Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In addition to the rapidly acting mechanisms for regulation of arterial pressure discussed in Chapter 18 , the body also has powerful mechanisms for regulating arterial pressure week after week and month after month. This long-term control of arterial pressure is closely intertwined with homeostasis of body fluid volume, which is determined by the balance between fluid intake and output. For long-term survival, fluid intake and output must be precisely balanced, a task that is performed by multiple nervous and hormonal controls and by local control systems in the kidneys that regulate their excretion of salt and water. In this chapter, we discuss these renal–body fluid systems that play a major role in long-term blood pressure regulation.
The renal–body fluid system for arterial pressure control acts slowly but powerfully, as follows. If blood volume increases and vascular capacitance is not altered, arterial pressure will also increase. The rising pressure, in turn, causes the kidneys to excrete the excess volume, thus returning the pressure back toward normal.
In the phylogenetic history of animal development, this renal–body fluid system for pressure control is a primitive one. It is fully operative in one of the lowest of vertebrates, the hagfish. This animal has a low arterial pressure, only 8 to 14 mm Hg, and this pressure increases almost directly in proportion to its blood volume. The hagfish continually drinks sea water, which is absorbed into its blood, increasing the blood volume and blood pressure. However, when the pressure rises too high, the kidney excretes the excess volume into the urine and relieves the pressure. At low pressure, the kidney excretes less fluid than is ingested. Therefore, because the hagfish continues to drink, extracellular fluid volume, blood volume, and pressure all build up again to the higher levels.
This primitive mechanism of pressure control has survived throughout the ages, but with the addition of multiple nervous system, hormones, and local control systems that also contribute to the regulation of salt and water excretion. In humans, kidney output of water and salt is just as sensitive—if not more so—to pressure changes as in the hagfish. Indeed, an increase in arterial pressure in the human of only a few millimeters of Hg can double the renal output of water, a phenomenon called pressure diuresis , as well as double the output of salt, called pressure natriuresis .
In humans, just as in the hagfish, the renal–body fluid system for arterial pressure control is a fundamental mechanism for long-term arterial pressure control. However, through the stages of evolution, multiple refinements have been added to make this system much more precise in its control. An especially important refinement, as discussed later, has been the addition of the renin-angiotensin mechanism.
Figure 19-1 shows the approximate average effect of different arterial pressure levels on the renal output of salt and water by an isolated kidney, demonstrating markedly increased urine output as the pressure rises. This increased urinary output is the phenomenon of pressure diuresis . The curve in this figure is called a renal urinary output curve or a renal function curve. In humans, at an arterial pressure of 50 mm Hg, the urine output is essentially zero. At 100 mm Hg, it is normal and, at 200 mm Hg, it is 4 to 6 times normal. Furthermore, not only does increasing the arterial pressure increase urine volume output, but it also causes an approximately equal increase in sodium output, which is the phenomenon of pressure natriuresis .
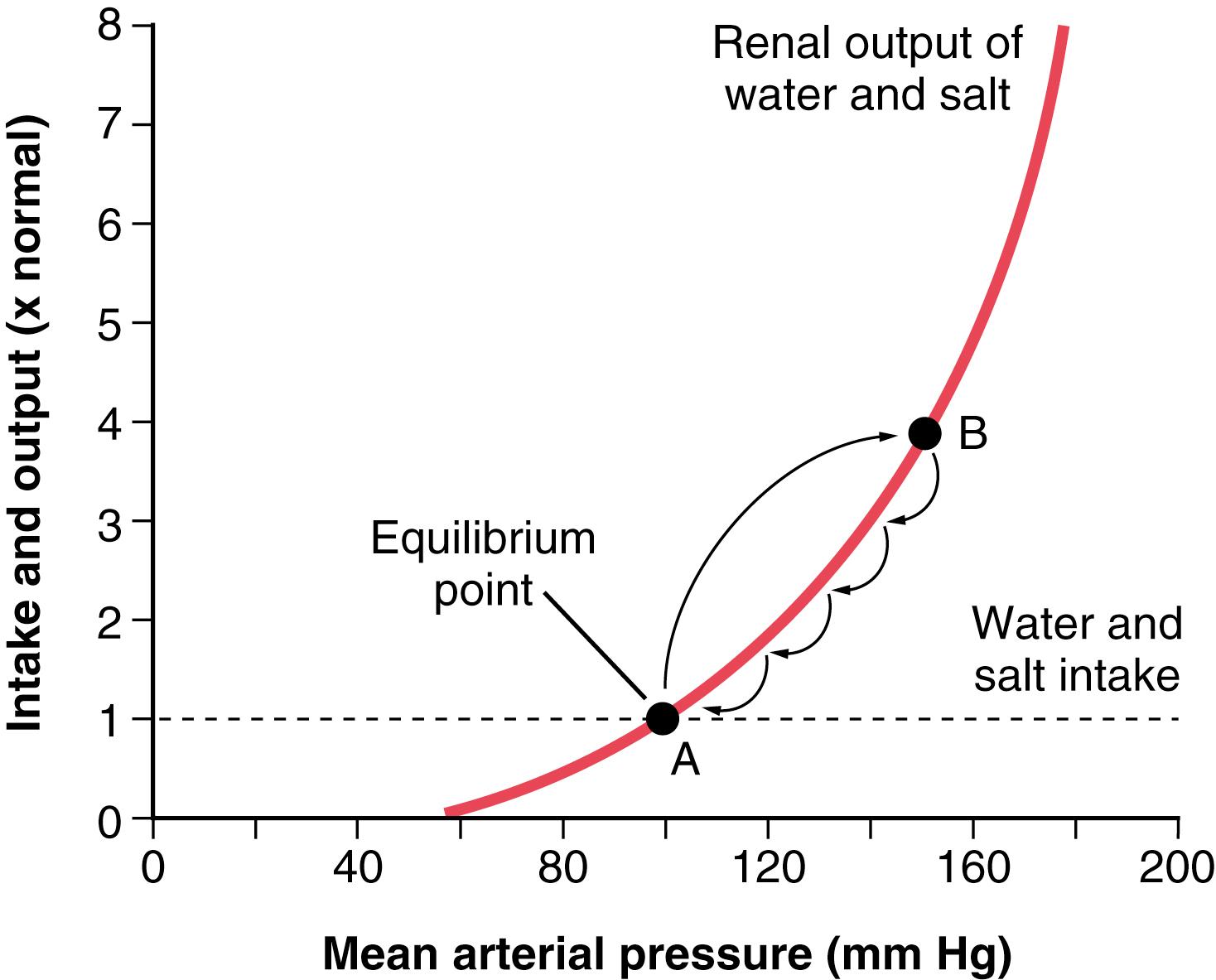
Figure 19-2 shows the results of an experiment in dogs in which all the nervous reflex mechanisms for blood pressure control were first blocked. Then, the arterial pressure was suddenly elevated by infusing about 400 ml of blood intravenously. Note the rapid increase in cardiac output to about double normal and the increase in mean arterial pressure to 205 mm Hg, 115 mm Hg above its resting level. Shown by the middle curve is the effect of this increased arterial pressure on urine output, which increased 12-fold. Along with this tremendous loss of fluid in the urine, both the cardiac output and arterial pressure returned to normal during the subsequent hour. Thus, one sees an extreme capability of the kidneys to eliminate excess fluid volume from the body in response to high arterial pressure and, in so doing, to return the arterial pressure back to normal.
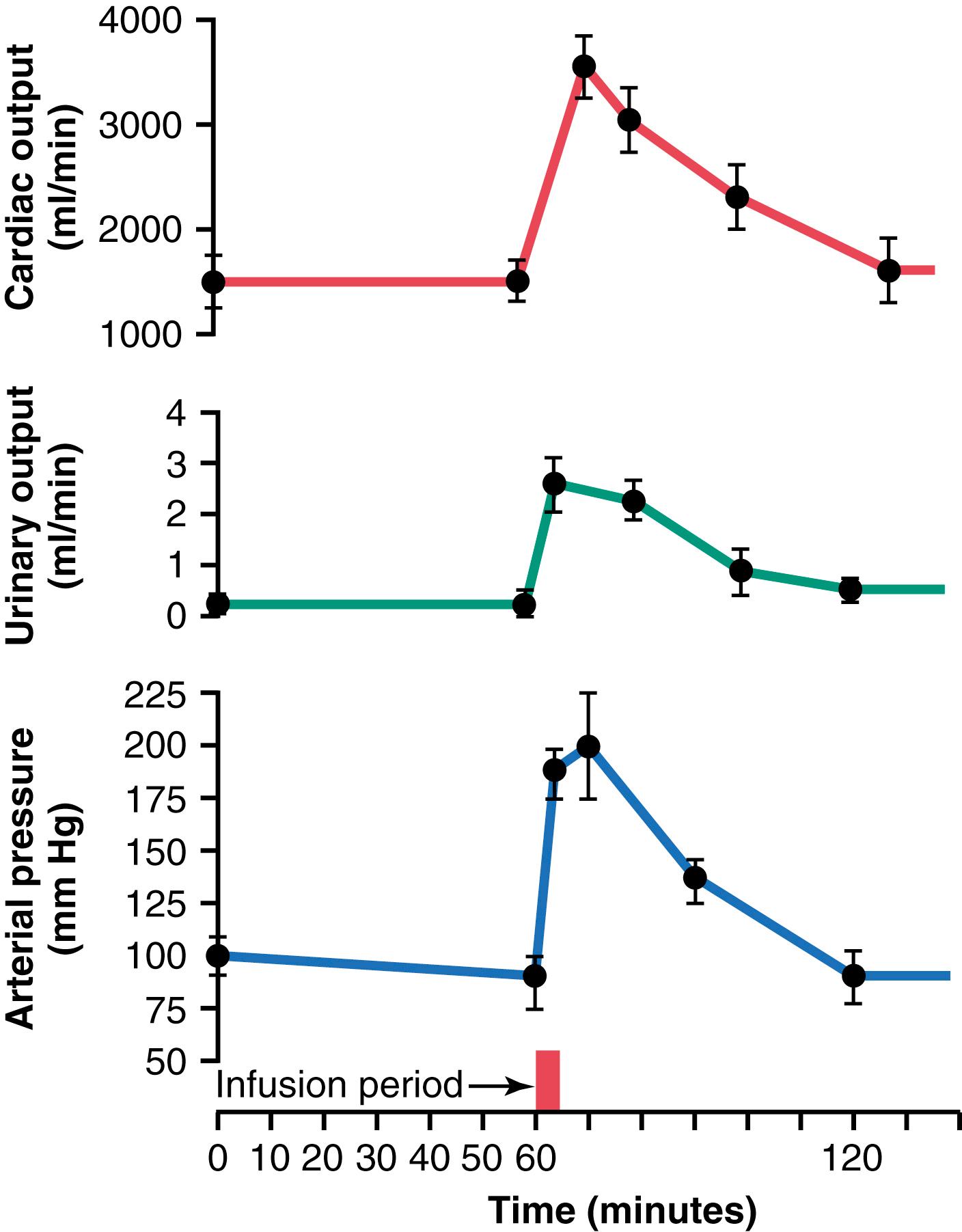
Figure 19-1 shows the relationship of the following: (1) the renal output curve for water and salt in response to rising arterial pressure; and (2) the line that represents the net water and salt intake. Over a long period, the water and salt output must equal the intake. Furthermore, the only point on the graph in Figure 19-1 at which output equals intake is where the two curves intersect, called the equilibrium point (point A). Let us see what happens if the arterial pressure increases above or decreases below the equilibrium point.
First, assume that the arterial pressure rises to 150 mm Hg (point B). At this level, the renal output of water and salt is almost three times as great as intake. Therefore, the body loses fluid, the blood volume decreases, and the arterial pressure decreases. Furthermore, this negative balance of fluid will not cease until the pressure falls all the way back exactly to the equilibrium level. Even when the arterial pressure is only a few mm Hg greater than the equilibrium level, there still is slightly more loss of water and salt than intake, so the pressure continues to fall that last few mm Hg until the pressure eventually returns to the equilibrium point .
If the arterial pressure falls below the equilibrium point, the intake of water and salt is greater than the output. Therefore, body fluid volume increases, blood volume increases, and the arterial pressure rises until once again it returns to the equilibrium point. This return of the arterial pressure always back to the equilibrium point is known as the near-infinite feedback gain principle for control of arterial pressure by the renal–body fluid mechanism.
In Figure 19-1 , one can also see that two basic long-term factors determine the long-term arterial pressure level. As long as the two curves representing the renal output of salt and water and the intake of salt and water remain exactly as they are shown in Figure 19-1 , the mean arterial pressure level will eventually readjust to 100 mm Hg, which is the pressure level depicted by the equilibrium point of this figure. Furthermore, there are only two ways in which the pressure of this equilibrium point can be changed from the 100 mm Hg level. One is by shifting the pressure level of the renal output curve for salt and water, and the other is by changing the level of the water and salt intake line. Therefore, expressed simply, the two primary determinants of the long-term arterial pressure level are as follows:
The degree of pressure shift of the renal output curve for water and salt
The level of the water and salt intake
Operation of these two determinants in the control of arterial pressure is demonstrated in Figure 19-3 . In Figure 19-3A , some abnormality of the kidneys has caused the renal output curve to shift 50 mm Hg in the high-pressure direction (to the right). Note that the equilibrium point has also shifted to 50 mm Hg higher than normal. Therefore, one can state that if the renal output curve shifts to a new pressure level, the arterial pressure will follow to this new pressure level within a few days.
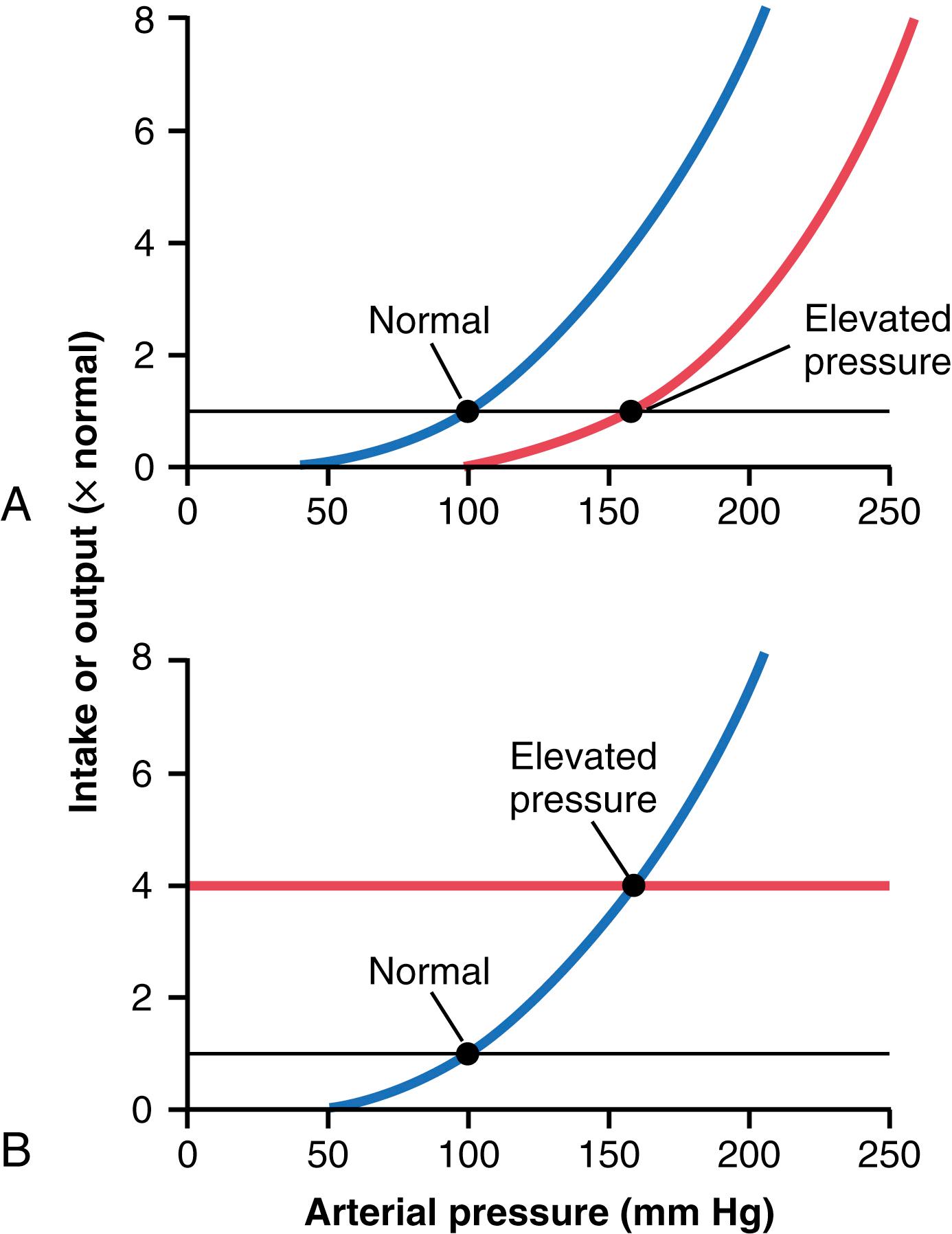
Figure 19-3B shows how a change in the level of salt and water intake also can change the arterial pressure. In this case, the intake level has increased fourfold, and the equilibrium point has shifted to a pressure level of 160 mm Hg, 60 mm Hg above the normal level. Conversely, a decrease in the intake level would reduce the arterial pressure.
Thus, it is impossible to change the long-term mean arterial pressure level to a new value without changing one or both of the two basic determinants of long-term arterial pressure, either (1) the level of salt and water intake or (2) the degree of shift of the renal function curve along the pressure axis. However, if either of these is changed, one finds the arterial pressure thereafter to be regulated at a new pressure level, the arterial pressure at which the two new curves intersect.
In most people, however, the renal function curve is much steeper than that shown in Figure 19-3 , and changes in salt intake have only a modest effect on arterial pressure, as discussed in the next section.
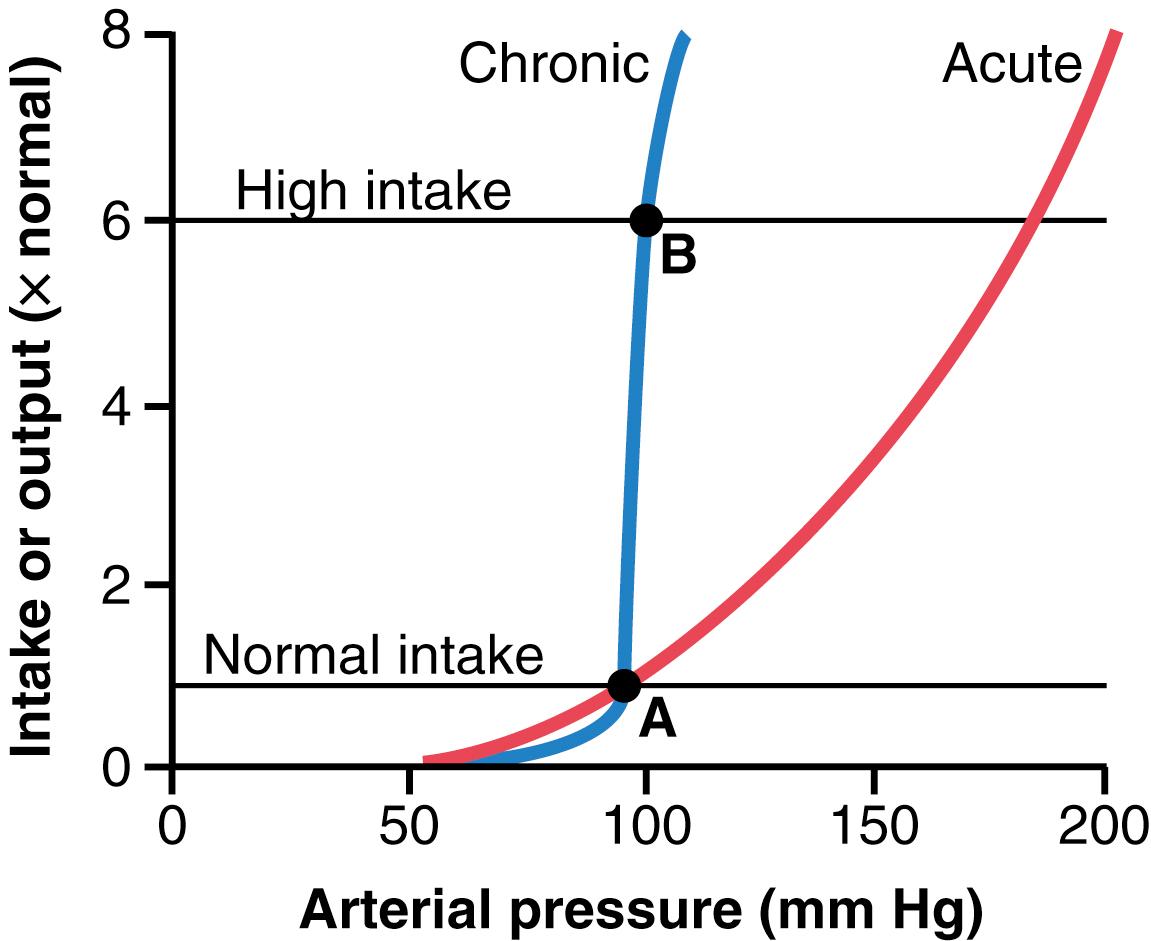
An important characteristic of pressure natriuresis (and pressure diuresis) is that chronic changes in arterial pressure, lasting for days or months, have a much greater effect on the renal output of salt and water than that observed during acute changes in pressure ( Figure 19-4 ). Thus, when the kidneys are functioning normally, the chronic renal output curve is much steeper than the acute curve.
The powerful effects of chronic increases in arterial pressure on urine output occur because increased pressure not only has direct hemodynamic effects on the kidney to increase excretion, but also has indirect effects mediated by nervous and hormonal changes that occur when blood pressure is increased. For example, increased arterial pressure decreases activity of the sympathetic nervous system, partly through the baroreceptor reflex mechanisms discussed in Chapter 18 , and by reducing formation of various hormones such as angiotensin II and aldosterone that tend to reduce salt and water excretion by the kidneys. Reduced activity of these antinatriuretic systems therefore amplifies the effectiveness of pressure natriuresis and diuresis in raising salt and water excretion during chronic increases in arterial pressure (see Chapters 28 and 30 for further discussion).
Conversely, when blood pressure is reduced, the sympathetic nervous system is activated, and formation of antinatriuretic hormones is increased, adding to the direct effects of reduced pressure to decrease renal output of salt and water. This combination of direct effects of pressure on the kidneys and indirect effects of pressure on the sympathetic nervous system and various hormone systems make pressure natriuresis and diuresis extremely powerful factors for long-term control of arterial pressure and body fluid volumes.
The importance of neural and hormonal influences on pressure natriuresis is especially evident during chronic changes in sodium intake. If the kidneys and nervous and hormonal mechanisms are functioning normally, chronic increases in intakes of salt and water to as high as six times normal are usually associated with little effect on arterial pressure. Note that the blood pressure equilibrium point B on the curve is nearly the same as point A, the equilibrium point at normal salt intake. Conversely, decreases in salt and water intake to as low as one-sixth normal typically have little effect on arterial pressure. Thus, many persons are said to be salt-insensitive because large variations in salt intake do not change blood pressure more than a few mm Hg.
Persons with kidney injury or excessive secretion of antinatriuretic hormones such as angiotensin II or aldosterone, however, may be salt-sensitive, with an attenuated renal output curve similar to the acute curve shown in Figure 19-4 . In these cases, even moderate increases in salt intake may cause significant increases in arterial pressure.
Some of the factors that cause blood pressure to be salt-sensitive include loss of functional nephrons due to kidney injury and excessive formation of antinatriuretic hormones such as angiotensin II or aldosterone. For example, surgical reduction of kidney mass or injury to the kidney due to hypertension, diabetes, or various kidney diseases all cause blood pressure to be more sensitive to changes in salt intake. In these cases, greater than normal increases in arterial pressure are required to raise renal output sufficiently to maintain a balance between the intake and output of salt and water.
There is evidence that long-term high salt intake, lasting for several years, may actually damage the kidneys and eventually makes blood pressure more salt-sensitive. We will discuss salt sensitivity of blood pressure in patients with hypertension later in this chapter.
Recalling the basic equation for arterial pressure— arterial pressure equals cardiac output times total peripheral resistance— it is clear that an increase in total peripheral resistance should elevate the arterial pressure. Indeed, when the total peripheral resistance is acutely increased, the arterial pressure does rise immediately. Yet, if the kidneys continue to function normally, the acute rise in arterial pressure usually is not maintained. Instead, the arterial pressure returns all the way to normal within about 1 or 2 days. Why?
The reason for this phenomenon is that increasing vascular resistance everywhere else in the body besides in the kidneys does not change the equilibrium point for blood pressure control as dictated by the kidneys (see Figures 19-1 and 19-3 ). Instead, the kidneys immediately begin to respond to the high arterial pressure, causing pressure diuresis and pressure natriuresis. Within hours, large amounts of salt and water are lost from the body; this process continues until the arterial pressure returns to the equilibrium pressure level. At this point, blood pressure is normalized, and extracellular fluid volume and blood volume are decreased to levels below normal.
Figure 19-5 shows the approximate cardiac outputs and arterial pressures in different clinical conditions in which the long-term total peripheral resistance is much less than or much greater than normal, but kidney excretion of salt and water is normal. Note in all these different clinical conditions that the arterial pressure is also normal.
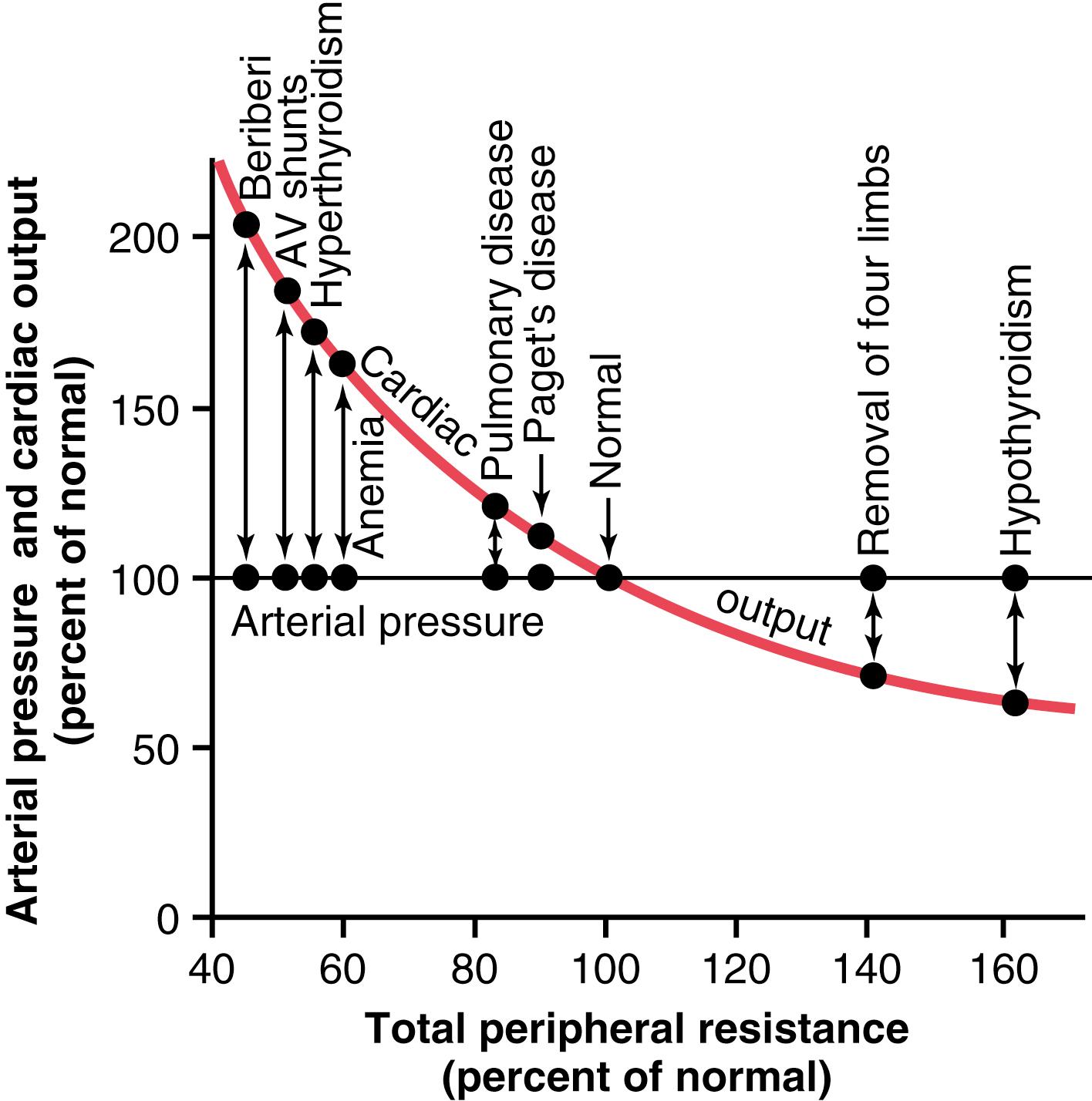
A word of caution is necessary at this point in our discussion. Often, when the total peripheral resistance increases, this also increases the intrarenal vascular resistance at the same time, which alters the function of the kidney and can cause hypertension by shifting the renal function curve to a higher pressure level, as shown in Figure 19-3A . We will see an example of this mechanism later in this chapter when we discuss hypertension caused by vasoconstrictor mechanisms. However, it is the increase in renal resistance that is the culprit, not the increased total peripheral resistance —an important distinction.
The overall mechanism whereby increased extracellular fluid volume may elevate arterial pressure, if vascular capacity is not simultaneously increased , is shown in Figure 19-6 . The sequential events are as follows: (1) increased extracellular fluid volume, which (2) increases the blood volume, which (3) increases the mean circulatory filling pressure, which (4) increases venous return of blood to the heart, which (5) increases cardiac output, which (6) increases arterial pressure. The increased arterial pressure, in turn, increases the renal excretion of salt and water and may return extracellular fluid volume to nearly normal if kidney function is normal and vascular capacity is unaltered.
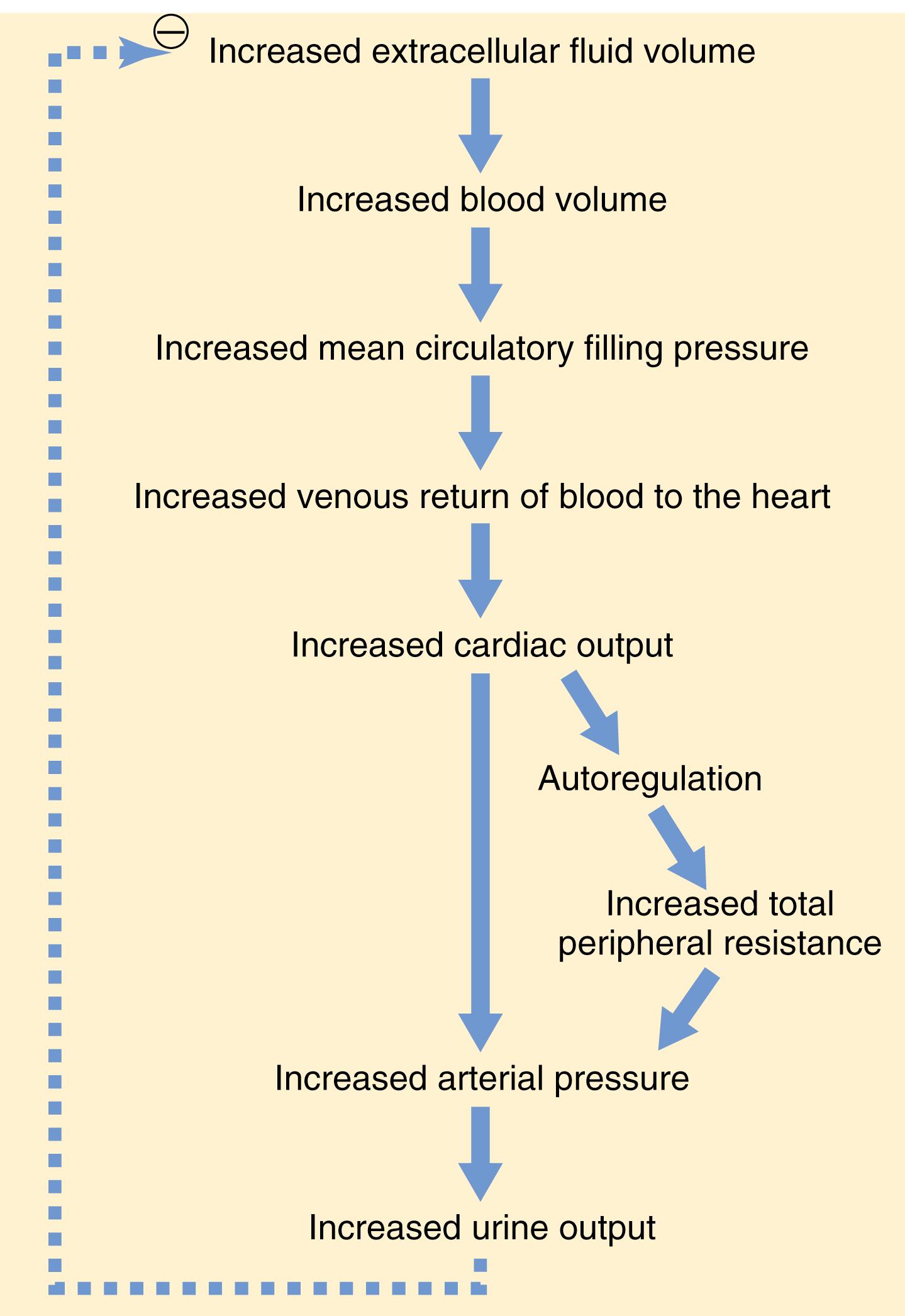
Note especially in this case the two ways in which an increase in cardiac output can increase the arterial pressure. One of these is the direct effect of increased cardiac output to increase the pressure, and the other is an indirect effect to raise total peripheral vascular resistance through autoregulation of blood flow. The second effect can be explained as follows.
Referring to Chapter 17 , let us recall that whenever an excess amount of blood flows through a tissue, the local tissue vasculature constricts and decreases the blood flow back toward normal. This phenomenon is called autoregulation, which simply means regulation of blood flow by the tissue itself. When increased blood volume raises the cardiac output, blood flow tends to increase in all tissues of the body; if the increased blood flow exceeds the metabolic needs of the tissues, the autoregulation mechanisms constricts blood vessels all over the body, which in turn increases the total peripheral resistance.
Finally, because arterial pressure is equal to cardiac output times total peripheral resistance, the secondary increase in total peripheral resistance that results from the autoregulation mechanism helps increase the arterial pressure. For example, only a 5% to 10% increase in cardiac output can increase the arterial pressure from the normal mean arterial pressure of 100 mm Hg up to 150 mm Hg when accompanied by an increase in total peripheral resistance due to tissue blood flow autoregulation or other factors that cause vasoconstriction. The slight increase in cardiac output is often not measurable.
Although the discussions thus far have emphasized the importance of volume in regulation of arterial pressure, experimental studies have shown that an increase in salt intake is far more likely to elevate the arterial pressure, especially in people who are salt-sensitive, than is an increase in water intake. The reason for this finding is that pure water is normally excreted by the kidneys almost as rapidly as it is ingested, but salt is not excreted so easily. As salt accumulates in the body, it also indirectly increases the extracellular fluid volume for two basic reasons:
Although some additional sodium may be stored in the tissues when salt accumulates in the body, excess salt in the extracellular fluid increases the fluid osmolality. The increased osmolarity stimulates the thirst center in the brain, making the person drink extra amounts of water to return the extracellular salt concentration to normal and increasing the extracellular fluid volume.
The increase in osmolality caused by the excess salt in the extracellular fluid also stimulates the hypothalamic–posterior pituitary gland secretory mechanism to secrete increased quantities of antidiuretic hormone (discussed in Chapter 29 ). The antidiuretic hormone then causes the kidneys to reabsorb greatly increased quantities of water from the renal tubular fluid, thereby diminishing the excreted volume of urine but increasing the extracellular fluid volume.
Thus, the amount of salt that accumulates in the body is an important determinant of the extracellular fluid volume. Relatively small increases in extracellular fluid and blood volume can often increase the arterial pressure substantially. This is true, however, only if the excess salt accumulation leads to an increase in blood volume and if vascular capacity is not simultaneously increased. As discussed previously, increasing salt intake in the absence of impaired kidney function or excessive formation of antinatriuretic hormones usually does not increase arterial pressure much because the kidneys rapidly eliminate the excess salt, and blood volume is hardly altered.
When a person is said to have chronic hypertension (or high blood pressure), this means that his or her mean arterial pressure is greater than the upper range of the accepted normal measure. A mean arterial pressure greater than 110 mm Hg (normal is ≈90 mm Hg) is considered to be hypertensive. (This level of mean pressure occurs when the diastolic blood pressure is greater than ≈90 mm Hg and the systolic pressure is greater than ≈135 mm Hg.) In persons with severe hypertension, the mean arterial pressure can rise to 150 to 170 mm Hg, with diastolic pressure as high as 130 mm Hg and systolic pressure occasionally as high as 250 mm Hg.
Even moderate elevation of arterial pressure leads to shortened life expectancy. At severely high pressures—that is, mean arterial pressures 50% or more above normal—a person can expect to live no more than a few more years unless appropriately treated. The lethal effects of hypertension are caused mainly in three ways:
Excess workload on the heart leads to early heart failure and coronary heart disease, often causing death as a result of a heart attack.
The high pressure frequently damages a major blood vessel in the brain, followed by death of major portions of the brain; this occurrence is a cerebral infarct. Clinically, it is called a stroke. Depending on which part of the brain is involved, a stroke can be fatal or cause paralysis, dementia, blindness, or multiple other serious brain disorders.
High pressure almost always causes injury in the kidneys, producing many areas of renal destruction and, eventually, kidney failure, uremia, and death.
Lessons learned from the type of hypertension called volume-loading hypertension have been crucial in understanding the role of the renal–body fluid volume mechanism for arterial pressure regulation. Volume-loading hypertension means hypertension caused by excess accumulation of extracellular fluid in the body, some examples of which follow.
Figure 19-7 shows a typical experiment demonstrating volume-loading hypertension in a group of dogs with 70% of their kidney mass removed. At the first circled point on the curve, the two poles of one of the kidneys were removed, and at the second circled point, the entire opposite kidney was removed, leaving the animals with only 30% of their normal renal mass. Note that removal of this amount of kidney mass increased the arterial pressure by an average of only 6 mm Hg. Then, the dogs were given salt solution to drink instead of water. Because salt solution fails to quench the thirst, the dogs drank two to four times the normal amounts of volume, and within a few days, their average arterial pressure rose to about 40 mm Hg above normal. After 2 weeks, the dogs were given tap water again instead of salt solution; the pressure returned to normal within 2 days. Finally, at the end of the experiment, the dogs were given salt solution again, and this time the pressure rose much more rapidly to a high level, again demonstrating volume-loading hypertension.
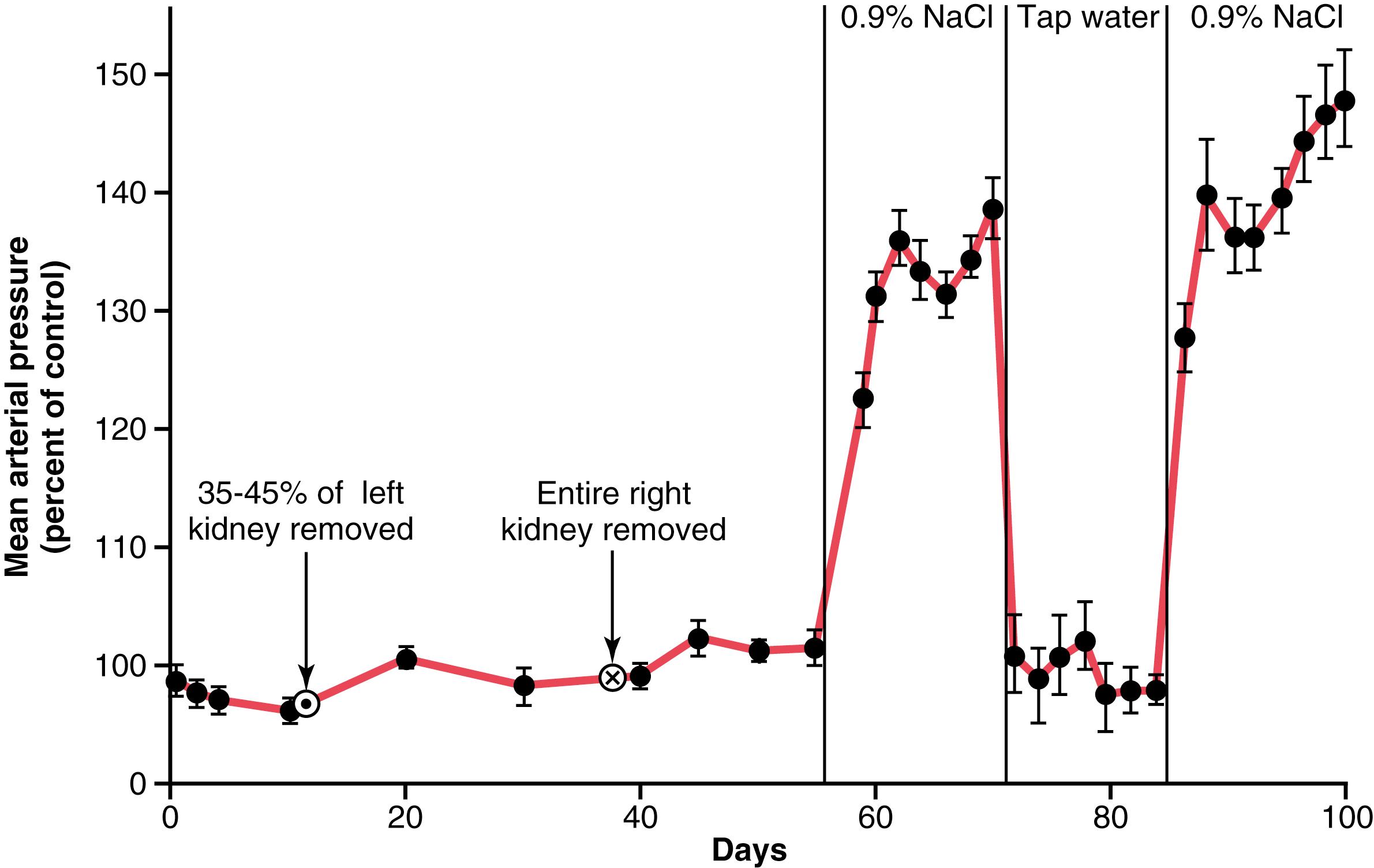
If one considers again the basic determinants of long-term arterial pressure regulation, it is apparent why hypertension occurred in the volume-loading experiment illustrated in Figure 19-7 . First, reduction of the kidney mass to 30% of normal greatly reduced the ability of the kidneys to excrete salt and water. Therefore, salt and water accumulated in the body and, in a few days, raised the arterial pressure high enough to excrete the excess salt and water intake.
It is especially instructive to study the sequential changes in circulatory function during progressive development of volume-loading hypertension ( Figure 19-8 ). A week or so before the point labeled “0” days, the kidney mass had already been decreased to only 30% of normal. Then, at this point, the intake of salt and water was increased to about six times normal and kept at this high intake thereafter. The acute effect was to increase extracellular fluid volume, blood volume, and cardiac output to 20% to 40% above normal. Simultaneously, the arterial pressure began to rise but not nearly so much at first as the fluid volumes and cardiac output. The reason for this slower rise in pressure can be discerned by studying the total peripheral resistance curve, which shows an initial decrease in total peripheral resistance. This decrease was caused by the baroreceptor mechanism discussed in Chapter 18 , which transiently attenuated the rise in pressure. However, after 2 to 4 days, the baroreceptors adapted (reset) and were no longer able to prevent the rise in pressure. At this time, the arterial pressure had risen almost to its full height because of the increase in cardiac output, even though the total peripheral resistance was still almost at the normal level.
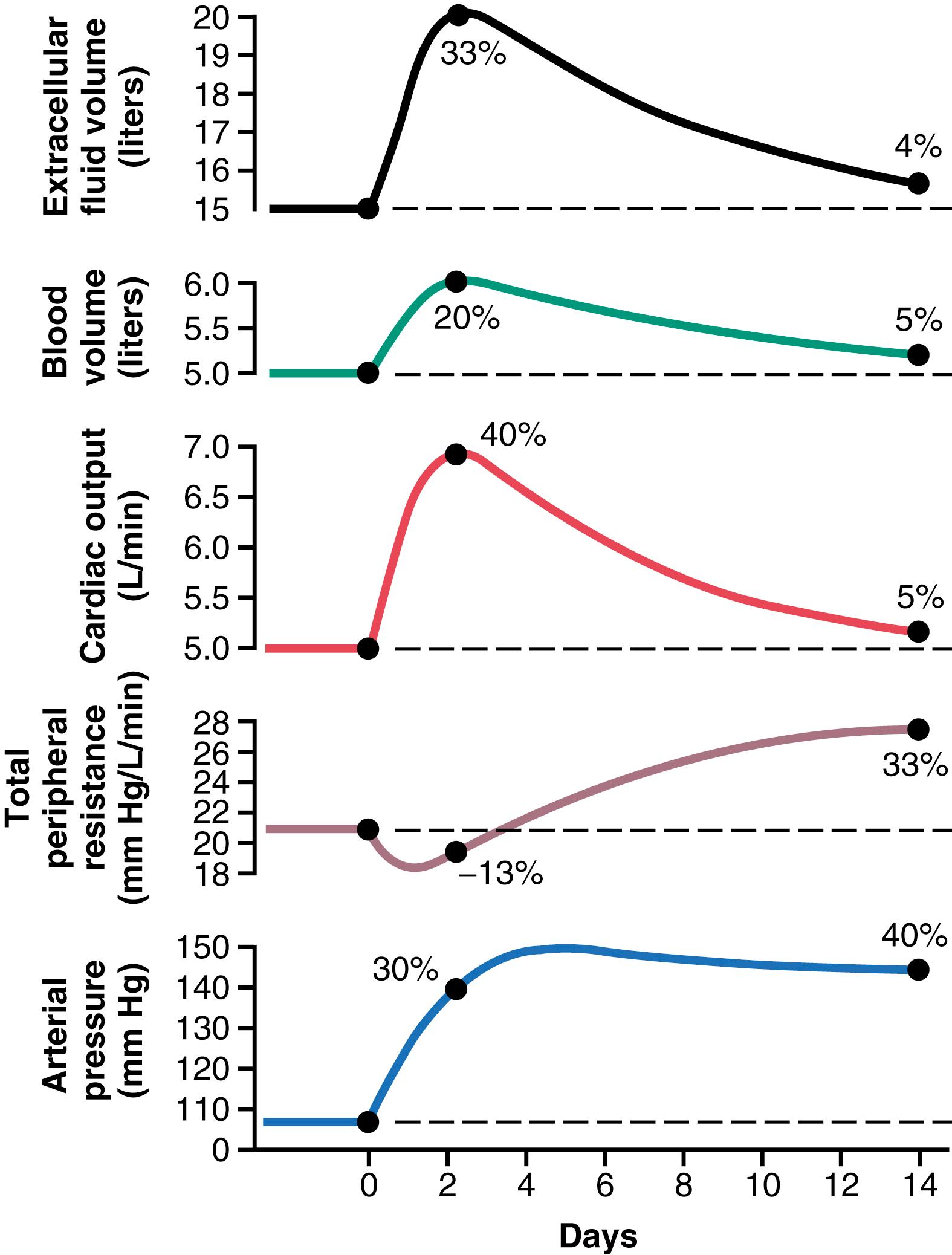
After these early acute changes in the circulatory variables had occurred, more prolonged secondary changes occurred during the next few weeks. Especially important was a progressive increase in total peripheral resistance, while at the same time the cardiac output decreased back toward normal, at least partly as a result of the long-term blood flow autoregulation mechanism discussed in Chapter 17 and earlier in this chapter. That is, after the cardiac output had risen to a high level and had initiated the hypertension, the excess blood flow through the tissues then caused progressive constriction of the local arterioles, thus returning the local blood flow in the body tissues and also the cardiac output toward normal while simultaneously causing a secondary increase in total peripheral resistance.
Note that the extracellular fluid volume and blood volume also returned toward normal along with the decrease in cardiac output. This outcome resulted from two factors. First, the increase in arteriolar resistance decreased the capillary pressure, which allowed the fluid in the tissue spaces to be absorbed back into the blood. Second, the elevated arterial pressure now caused the kidneys to excrete the excess volume of fluid that had initially accumulated in the body.
Several weeks after the initial onset of volume loading, the following effects were found:
Hypertension
Marked increase in total peripheral resistance
Almost complete return of the extracellular fluid volume, blood volume, and cardiac output back to normal
Therefore, we can divide volume-loading hypertension into two sequential stages. The first stage results from increased fluid volume causing increased cardiac output. This increase in cardiac output mediates the hypertension. The second stage in volume-loading hypertension is characterized by high blood pressure and high total peripheral resistance but return of the cardiac output so close to normal that the usual measuring techniques frequently cannot detect an abnormally elevated cardiac output.
Thus, the increased total peripheral resistance in volume-loading hypertension occurs after the hypertension has developed and, therefore, is secondary to the hypertension rather than being the cause of the hypertension.
When a patient is maintained with an artificial kidney, it is especially important to keep the patient’s body fluid volume at a normal level by removing the appropriate amount of water and salt each time the patient undergoes dialysis. If this step is not performed, and extracellular fluid volume is allowed to increase, hypertension almost invariably develops in exactly the same way as shown in Figure 19-8 . That is, the cardiac output increases at first and causes hypertension. Then, the autoregulation mechanism returns the cardiac output back toward normal while causing a secondary increase in total peripheral resistance. Therefore, in the end, the hypertension appears to be a high peripheral resistance type of hypertension, although the initial cause is excess volume accumulation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here