Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Ultrasonography with Doppler (US Doppler) is the best imaging modality for systematic follow-up of the liver allograft and for the detection and diagnosis of complications.
Grayscale mode allows for the analysis of graft parenchyma, vessels, and bile ducts and the detection of fluid collections ( Fig. 29.1 ). Color and pulsed Doppler modes allow the precise analysis of blood flow in the vessels and facilitate the diagnosis of bile duct dilation by showing tubular structures that are not color-encoded in contrast with the vessels ( Fig. 29.2 ).
There is no consensus in the literature concerning optimal systematic US screening during and after pediatric liver transplantation (LT). It is usually accepted that intraoperative US Doppler evaluation is helpful to detect very early vascular complication, thereby warranting immediate surgical repair. Routine follow-up varies among teams; some centers will recommend one US Doppler study every 12 hours, and others opt for every 24 hours, at least for the first few days after LT. After this period, weekly examinations are common and are then eventually limited to scheduled anniversary visits. The recommended systematic analysis with US Doppler is described in Tables 29.1 and 29.2 .
| Gray-scale imaging |
|
| Doppler study |
|
| Elastography (If available) |
|
| Normal | In case of stenosis | |
|---|---|---|
| Hepatic artery | Hepatopetal flow Velocity > 20 cm/s, no significant acceleration at the anastomosis RI > 0,50 |
Velocity < 20 cm/s; prolonged acceleration time (> 80 ms) = Tardus parvus wave RI < 0,50 Stenotic velocity elevated > 200 cm/s |
| Portal vein | Hepatopetal flow Velocity > 15 cm/s no significant acceleration at the anastomosis |
Slow velocity in intrahepatic branches < 10 cm/s Increased velocity at stenosis Intermittent or hepatofugal flow in the liver and upstream the portal anastomosis Presence of hepatopetal cavernoma, signs of portal hypertension Elevated spleen elasticity |
| Hepatic vein | Hepatofugal flow Velocity > 20 cm/s No significant acceleration at the anastomosis mono-, bi- or tri-phasic waveform |
Decreased velocity of hepatic veins < 10 cm/s Increased velocity at stenosis > 200 cm/s Decreased velocity or even reversed flow in portal vein Signs of portal hypertension Elevated liver and spleen elasticity |
The healthy graft has a homogeneous echostructure. Note that the cut surface may be heterogeneous in smaller grafts. The normal Doppler patterns for graft vessels are summarized in Table 29.1 and illustrated in Fig. 29.1 .
Computed tomography (CT) scans and magnetic resonance imaging (MRI) scans are not necessary in routine follow-up.
Biliary complications are frequent after LT and may be challenging to diagnose. Two types of complications exist—leaks and strictures. (See Chapter 21 for a more detailed discussion of biliary complications.)
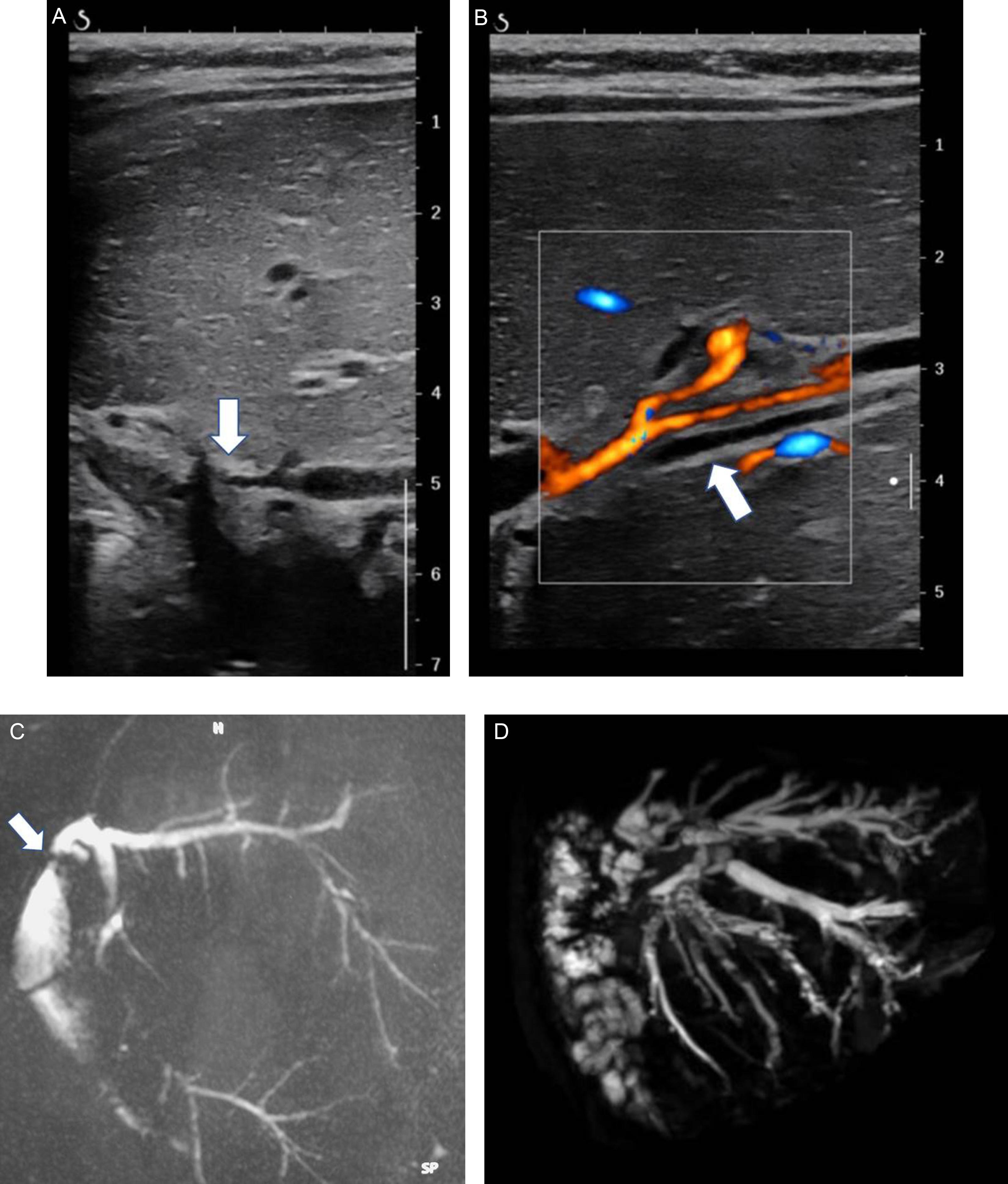
Leaks occur less than 30 days following transplantation and are mainly due to anastomotic disorders or the failure to anastomose an accessory bile duct at the time of transplantation. They usually appear on imaging as well-defined fluid collections, mainly anechoic. A rapid increase in size with time is very useful and may help differentiate bile leaks from a hematoma or ascites. In difficult cases, mainly for small leaks, magnetic resonance cholangiopancreatography (MRCP) with liver-specific contrast agent injection or hepatobiliary scintigraphy (HBS) can help identify the site of bile leakage.
Biliary strictures are more challenging to diagnose. In typical cases, the patients will have clinical and biological signs of cholestasis, and US will show dilated bile ducts (see Fig. 29.2 ). When there is an isolated biliary anastomotic stricture, bile ducts are dilated in all segments to the level of the anastomosis. If intrahepatic strictures exist, US may show suspended dilations of the bile ducts. US allows the depiction of thickened and/or irregular bile ducts walls and abnormal content, such as stones and sludge. Analysis of the entire biliary tree and the depiction of the exact site of obstruction are not always possible on US, however, and MRCP can be helpful for the exact mapping of the biliary tract (see Fig. 29.2 ).
Bile duct dilations may be absent on US in patients with biliary obstruction. Another important fact is that bile duct dilation is not well correlated with the severity of biliary strictures on percutaneous transhepatic cholangiography (PTC). In several studies, the sensitivity and specificity of US were between 79% and 93%, respectively. HBS has a better sensitivity (94%) and specificity (93%) but does not provide anatomical analysis of the bile ducts and anastomotic site. MRCP has the best diagnostic performance, with 100% sensitivity and 92% specificity. Moreover, it can show all the bile ducts and site of obstruction(s) precisely. However, in young children, MRCP is more challenging and may require sedation. Contrast-enhanced CT may show dilated bile ducts, but it is less effective than MRCP and exposes young patients to ionizing radiation. In daily practice, US is the best examination; MRCP or HBS is recommended for evaluating patients with possible biliary obstruction, regardless of findings on US.
Hepatic artery (HA) complications are the most frequent vascular complications after LT, occurring in 1% to 26% of cases in published series. (See Chapter 20 for a more detailed discussion of arterial complications.)
Stenosis and thrombosis may occur any time after transplantation, but early thrombosis, within the first month after LT, is the most frequent and threatening complication. HA thrombosis (HAT) can lead to graft necrosis, deterioration of liver function, and/or biliary complications that are frequently delayed and appear after a few weeks. The mortality rate has been reported to be as high as 55.6% in cases of early occlusion, compared with 15% to 22.6% with late occlusion. The site of stenosis or thrombosis is usually the arterial anastomosis.
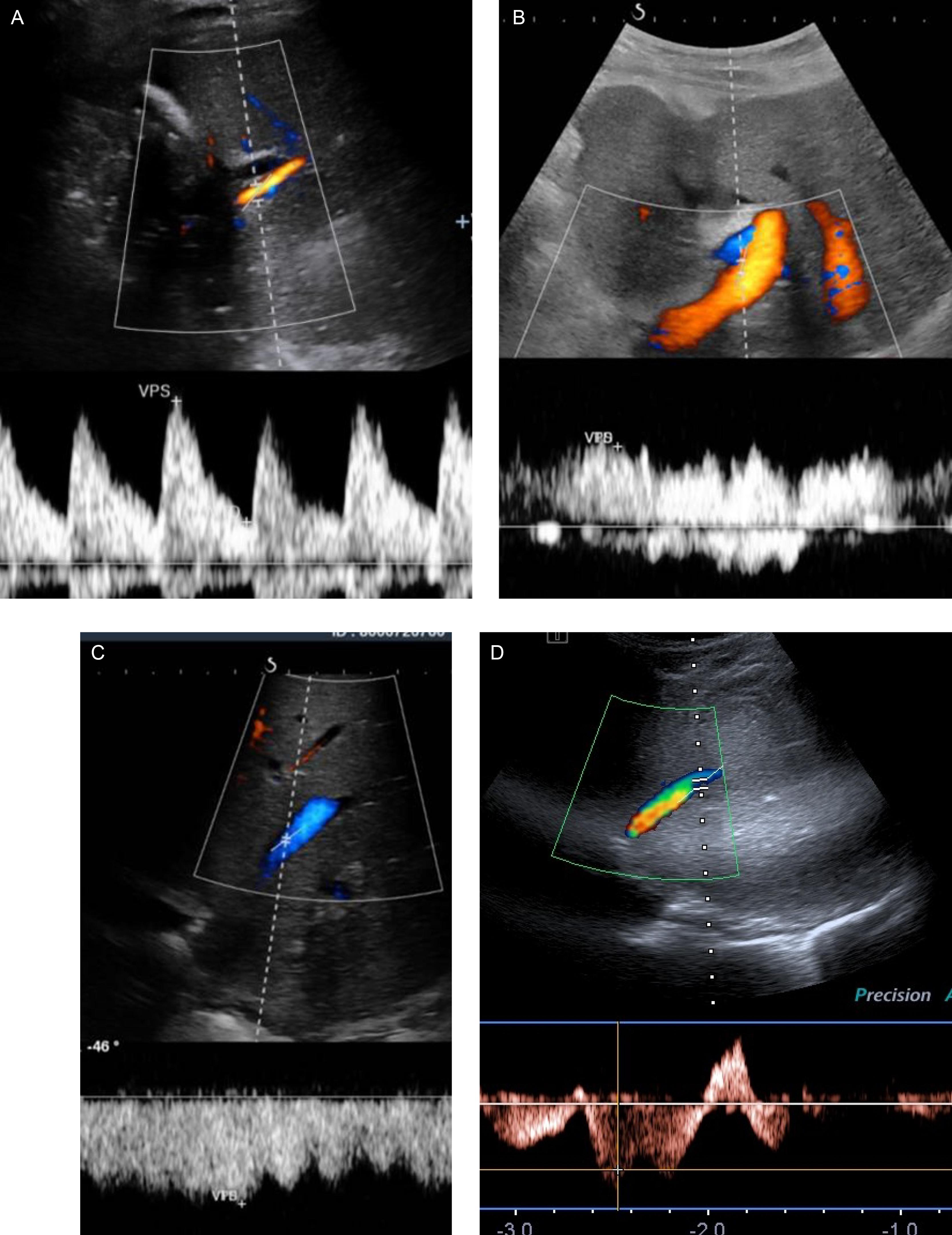
Early diagnosis is mandatory for emergent treatment to limit complications. HAS diagnosis relies on a Doppler US examination. CT angiography (angio-CT) or MR angiography (angio-MR) can confirm the diagnosis. HAS has a characteristic appearance on Doppler US. The intrahepatic arterial Doppler signal distal to the stenosis may show a tardus parvus pattern, with a decreased resistivity index (< 0.50) and a prolonged acceleration time (> 80 ms). At the site of narrowing, there is a focally accelerated velocity greater than 200 cm/s ( Fig. 29.3 ; see Table 29.1 ).
Of note, a decreased resistivity index can be a normal finding in patients with tachycardia. During the early phase of HAT, Doppler US will typically show the absence of arterial flow in the liver. However, within 2 weeks of the thrombotic event, collateral arteries develop, which may mimic normal arterial flow. They typically have a low resistive index and low peak systolic velocity, but a normal Doppler spectrum may be present.
Portal vein complications include portal vein stenosis and thrombosis. They are explained in detail in Chapter 21 on vascular complications. Diagnosis relies mainly on Doppler US. Angio-CT or angio-MR is useful to confirm diagnosis.
Portal vein stenosis occurs at the anastomosis in the vast majority of the cases. The diagnosis may be quite difficult in some cases because of caliber mismatch between donor and recipient portal veins, something expected especially in very young children. This common finding is frequently associated with turbulence and even acceleration of the portal flow immediately downstream of the anastomosis, and it should not be mistaken as a stenosis. In case of portal stenosis, there is at least a 50% reduction of the diameter of the vessel lumen compared with the diameter of the prestenotic vessel using grayscale US. Cutoffs for the diameter of the anastomosis have been proposed ranging from less than 4 to 2.5 mm at the site of the narrowing.
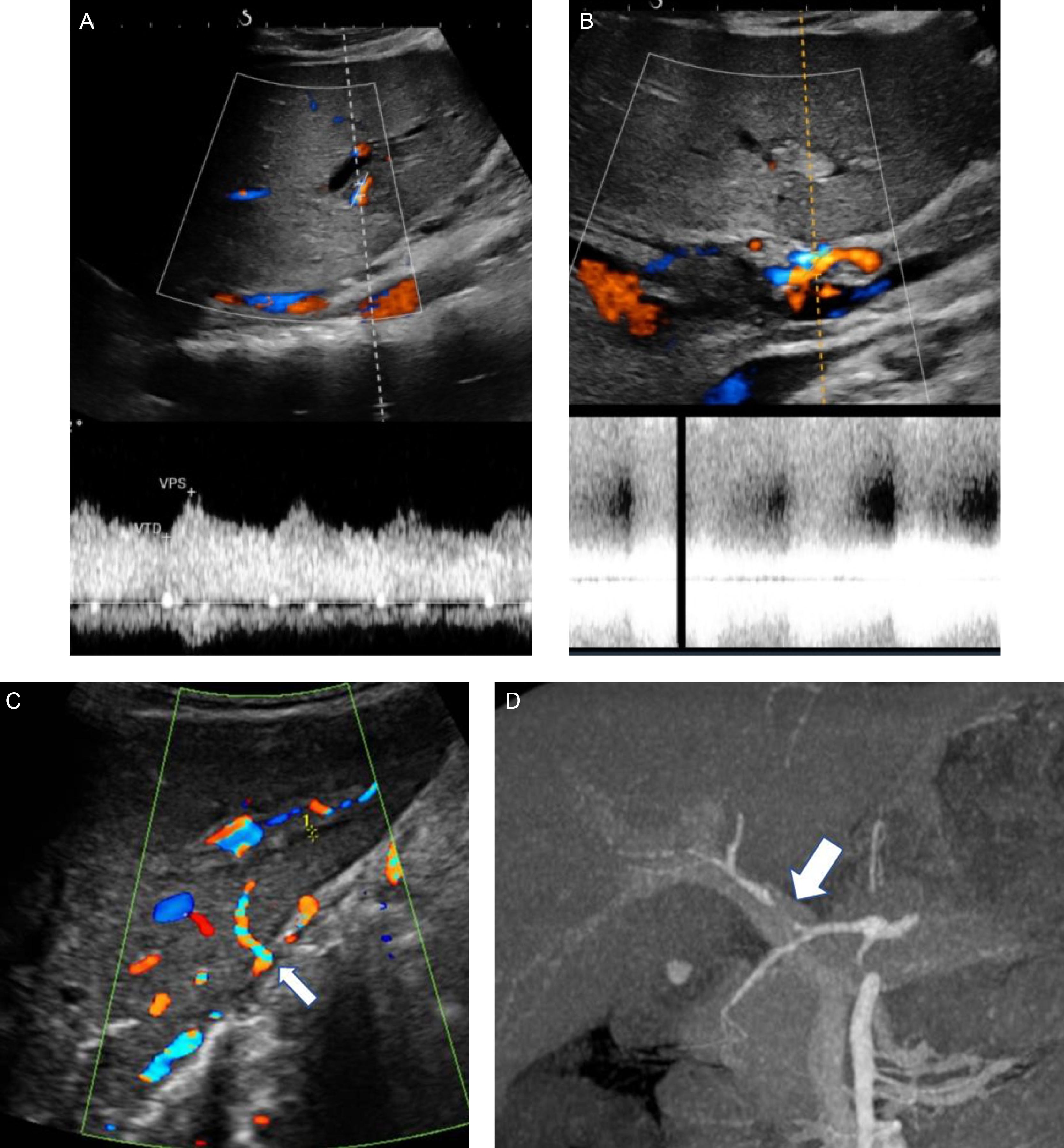
Color Doppler US shows focal color aliasing at the site of the stenosis. A pulsed Doppler study shows a marked acceleration of the portal flow at the site of the narrowing. A cutoff value of 125 cm/s allows a 95% specificity with a 73% sensitivity. The comparison of pre-stenotic and post-stenotic portal flow velocity may also help; a ratio of 2 to 4 or a difference of 30 cm/s may reflect stenosis. The post-stenotic jet is usually fast and could explain the increased width of the portal vein. It has been suggested that portal vein stenosis is associated with a Rex recessus diameter >1.5cm (sensitivity 88%, specificity 83%). Portal flow velocity in the liver is slow. Slow portal flow in pre-stenotic portal vessels (portal, mesenteric, and splenic veins) argues in favor of a significant stenosis. Reverse flow upstream from the stenosis may be observed in severe cases. The presence of a venous cavernoma may also be helpful for the diagnosis of significant portal stenosis or thrombosis. Signs of portal hypertension, including splenomegaly and the presence of portosystemic shunts, are synonymous with venous congestion and, as such, may both facilitate diagnosis and follow-up after an intervention ( Fig. 29.4 ).
Angio-MR and angio-CT are useful to confirm diagnosis, study the portal vein in detail, and assess for signs of portal hypertension. Calcifications of the portal vein walls are better depicted on CT.
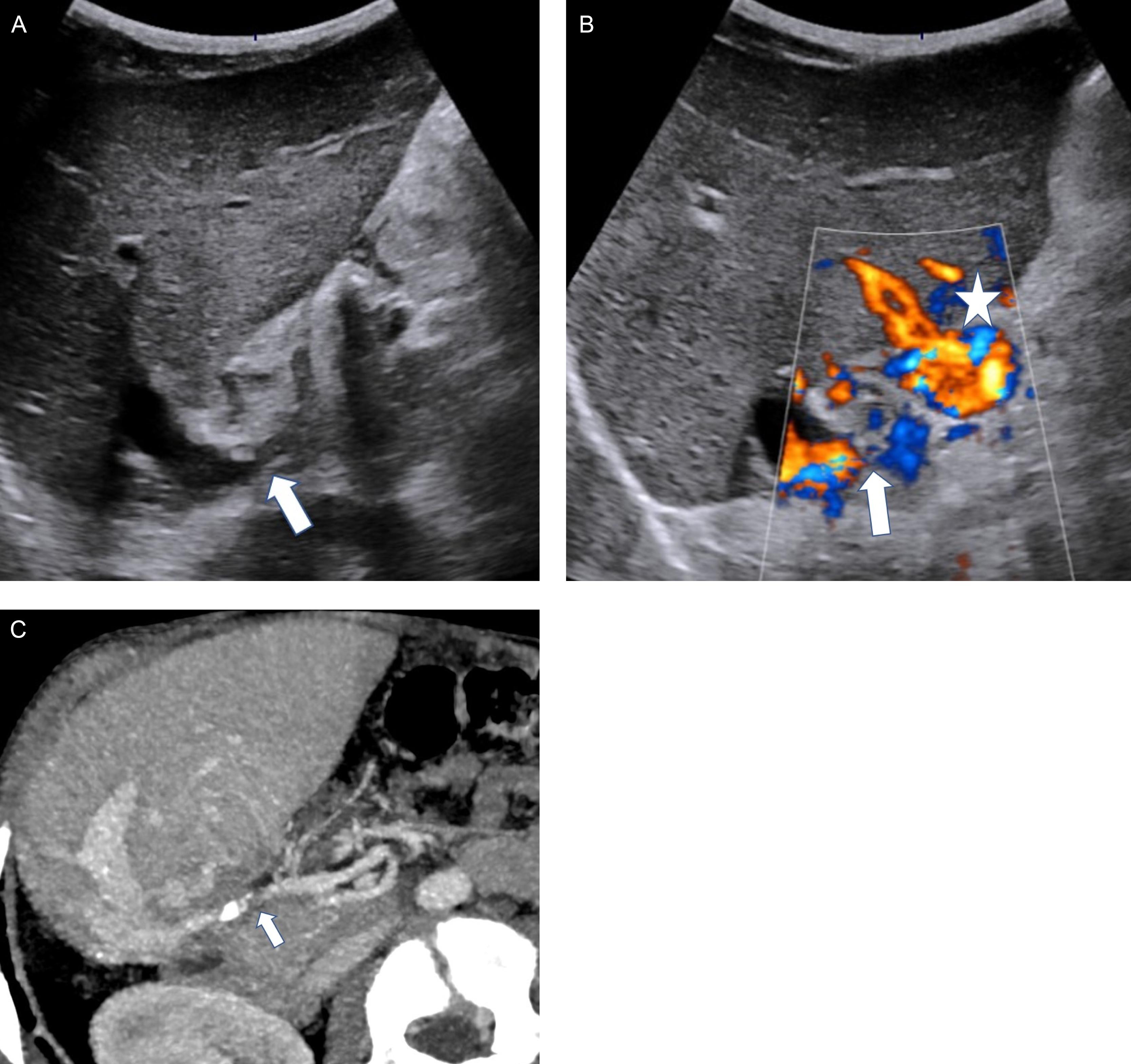
Acute portal vein thrombosis may be difficult to depict on a grayscale study because the thrombus is frequently anechoic. The absence of detectable flow in the portal vein is confirmatory. The intrahepatic portal flow will be either hepatopetal or reversed. Progressive narrowing or the portal lumen in the liver can be observed in two conditions: when the hepatopetal cavernoma is not efficient or if thrombosis has extended to the intrahepatic portal branches.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here