Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Any unexplained salivary gland mass is an indication for fine-needle aspiration (FNA). FNA is the preferred biopsy method because incisional biopsy is associated with an increased risk of infection and potential contamination of surgical planes. FNA, in contrast, is a cost-effective technique that poses minimal risk to the patient. The few relative contraindications to FNA are a bleeding disorder (which might result in a hematoma) and acute sialadenitis (associated with aspiration-related pain); obscuring blood or inflammatory cells, in these settings, can limit diagnostic interpretation.
Clinical and imaging findings cannot always establish the origin of tumors in the head and neck region. Thus a primary goal of FNA is to distinguish salivary gland lesions from nonsalivary head and neck masses, especially those of lymph node origin, but also soft tissue lesions and skin and skin adnexal masses. Because the normal parotid gland contains more than 20 intraparotid and periparotid lymph nodes, primary lymphomas and metastatic tumors mimicking salivary gland neoplasms are commonly seen.
Some authors have argued that FNA is superfluous because mass lesions in the head and neck always require excision. Such an approach would result in the unnecessary excision of a number of lesions (up to one-third of salivary gland masses) that in fact do not require surgery. Some patients have a nonneoplastic lesion (e.g., chronic sialadenitis, granulomatous disease, a lymphoepithelial cyst); others have a benign neoplasm, and, for those who are poor surgical candidates, knowledge of its benignity provides reassurance that surgery can be avoided. Still others have a malignancy for which surgical excision is not appropriate (e.g., lymphoproliferative disease, metastasis). Even when surgery is indicated, FNA guides preoperative strategy (e.g., partial or total parotidectomy, facial nerve resection, neck dissection), providing reassurance to both the patient and surgeon. Surgical management is often influenced by cytologic findings. High-grade malignancies are treated more aggressively than low-grade malignancies and benign neoplasms. In the superficial parotid gland, the most common site of salivary gland neoplasms, benign tumors and low-grade malignancies are treated with superficial parotidectomy alone, whereas high-grade carcinomas are treated with total parotidectomy and, potentially, facial nerve sacrifice. Furthermore, lymph node neck dissection and neoadjuvant therapy are often indicated for high-grade tumors.
Aspirations are performed using a 25- or 23-gauge needle, small enough to reduce the risk of tissue trauma but large enough to obtain an adequately cellular sample. Rapid on-site evaluation of smears can determine the number of passes required to ensure adequacy and facilitate appropriate triage of the specimen, like allocation of material for flow cytometry (in the case of lymphoid lesions) or cell block preparation (in the case of diagnostically challenging lesions like oncocytic, spindle cell, and clear cell tumors).
Although FNA was first described in the 1930s, salivary gland FNA has gained wide acceptance only in the last 40 years. Meta-analysis of published series indicates 96% sensitivity and 98% specificity for neoplasia, whereas distinction between benign and malignant neoplasms has 79% sensitivity and 96% specificity. Because accuracy varies greatly among published studies, the utility of salivary gland FNA is highly practitioner dependent. For nondiagnostic and indeterminate aspirates, results improve with repeat FNA and ultrasound guidance. Limited data indicate core biopsy offers improved accuracy but at a cost: more anesthesia, more discomfort for the patient, and a greater risk of complications, including nerve damage, tumor spillage, and needle tract seeding.
Evidence-based criteria for assessing adequacy have yet to be established, but a minimum of 60 lesional cells has been proposed. False-negative diagnoses are principally due to inadequate sampling of the lesion, most frequently those that are cystic. Cystic neoplasms (e.g., Warthin tumor, low-grade mucoepidermoid carcinoma, metastatic squamous cell carcinoma) are the most likely causes of false-negative diagnoses. A distinction should be made between mucinous and nonmucinous cysts because mucinous cysts raise concern for mucoepidermoid carcinoma. When a cystic salivary gland lesion is aspirated, any residual mass should be resampled after fluid is withdrawn. Surgical excision is indicated if the cyst does not resolve by aspiration or if it recurs.
False-negative FNAs resulting from an interpretation error are most common with low-grade mucoepidermoid carcinoma, adenoid cystic carcinoma, and non-Hodgkin lymphoma. False-positive diagnoses are seen with cystic lesions, particularly Warthin tumor, which sometimes contains atypical squamous cells, and pleomorphic adenoma, in which nuclear atypia or stromal spheres result in an incorrect diagnosis of carcinoma ex pleomorphic adenoma or adenoid cystic carcinoma, respectively. Multiple passes help to minimize sampling and interpretative errors resulting from variation in cytologic atypia and cellular constituents within a tumor.
Complications are infrequent. The most common are a hematoma, infection, and facial nerve pain, but these occur in fewer than 1% of patients. Tumor seeding of the needle tract is extremely rare. In some cases, FNA leads to partial or, rarely, complete infarction of the neoplasm. Particularly susceptible neoplasms are oncocytoma, Warthin tumor, and acinic cell carcinoma. When using a 25-gauge needle, significant infarction or hemorrhage is seen in up to 10% of cases, but such changes rarely hinder histologic diagnosis.
Both Romanowsky-type and Papanicolaou stains are important because many neoplasms contain a combination of epithelial and stromal components. Air-dried Romanowsky-stained smears highlight diagnostically useful features of the stromal component that are poorly visualized in alcohol-fixed preparations of lesions such as pleomorphic adenoma, basal cell tumors, and adenoid cystic carcinoma. Romanowsky stains also aid in the evaluation of lymphoid lesions and cytoplasmic vacuolization in acinic cell carcinomas. Papanicolaou-stained preparations are especially useful for evaluating nuclear features and cytoplasmic differentiation.
Either smears or liquid-based preparations can be used, but smears are preferred. With liquid-based preparations, extracellular constituents are less prominent, cellular shrinkage is greater, and tissue fragmentation is more pronounced, with possible decreased sensitivity and specificity. A cell block is valuable for both histochemical and immunohistochemical stains as well as providing material for molecular studies. Cell block preparations can also better demonstrate architectural patterns and some cellular features, particularly serous acinar differentiation. In challenging cases, cytogenetic evaluation is also valuable. Characteristic chromosomal translocations have been identified in pleomorphic adenoma, mucoepidermoid carcinoma, adenoid cystic carcinoma, secretory carcinoma, and clear cell carcinoma. Surrogate immunohistochemical markers, in situ hybridization testing, or next generation sequencing platforms for these translocations are diagnostically useful in challenging cases ( Table 11.1 ).
| Tumor | Genetic Alteration | Genes Involved | FISH Probe | IHC Markers |
|---|---|---|---|---|
| Pleomorphic adenoma and carcinoma ex PA | t(8q12) t(12q13-15) |
PLAG1 HMGA2 |
PLAG1 HMGA2 |
PLAG1 HMGA2 |
| Adenoid cystic carcinoma | t(6;9)(q22-23;p23-24) | MYB-NFIB | MYB | MYB |
| Mucoepidermoid carcinoma | t(11;19)(q21;p13) t(11;15)(q21;q26) |
CRCT1-MAML2 CRCT3-MAML2 |
MAML2 | p63, p40 |
| Secretory carcinoma | t(12;15)(p13;q25) | ETV6-NTRK3 | ETV6 | S100, mammaglobin |
| Clear cell carcinoma | t(12;22)(q13;q12) | EWSR1-ATF1 | EWSR1 |
The Milan System for Reporting Salivary Gland Cytopathology recommends that every case be categorized into one of six diagnostic categories ( Table 11.2 ). Each has an implied risk of malignancy (ROM) that ranges from less than 5% for “Neoplasm: Benign” to 90% or higher for “Malignant.”
| Diagnostic category | Risk of malignancy (%) |
|---|---|
| Nondiagnostic | 25 |
| Non-neoplastic | 10 |
| Atypia of undetermined significance | 20 |
| Neoplasm: Benign | <5 |
| Neoplasm: SUMP | 35 |
| Suspicious for malignancy | 60 |
| Malignant | >90 |
The Nondiagnostic category is for significantly limited specimens: those judged as nonrepresentative of the target and those with scant cellularity or an obscuring artifact. Examples include non-mucinous cyst contents and “normal-appearing” salivary gland elements in the setting of a clinically and radiologically defined mass. Excluded from the nondiagnostic category are mucinous cyst contents, aspirates with atypia, and specimens with abundant acellular matrix. The rate of nondiagnostic salivary gland FNAs should be less than 10%.
The Nonneoplastic category includes acute, chronic, and granulomatous sialadenitis. Another common entity in this category is the reactive lymph node. The ROM for this category is less than 10% if strict criteria are applied.
Atypia of Undetermined Significance (AUS) is for cases that contain some atypical feature(s), but they do not fulfill criteria for the other 5 categories. The category is heterogeneous and has a ROM of approximately 20%. It is recommended that no more than 10% of salivary gland FNAs be interpreted as AUS.
The Neoplasm category is divided into two subcategories: (1) Neoplasm: Benign and (2) Neoplasm: Salivary Gland Neoplasm of Uncertain Malignant Potential (SUMP). The Neoplasm: Benign subcategory is for benign neoplasms that are reliably recognized by conventional cytomorphology, most commonly pleomorphic adenoma (PA) and Warthin tumor. The ROM is less than 5%. The Neoplasm: SUMP category is for cases with the cytomorphologic features of a neoplasm, but a specific entity cannot be established and, importantly, a malignant neoplasm cannot be excluded.
The Suspicious for Malignancy category is for cases highly suggestive of a malignancy, but the cytomorphologic features are not conclusive. Many such specimens benefit from ancillary studies.
The Malignant category includes a broad range of primary malignant neoplasms of the major and minor salivary glands as well as metastatic carcinomas to salivary gland lymph nodes. Whenever possible, aspirates classified as Malignant should be graded as low- or high-grade, given the impact of tumor grade on clinical management.
Precise classification of salivary gland neoplasms by FNA is possible for many of the commonly encountered lesions but remains problematic for the less common entities. The cytologist is faced with five distinct challenges. First, there are more than 30 salivary gland tumors of epithelial type, many of which are rare, placing familiarity with all of them out of reach for most practitioners. Second, most salivary gland malignancies are low-grade, displaying few overt cytologic features of malignancy. Third, the less common, high-grade malignancies, although readily recognizable as malignant, are difficult to distinguish from one another. Fourth, some benign tumors (e.g., basal cell adenoma) have a malignant counterpart (e.g., basal cell adenocarcinoma) that is morphologically identical except that there is an infiltrative growth pattern, something that cannot be assessed cytologically. Finally, many different salivary gland tumors share similar cellular constituents; it is the architectural relationship and relative abundance of these constituents that ultimately determines the tumor type. With some tumors such as pleomorphic adenoma, there is great variability not just within a given tumor but from one tumor to the next.
Fortunately, there are two mitigating factors. First, the two most common neoplasms, pleomorphic adenoma and Warthin tumor, together comprise more than 80% of salivary gland tumors and, with their distinctive cytomorphologic features, are readily identified. Second, conservative excision is used for both benign tumors and low-grade malignancies. In contrast, radical surgical approaches with combined modality therapy are reserved for high-grade malignancies. Thus, although a specific diagnosis may not be feasible, low-grade neoplasms usually can be distinguished from high-grade ones, and an appropriate differential diagnosis is sufficient for clinical management.
The large group of so-called “basaloid” neoplasms are a special case, however. The basaloid neoplasms encompass the entire spectrum of biologic behavior, from benign neoplasms, through low-grade malignancies, to the aggressive solid variant of adenoid cystic carcinoma. A precise cytologic diagnosis is not possible with many FNAs that show basaloid cells only. A frozen section is often necessary to guide appropriate surgical management. This and other diagnostic dilemmas are listed below. Suggested diagnostic approaches are discussed in detail in the remainder of the text.
Basaloid neoplasms , especially basal cell adenoma and the solid variant of adenoid cystic carcinoma
Oncocytic lesions , especially oncocytoma and acinic cell carcinoma
Mucus-containing cysts , especially mucoepidermoid carcinoma, mucocele, and mucinous metaplasia
High-grade carcinomas , including mucoepidermoid carcinoma, salivary duct carcinoma, and carcinoma ex pleomorphic adenoma
Clear cell neoplasms : myoepithelioma, epithelial-myoepithelial carcinoma, acinic cell carcinoma, and clear cell carcinoma
Spindle cell lesions , especially myoepithelioma and schwannoma
Clinical information is often helpful. It is useful to know whether the patient has had a malignancy elsewhere. Aggressive signs, symptoms, and imaging findings, such as rapid growth, pain (suggestive of neural invasion), and infiltrative growth on radiological studies, are indicative of malignancy. Most salivary gland neoplasms are firm and painless; Warthin tumor has a characteristically doughy consistency. Cystic change and bilaterality also help narrow the diagnostic possibilities.
Warthin tumor
Mucoepidermoid carcinoma
Acinic cell carcinoma
Sialadenitis
Amyloidosis
Lymphoepithelial cyst
Warthin tumor
Acinic cell carcinoma
Lymphoma
The epidemiological features of salivary gland neoplasms are also helpful. Most salivary gland neoplasms are more common in women, but Warthin tumor, salivary duct carcinoma, and metastatic squamous cell carcinoma occur more frequently in men. Whereas 68% to 85% of parotid gland tumors are benign, 80% to 90% of sublingual and minor salivary gland neoplasms are malignant. Some tumors are almost site-specific (e.g., Warthin tumor in the parotid gland, polymorphous adenocarcinoma in the minor salivary glands of the palate).
Attention to the constituents of an aspirate is the key to the neoplastic, inflammatory, lymphoid, or cystic nature of a lesion. Hypercellular specimens are typical of neoplasms. Inflammatory cells are prominent in sialadenitis and cystic lesions. Stone fragments are diagnostic of sialolithiasis. Abundant lymphoid cells are seen in a variety of salivary gland lesions, not all of them lymphoid in nature.
Intraparotid or periparotid lymph node
Lymphadenoma
Lymphoma
Lymphoepithelial cyst
Warthin tumor
Mucoepidermoid carcinoma
Acinic cell carcinoma
Lymphoepithelial carcinoma
The presence and character of matrix material provides important diagnostic information. Mucin (pale magenta in Romanowsky preparations, translucent blue/purple on Papanicolaou smears) suggests a mucoepidermoid carcinoma, mucocele, retention cyst, or mucinous metaplasia. A chondromyxoid matrix is characteristic of pleomorphic adenoma and stromal spheres are typical of adenoid cystic carcinoma, but neither finding is entirely specific. Such findings, along with a more detailed impression of the cell type(s) seen (myoepithelial, duct-lining epithelial, basaloid, oncocytic, mucinous, squamous, acinic, sebaceous, and clear cells) enable one to refine the differential diagnosis.
A variety of crystalloids are seen in the salivary glands, but none of them are specific for any particular salivary gland lesion or neoplasm. Tyrosine crystalloids are floret-shaped and most often encountered in pleomorphic adenomas, but they can be seen in other lesions, benign and malignant ( Fig. 11.1A ). Amylase crystalloids (“nontyrosine crystalloids”) are polygonal, plate-like, or needle-shaped and are most often associated with benign, nonneoplastic conditions, especially infections and cysts ( Fig. 11.1B ).
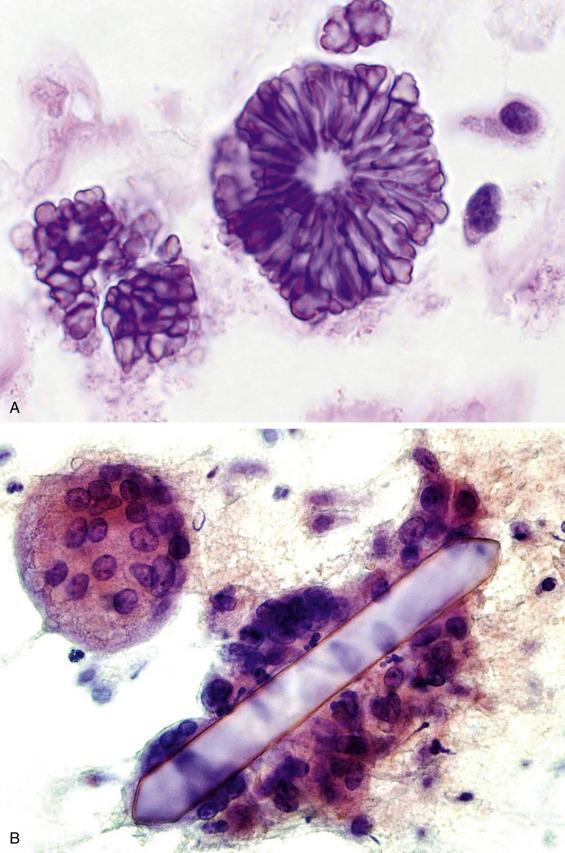
In some instances, often due to sampling error, the FNA specimen shows only normal salivary gland elements.
Rounded clusters of serous or mucinous acinar cells
Flat sheets and tubules of ductal cells
Adipose tissue
Aspirates of normal salivary gland tissue are comprised of acinar cells, ductal cells, and adipose tissue ( Fig. 11.2A ). Occasionally, naked acinar nuclei that mimic lymphocytes and scattered myoepithelial cells are present. Acinar cells are usually arranged in tight grape-like clusters. Ductal cells are smaller and less conspicuous, arranged as tubules or honeycomb-like flat sheets. The acinar cells are of serous type in the parotid gland, a mixture of serous and mucinous types in the submandibular gland, and predominantly mucinous in the minor salivary glands. The serous-type acinar cells ( Fig. 11.2B ) are evenly spaced, with basally placed nuclei. They are large pyramidal cells with abundant foamy and granular, basophilic cytoplasm and a small, eccentrically placed, round to oval nucleus with an indistinct nucleolus. Normal (and neoplastic) acinar cells have characteristic coarse, basophilic, PAS-positive and diastase-resistant cytoplasmic zymogen granules. In contrast to the pyramidal serous cells, mucinous cells are columnar, with pale cytoplasm that indents a bland nucleus. Ductal cells come in several varieties. Intercalated duct cells are uniform, small, and cuboidal, with scant, dense cytoplasm and uniform nuclei; occasional large branching ductal fragments are present. Ductal cells derived from the larger striated ducts are oncocytic, whereas those from the collecting ducts are columnar and ciliated. Ductal cells sometimes exhibit mucinous or squamoid changes. Mature lymphocytes can also be seen, owing to the abundance of intraparotid and periparotid lymphoid tissue. A mixture of ductal cells, adipose tissue, and acinar cells distinguishes normal salivary gland tissue from acinic cell carcinoma, which usually consists of a monomorphous population of neoplastic acinar cells.
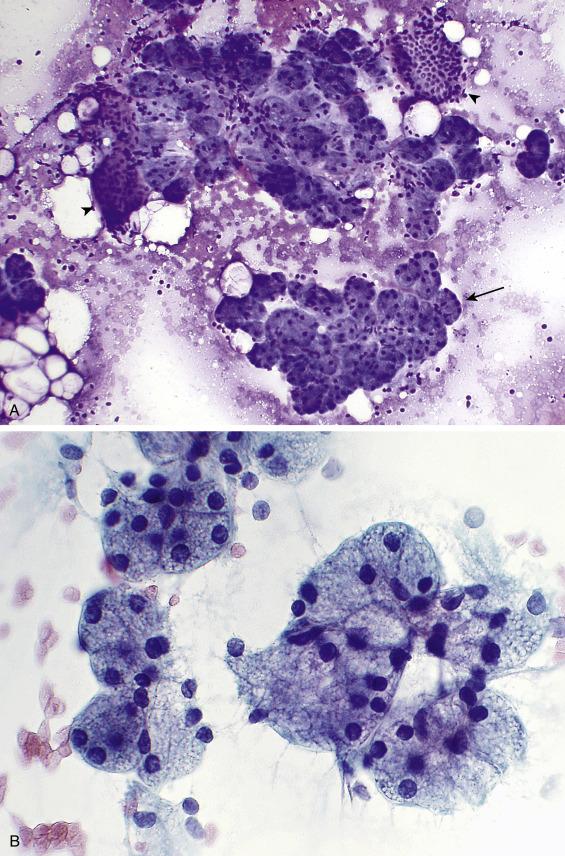
Up to 20% of salivary gland aspirates yield only normal tissue. FNA of a palpable or radiographic mass that reveals only normal salivary gland elements is reported as Nondiagnostic. Besides sampling error, other explanations for a normal-elements-only result include a prominent but normal salivary gland, sialadenosis, hamartoma, and lipoma.
Acute sialadenitis is rarely aspirated because it is usually diagnosed clinically as a postoperative complication, a viral or fungal infection, or a secondary bacterial infection due to obstruction caused by sialolithiasis ( Fig. 11.3 ). There is usually no discrete mass, and most cases affect the parotid gland.
Neutrophils, necrotic debris
Stone fragments (if sialolithiasis also present)
Scant ductal cells
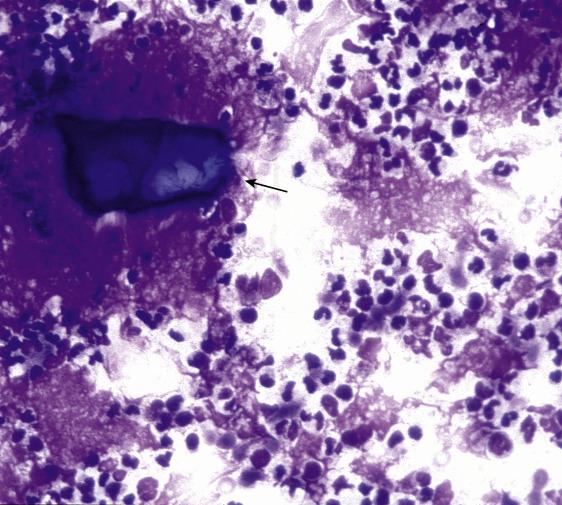
Fine-needle aspirates of acute sialadenitis show abundant neutrophils, necrotic cells, and fibrin. Small groups of ductal cells, some with reactive atypia, are present. A malignant neoplasm is excluded based on the hypocellularity and limited atypia. When an infectious cause is suspected, a portion of the material should be sent for a microbiologic workup. If there is clinical suspicion of a neoplastic process, reaspiration after treatment and resolution of the acute infection might be helpful.
As with acute sialadenitis, FNA of chronic sialadenitis can be associated with pain. Chronic sialadenitis is more likely to present as a clinically discrete mass, often in the submandibular gland. Common causes include sialolithiasis and radiation therapy for head and neck cancer (usually squamous cell carcinoma).
Scant cellularity
Small sheets and tubules of basaloid ductal cells with sharp borders
Paucity of acinar elements
Blood, proteinaceous debris, mature lymphocytes
Fragments of fibrous tissue
Aspirates of chronic sialadenitis are sparsely cellular and usually contain scant clusters of basaloid cells of ductal origin. So-called “basaloid” cells are small or intermediate-sized cells with scant cytoplasm, often tightly clustered together ( Fig. 11.4 ). In chronic sialadenitis, they are admixed with blood, proteinaceous debris, mature lymphocytes, and small amounts of fibrous tissue. Acinar cells are sparse or absent. Sialolithiasis can be diagnosed when stone fragments are identified (see Fig.11.3 ). Atypical squamous metaplasia, mucinous metaplasia, radiation atypia, abundant histiocytes, extracellular mucin, crystals, and (rarely) psammoma bodies may be present. Chronic sclerosing sialadenitis (Kuttner tumor) is a form of chronic sialadenitis affecting the submandibular gland, manifesting clinically as a firm mass and mimicking a neoplasm. The morphologic findings are nonspecific and similar to those of a conventional chronic sialadenitis. In some cases, lymphoid cells are abundant, and lymphoma must be excluded. Chronic sclerosing sialadenitis has an increased proportion of IgG4-positive plasma cells and is a form of systemic IgG4-related disease.
Normal salivary gland
Lymphoepithelial sialadenitis (LESA)
Warthin tumor
Basaloid neoplasms
Mucoepidermoid carcinoma
Squamous cell carcinoma
Normal salivary gland has a greater proportion of acinar to ductal cells than chronic sialadenitis. Chronic sialadenitis is distinguished from LESA by the absence of lymphoepithelial islands and germinal center fragments. Unlike Warthin tumor, chronic sialadenitis lacks cohesive groups of oncocytes. The basaloid ductal cells of chronic sialadenitis resemble the cells of basaloid neoplasms but are less numerous and arranged in smaller groups than those of neoplasms. The intermediate cells of a mucoepidermoid carcinoma resemble the basaloid ductal cells of chronic sialadenitis, but mature squamous cells and mucus cells are absent. Chronic sialadenitis is distinguished from a squamous cell carcinoma by its scant cellularity; the absence of marked atypia, mitotic activity, and necrotic tumor cells; and the paucity of isolated epithelial cells.
Granulomatous sialadenitis can be caused by infection (fungi, mycobacteria, toxoplasmosis, and cat scratch disease), sarcoidosis, cyst rupture, and rarely neoplasia (Hodgkin lymphoma, T-cell lymphoma, or metastatic carcinoma). Aspirates are characterized by a variable background of granular, necrotic cell debris and inflammation, together with aggregates of epithelioid histiocytes, frequently with multinucleated forms . Epithelioid histiocytes have abundant eosinophilic cytoplasm and cytologically bland, elongated, folded nuclei with indistinct nucleoli. Asteroid bodies, Schaumann’s bodies, and calcium oxalate crystals can be present in sarcoidosis, but these findings are not specific, and an infectious cause must be excluded by special stains or microbiologic cultures.
Sialadenosis is a nonneoplastic, noninflammatory enlargement of the salivary gland that more commonly affects the parotid gland and is often bilateral. It results from acinar cell hypertrophy and has been associated with a wide range of causes, including endocrine abnormalities such as diabetes mellitus, nutritional deficiencies, alcoholism, cirrhosis, and certain drugs, especially antihypertensives.
Aspirates of sialadenosis appear normal except that the constituent acinar cells are significantly larger than normal acinar cells, and inflammatory cells tend to be absent. Normal acinar cells are approximately 50 μm in diameter, whereas acinar cells of sialadenosis can measure up to 100 μm. In practice, these size differences are difficult to assess. Excluding a discrete mass is essential for a diagnosis of sialadenosis (under the “Nonneoplastic” heading). Otherwise, the case should be reported as “Nondiagnostic” because of the strong likelihood of a sampling error.
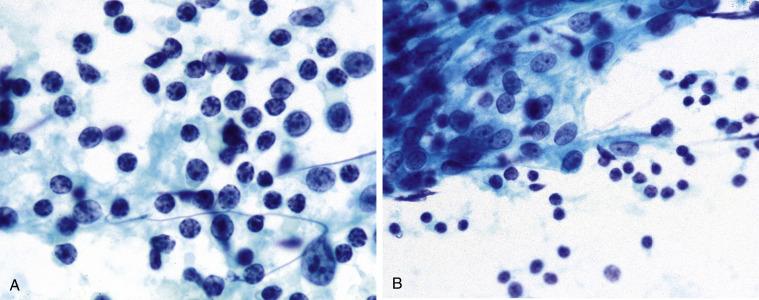
This lesion has been known by a variety of names, including Mikulicz disease, benign lymphoepithelial lesion, and myoepithelial sialadenitis. Resulting in part from the discovery that the cells comprising the lymphoepithelial islands (formerly called epimyoepithelial islands ) of this disorder are almost entirely epithelial, the term lymphoepithelial sialadenitis (LESA) has emerged as the preferred terminology. LESA most frequently affects women and manifests as a diffuse, often bilateral, enlargement of the parotid gland; the submandibular gland is also involved in a minority of cases. Minor salivary glands show chronic inflammatory changes related to LESA but lack the lymphoepithelial islands. LESA is an autoimmune disorder and is seen in virtually all patients with Sjögren syndrome. Approximately 50% of patients with LESA do not have Sjögren syndrome, however. They may have some other connective tissue disorder or no disease whatever.
Cellular aspirate
Mixed population of lymphocytes, plasma cells, tingible-body macrophages
Germinal center fragments
Lymphoepithelial islands
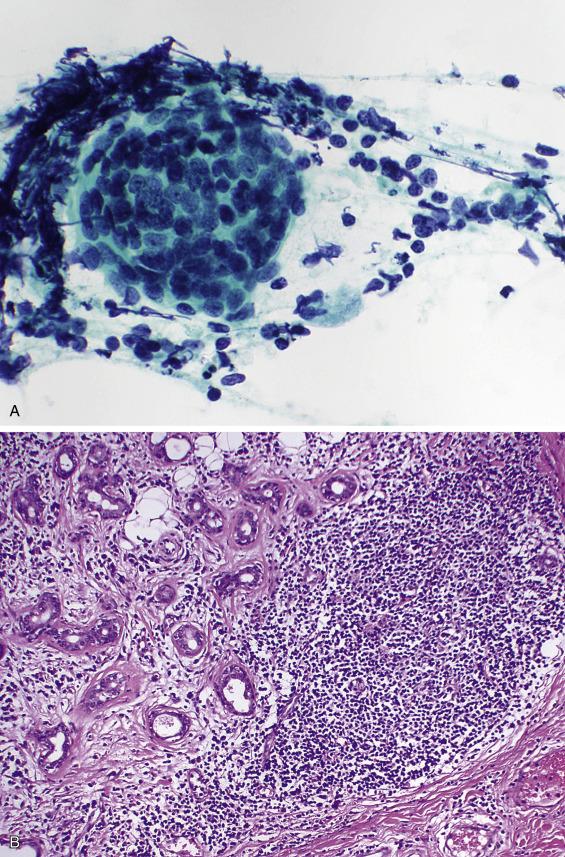
Aspirates are cellular and show a mixed population of mature lymphocytes, plasma cells, tingible-body macrophages, germinal center fragments, and characteristic lymphoepithelial islands: sheets of pale, overlapping, ductal-type cells infiltrated by lymphocytes ( Fig. 11.5 ). The ductal cells often exhibit reactive and squamous metaplastic changes. Acinar cells are rarely present.
Chronic sialadenitis
Simple lymphoepithelial cyst
Human immunodeficiency virus (HIV)–associated cystic lymphoepithelial lesions
Warthin tumor
Metastatic carcinoma to a lymph node
Extranodal marginal zone B-cell lymphoma
Chronic sialadenitis tends to be sparsely cellular, with fewer lymphocytes and germinal center fragments, and lacks the characteristic lymphoepithelial islands of LESA. Simple lymphoepithelial cysts and HIV-associated cystic lymphoepithelial lesions are cytologically similar to the cystic form of LESA and are discussed further in the next section. Warthin tumor is distinguished from LESA because the former contains oncocytic epithelium. The epithelial component of LESA lacks the pleomorphism, mitotic activity, and necrosis of a metastatic carcinoma. Perhaps most importantly, an extranodal marginal zone lymphoma should be suspected if large numbers of monocytoid B-cells are encountered. Ancillary studies to assess for clonality (flow cytometry, immunocytochemistry) can be invaluable in such circumstances.
Cystic lesions account for approximately 5% of salivary gland FNAs. A wide variety of non-neoplastic and neoplastic lesions can be cystic. Non-neoplastic cysts, both congenital and acquired, occur either adjacent to or within the salivary glands. Diagnostically, these cysts can be broadly categorized into squamous-lined cysts and mucus-containing cysts.
Squamous-lined cysts include congenital cysts , encompassing dermoid and branchial cleft cysts. Also in this category are the sporadic simple lymphoepithelial cysts , usually found within the parotid glands of middle-aged men. Simple lymphoepithelial cysts are not related to HIV infection or Sjögren syndrome. Typically unilateral and solitary, they probably arise from either entrapped salivary duct tissue within intraparotid lymph nodes or from branchial cleft remnants. In contrast, HIV-associated cystic lymphoepithelial lesions are usually multiple and often bilateral. Squamous-lined cysts yield clear to turbid yellow-brown fluid.
Cellular aspirate
Histiocytes
Keratin debris and anucleate squames
Small clusters of squamous (or columnar) cells
Mixed population of lymphocytes
With or without germinal center fragments with tingible-body macrophages
Absence of lymphoepithelial islands (except in HIV-associated cases and cystic LESA)
The aspirate findings are non-specific, showing a mixed population of lymphocytes, often with germinal center fragments, tingible-body macrophages, keratin debris, squamous or columnar cyst lining cells, histiocytes, and proteinaceous debris ( Fig. 11.6A ). Within this group of lesions, finding epithelial cell clusters with interspersed lymphocytes (called lymphoepithelial islands ) is indicative of either a cystic LESA or an HIV-associated cystic lymphoepithelial lesion. Clinical and radiographic correlation is helpful in this distinction.
Pure lymphoid lesions (intraparotid lymph node, lymphoma)
Cystic salivary gland neoplasms (especially Warthin tumor, acinic cell carcinoma, and mucoepidermoid carcinoma)
Cystic metastatic squamous cell carcinoma
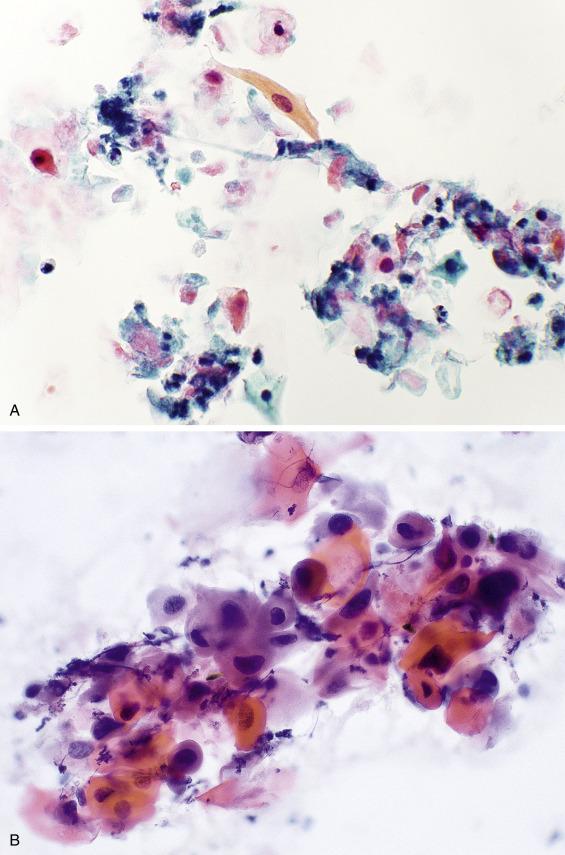
The presence of an epithelial component distinguishes these cysts from a lymph node. Lymphoid elements do not help in the distinction from a cystic salivary gland neoplasm, because some neoplasms have a prominent lymphoid infiltrate. Lymphoma can be excluded using flow cytometry, which demonstrates a polyclonal population of lymphoid cells in these benign cystic lesions. Distinction from a Warthin tumor can be especially difficult, because the oncocytes of a Warthin tumor can show extensive squamoid differentiation (see Fig. 11.15D ). Identification of a salivary gland neoplasm rests on the recognition of the characteristic cell type(s) associated with that tumor (e.g., oncocytes, acinar cells, mucus, intermediate, and squamous cells). Most importantly, a cystic metastatic squamous cell carcinoma must also be considered. Although squamous-lined non-neoplastic cysts can exhibit reactive atypia, metastatic squamous cell carcinoma ( Fig. 11.6B ) contains necrotic cells, mitotic activity (including atypical mitoses), and more severe nuclear atypia. Nevertheless, a cystic well-differentiated squamous cell carcinoma is occasionally difficult to distinguish from a benign squamous-lined cyst. Beware of diagnosing a nonmidline developmental cyst in an older adult. Such aspirates should be thoroughly screened to exclude malignant features. Even in the absence of definitive evidence of malignancy, it is prudent to report benign-appearing squamous-lined cysts descriptively and include the differential diagnosis of a developmental and lymphoepithelial cyst. An accompanying explanatory note can emphasize the need for clinical correlation to exclude a more significant lesion and a recommendation that any persistent mass be excised to exclude malignancy. The tonsil is often the primary site for a squamous cell carcinoma presenting as a cystic lesion (see Fig. 12.32 ). Because tonsillar squamous cell carcinomas are frequently associated with human papillomavirus (HPV) infection, HPV testing of the aspirate can be helpful both in confirming the diagnosis and in identifying a likely primary site.
The umbrella term mucin-containing cysts refers to a heterogeneous group of lesions that include a malignant neoplasm (mucoepidermoid carcinoma), inflammatory conditions (chronic sialadenitis with mucinous metaplasia), and acquired cysts. Acquired cysts, comprising the mucocele and retention cyst, occur more commonly in the submandibular and sublingual glands than the parotid. Mucoceles are pseudocysts because they lack an epithelial lining, whereas retention cysts are lined by squamous, columnar, or oncocytic epithelium. Both result from obstruction, usually caused by stones.
Sparsely cellular
Extracellular mucin
Histiocytes
Amylase crystalloids (nonspecific)
Scattered inflammatory cells
Aspirates are sparsely cellular and notable for extracellular mucin ( Fig. 11.7 ). There may be histiocytes, some with intracellular mucin (“muciphages”), granular debris, and amylase crystalloids (see Fig. 11.1B ). Occasional metaplastic epithelial cells and normal salivary gland cells are also seen.
Mucocele
Retention cyst
Chronic sialadenitis with mucinous metaplasia of ducts
Mucoepidermoid carcinoma

Chronic sialadenitis with mucinous metaplasia of ducts has a similar appearance to the acquired cysts; ciliated columnar cells suggest the diagnosis. The nonspecific constellation of findings described herein is also seen in some low-grade mucoepidermoid carcinomas , which, like mucoceles and retention cysts, contain muciphages. The following features, typical of a low-grade mucoepidermoid carcinoma, can help distinguish it from one of its benign mimics: a residual mass after aspiration; greater cellularity; more severe cytologic atypia; and at least an occasional cluster of intermediate and epidermoid cells.
Whenever a specimen contains extracellular mucin but minimal (or even absent) cellular atypia (see Fig. 11.7 ), an AUS interpretation is warranted. Mucoepidermoid carcinoma can be mentioned in the differential diagnosis, with an educational note. This approach helps avoid a false-negative interpretation in the case of a low-grade mucoepidermoid carcinoma.
ATYPIA OF UNDETERMINED SIGNIFICANCE.
Abundant extracellular mucin (see NOTE).
NOTE: The differential diagnosis includes a mucocele, mucus retention cyst, chronic sialadenitis with mucinous metaplasia, and a low-grade mucoepidermoid carcinoma. If the lesion persists or recurs, excision should be considered.
Amyloidosis is a rare, often bilateral cause of focal or diffuse salivary gland enlargement. Amorphous extracellular material (see Figs. 10.11 and 17.43) stains pale red with the Congo red stain and exhibits a characteristic apple-green birefringence under polarized light (see Fig. 2.23 ). The remainder of the smear is often hypocellular, with scant or absent acinar cells and scattered groups of ductal cells as are seen in chronic sialadenitis. In the salivary gland, amyloid is also encountered in association with an extramedullary plasmacytoma.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here