Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Intraoperative neurophysiology normally improves patient safety, but like any medical procedure it can cause inadvertent harm. This chapter reviews electrical and procedure-specific safety, infection control, and essential performance in terms of potential hazards and their means of protection.
Electrical dangers inherent to the devices, electrodes, stimuli, and materials employed for neurophysiologic testing include shock, hazardous output, electrode burns, and fire.
An electric shock consists of current passing through the body between two or more contacts and stimulating excitable tissue. The effects range from perception to pain, muscle contractions, tissue damage, convulsions, and ventricular fibrillation with cardiac failure that is the usual cause of death from electrocution .
The risk of cardiac failure varies with current type, frequency, origin, and strength . Alternating is more dangerous than direct current and is most hazardous between 10 and 200 Hz, including 50–60 Hz power line frequency. Intracardiac is riskier than transthoracic origin, because current strength drops rapidly with distance through body tissues. The likelihood of ventricular fibrillation with a 50–60 Hz shock from an intracardiac electrode is 100% at only 500 µA and drops to 1% at 50 µA; there is no absolutely safe minimum current. It takes much larger transthoracic shocks causing strong muscle contractions to affect cardiac rhythm: 1 A would generate about 50 µA at the heart. Imperceptible 500 µA transthoracic current would produce only about 0.025 µA at the heart and no real risk of ventricular fibrillation, while the same current directly to the heart could be fatal.
Obviously, neurophysiologic electrical devices are not designed for intracardiac electrode connections and must never have a direct conductive pathway to the heart. However, this could happen accidentally through leakage current shocks.
All medical electrical devices produce three types of leakage current: (1) chassis leakage current that flows from the device enclosure through the patient or operator to ground or another enclosure part; (2) patient leakage current that flows from patient connections to ground and may originate from an external voltage source on the patient; and (3) patient auxiliary current that flows between patient connections, for example, impedance testing or amplifier bias . Avoiding excessive transthoracic or direct cardiac conduction of otherwise minor leakage current is critical.
Intraoperative neurophysiological devices commonly have limb and multiple connections that create transthoracic paths and increase total leakage current through summation . In addition, other operating room electrical device connections introduce external voltage sources and ground paths. Furthermore, internal jugular or subclavian central lines and their tracks are potential conductive pathways to the heart. Consequently, the monitoring device, the operator who may be in contact with it, and patient connections should not touch these lines . Erb’s point is a nearby patient connection that could possibly make contact and may therefore be safest to omit, even though there are no reports of such an event so far . Certainly, one must prevent the worst cases of accidental direct cardiac leakage current shocks or power mains voltage at a patient connection.
All medical electrical devices have at least two built-in means of protection against leakage current shocks. These may be protective grounding, insulation, or impedance satisfying detailed technical specifications .
Devices must also be single fault safe, meaning that the failure of one means of protection does not cause unacceptable risk . Single faults arise spontaneously or from the stress of use; a broken power cord ground is a common example. Some single faults trigger a warning, while others are silent and their safety depends on periodic inspection and repair to minimize the chance of a second fault before the next inspection.
Biomedical testing confirms that leakage currents between any accessible part of the device and ground or another accessible part are within safe limits for the intended use. Intraoperative neurophysiology devices must be safe for external and internal noncardiac connections. Presently, the relevant International Electrotechnical Commission alternating current limits in normal and single fault conditions are 100 and 500 µA for individual leakage currents and 500 and 1000 µA for total leakage current . In addition, leakage current must not exceed 5000 µA in the special condition of an external voltage (including power mains) patient connection. This could generate about 0.25 µA at the heart, which still has a low risk of ventricular fibrillation—unless there is a direct cardiac path . Note that limits undergo periodic revision and may vary between jurisdictions.
Device testing must be done after manufacture, installation, signs of damage, repair, and every 6 months or more often if indicated by the manufacturer, local rules, or hard use . The biomedical engineer must test interconnected devices supplied by one power cord as a unit, as well as custom-built or modified devices. Neglect of periodic testing increases the risk of a seriously hazardous double fault.
To avoid loss of protective grounding, power outlets should be well-constructed without tin soldering that loosens over time, and power cords must be heavy duty with strain relief at both ends and no strain prone right-angle plugs . While discouraged, extension cords meeting the same standards may be acceptable . Power and extension cord inspection should be part of periodic testing.
Patient leads with unprotected pin connectors can result in electrocution from accidental power contact . Consequently, patient leads must have “touch proof” connectors that cannot make potentially dangerous electrical contacts when not seated in their intended receptacles .
Neurophysiologic testing normally applies intentional controlled shocks to selected nervous system targets and excessive output could damage neural or intervening tissue . Safe practice requires an understanding of stimulus parameters, strength–duration properties, and means of protection against hazardous output.
The electrical stimuli are monophasic or biphasic pulses and are normally cathodic or cathodic-first, with the exception of anodic or anodic-first pulses for transcranial electric stimulation (TES) and direct cortical stimulation (DCS) motor-evoked potentials (MEPs). Each pulse phase ( ph ) has its duration D in ms, time-varying current intensity I in mA, charge Q in µC that is the integral of current over phase duration time, and energy E in mJ given by the integral of squared current over phase duration time and multiplied by resistance R in kΩ:
These calculations simplify to
and
for monophasic rectangular pulses that are most common ( Fig. 41.1A ). Charge-balanced pulses follow the stimulus phase charge Q S with an equal opposite-polarity reversal phase charge
. Asymmetric balancing applies a smaller and slowly decaying reversal or balancing phase ( Fig. 41.1B and E ). Symmetric biphasic pulses have an equally shaped opposite-polarity reversal phase ( Fig. 41.1C ). Charge balancing can reduce electrochemical toxicity and electrode corrosion but is not always available in monitoring devices.
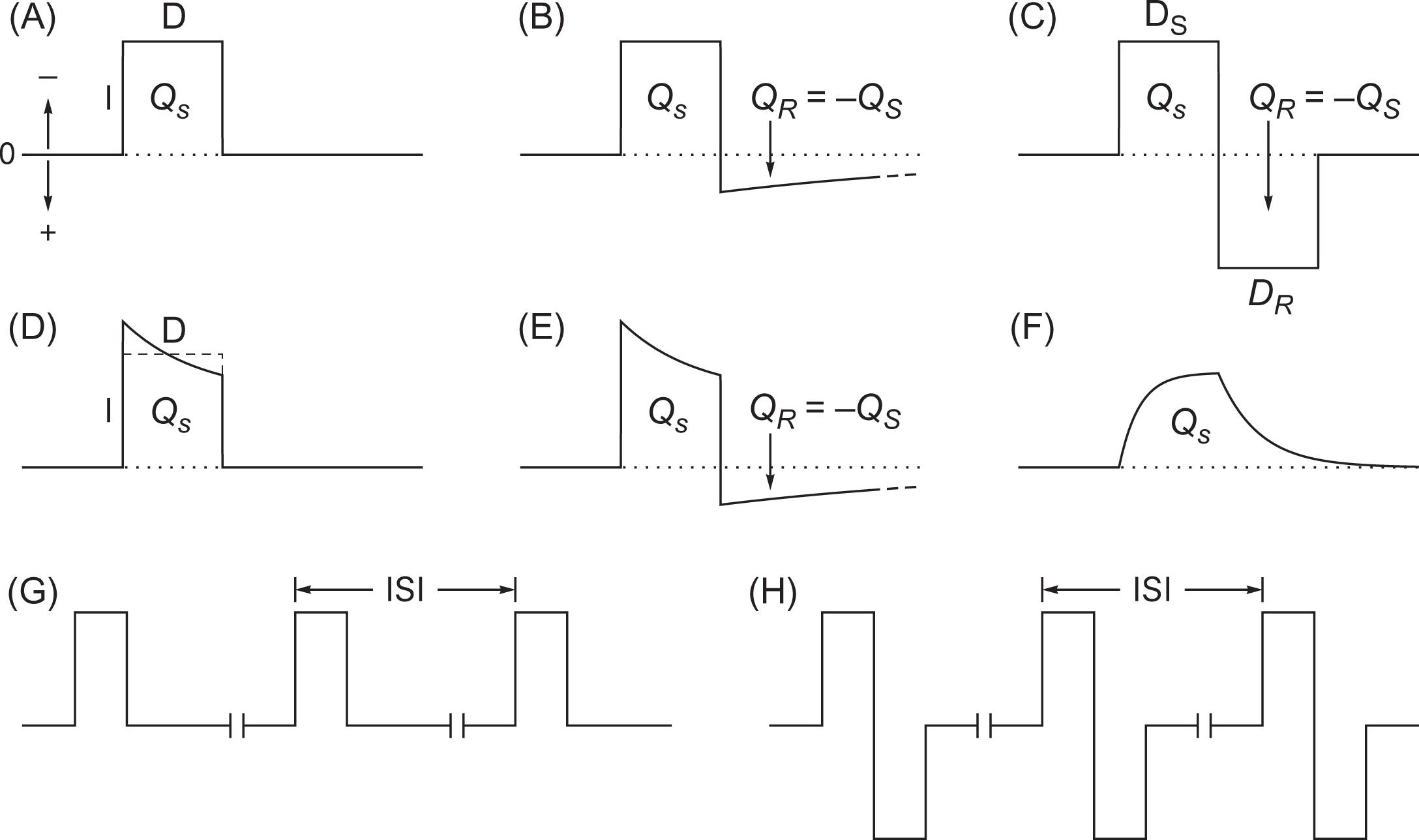
Constant-current stimulators deliver operator-selected current by adjusting voltage according to Ohm’s law
. This compensates for resistance variations to enable predictable current and thus promote response stability . Failure to reach selected current occurs when resistance is excessive for available voltage, and the device should indicate success or failure with an obvious signal (e.g., yellow or red). While constant-current pulses have minor rise and fall time distortions and can show some current decay when stressed by long 0.5–1 ms pulses at high frequency , they are approximately rectangular.
Constant-voltage stimulators deliver operator-selected voltage and are subject to resistance variations that can cause current and response instability; a delivered current readout may aid interpretation. They also complicate charge and energy estimation because the pulses are not rectangular. Instead, there may be an initial current peak that decays exponentially ( Fig. 41.1D ) or other distortions under load. For example, Digitimer D185 constant-voltage pulses show negative exponential rising current and then exponential decay after pulse termination ( Fig. 41.1F ) .
Trains are repetitive pulses characterized by their frequency in Hz or interstimulus interval (ISI) in ms defined as the time between successive pulse onsets and equal to 1000/frequency ( Fig. 41.1G and H ) . For example, traditional Penfield cortical stimulation applies 50–60 Hz (20–16.7 ms ISI) trains lasting 1–5 seconds , and 5-pulse trains with a 4 ms ISI (250 Hz) appear generally optimal for TES or DCS muscle MEPs . Total charge and energy are the sums of all pulses in the train.
The stimulus strength required to initiate action potentials and evoke a threshold response varies with pulse duration and the neural target’s rheobase ( Rb ) and chronaxie ( Cx ). Rheobase is asymptotic threshold current at infinite pulse duration, and chronaxie is an excitability time constant defined as the pulse duration at which threshold current is twice the rheobase . Classical threshold ( th ) strength–duration equations, developed over a century ago , show excellent fit to single-pulse and pulse train neurophysiologic results :
Logically, parameters that minimize threshold stimulus strength are safest. However, current, charge, and energy are related but opposing strength measures having different potential injury mechanisms . Threshold current decreases with pulse duration and can cause electrochemical damage. Charge increases with pulse duration and is an excitotoxic factor. Energy is high with short or long pulses but minimal at the chronaxie and generates heat that can cause thermal injury. Fig. 41.2 plots generic strength–duration curves and shows that chronaxie is the safest pulse duration, because it minimizes energy while balancing modest current and charge .
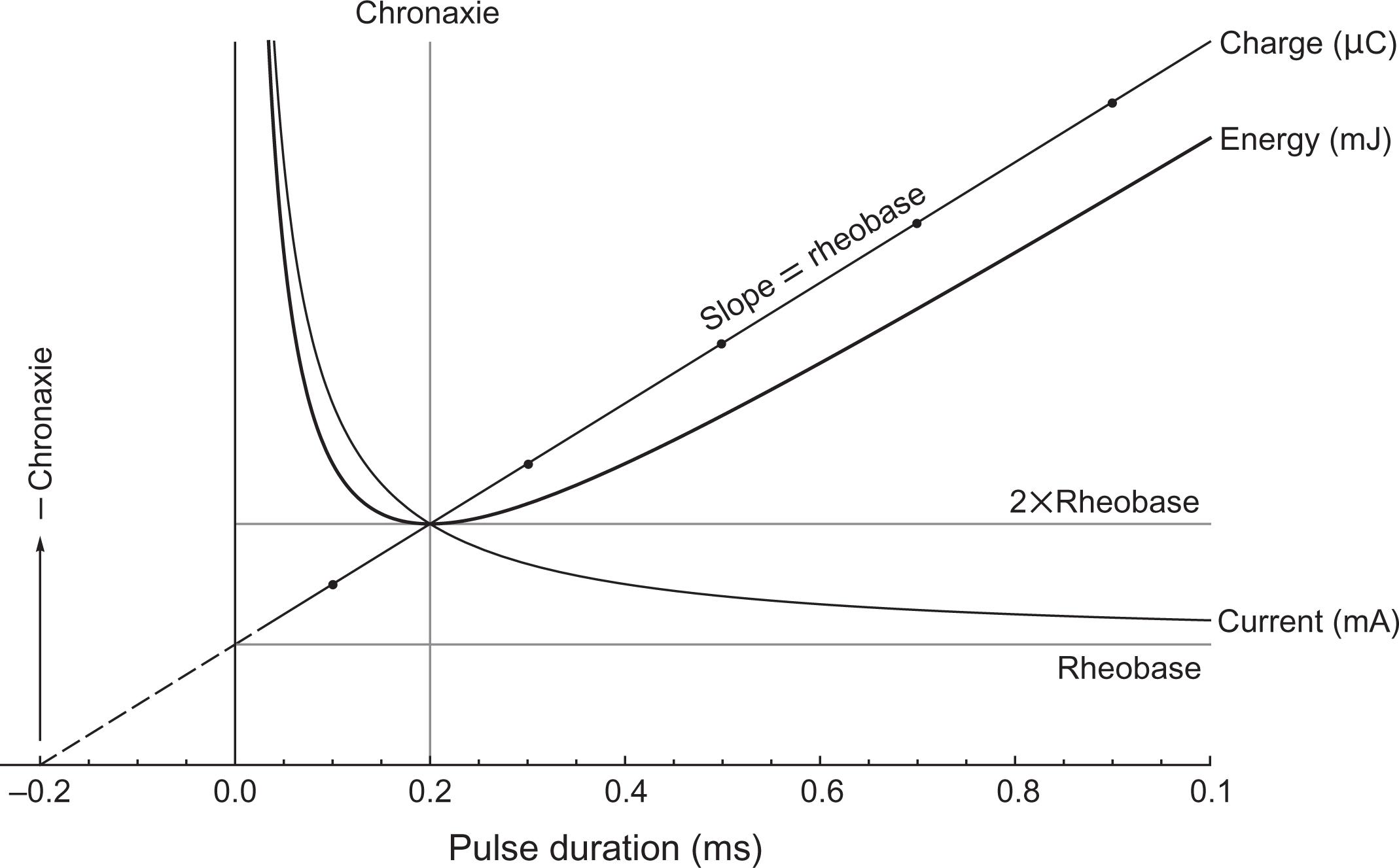
Thermal skin or neural injury due to excessive pulse energy is a concern for external or direct nervous system stimulation . Setting pulse duration to the chronaxie if known would help to minimize this risk ( Fig. 41.2 ). There is good strength–duration evidence that mean DCS MEP chronaxie is about 0.2 ms . There is some evidence that the same may be true for TES MEPs , but currently available TES stimulators either do not provide or have insufficient output for consistent success at 0.2 ms pulse duration. There is a lack of strength–duration data for most other techniques.
Thermal skin damage is possible if energy exceeds an empirical 50 mJ safety limit for external stimulation . This could happen with TES using 0.05 ms pulses that require much higher current than longer pulses. For example, >1000 mA 0.05 ms pulses could exceed 50 mJ assuming 1 kΩ resistance, and a TES safety review identified two possibly but not definitely related scalp burns .
For surface stimulation over peripheral nerves, pulse duration is usually 0.2 ms with 100 mA maximum output, which would generate only 2 mJ assuming 1 kΩ resistance. Even if pulse duration was increased to 1 ms, energy would still be acceptable at 10 mJ. In addition, supramaximal intensity (the point at which responses stop increasing with stronger stimuli) is usually far below maximum output. Thus these techniques are unlikely to cause thermal injury. Greater caution is advisable for subcutaneous needle stimulation because of closer neural proximity and special care should be taken not to exceed supramaximal intensity.
For direct cortical or neural stimulation, there is a longstanding recommendation to employ at least 0.1 ms pulse duration, based on observations of thermal cortical damage from highly energetic very brief 0.015 ms pulses in experimental animals . There are no clinical reports of neural tissue thermal injury in humans so far. However, damage might not produce obvious clinical signs and there is only one human histologic study .
Means of protection against thermal injury include (1) setting pulse duration to the chronaxie when known and feasible (e.g., 0.2 ms for DCS MEPs); (2) ensuring that external stimuli do not exceed 50 mJ; (3) avoiding very high TES intensity with 0.05 ms pulses; (4) avoiding intensity beyond that needed for adequate or when appropriate, supramaximal responses and in any case below published limits that appear clinically safe; and (5) avoiding <0.1 ms pulse duration for direct nervous system stimulation.
Excitotoxicity is a concern with direct cortical or neural stimulation and possibly TES . Again, there are no clinical reports of this kind of injury so far, but experimental animal models clearly demonstrate excitotoxic damage . These models apply 50 Hz biphasic pulse trains lasting hours or days and their relevance to much briefer stimuli used for neurophysiologic testing is uncertain. Nevertheless, they indicate that charge and charge density
are reciprocal excitotoxic cofactors with an empirical injury threshold of approximately
. Histologic lesions appear only above this threshold and worsen with total stimulation time.
Fig. 41.3 compares parameter limits of several human applications to the experimental injury threshold. Evidently, most DCS techniques are potentially excitotoxic, including Penfield stimulation with 2 mm probes and DCS MEPs with 0.5 ms pulses and 2–3 mm diameter or 1 cm 2 disc electrodes. The absence of clinical injury might be explained by stimulation brevity but does not necessarily exclude subclinical lesions, even though the only available histologic study argues against this . A notable DCS MEP exception could be 0.2 ms pulses with a 1 cm 2 electrode that should be safely below the injury threshold . Maximal reported TES MEP charges should also be safe based on estimated 20:1 skull dispersion . For comparison, electroconvulsive therapy limits do exceed the threshold .
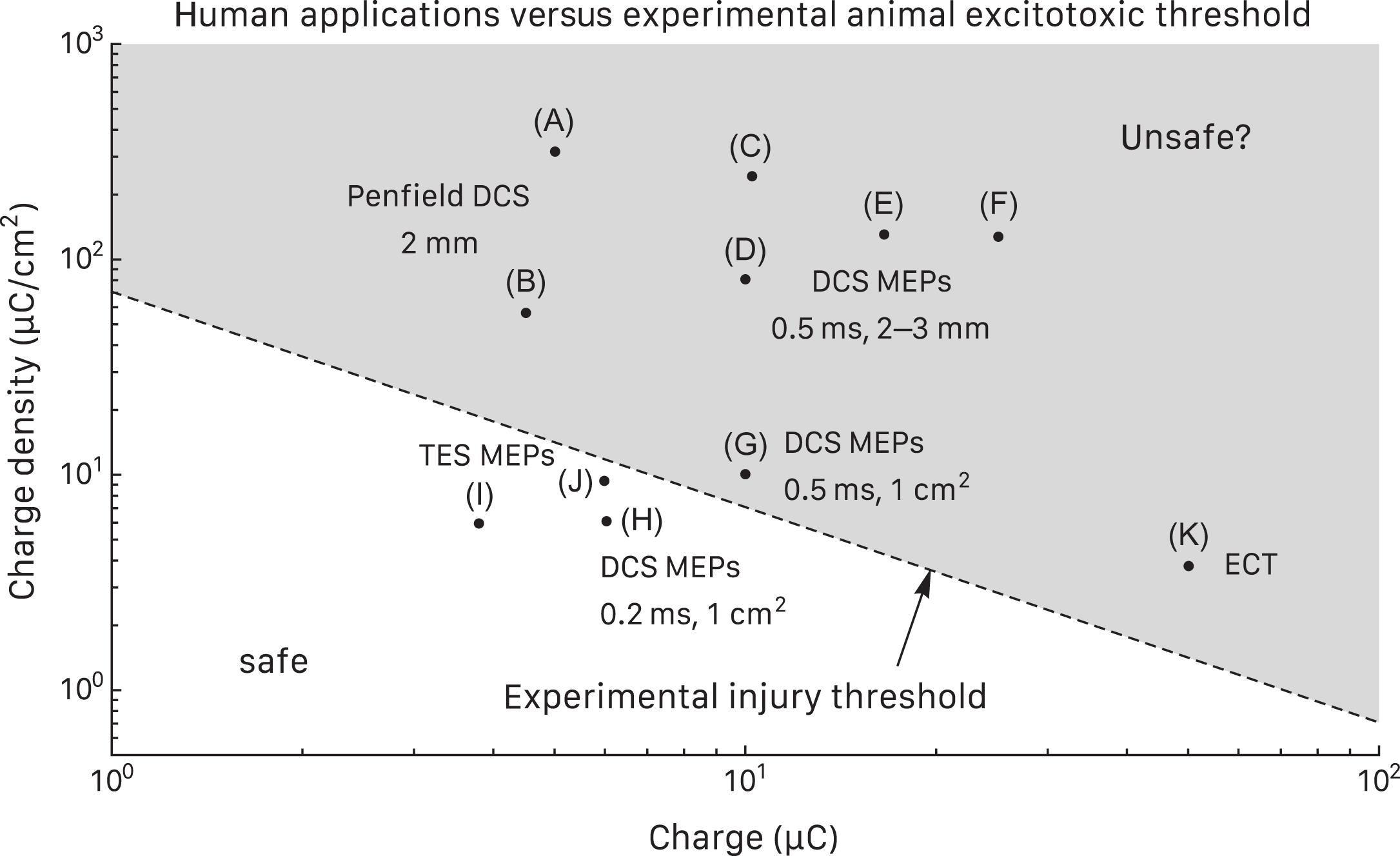
Means of protection against excitotoxicity include (1) ideally, keeping charge and charge density below the experimental injury threshold to make excitotoxicity virtually impossible; (2) considering 1 cm 2 DCS electrodes to reduce charge density ; (3) using 0.2 ms pulses for DCS MEPs to limit charge compared to longer pulses ; and (4) in any case, avoiding stimulus intensity beyond that needed for adequate responses and staying below published limits that appear clinically safe .
Electrochemical toxicity is a concern for direct nervous system stimulation, because it occurs at the electrode-tissue interface . An inactive metal electrode in contact with biological tissue forms an equilibrium potential. A cathodic pulse causes an accumulation of electrons that shift the electrode potential negative, thereby initiating charge transfer into tissue. The transfer is capacitive near the electrode’s equilibrium potential, meaning that cations in the extracellular fluid flow toward the electrode and anions flow away. Reversing stimulus polarity shifts the electrode potential positive and reverses ion flow. No charge is lost and the process is nontoxic ( Fig. 41.4 , left panel) .
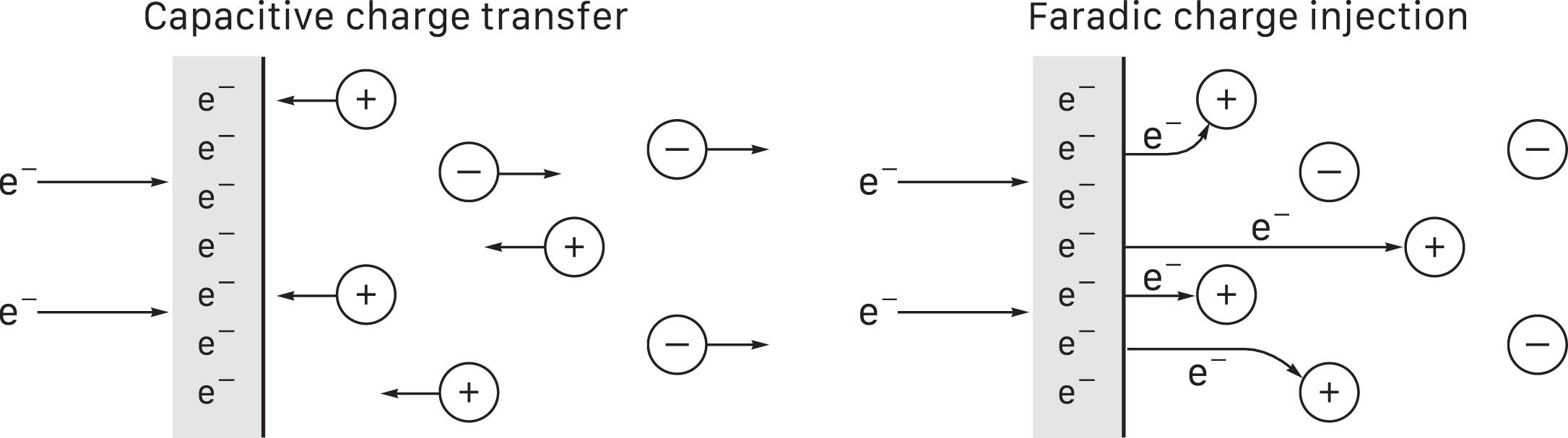
An excessively strong or >1 ms cathodic pulse shifts the electrode potential negative beyond its capacitive limit and initiates faradic charge injection that forces electrons into tissue ( Fig. 41.4 , right panel) . The electrons form reductive electrochemical reactions with various molecules, releasing potentially toxic byproducts. Reversing polarity partially reverses the process but does not recover all injected charge because some reactions are irreversible. An excessively strong or >1 ms anodic pulse shifts the electrode potential positive beyond its capacitive limit and initiates faradic charge removal, pulling electrons out of tissue through oxidative reactions that are similarly potentially toxic and partially irreversible.
Monophasic pulse trains are especially hazardous because they do not allow enough time for the electrode potential to recover to equilibrium before the next pulse . This progressively shifts prepulse electrode potentials away from equilibrium until a steady state with predominantly hazardous faradic charge transfer ( Fig. 41.5 , left panel) . Symmetric biphasic trains may be safer because they gradually shift prepulse electrode potentials in the opposite direction until a steady state with mostly safe capacitive charge transfer and small bidirectional faradic transfers ( Fig. 41.5 , right panel) . In fact, experimental animals subjected to 50 Hz DCS for hours or days exhibit electrochemical lesions with monophasic, but not symmetric biphasic pulses . Again, the relevance for briefer trains in humans is uncertain. Tissue can buffer some toxic byproducts and it seems unlikely that very brief DCS trains for MEPs could cause electrochemical injury . On the other hand, traditional 50–60 Hz trains lasting seconds might be a concern.
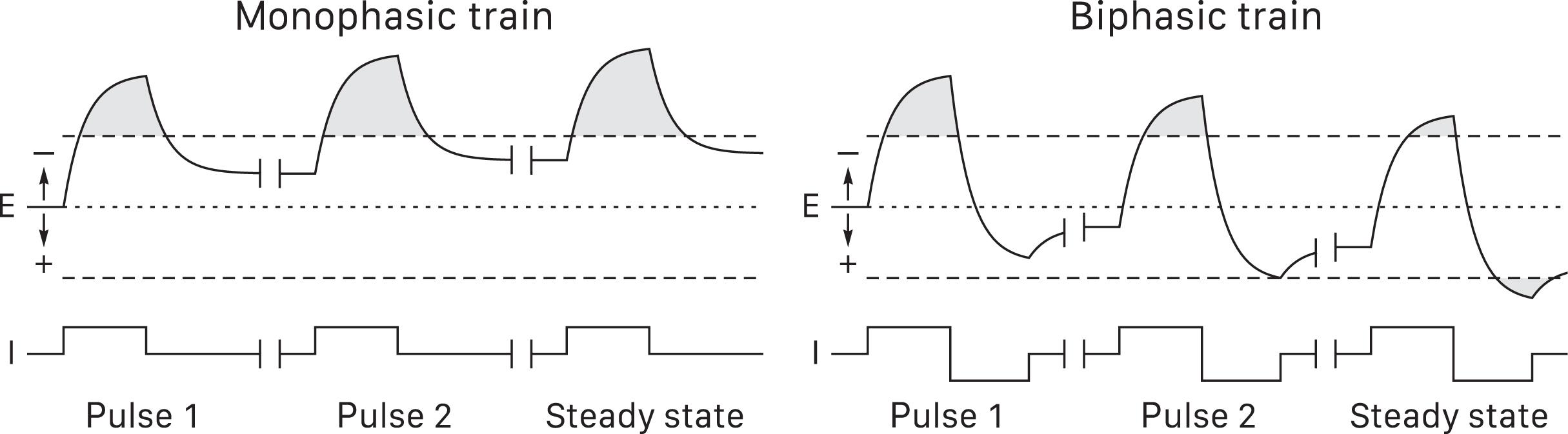
Means of protection against direct nervous system stimulation electrochemical toxicity include (1) not permitting >1 ms pulses; (2) avoiding stronger stimuli than needed for adequate responses and staying below published limits that appear clinically safe; (3) applying charge-balanced pulses if available ( Fig. 41.1B and E ); and (4) using symmetric biphasic pulses ( Fig. 41.1C ) for 50–60 Hz trains lasting seconds. Very brief monophasic pulse trains are probably acceptable.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here