Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Chronic progressive nephropathies, independent of the type of the initial insult, have in common persistently high levels of urinary protein excretion, chronic tubulointerstitial lesions, and glomerular sclerosis. It has become clear that in otherwise comparable groups of patients, those with higher levels of proteinuria are at a greater risk of progression. It has been long known that the severity of tubular interstitial damage is highly correlated to the degree of deterioration of renal failure, even better than the glomerular lesions do . The recognition that proteinuria is an independent predictor and a possible contributor of progression rather than just a marker of the severity of glomerular damage represents a major change in our concepts of progressive kidney disease. Proteinuria is enlisted among modifiable risk factors of progressive renal insufficiency, and antiproteinuric treatments are consistently renoprotective. Proteinuria reduction must therefore be considered as a target of therapeutic intervention in managing the patient with a chronic proteinuric nephropathy.
Among the cellular mechanisms that may contribute to determine progression, proteins that gain access to the glomerular filtrate as a consequence of altered glomerular permeability reach the Bowman’s space and the tubular lumen and act as trigger of interstitial inflammatory and fibrotic reactions through the tubular synthesis of bioactive mediators. In vitro studies using polarized proximal tubular epithelial cells as a model system to mimic the effects of prolonged exposure to proteins have highlighted specific mechanisms underlying renal cell activation and renal interstitial injury.
The main focus of this chapter is on pathways of interstitial inflammation and fibrosis activated by ultrafiltered proteins that play major roles in the multifactorial process ultimately responsible for kidney scarring and loss of function ( Figure 87.1 ).
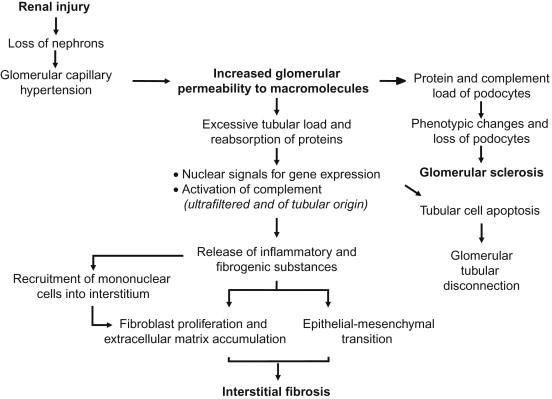
A relentless decline in glomerular filtration rate can follow initial insults to the kidney through a process termed as ‘renal disease progression’ by nephrologists since 1982. Studies in the early 19 th and 20 th centuries contributed to link a disparate body of knowledge about patients with albuminuria and pathological evidence of diseased kidneys. By summarizing his concept in term of renal ‘osmotic work’, Addis calculated how this work would vary with the amount of proteins in the diet and suggested that dietary protein restriction could possibly help patients with renal impairment.
When glomerular permeability is altered, as occurs in many glomerular diseases, considerable quantities of circulating proteins are abnormally filtered in the urinary space. Volhard and Fahr and subsequently Mollendorf and Stohr provided the early descriptions of hyalin droplets in the cytoplasm of proximal tubular cells and suggested that they reflected renal damage related to severe proteinuria. It was then proposed that such changes could represent the initial phase of activation of cellular pathways leading to cell necrosis. The appearance of droplets was also suggested to be associated with impairment of normal reabsorbption and degradation of plasma proteins by proximal tubular cells.
Hints about a possible association between proteinuria and renal function impairment formally emerged in the late 1970s. Cameron and colleagues showed that among patients with focal and segmental glomerulosclerosis those with nephrotic syndrome progressed more rapidly than those who had never been nephrotics. This was also true for mesangiocapillary glomerulonephritis and membranous nephropathy. Their speculation was that during the course of prolonged nephrotic syndrome intracapillary platelet thrombi induced segmental sclerosis, or that these glomerular lesions were a consequence of proteinuria. These observations were in agreement with previous findings that none of patients with focal and segmental glomerulosclerosis who responded to corticosteroid treatment with reduction of proteinuria developed renal failure.
In 1981 Brenner and associates introduced the concept that glomerular hemodynamic changes (glomerular hyperfiltration) ensuing as compensatory adaptation to nephron loss can cause progressive deterioration of remaining nephrons. Instrumental in clarifying the pathophysiology of renal adaptation to nephron loss was the model of renal ablation in the rat. After removal of a critical portion of renal mass, remnant nephrons undergo sudden hypertrophy with concomitant lowering of arteriolar resistance and increased glomerular plasma flow. Since the vascular tone drops more in afferent arterioles than in the efferent ones, glomerular capillary hydraulic pressure rises and more filtrate is formed per nephron. While such changes serve to enhance the filtration capacity of remaining nephron units, thus minimizing the functional consequences of nephron loss, they are ultimately detrimental. Therapies that attenuate such adaptive changes limit GFR decline and structural damage. In harmony with the data obtained in animal studies, clinical studies showed markedly reduced rates of GFR decline in patients with diabetes receiving antihypertensive agents. Elevated intraglomerular capillary pressure was suggested to act as a noxious stimulus to glomerular cells. Studies of mathematical models of the glomerular filtering membrane documented that the high glomerular capillary pressure acted to enlarge the radii of the pores perforating the glomerular membrane by a mechanism at least partly mediated by angiotensin II. This impairs the size-selective function of the barrier resulting in protein ultrafiltration. The association of protein ultrafiltration and progression of renal disease was underscored by Mathiesen and coworkers who reported that in diabetic patients the higher the microalbuminuria the faster the rate of GFR decline.
In 1986, it was suggested for the first time that proteins abnormally filtered through the glomerular capillary might have intrinsic renal toxicity that is relevant to the progression of renal damage. In the kidneys of proteinuric rats with adriamycin nephrosis, the accumulation of filtered proteins in the cytoplasm of proximal tubular cells and in luminal casts was associated with focal breaking of the tubular basement membrane and extravasation of the tubular content into the renal interstitium. Aging is associated with progressive proteinuria and glomerulosclerosis, which lead to the renal insufficiency of certain strains of rats. The severity of proteinuria predicted the development of glomerulosclerosis and tubulointerstitial changes. Implications of these studies were that the enhanced protein uptake may activate proximal tubular epithelial cells to promote the accumulation of inflammatory cells into the interstitium, in turn responsible for renal scarring. Studies using animal models and pharmacologic manipulations found that the dysfunction of the glomerular capillary barrier to proteins can precede the onset of structural lesions in the absence of glomerular hemodynamic changes, consistent with the possibility that—at least in certain settings—protein load of the nephron may contribute independently to progressive renal damage before maladaptive hemodynamic changes can take place or be measurable within the glomerulus.
Cause-and-effect relationships between enhanced transtubular protein passage and interstitial inflammation were investigated by Eddy in a rat model of protein overload. Repeated injections of albumin increased the permeability of the glomerular barrier leading to proteinuria and tubular changes, including the accumulation of injected albumin in intracellular droplets of tubular cells. These changes occurred in advance of heavy infiltration of macrophages and T lymphocytes into the renal interstitium. Excessive proteinuria was also induced in rats by transplanting a pituitary tumor (MtT SA5) that causes liver hyperplasia with both overproduction and abnormally high urinary excretion of albumin, followed by tubular damage and interstitial inflammation. Models of protein overload contributed to suggest that the pathogenic potential of ultrafiltered plasma proteins is not confined to pathways underlying tubular and interstitial damage. The availability of cultured cells with features of differentiated podocytes revived investigation on the effects of plasma proteins on the function of glomerular epithelial cells, currently recognized to play a key role in the progression of lesions toward glomerular sclerosis. Plasma proteins that are filtered accumulate as protein droplets within podocytes from the early stages of non-immunologically-induced glomerular damage . At the same stage, podocytes show signs of dedifferentiation and injury . These abnormalities are likely to play role in the development of glomerular sclerotic lesions. They will be discussed in the light of findings indicating another important pathway, i.e., the activation of complement upon excess protein ultrafiltration.
Proteins that reach the tubular lumen are reabsorbed in the proximal segments by receptor-mediated endocytosis, via two multiligand-binding receptors, megalin and cubilin. Megalin, a 600-kDa transmembrane glycoprotein belonging to the low density-lipoprotein receptor family, represents the most abundant endocytic receptor in the proximal tubule, being concentrated in clathrin-coated pits (CCPs) and vesicles in the brush border region. It binds different ligands including albumin, hormones such as insulin, angiotensin II and prolactin, and vitamin-binding proteins. Megalin’s endocytic function is regulated by the G protein–mediated signaling pathway that includes Gαi3, GAIP and GIPC, the latter interacting with the cytoplasmic tail of megalin ( Figure 87.2 ). This portion contains recognition motifs for intracellular adaptor proteins and protein kinases involved in endocytosis, apical sorting of receptor, and signaling.
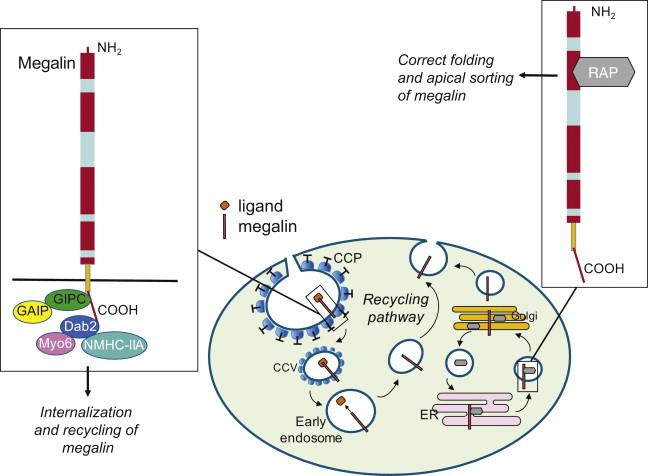
Among adaptor proteins, Disabled protein 2 (Dab2) colocalizes with megalin in CCPs and vesicles of renal proximal tubules where it binds the second endocytic motif of megalin, and, by interacting with the motor protein nonmuscle myosin heavy chain IIA, regulates trafficking of megalin through the endocytic/recycling pathway ( Figure 87.2 ). Suboptimal trafficking of megalin in Dab2 knockout mice leads to decreased megalin levels, possibly due to increased protein shedding and degradation, and impaired endocytosis as documented by urinary loss of megalin ligand. Association of motor proteins with adaptor proteins is now emerging to be instrumental for renal proximal tubular endocytosis. Myosin VI (Myo6) is highly expressed in the brush border where it associates with the CCP via its tail domain binding to Dab2 ( Figure 87.2 ). Myo6 functional null mice show increased urinary albumin excretion, similar to megalin- and cubilin-deficient animals, and reduced and delayed endocytic uptake and trafficking of horseradish peroxidase consistent with impaired endocytosis. This defect is associated with structural and phenotypic changes of tubular cells and fibrosis.
The expression and subcellular distribution of megalin is controlled by the chaperone receptor-associated protein RAP that prevents ligand–induced endoplasmic reticulum (ER) retention and degradation of the receptor and is possibly involved in its proper folding ( Figure 87.2 ). RAP knockout mice are characterized by both reduced expression of megalin and its preferential subcellular distribution to the ER. RAP deficiency is also associated with leakage of proteins in urine s due to impaired megalin - dependent tubular reabsorption. Pro-inflammatory and pro-fibrotic mediators such as transforming growth factor beta (TGF-β) and angiotensin II as well as nephrotoxicants also down-regulate megalin expression in proximal tubular cells.
Cubilin or intrinsic-factor B12 receptor, is a 460-kDa peripheral membrane protein that binds albumin, transferrin, IgG light chains and RAP, but lacks the sites for interaction with adaptor proteins or other mediators of clathrin-dependent endocytosis. Since megalin binds cubilin with high affinity, it has been hypothesized that megalin mediates endocytosis and intracellular trafficking of cubilin. In vitro , the uptake of the cubilin-specific ligands, including transferrin, was inhibited both by anti-megalin antibodies and by megalin anti-sense oligonucleotides. Furthermore, in megalin-deficient mice transferrin accumulates on the luminal membrane of the proximal tubule without being internalized. Cubilin binds amnionless (AMN), a 50-kD transmembrane protein that is required for membrane targeting and may permit internalization of cubilin. Patients with Imerslund-Grasbesk syndrome (IGS), a rare autosomal recessive disease caused by inheritable cubilin or AMN gene defect, show reduced protein reabsorption and proteinuria. Consistently, inappropriate apical membrane insertion of cubilin in the proximal tubule leads to proteinuria in a model of IGS in dog s with a mutation of the AMN gene. Kidney specific AMN knockout mice also show increased urinary excretion of transferrin.
The generation of mice with the genetic ablation of cubilin, with or without ablation of megalin, disclosed a mutual dependency of cubilin and AMN to form a functional membrane receptor complex. Cubilin is indispensable for albumin reabsorption in renal proximal tubule under physiological conditions, whereas megalin drives the internalization of cubilin-albumin complexes.
Megalin undergoes regulated intramembrane proteolysis with protease-mediated ectodomain shedding, producing a membrane–associated C-terminal fragment, which is in turn the substrate for the γ-secretase activity of a multimolecular complex of proteins, including the so-called presenilins, that release the C-terminal soluble cytosolic domain predicted to target the nucleus. In the rat kidney, the brush border exhibits both γ-secretase activity and presenilin-1 expression. In a cell line derived from opossum proximal tubule (OK cells), metalloprotease activity sheds the ectodomain of megalin, producing a membrane-bound megalin COOH-terminal fragment with the same size as a major fragment of megalin found in the kidney. This fragment is further cleaved by γ-secretase into a soluble intracellular domain leading to down-regulation of megalin and Na + /H + exchanger 3 (NHE-3) transcripts. This domain does not affect megalin expression and endocytic function in vivo at least under physiological conditions, although a regulatory effect on renal gene transcription under stress cannot be excluded.
Reduced expression of megalin is a common feature to settings of proteinuria and defective tubular uptake of filtered proteins, such as early stages of experimental diabet es or polycystic kidney disease, Fanconi syndrome and Dent’s disease in humans. Dent’s disease is caused by a mutation of the gene CLCN5 encoding the 2 chloride (Cl − )/proton (H + ) exchanger ClC-5. Inactivating mutations of ClC-5 in Dent’s disease and the deletion of CLCN5 in knockout mice lead to low - molecular - weight proteinuria due to reduced endocytic uptake of filtered proteins in the proximal tubule. This defect was initially attributed to impaired acidification of apical endosomes in these cells, but subsequent data indicate selective loss of expression of megalin and cubilin at the brush border as a major cause of defective protein endocytosis in this disease. Along this line, reduced megalin and cubilin levels have been found in tubules with the uncoupling E211A (unc) mutation that converts CLC-5 into a pure Cl − conductor. Despite maintaining active endosomal acidification, ClCN5 unc mice show impaired proximal tubular endocytosis and resultant low-molecular-weight proteinuria as found in CLC-5 knockout mice and in patients with Dent’s disease. These results indicate that endosomal chloride-proton exchange rather than chloride conductance is crucial for tubular endocytosis ( Figure 87.3 ).
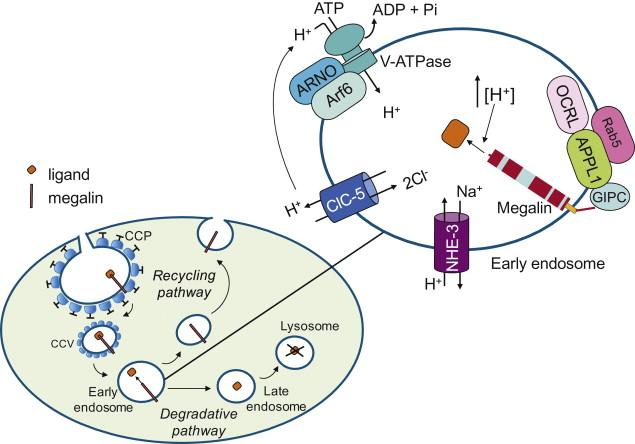
During receptor-mediated endocytosis, ligand-receptor complexes are internalized and transported via clathrin-coated vesicles to early endosomes where they dissociate. The receptor is sorted to the plasma membrane (recycling pathway) and the endocytosed proteins are transported further into the degradative pathway. The endocytic pathway is regulated by a protein network. The inositol 5’-phosphatase OCRL -whose mutations are responsible for OculoCerebroRenal Syndrome of Lowe , an X-linked disorder characterized by cataract, mental retardation, and renal Fanconi syndrome- is present throughout the early endocytic pathway including CCPs and interacts with adaptor molecules involved in the early endocytic traffic of receptors in brain and kidney. On peripheral early endosomes OCRL binds the Rab5 effector APPL1, an adaptor/signaling protein that binds several transmembrane receptors and also the adaptor protein GIPC ( Figure 87.3 ). Since GIPC directly binds megalin (see above), loss of the interaction of OCRL with APPL1 due to disease mutation may have a causal role in the reabsorptive defect of kidney resulting in low-molecular-weight proteinuria in Lowe syndrome patients and in a subset of Dent’s disease patients.
Another protein, the transporter NHE - 3 contributes to the early phase of the apical endocytic pathway at least in part due to its involvement in endocytic vesicle fusion. NHE - 3 is associated with megalin and dipeptidyl IV in proximal tubule endosomes where it also participates in the acidification process ( Figure 87.3 ). Acidification of endosomal compartment is crucial not only for ligand-receptor dissociation and receptor recycling, but also for a correct protein endocytic trafficking from early to late endosomes, and finally lysosomes. The acidification of proximal tubule endosomes is driven by the vacuolar H + -ATPase (V-ATPase), an electrogenic pump that translocates protons from the cytoplasm to the endosomal lumen and in conjunction with a parallel chloride conductance generates the acidic milieu of the endosomal compartment ( Figure 87.3 ). V-ATPase acts as an endosomal pH sensor that recruits the small GTPase Arf6 (ADP-ribosylation factor) and its cognate GDP/GTP exchange factor ARNO (ADP-ribosylation factor nucleotide site opener) from the cytosol to the endosomal membranes by direct binding these proteins upon vesicle acidification ( Figure 87.3 ). Interaction of both Arf6 and ARNO with V-ATPase allows correct vesicular trafficking from early to late endosomes. By contrast, inhibition of V-ATPase or uncoupling of endosomal acidification impairs V-ATPase/ARNO/Arf6 interaction leading to accumulation of endocytosed proteins in early endosomes, and ultimately, to inhibition of endocytosis. An intact actin cytoskeleton is important for correct assembly and function of the V-ATPase. The disorganization of the actin cytoskeleton induced by statins through reduced prenylation of the small GTP-binding proteins result s in a defect of albumin endocytosis in proximal tubular cells.
Insights into mechanisms linking the excess traffic of plasma proteins and interstitial injury have come from in vitro studies using polarized proximal tubular cells as a model to assess effects of apical exposure to proteins ( Figure 87.4 ). Challenge of cultured proximal tubular cells with high concentrations of proteins (delipidated or lipid-enriched albumin, IgG and transferrin) induced the synthesis of the vasoconstrictor peptide endothelin-1, a mediator of progressive renal injury due to its ability to stimulate renal cell proliferation and extracellular matrix production and to attract monocytes. Monocyte chemoattractant protein-1 (MCP-1 or CCL2) and Regulated upon Activation, Normal T cell Expressed and Secreted (RANTES or CCL5), potent chemoattractant s for monocytes/macrophages and T-lymphocytes, were upregulated in proximal tubular cells challenged with albumin and other plasma proteins. Albumin overload also induced tubular gene expression and production of the C-X-C chemokine interleukin-8 (IL-8), a potent chemoattractant for neutrophils and lymphocytes. Endothelin-1 and chemokines induced by plasma proteins were released mainly toward the basolateral compartment of the cell, which is indicative of a directional secretion potentially responsible for the tubulointerstitial inflammatory reaction as found in proteinuric nephropathies. Co-culture systems of proximal tubular epithelial cells and monocytes/T cells proved useful to document that the release of MCP-1 and RANTES upon apical exposure of tubular cells to albumin was further increased in the presence of monocytes or T cells, either through a cell-to-cell contact mechanism or mediated by soluble factors such as interleukin-1 and tumor necrosis factor. Moreover, the conditioned medium of tubular cells activated by albumin induced distinct patterns of chemokine receptor expression on T cells or monocytes, thereby implying that different arrays of chemokines may mediate the chemotactic activity of leukocytes in the tubulointerstitium during the proteinuric state.
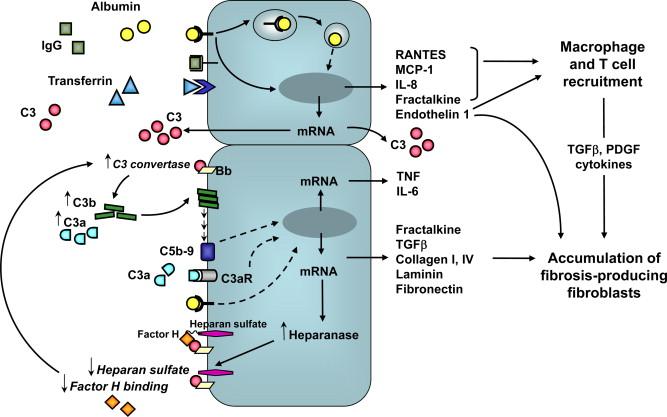
Protein overload promotes the tubular expression of fractalkine/CX3CL1, a cell-membrane anchored chemokine that serves as an adhesive molecule to promote firm adhesion of mononuclear cells expressing the specific receptor CX3CR1, in addition to functioning in its cleaved soluble form as a chemoattractant. Upregulation of fractalkine mRNA and increased synthesis of both membrane-bound and soluble forms of the protein occurred upon stimulation of human proximal tubular cells with albumin. In a murine model of protein overload proteinuria, fractalkine mRNA was overexpressed in the kidney, and fractalkine staining was detected in tubular epithelial cells in a focal distribution. Treatment of mice with an antibody against CX3CR1 limited the accumulation of monocytes/macrophages in the renal interstitium. These data suggest that in proteinuric conditions, fractalkine overproduction might contribute to direct mononuclear cells into the peritubular interstitium and possibly enhance their adhesive property.
TGFβ is a profibrogenic cytokine capable of directly stimulating the proliferation of fibroblasts and the synthesis of matrix proteins, in addition to exerting indirect stimulatory effects via inflammatory infiltrating cells. TGFβ acts as a key stimulus for epithelial-to-mesenchymal transition (EMT), by which tubular cells acquire features of fibroblasts. High concentrations of albumin induced in cultured proximal tubular cells the transcription of the TGFβ gene resulting in the enhanced release of the cytokine in the cell supernatant . Albumin upregulated TGFβ receptor type II expression in proximal tubular cells, which became more susceptible to the matrix-stimulatory actions of TGFβ. Albumin stimulated the accumulation of extracellular collagen type IV, laminin, and fibronectin by proximal tubular cells through a post-transcriptional mechanism. A reduced degradation could be responsible for the increased accumulation of extracellular matrix protein components, as indicated by induction of tissue inhibitors of metalloproteinases (TIMP)-1 and TIMP-2, in response to albumin.
Advances have been made in clarifying the mechanisms of albumin-induced TGFβ production in proximal tubular cells. In vitro , albumin-induced secretion of TGFβ1 by proximal tubular cells could occur in the absence of albumin endocytosis. Thus two cell lines, opossum kidney (OK) proximal tubular cells and HKC-8 proximal tubular cells, which display different degrees of endocytosis, produced an equivalent amount of TGFβ1 when exposed to albumin. Moreover, inhibiting albumin endocytosis in OK cells with two agents with different mechanisms of action as EIPA (a sodium/hydrogen exchanger-3 inhibitor) or simvastatin (a 3-hydroxy-3-methylglutaryl CoA reductase inhibitor) did not reduce albumin-induced TGF-β1 secretion. On the other hand, RAP, a known inhibitor of binding of albumin to megalin, inhibited albumin endocytosis but it did not affect TGFβ1 production, suggesting that albumin-induced secretion of TGFβ1 is not via megalin signaling . Along this line, further studies showed that primary cultures of mouse proximal tubular cells exhibited substantial albumin endocytosis that could be similarly inhibited by both statins and thiazolinediones, but only statins had the ability to limit albumin-stimulated MCP-1 production. It was concluded that inhibition of albumin endocytosis alone was insufficient to attenuate chemokine production and therefore to protect proximal tubular cells from the toxic effects of albumin.
The contact between tubular cells and interstitial matrix is operated by specialized adhesion molecules, the integrins. They are α/β transmembrane heterodimers that mediate adhesion of the basal surface of cells to the underlying substratum by recognizing extracellular matrix components and by interacting with different cytoskeletal molecules. In vitro these molecules are clustered in specialized structures, called focal contacts. In tubular cells the expression of integrins can be regulated by cytokines, including TGFβ, that either directly influence mRNA transcription of integrins or modulate the synthesis of interstitial matrix and the consequent rearrangement of integrins at focal contacts. On the other hand, matrix proteins can modulate integrin expression and via integrin-generated signals, regulate the synthesis, degradation and organization of the matrix itself. Therefore, alterations in the expression and/or functional status of tubular cell integrins may play roles in the events leading to tubulointerstitial fibrosis. In cultured tubular cells the addition of albumin increased membrane expression of αvβ5 integrin which became organized in typical focal contacts dose-fashion related.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here