion. Recently the expression of neurotrophin-3 (NT3) and its receptor TrkC/NTRK3 was demonstrated in ACCs. These tumor-innervating nerve cells may release neurotransmitters that function as proliferative and promigratory signals for the tumor cells. Furthermore, nerve fibers are used as routes for tumor cell dissemination. For pancreatic carcinoma, detailed studies of serial sections have demonstrated that tumor cells can progress along branching nerve fascicles in a continuous fashion.
Therapeutically these insights offer the potential for the development of small molecule inhibitors, such as Trk tyrosine kinase inhibitors, especially for ACCs in hopes of addressing this pattern of spread. Importantly, radiation recently has been shown to impair PNI not only through direct cancer cytotoxicity but also with prophylactic treatment of the nerve, disruption of paracrine signaling preventing perineural spread in an animal model system. Such observations are important because they have potential radiotherapy treatment planning implications.
In contrast to other solid malignancies of the head and neck, the role of tumor hypoxia has not been as extensively evaluated in MSGTs. This is an important consideration for MSGTs given their relative resistance to radiotherapy and the improved results seen with neutron radiation, which is less oxygen dependent.
Preliminary investigations using methodologies such as immunohistochemical staining for the oxygen-dependent binding of 2-nitroimidazoles suggest that salivary gland malignancies may be well-oxygenated tumors. This observation would not support the hypothesis that an underlying mechanism for not only the radioresistance but also high rates of distant metastasis characterizing MSGTs is the presence of tumor hypoxia. In a study of 12 patients receiving surgery, the hypoxia-marker pimonidazole was administered preoperatively. Of the 8 patients yielding sufficient tumor specimen for analysis, immunohistochemical staining was not seen for pimonidazole and for the hypoxia inducible factor (HIF)-α or the HIF-1α regulated carbonic anhydrase (CA) IX and glucose transporters (GLUT) 1 and 3. Given that salivary gland malignancies are rare and of diverse histologies, further evaluation is needed before more generalized conclusions can be made.
Mucoepidermoid carcinoma can be divided into high grade versus low grade or high, intermediate, and low grade with the treatment implication being that low-grade cancers did not need elective nodal irradiation. In a classic paper by Brandwein et al., 20 mucoepidermoid cancer slides were shared with five expert pathologists and they were asked to grade them using their own criteria as well as a proposed, standardized Armed Forces Institute of Pathology (AFIP) criteria. The average kappa for their own criteria and proposed AFIP standardized grading was only 0.49 and 0.61. It is tantalizing to think that genetic information could help us more accurately subtype these cancers.
In 2003, the recurrent and hallmark t(11;19)(q21;p13) translocation involving CRTC1 at 19p13 and MAML2 at 11q21 was described by several groups demonstrating it to be a key pathogenic event that underlies the development of mucoepidermoid carcinomas (MECs). Although diagnostically useful, there was conflicting data from other groups on the prognostic significance of the MAML2 rearrangement.
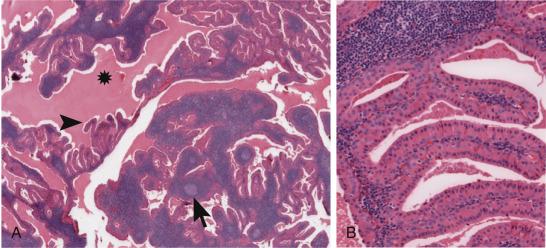
The identification of the CRCT1/3-MAML2 translocation, in some instances, might represent the most direct pathway to a confident diagnosis of the so-called ‘Warthin-like’ mucoepidermoid carcinoma, a low grade variant of mucoepidermoid carcinoma that shares morphologic and immunohistochemical overlap with a benign Warthin tumor ( Fig. 43.1 ). Identification of the characteristic fusion offers not only the promise of future risk stratified treatment approaches but also insights into the development of novel therapeutic targets. For example, unfavorable fusion-positive MECs are believed to have acquired further somatic mutations conferring increased invasiveness such as deletion in the CDKN2A (p16) gene.
It has been observed that low/intermediate mucoepidermoid cancers have fewer copy-number-aberrations when compared with high-grade mucoepidermoid cancers. Translocation-negative tumors were found to have more copy-number-aberrations, suggesting that mucoepidermoid cancers be divided into t(11:19)(q21: p13) translation-positive tumors with no or few chromosomal mutations versus translation-negative tumors with multiple positive chromosomal aberrations.
The parotid is a purely serous gland, therefore no mucin should be produced. A number of mucins were expressed in mucoepidermoid cancers and the difference in the expression of various mucins may also aid in more accurate grading of mucoepidermoid cancers.
Epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor 2 (erbB2 or HER2/neu) are members of the EGFR transmembrane receptor family and, when activated, transduce mitogenic signals. Several studies have reported high immunohistochemical expression levels that may be more dominantly expressed in non-ACCs. However, the clinical significance of EGFR protein overexpression remains controversial with some multivariate analyses demonstrating both an independent association with poor survival and others demonstrating no independent association with poor survival.
Similarly, erbB2 overexpression has been reported for MSGTs. These include strong overexpression in some MECs and in salivary duct carcinomas (SDCs), but rarely for ACCs. Univariate and multivariate independent prognostic significance has been reported for erbB2 overexpression. Agulnik et al. suggest that greater clinical activity with the dual EGFR and erbB2 inhibitor lapatinib may be possible in tumors with both high EGFR and erbB2 expression and that erbB2 may be especially important. Recently, patients with SDCs and erbB2 amplification treated with a trastuzumab-containing regimen containing other targeted agents based on actionable mutations were found to have improved survival compared with patients who did not have actionable mutations. Identifying subgroups of patients more dependent on erbB2 signaling may be important in further defining the therapeutic benefit with erbB2 inhibition because tailoring therapies based on molecular abnormalities may be have therapeutic benefits.
The classification of SGTs is primarily based on microscopic morphologic criteria, with ancillary testing, such as immunohistochemical staining, and more recently, the utilization of fluorescence in situ hybridization (FISH) to detect characteristic chromosomal rearrangements in support of the morphologic interpretation. Two general classification systems of salivary gland tumors have been recognized and includes the AFIP and the World Health Organization (WHO). In 2017 the WHO updated their 2005 classification of salivary gland tumors ( Table 43.4 ). Several benign epithelial and mesenchymal lesions of the salivary glands are newly included with the fourth edition of the WHO Classification of Head and Neck Tumors. Among epithelial lesions, sclerosing polycystic adenosis is a rare but benign lesion that can be multifocal and occasionally recur. Intercalated duct hyperplasia is highlighted here as it may represent a precursor salivary lesion, particularly to basal cell adenoma and possibly to epithelial-myoepithelial carcinoma. Newly included mesenchymal lesions of salivary gland include hemangioma, lipoma, and nodular fasciitis. Mammary analog secretory carcinoma originally described in 2010, is a new classification within the fourth edition of the WHO classification, and the name is now simplified to secretory carcinoma. Prior to its recognition as a distinct salivary gland tumor, examples of secretory carcinoma were most likely designated a variant of acinic cell carcinoma, mucoepidermoid carcinoma, or adenocarcinoma not otherwise specified when encountered in practice. Secretory carcinoma is morphologically analogous to secretory carcinoma of the breast, and both the salivary and breast carcinoma share the ETV6-NTRK3 gene fusion. Although secretory carcinoma is generally regarded as a low-grade salivary gland malignancy, prognosis can be influenced by stage, and the presence of high-grade transformation, which has been described in rare cases. Accurate identification of secretory carcinoma may be important if a selective TRK inhibitor is under therapeutic consideration.
| Descriptor | ICD-O Codes a |
|---|---|
| Malignant tumors | |
| Mucoepidermoid carcinoma | 8430/3 |
| Adenoid cystic carcinoma | 8200/3 |
| Acinic cell carcinoma | 8550/3 |
| Polymorphous adenocarcinoma | 8525/3 |
| Clear cell carcinoma | 8310/3 |
| Basal cell adenocarcinoma | 8147/3 |
| Intraductal carcinoma | 8500/2 |
| Adenocarcinoma, NOS | 8140/3 |
| Salivary duct carcinoma | 8500/3 |
| Myoepithelial carcinoma | 8982/3 |
| Epithelial-myoepithelial carcinoma | 8562/3 |
| Carcinoma ex pleomorphic adenoma | 8941/3 |
| Secretory carcinoma | 8502/3 |
| Sebaceous adenocarcinoma | 8410/3 |
| Carcinosarcoma | 8980/3 |
| Poorly differentiated carcinoma | |
| Undifferentiated carcinoma | 8020/3 |
| Large cell neuroendocrine carcinoma | 8013/3 |
| Small cell neuroendocrine carcinoma | 8041/3 |
| Lymphoepithelial carcinoma | 8082/3 |
| Squamous cell carcinoma | 8070/3 |
| Oncocytic carcinoma | 8290/3 |
| Uncertain malignant potential | |
| Sialoblastoma | 8974/1 |
a The morphology codes are from the International Classification of Diseases for Oncology (ICD-O). Behavior is coded /0 for benign tumors; /1 for unspecified, borderline, or uncertain behavior; /2 for carcinoma in situ and grade III intraepithelial neoplasia; and /3 for malignant tumors.
In addition to new tumor entries, several conceptual changes regarding salivary gland tumor classification were introduced in the 4 th edition of the WHO classification. The name polymorphous low-grade adenocarcinoma (PLGA) has now been shortened to polymorphous adenocarcinoma (PAC). The rationale for modification of the tumor name lies first in recognizing that not all polymorphous “low-grade” adenocarcinoma behave in such a fashion. Removing the default terminology “low-grade” from the name is important in allowing the pathologist to characterize examples, although uncommon, where the tumor deviates from the typical low-grade characterization. Although highly controversial, the name change to PAC additionally serves as a unifying diagnosis between two previously separate entities; “PLGA” and the morphologically and molecularly similar tumor known as cribriform adenocarcinoma of minor salivary gland (CAMSG). Although the latter is regarded as having a higher incidence of regional metastases, the identification of shared genes within the same family suggests “PLGA” and “CAMSG” may be variants within a spectrum. As always, polymorphous adenocarcinoma may have a microscopic appearance that can on small biopsy samples mimic that of adenoid cystic carcinoma. The indolent clinical course more commonly described with PAC makes accurate distinction from adenoid cystic carcinoma important.
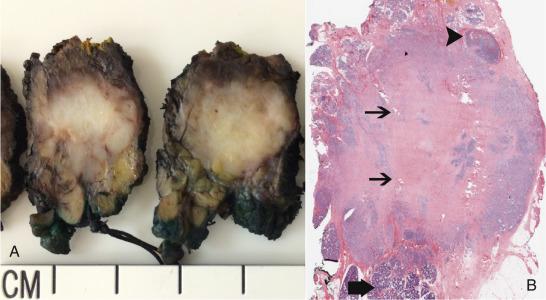
Changes to the section on pleomorphic adenoma include the addition of the new subheading metastasizing pleomorphic adenoma, which despite the name, is reclassified from a malignancy because its histologic pattern is identical to benign pleomorphic adenoma. Nonetheless, metastasizing pleomorphic adenoma should still be regarded as having the potential for aggressive biologic behavior. In the classification of infiltrating carcinoma ex-pleomorphic adenoma it is important to note that the terminology “carcinoma ex pleomorphic adenoma” should no longer be utilized as a stand-alone diagnosis. The malignancy identified as arising in association with the pleomorphic adenoma component should additionally be classified. The most commonly recognized subtypes arising ex-pleomrphic adenoma are salivary duct carcinoma, myoepithelial carcinoma, epithelial-myoepithelial carcinoma, and adenocarcinoma not otherwise specified ( Fig. 43.2 ).
Also new in the WHO 4 th edition is incorporation of the terminology “high-grade transformation” (HGT), which represents the preferred terminology over “dedifferentiation.” Acinic cell carcinoma, adenoid cystic carcinoma, and epithelial-myoepithelial carcinoma are tumors in which the phenomenon of HGT is well known. A tumor with HGT is expected to show more aggressive behavior.
Practically, histologies may be categorized as high-grade histologies with at a greater risk of nodal and distant metastases or low-grade histologies with a lower risk of nodal metastasis. The former includes high-grade MEC, high-grade adenocarcinoma–NOS, carcinoma ex pleomorphic adenoma, SDCs, certain variants of carcinoma ex pleomorphic adenoma, the presence of HGT and squamous cell carcinomas. Low-grade histologies include low-grade MECs, acinic cell carcinomas, secretory carcinoma, and PAC. In adenoid cystic carcinoma, tubular and cribriform patterns of growth are cytologically low grade, but identification of solid pattern growth portends a more aggressive course. An intermediate group has also been described consisting of intermediate MECs.
For MECs, the histologic grading has been shown to be of prognostic significance. High-grade disease also increases the risk of nodal metastasis sufficient to warrant elective management (e.g., neck dissection). Despite this, the risk of nodal metastasis with low grade (LG) and especially intermediate grade (IG) can still be significant. Ozawa et al. reported that, using the Goode (a.k.a. AFIP) grading schema, low- and intermediate-grade MECs had nodal metastases in 24% and 30%, respectively. High-grade (HG) MECs demonstrated nodal metastases in 56% of patients. In the modified Healey grading schema, lymph node involvement was 0% (LG), 22% (IG), and 72% (HG). T category also is associated with an increased risk of nodal metastasis for both major and minor salivary gland MECs. T1 HG disease may be at low risk for nodal metastasis in major salivary glands, that is, anatomic sites not involving the mucosa.
When considering all histologies together, the risk of lymph node metastasis is increased with T3 and T4 disease, involvement of a pharyngeal site and high-grade MECs, adenocarcinoma–NOS, and salivary duct carcinoma. Similar observations have been reported with T stage, anatomic site, and histology ( Table 43.5 ). For minor salivary glands, the density of the lymphatics in the anatomic site has a significant influence on the risk of nodal metastases. In general, cancers arising within the oropharynx or nasopharynx have about a 60% incidence of lymph node metastasis compared with 5% to 10% for hard palate and paranasal sinus sites. Minor salivary gland cancers arising within the tongue and floor of the mouth have an approximately 40% incidence of lymph node metastasis; nasal cavity, buccal mucosa, and lip have a 15% incidence or less. For minor salivary gland tumors, independent risk factors for nodal involvement include male gender, T3-T4 stage, pharyngeal site of primary malignancy, and high-grade adenocarcinoma or high-grade mucoepidermoid carcinomas.
| Total Score (T Stage Plus Histologic Type) | Parotid Gland (%) | Submandibular Gland | Oral Cavity | Other Locations |
|---|---|---|---|---|
| 2 | 4 | 0 | 4 | 0 |
| 3 | 12 | 33 | 13 | 29 |
| 4 | 25 | 57 | 19 | 56 |
| 5 | 33 | 60 | — | — |
| 6 | 38 | 50 | — | — |
ACCs at the primary site have a predilection for PNI as a unique pattern of local disease extension. ACCs occur most commonly in the palate and frequently spread by PNI. PNI may be seen in more than 50% of cases and spread may occur in both directions along the nerve. Growth along the nerve has been shown to have “skip” areas of involvement and noninvolvement; however, “skip” metastases along nerves have not been substantiated in studies of squamous cancers of the skin and careful anatomic studies of adenoid cystic cancer may yield to the same conclusion.
The risk of distant metastases is increased with the presence of lymph node metastasis, skull-base involvement, and high-grade histology. Common distant metastatic sites, which include lung, liver, and brain, are also associated with the histologic grade.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here