Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Rheumatoid arthritis is a chronic systemic inflammatory disease of unknown etiology that primarily targets synovial tissues. The primary target of rheumatoid arthritis is the joint, but systemic inflammation is thought to be responsible for a variety of coexistent comorbid conditions, including cardiovascular disease, osteoporosis, cognitive dysfunction, psychiatric disease, and cancer.
Rheumatoid arthritis is a global disease with a variable geographic prevalence of 0.5 to 1% of adults. The prevalence is about three times greater in women before menopause than in men. Rheumatoid arthritis can occur at any age. Overall, the annualized incidence of rheumatoid arthritis is approximately 40 per 100,000 for women and about half that for men. Because rheumatoid arthritis is a lifelong disease and its incidence increases or is stable with age, the prevalence of rheumatoid arthritis increases with each decade. The incidence of rheumatoid arthritis may be decreasing, perhaps reflecting reductions in smoking. Geographic variations in prevalence and phenotype can be remarkable. Most notably, in rural Nigeria, cohorts in which no individuals are affected with rheumatoid arthritis have been described, though case ascertainment bias needs to be excluded. In contrast, a prevalence of 5% has been found in some studies of Chippewa, Yakima, and Inuit Native Americans. Such extreme phenotypes are poorly understood but likely reflect the impact of genetics, the gastrointestinal or respiratory microbiome, or other environmental influences.
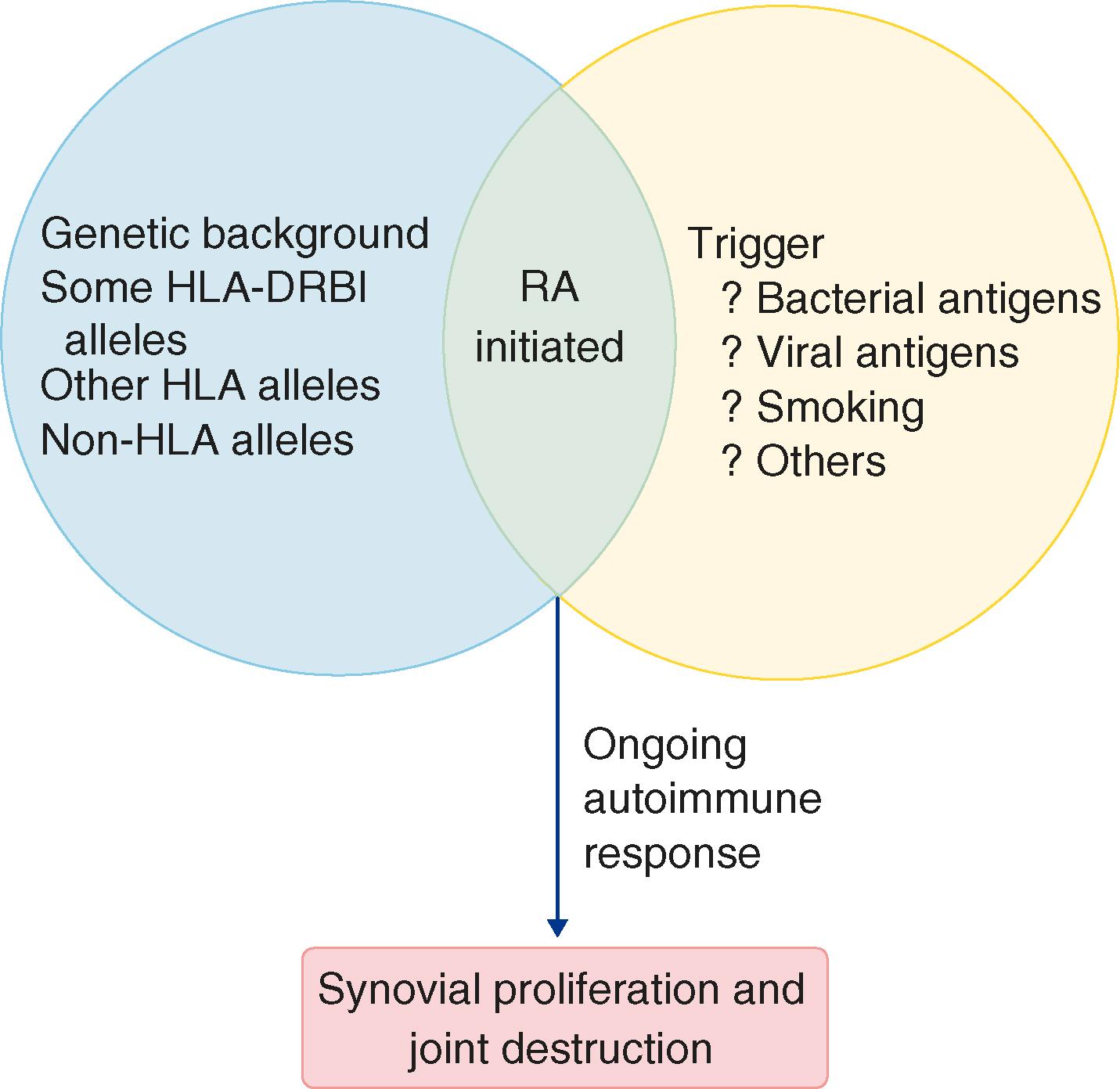
A combination of candidate association, twin, and genome-wide association studies (GWAS) has established a strong genetic component to the risk for developing rheumatoid arthritis and the severity of the disease. Twin studies reveal a concordance rate of approximately 12 to 15% for monozygotic twins and 2 to 5% for dizygotic twins. Heritability is reported to be around 60%, with lower values for patients who are seronegative for autoantibodies.
GWAS in European, North American, and recently Asian populations, combined with subsequent meta-genome analyses, demonstrate that rheumatoid arthritis is a polygenic disorder. The majority of more than 100 thus far identified informative single nucleotide polymorphisms (SNPs) implicate immune genes, indicative of a primary immunologic etiology. Around 40% of genetic risk accrues in the HLA region. Certain HLA-DR alleles (e.g., DRB∗0401, DRB∗0404, DRB∗0101, DRB∗1402) associate with an increased risk for developing rheumatoid arthritis and thereafter progressing to more severe disease. Hypervariable regions on DR molecules are particularly important for antigen recognition by binding to antigenic peptides and presenting them to the T-cell receptor (TCR). The amino acid sequence of disease associated DRβ1 chains share a common structural motif, called the shared epitope ( E-Table 243-1 ).
Peptides derived from post-translationally modified proteins (e.g., via citrullination, acetylation, carbamylation) may bind with altered avidity to the shared epitope, thereby providing a potential mechanism whereby this genetic factor can mediate the risk of disease at the molecular level. Thus, altered binding of such peptides to the HLA molecule (expressed on dendritic cells or B cells) may alter the dynamics of HLA-TCR interactions and thereby confer loss of self-tolerance upon (self-reactive) T cells inappropriately activated by this abnormal receptor engagement.
Many other immune related genes have been implicated and add considerable depth to the understanding of pathogenetic pathways. Although individual SNPs usually exhibit modest risk contribution (odds ratios ≈1.05 to 1.2-fold), they nevertheless could confer significant functional impact. Risk genes can be usefully defined on the basis of their contribution to functional immune system compartments. Adaptive immunity-associated SNPs include a functional polymorphism in protein tyrosine phosphatase nonreceptor 22 ( PTPN22 ), a polymorphism that has been reproducibly associated with rheumatoid arthritis and a number of other autoimmune diseases, including type 1 diabetes, systemic lupus erythematosus, Graves disease, and Hashimoto thyroiditis. The costimulation receptors, CTLA, CD28, CD40, and signal protein kinase TYK2 have similar disease associations. An association with peptidyl arginase deiminase also has been recognized. Because this enzyme converts arginine to citrulline, it may confer a genetically predisposed risk via increased generation of a major rheumatoid arthritis autoantigen. Pathways that regulate innate immune pathways are also identified (e.g., TRAF1-C5, STAT4 , TNF-AIP3, IRAK1) together with pathways concerned with cell migration (ELMO1) and fetal development (LBH). Several SNPs identify cytokine or cytokine receptor coding loci, including TNF and IL-6R.
Finally, there is increased interest in the role of epigenetics in disease risk and especially propagation. Altered DNA methylation, histone modification (e.g., via acetylation), and microRNA expression patterns associate with increased or perpetuation of inflammation. These patterns have been best defined in fibroblast-like synoviocytes (e.g., differential methylation and thus gene expression has been found particularly for pathways related to cell growth and differentiation). The presence of differentially expressed microRNAs has also attracted attention in myeloid lineage cells, T cells, and fibroblast-like synoviocytes. For example, dysregulation of miR146 and miR155 promotes cytokine and inflammatory pathways in macrophages.
| HLA TYPES (ALLELES) AND METHODS OF DETECTION | THIRD HYPERVARIABLE REGION AMINO ACID SEQUENCES | |||||||
|---|---|---|---|---|---|---|---|---|
| ALLOANTISERA (DR) | MLC (DW) | DNA (DRβ1) | 70 | 71 | 72 | 73 | 74 | MOST COMMON ETHNIC GROUPS |
| ASSOCIATED WITH RA | ||||||||
| DR4 | Dw4 | ∗0401 | Q | K | R | A | A | Whites (Western Europe) |
| DR4 | Dw14 | ∗0404 | R | Whites (Western Europe) | ||||
| DR4 | Dw15 | ∗0405 | R | Japanese, Chinese | ||||
| DR1 | Dw1 | ∗0101 | R | Asian Indians, Israelis | ||||
| DR6 (14) | Dw16 | ∗1402 | R | Yakima Native Americans | ||||
| DR10 | — | ∗1001 | R | R | Spanish, Greeks, Israelis | |||
| NOT ASSOCIATED WITH RA | ||||||||
| DR4 | Dw10 | ∗0402 | D | E | Whites (Eastern Europe) | |||
| DR4 | Dw13 | ∗0403 | R | E | Polynesians | |||
| DR2 | Dw2 | ∗1501 | D | A | Whites | |||
| DR3 | Dw3 | ∗0301 | G | R | Whites | |||
Increasing evidence supports a substantial role for environmental factors including smoking, other pulmonary exposures (e.g., silica), obesity, vitamin D deficiency, and lower educational attainment as risk factors for rheumatoid arthritis. Low levels of alcohol intake may be protective ( E-Fig. 243-1 ). Smoking in particular has long been associated with a significantly increased risk for developing rheumatoid arthritis, but only for anti-citrullinated peptide antibody (ACPA)-positive disease, especially in patients who have the shared epitope. Induction of epigenetic changes by smoking provides the most likely explanation for this etiologic link. Infectious triggers are also implicated, such as Mycobacteria, Streptococcus, Mycoplasma, Escherichia coli, Helicobacter pylori , and viruses (rubella, Epstein-Barr, parvovirus). The mechanisms whereby infections drive disease are poorly understood, but rheumatic fever ( Chapter 269 ), reactive arthritis ( Chapter 244 ), and Lyme arthritis ( Chapter 296 ) are prime examples. Reactive arthritis in particular may occur following one of a myriad of different but specific infectious triggers presented to a specific location in the body (e.g., the gastrointestinal or genitourinary tract) on a predisposed genetic background, in most cases human leukocyte antigen (HLA)-B27. In this syndrome, age and gender and hence the maturity of the immune system may be critical in the development of clinical disease, which occurs primarily between the ages of 15 and 40 years in males. Many other examples exist in animal models of arthritis, including arthritis induced by mycobacteria and streptococci. Studies of the gastrointestinal and oral microbiome (the latter particularly in periodontal disease) have identified dysbiosis associated with early rheumatoid arthritis. Specific microbes (e.g., Porphyromonas gingivalis , Prevotella copri, Aggregatibacter actinomycetemcomitans ) have been implicated. Whether these associations represent molecular mimicry, altered immune homeostasis (the gastrointestinal tract is a vital area for maintenance of peripheral immunologic tolerance), or direct stimulation of immune function (e.g., via enhanced citrullination) remains under investigation.
The use of oral contraceptives has been associated with a decrease in the incidence of rheumatoid arthritis. Because the effect appears to be strongest for oral contraceptives that have high estrogen content, it is postulated that estrogen is responsible for this protective effect.
The overarching pathogenesis of rheumatoid arthritis remains unknown. The earliest signs of inflammation often occur in the lung: abnormal high-resolution CT scans of pre-rheumatoid arthritis patients have been reported. Pulmonary mucosal citrullination is increased, perhaps reflecting smoking or other pulmonary irritants. Increased local inflammation supports initial B-cell and then T-cell activation, with consequent “breach of tolerance” to post-translationally modified self proteins. Similar events in the oral mucosa, in periodontal disease, or conceivably in the gastrointestinal tract may also contribute in some patient subgroups. The physico-chemical properties of these self-protein derived peptides that confer increased immune activation presumably arise within the genetic architecture described above and especially the structure of the shared epitope. A prearticular clinical phase (pre-rheumatoid arthritis) ensues and may persist for up to several years, during which rheumatoid factor and autoantibodies against post-translationally modified proteins (AMPA), particularly anti-citrullinated peptide antibodies (ACPA), rise in titer and with increasingly broad specificity (epitope spreading). ACPAs, for example, can recognize citrullinated residues on a variety of self-proteins (e.g., type II collagen, vimentin, α-enolase, fibronectin, fibrinogen, and histones). The abnormalities thus appear to represent a broad failure to regulate immune homeostasis, rather than one individual (auto)antigen driving disease overall. One autoantigen may, however, serve as a trigger. In parallel, elevated levels of serum cytokines and chemokines are detected, indicative of generalized increased levels of systemic inflammation, along with lipid dysmetabolism. Biopsies of synovial tissue during this pre-rheumatoid arthritis phase are essentially normal, consistent with the systemic nature of this disease phase.
Thereafter, a transitional event heralds the onset of clinically detectable arthritis. Systemic disease becomes localized to the joint. ACPA specificities are broadest (they expand before the onset of rheumatoid arthritis), and cytokine concentrations are highest immediately before the onset of disease (imminent rheumatoid arthritis). Altered ACPA glycosylation also precedes clinical disease. Early changes in synovial vascularization, deposition of immune complexes, altered neurologic supply, local infection, and microtrauma have all been postulated as articular localizing mechanisms.
Established rheumatoid arthritis is associated with the development of a dense synovial cellular infiltrate that has some degree of organization ( E-Fig. 243-2 ). A lining layer of 4 to 8 cells in depth includes macrophages and fibroblast-like synoviocytes. The interstitial area contains large numbers of T cells, B cells, plasma cells, mast cells, and fibroblast-like synoviocytes. In some patients, lymphocytic aggregates form (ectopic germinal centers), likely serve as a source of autoantibody production, and confer a poorer clinical prognosis. Biopsy studies have identified discrete synovial appearances across patient subgroups; myeloid, lymphocytic, and fibroblastic pathotypes have been described at the transcriptional and histologic level. These pathotypes may represent clinically discrete endotypes that, in the future, could define useful biomarkers to determine optimal choices of immune targeted therapies.
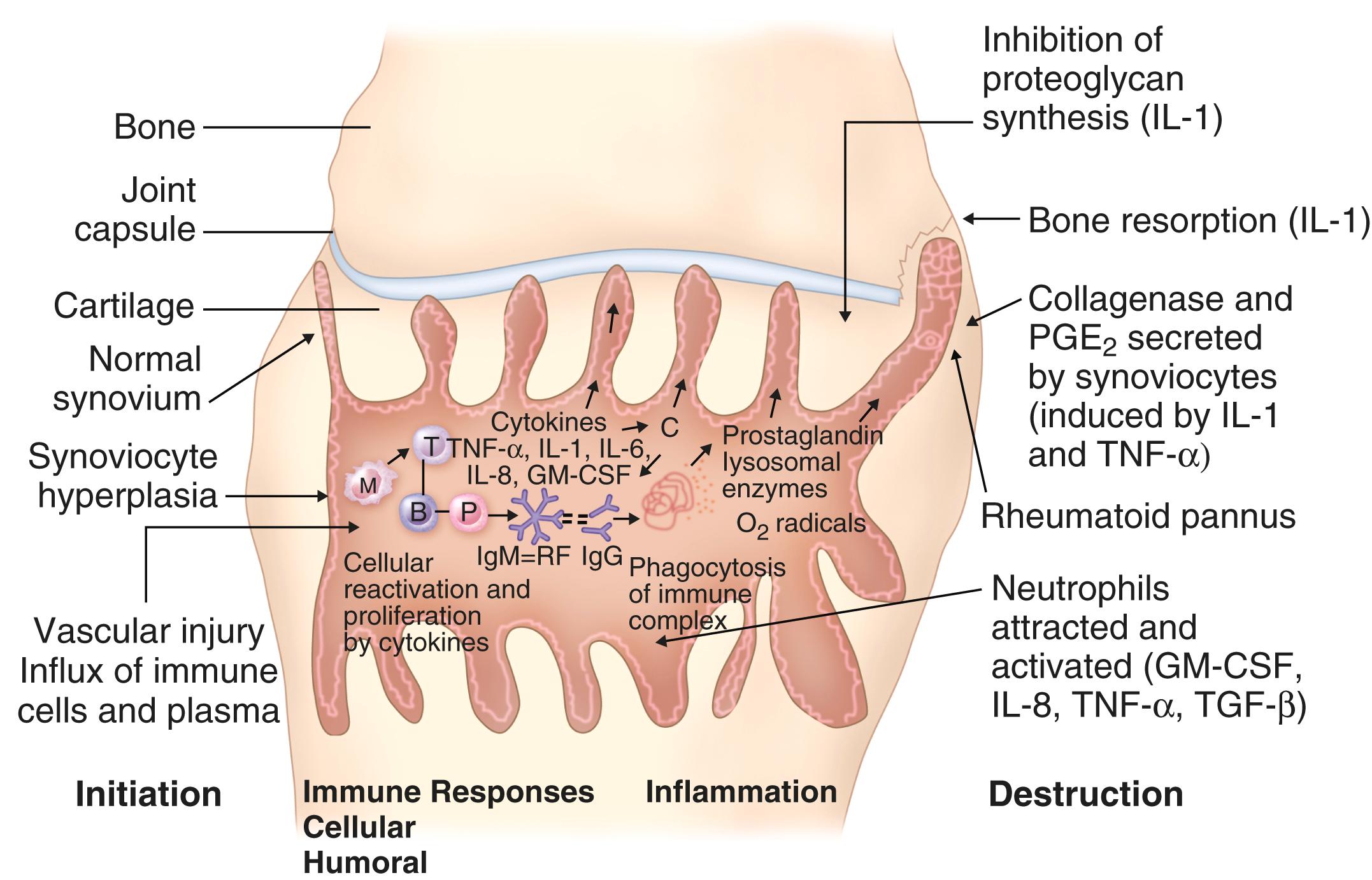
The synovial T-cell response is primarily of T H 1 and T H 17 type. T cells are activated via antigen presented in a CD28/CD80/86 dependent manner by macrophages, B cells, fibroblast-like synoviocytes, the local cytokine milieu (e.g., IL-7, IL-15, TNF, IL-6), or cognate cellular activation through cell contact with macrophages. The T cells secrete cytokines (e.g., IL-17 and GM-CSF) that drive further synovial proliferation. The predominant source of synovial cytokines is, however, macrophages, mast cells, and fibroblast-like synoviocytes. Macrophage-derived cytokines, particularly interleukin-1 (IL-1), IL-6, and tumor necrosis factor-α (TNF-α), play central roles in this ongoing inflammatory process. TNF and IL-6 in particular appear to have hierarchical functional dominance, as reflected in successful therapeutic targeting (e.g., TNF inhibitors, tocilizumab, and JAK inhibitors).
The humoral immune system also plays a role, as reflected in the success of rituximab therapy. Rheumatoid factor and the presence of ACPAs correlate with more severe disease, including erosions of bone, and with the presence of extra-articular features. Rheumatoid factor, which is an antibody that recognizes the Fc portion of immunoglobulin G as its antigen, and ACPAs likely have a pathologic role. Via immune complex formation or perhaps functioning in isolation, these factors increase complement activation, promote macrophage activation to release lysosomal enzymes, kinins, prostanoids, and oxygen/nitrogen free radicals via Fc receptor (FcR) binding and may activate osteoclasts. ACPAs also activate macrophages via TLR/FcR cross-talk mediated via citrullinated fibronectin.
Articular damage is driven primarily by fibroblast-like synoviocytes and osteoclasts. Fibroblast-like synoviocytes are partially transformed cells that have a distinct epigenetic profile and that exhibit anchorage independence, loss of contact inhibition, low-grade proliferation, and TLR expression, thereby rendering them immunologically competent to sense tissue damage. Single cell sequencing studies have shown functionally distinct subsets of fibroblast-like synoviocytes, some with bone erosive propensity and others with a more inflammatory phenotype. Fibroblast-like synoviocyte subsets release prostanoids, cytokines, chemokines, and matrix metalloproteinases (MMPs) such as MMP1, MMP3, and MMP13, in disproportion to the release of tissue inhibitors of MMPs (TIMPs). In consequence, fibroblast-like synoviocytes promote cartilage damage; this local tissue damage area is known as the cartilage-pannus junction . Macrophages and mast cells are also likely to contribute to this local process. Subsets of macrophages can be defined by single cell sequencing that clearly differentiates synovitis in active disease as compared with remission. Bone damage requires cells with capacity to acidify the local milieu—osteoclast maturation and activation is a localized feature of rheumatoid arthritis synovium, arising as a consequence of RANKL, IL-1, TNF, and IL-17 activity. Thus activated osteoclasts localize in periarticular bone and in the adjacent bone marrow, thereby leading to the characteristic erosions detected on plain radiography. The inflammatory process underlying erosion is detected as “bone edema” on MRI. Synovitis thus results in the destruction of cartilage in marginal bone and in the stretching or rupture of the joint capsule or tendons and ligaments. Damage is directly related to disability.
Finally, generalized inflammation promotes systemic comorbidities. Circulating cytokines and immune complexes are proposed to activate endothelium and accelerate atherosclerosis, may provoke systemic osteoporosis, and can drive cognitive impairment, fatigue, and frank psychiatric presentations (e.g., depression). Inhibition of such processes should reduce comorbidities in the clinic.
Although the presentation is variable, most patients with rheumatoid arthritis exhibit insidious onset of pain, stiffness, or swelling in multiple small joints over the course of weeks to months. Systemic features such as fatigue, low-grade fevers, and weight loss also may be present. Less commonly, the onset can be fulminant, occurring almost overnight, or patients may exhibit persistent monoarthritis or oligoarthritis for prolonged periods before manifesting the more typical pattern of joint involvement. Rarely, particularly men develop extra-articular features of rheumatoid arthritis, especially lung disease, before the joint problems appear. Periodic flares of disease are common and may be associated with an increase in circulating pre-inflammatory mesenchymal cells. ,
Morning stiffness is a hallmark of inflammatory arthritis and is a prominent feature of rheumatoid arthritis. Patients are characteristically at their worst in the morning or after prolonged periods of rest. This stiffness in and around joints often persists for hours, and quantifying it is one way to measure impact of an intervention.
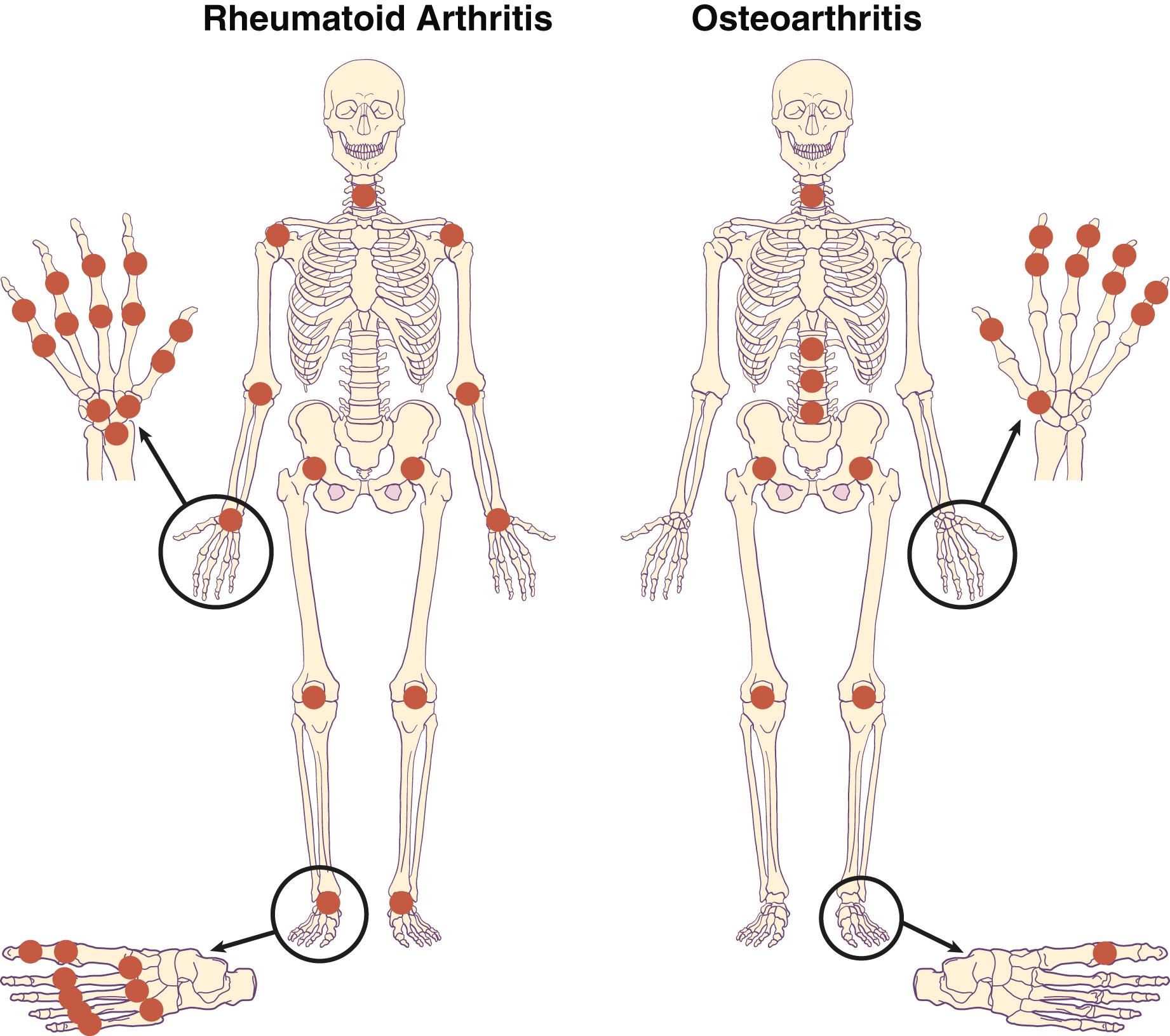
Rheumatoid arthritis can affect any of the synovial (diarthrodial) joints ( Fig. 243-1 ). Most commonly, clinically apparent disease starts in the metacarpophalangeal (MCP), proximal interphalangeal (PIP), and metatarsophalangeal (MTP) joints, followed by the wrists, knees, elbows, ankles, hips, and shoulders, in roughly that order. Early treatment limits the extent of joints involved. Less commonly, and usually later, rheumatoid arthritis may involve the temporomandibular, cricoarytenoid, and sternoclavicular joints. Rheumatoid arthritis may involve the upper part of the cervical spine, particularly the C1-C2 articulation ( Fig. 243-2 ), but, unlike the spondyloarthropathies ( Chapter 244 ), it rarely involves the remainder of the spine. Patients are also at an increased risk for osteoporosis ( Chapter 225 ), and this risk should be considered and dealt with early in both women and men.
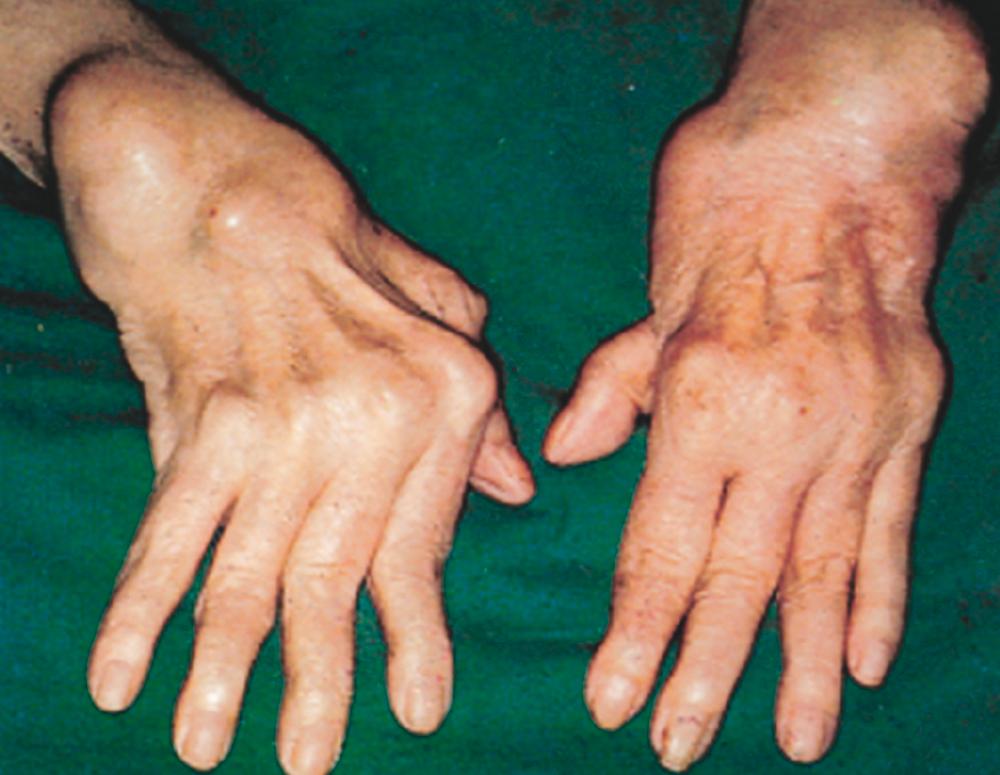
A significant proportion of the disability caused by rheumatoid arthritis is due to damage and dysfunction of the hands. Typically disease starts with swelling of the PIPs and MCPs. The distal interphalangeal (DIP) joints are almost never involved; significant involvement of the DIP joints should suggest the possibility of a different diagnosis (e.g., osteoarthritis or psoriatic arthritis). Manifestations that can be seen late in the disease but are rarely seen in treated patients include the classic ulnar deviation of the MCP joints ( Fig. 243-3 ), and swan-neck deformities (hyperextension of the PIP joints). Boutonnière (or buttonhole) deformities also occur as a result of hyperflexion of the PIP joints. If the clinical disease remains active, hand function deteriorates. Sudden loss of function of individual fingers may occur as a result of tendon rupture.
Feet, particularly the MTP joints, are involved early in most patients and are often an initial site of radiographic erosion. Subluxation of the toes is common and leads to the dual problem of breakdown of the skin and ulcers on the top of the toes and malalignment of the MTP heads. Painful ambulation is caused by dislocation of the cushioning pads that usually protect the heads of the MTP joints.
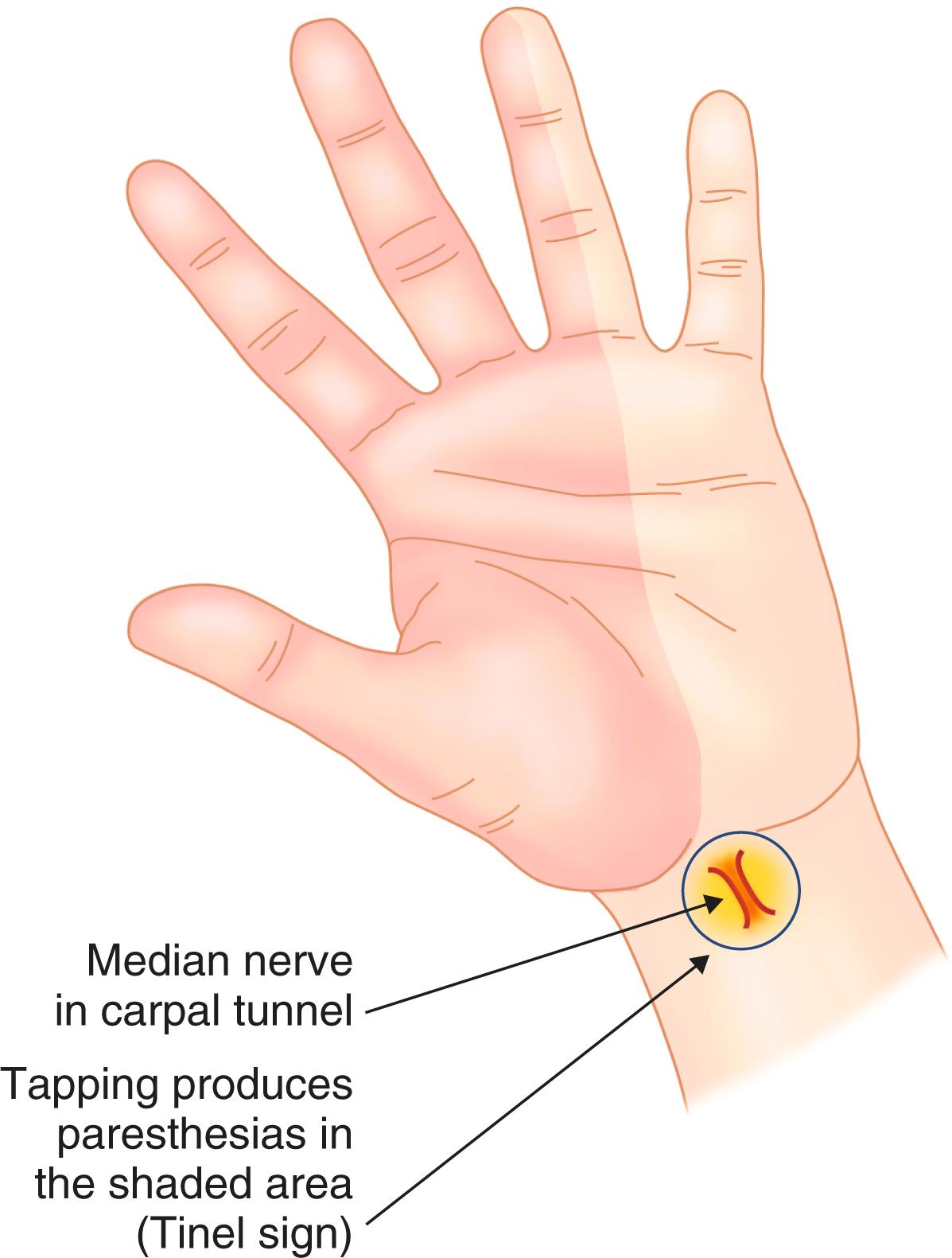
The wrist joints are involved in most patients with rheumatoid arthritis. Radial deviation is the rule, and patients with severe involvement may progress to volar subluxation. Even early in the course of the disease, synovial proliferation in and around the wrists may compress the median nerve, thereby causing carpal tunnel syndrome ( Fig. 243-4 ). Later, this synovial proliferation may invade tendons and lead to rupture, most commonly of extensor tendons.
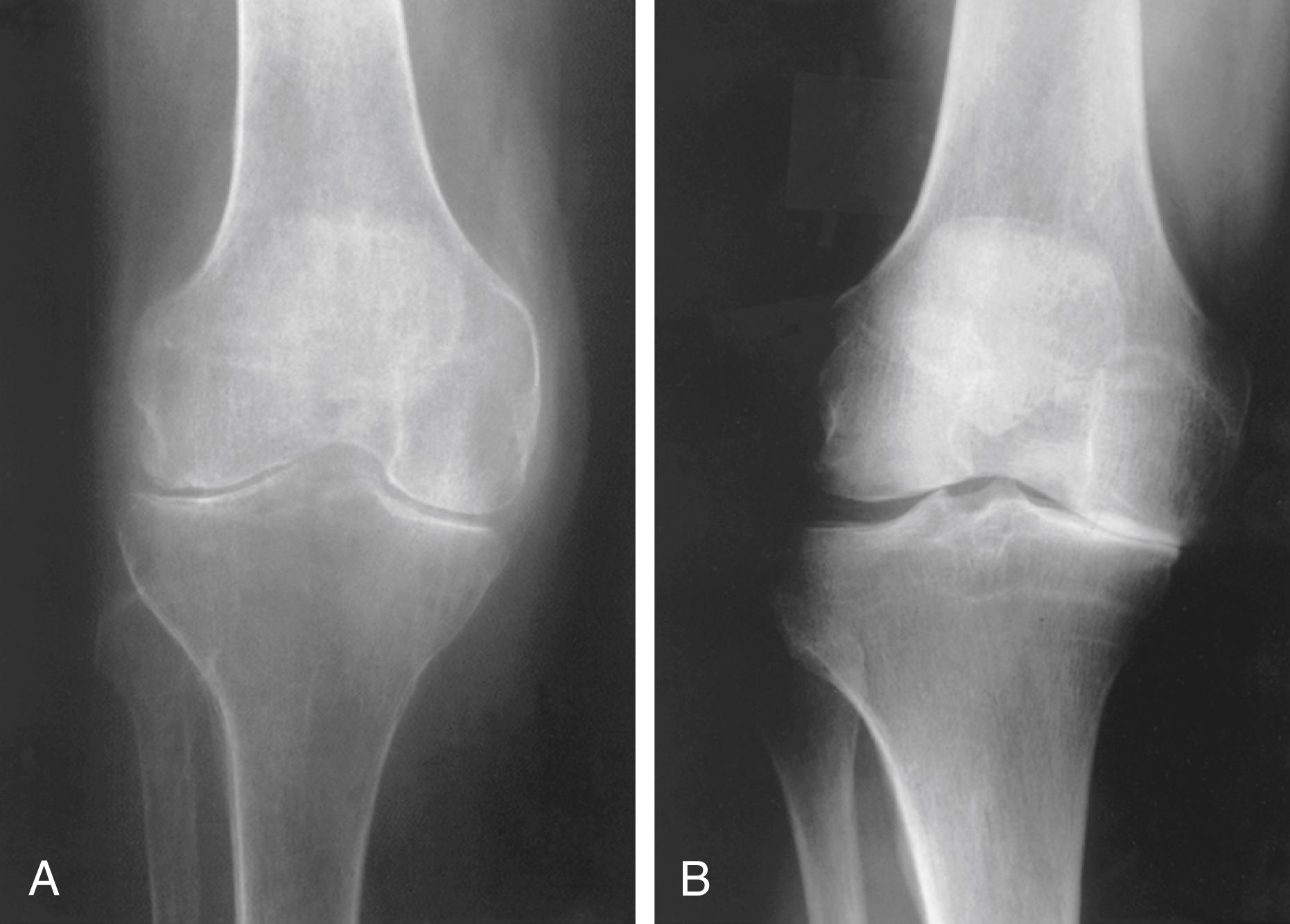
Involvement of knees, ankles, elbows, hips, and shoulders is common. Characteristically, the whole joint surface is involved in a symmetrical fashion. Rheumatoid arthritis is symmetrical, not only from one side of the body to the other but also within the individual joint. In the knee ( Fig. 243-5A ), both the medial and lateral compartments may be narrowed. In contrast, in patients with osteoarthritis ( Fig. 243-5B ), only one compartment of the knee may be involved.
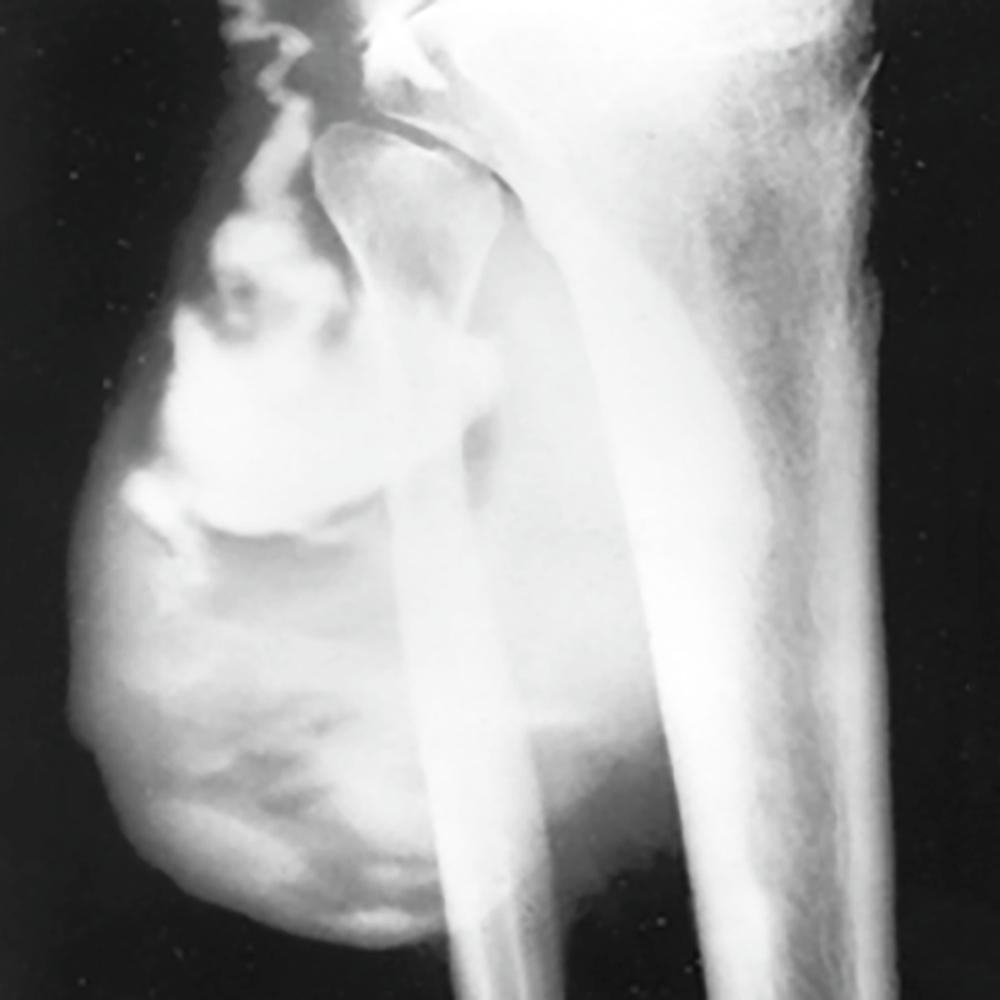
Synovial cysts, which may occur around any of the joints (large or small), occasionally manifest as soft, fluctuant masses that present diagnostic challenges. When the knee produces excess synovial fluid, it may accumulate in the popliteal fossa (popliteal or Baker cyst) ( E-Fig. 243-3 ). These cysts can compress the popliteal nerve, artery, or veins. Baker cysts may dissect into the tissues of the calf (usually posteriorly), or they may rupture. Dissection may produce only minor symptoms, such as a feeling of fullness; rupture of the cyst with extravasation of its inflammatory content produces significant pain and swelling and may be confused with thrombophlebitis, the so-called pseudothrombophlebitis syndrome ( Chapter 68 ).
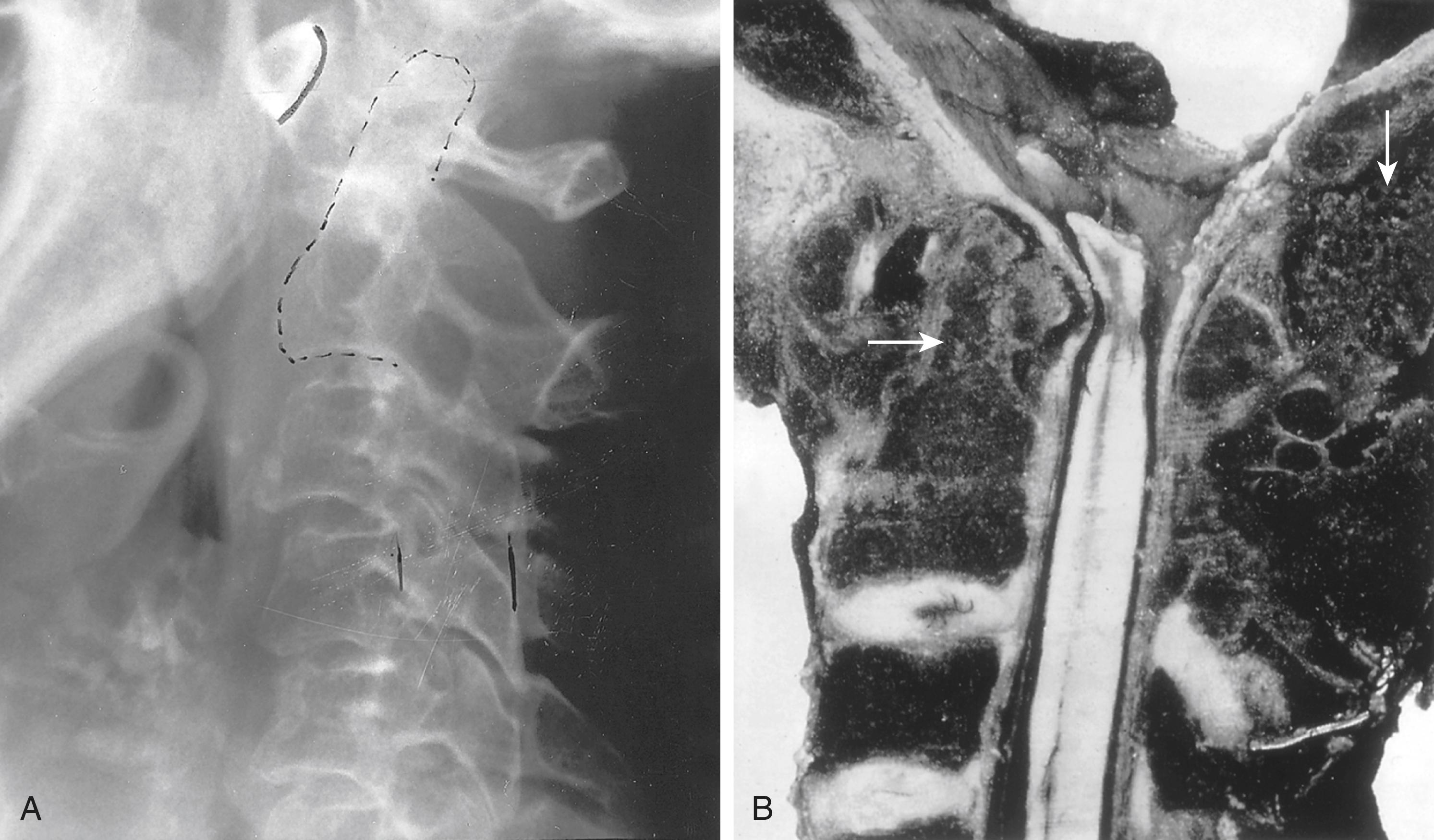
Although most of the axial skeleton is spared in rheumatoid arthritis, the cervical spine is commonly involved, particularly the C1-C2 articulation. Bony erosions and ligament damage can occur in this area and may lead to subluxation (see Fig. 243-2 ). Most often, subluxation at C1-C2 is minor and without accompanying symptoms; patients and caregivers need only be cautious and avoid actively forcing the neck into positions of flexion. Occasionally, subluxation at C1-C2 is severe and leads to compromise of the cervical cord with neurologic symptoms and even death.
Other joints that may be involved include the temporomandibular, cricoarytenoid, and sternoclavicular joints. The cricoarytenoid joint is responsible for abduction and adduction of the vocal cords, and involvement of this joint may lead to a feeling of fullness in the throat, to hoarseness, and, rarely when the cords are essentially fused in a closed position, acute respiratory distress with or without stridor.
Systemic features of rheumatoid arthritis such as fatigue, weight loss, and low-grade fevers occur frequently. All the other extra-articular features are more common in patients who are positive for rheumatoid factor, ACPA antibodies, or both ( Table 243-1 ).
| Skin | Nodules, fragility, vasculitis, pyoderma gangrenosum |
| Heart | Pericarditis, premature atherosclerosis, vasculitis, valve disease, and valve ring nodules |
| Lung | Pleural effusions, interstitial lung disease, bronchiolitis obliterans, rheumatoid nodules, vasculitis |
| Eye | Keratoconjunctivitis sicca, episcleritis, scleritis, scleromalacia perforans, peripheral ulcerative keratopathy |
| Neurologic | Entrapment neuropathy, cervical myelopathy, mononeuritis multiplex (vasculitis), peripheral neuropathy |
| Hematopoietic | Anemia, thrombocytosis, lymphadenopathy, Felty syndrome, large granular lymphocyte syndrome |
| Kidney | Amyloidosis, vasculitis |
| Bone | Osteopenia |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here