Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Exfoliative cytology was first used to study cells of the respiratory tract in 1845. The ability to diagnose pulmonary diseases cytologically was appreciated as early as 1919, but it was not until the 1950s and 1960s that pulmonary cytology came into its own as a diagnostic discipline. Its emergence was bolstered by the introduction of direct sampling methods via bronchoscopy and fine-needle aspiration (FNA), resulting in an impressive armamentarium of sampling techniques. Since then, the improved sensitivity (and common application) of thoracic imaging has created an ever-increasing need for the cytologic evaluation of pulmonary lesions.
The respiratory tract can be categorized into upper and lower compartments. The upper airway extends from the sinonasal region to the larynx. The lower respiratory tract, which is the major focus of diagnostic respiratory cytopathology, extends from the trachea to the lungs. The tracheobronchial tree divides into progressively smaller units: bronchi, bronchioles, and respiratory acini.
Upper respiratory tract:
Ciliated columnar cells
Squamous cells
Lower respiratory tract:
Trachea and bronchi
Ciliated columnar cells
Goblet cells
Basal/reserve cells
Neuroendocrine cells
Terminal bronchioles
Nonciliated cuboidal/columnar cells (club [previously “Clara”] cells)
Alveoli
Type I and II pneumocytes
Alveolar macrophages
The trachea and bronchi are lined by a pseudostratified epithelium. The predominant cell is the ciliated columnar cell , which has a basally placed nucleus with finely textured chromatin. The luminal surface has a thick terminal bar with cilia ( Fig. 2.1 ). Goblet cells , present in a ratio of approximately one per six ciliated cells, also have a basally located nucleus but lack cilia, and their cytoplasm is distended by mucus. Goblet cells secrete mucus, whereas ciliated cells move the mucus and entrapped contaminants up the airway. Adjacent to the basement membrane are basal or reserve cells : small, undifferentiated cells that are the presumed forerunners of the ciliated and goblet cells . Neuroendocrine cells , or Kulchitsky cells, are also present in the respiratory epithelium, but they are identified only with special stains or ultrastructural examination: they are argyrophil-positive and possess dense-core granules.
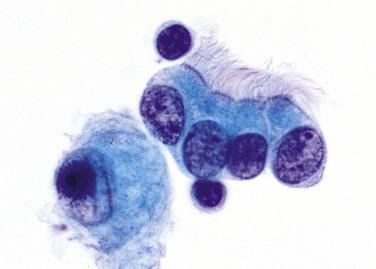
The terminal bronchioles are lined by nonciliated cuboidal to columnar cells called club cells or respiratory exocrine cells (previously called Clara cells ); they are not sufficiently distinctive with routine cytologic preparations and thus not specifically identified. The alveolar lining consists of type I and type II pneumocytes . Type I pneumocytes, which are more numerous, are paper thin and cover the gas exchange portion of the alveolar surface. The type II pneumocyte is more conspicuous: plump and cuboidal rather than flat. It secretes pulmonary surfactant, seen ultrastructurally as osmiophilic lamellar bodies. After lung injury, these cells function as reserve cells for the delicate type I pneumocyte. On cytologic preparations, type II pneumocytes are round and have vacuolated cytoplasm; they can be difficult to distinguish from macrophages.
Alveolar (pulmonary) macrophages vary in appearance depending on the amount and type of phagocytosed cytoplasmic material. In general, they have one or more round to oval nuclei and lacy or bubbly cytoplasm, often with small black particles from inhaled pollutants (“dust cells,” Fig. 2.2A ). After pulmonary hemorrhage, alveolar macrophages contain hemosiderin pigment, which is golden-brown rather than black. Numerous alveolar macrophages must be present for a sputum sample to be judged adequate.
Under normal circumstances, a few white blood cells, such as neutrophils and lymphocytes, are also found within the alveolar compartment. An increased number of inflammatory cells is abnormal: abundant neutrophils indicate an acute pneumonia, and numerous lymphocytes are usually associated with chronic inflammation.
Familiarity with the variety of sampling and preparation methods is crucial for cytologic interpretation because cytomorphology is different depending on the sampling and preparation method. The accuracy of respiratory cytology also varies depending on the specimen type.
As with other nongynecologic cytology specimens, respiratory tract diagnoses are typically reported as “negative for malignant cells,” “positive for malignant cells,” or “nondiagnostic (unsatisfactory),” each of which is associated with a different risk of malignancy. Inconclusive findings are commonly reported as “atypical cells present” (connoting a low degree of suspicion) or “suspicious for malignancy” (connoting a high degree of suspicion). Cancer is confirmed in 54% to 59% of “atypical” respiratory specimens and in 82% to 90% of those reported as “suspicious.” Atypical/suspicious cases usually remain inconclusive even after careful retrospective reexamination, which fails to reveal any morphologic features to reliably distinguish benign from malignant specimens.
Sputum consists of a mixture of cellular and noncellular elements that are cleared by the mucociliary apparatus. It was once the most common respiratory tract specimen because it is relatively easy to obtain, with little discomfort to the patient. Sputum cytology is generally reserved for symptomatic individuals; as a screening test (e.g., in symptom-free smokers), sputum cytology is not effective in decreasing rates of death from lung cancer. With the advent of bronchoscopy and FNA, its use as the mainstay in respiratory cytology has declined significantly.
Collecting multiple sputum samples over several days optimizes sensitivity. Early morning, deep cough specimens are preferred. If the patient is not able to expectorate adequately, expectoration can be induced by having the patient inhale nebulized water or saline solution. Sputum induction increases the detection of lung cancer. When prompt preparation of sputum is not possible, the patient can expectorate into a 70% ethanol solution, which prefixes the specimen.
A simple method of sputum preparation is known as the “pick and smear” technique, whereby fresh sputum is examined for tissue fragments, blood, or both. Smears are prepared from areas that contain these elements and immediately fixed in 95% ethanol. A modification of this is the Saccomanno method, which calls for sputum to be collected in 50% ethanol and 2% carbowax ; it must be performed in a biologic safety hood because of the risks of infection from aerosolization. The specimen is then homogenized in a blender and concentrated by centrifugation. Improved sensitivity has also been demonstrated by the use of dithiothreitol, N-acetyl-L-Cysteine, or CytoRich Red for mucolysis and homogenization. Smears are made from the concentrated cellular material. Sputum can also be processed using thin-layer methods or embedded in paraffin for cell block sections.
The adequacy of a sputum sample is established by finding numerous pulmonary macrophages. Specimens consisting merely of squamous cells, bacteria, and Candida organisms are unsatisfactory because they represent only oral contents. Even ciliated cells, which also line the sinonasal passages, do not guarantee that a sample is from the lower respiratory tract. The presence of numerous macrophages indicates that a satisfactory, deep cough specimen of the lower respiratory tract has been obtained. In an adequate sample they should not be difficult to find: if they are absent or few in number, the sample should be reported as unsatisfactory.
The sensitivity of sputum cytology for the diagnosis of malignancy increases with the number of specimens examined, from 42% with a single specimen to 91% with five specimens. The specificity of sputum examination is high, ranging from 96% to 99%, and the positive and negative predictive values are 100% and 15%, respectively. Thus negative sputum results do not guarantee the absence of a malignancy, especially in a patient suspected of having lung cancer. The sensitivity of sputum cytology depends also on the location of the malignant tumor: 46% to 77% for central lung cancers but only 31% to 47% for peripheral cancers. Sensitivity is independent of tumor stage and histologic type. Accuracy in tumor classification is 75% to 80% and is tumor-type dependent.
A pivotal improvement in sampling the lower respiratory tract occurred with the development of the flexible bronchoscope in the late 1960s. Today, any part of the respiratory mucosa can be sampled with this device.
Complications of bronchoscopy are rare (0.5% and 0.8% for major and minor complications, respectively) and include laryngospasm, bronchospasm, disturbances of cardiac conduction, seizures, hypoxia, and sepsis. The incidence of major complications is higher for transbronchial biopsy (6.8%).
Bronchial secretions can be aspirated directly from the lower respiratory tract through the bronchoscope, but an alternative (and more common) method is to “wash” the mucosa by instilling 3 to 10 mL of saline solution and suctioning the washings. The fluid is spun in a centrifuge, and the concentrate is used to make smears, thin-layer preparations, or cell blocks; the latter are particularly useful when special stains are needed.
Fiberoptic bronchoscopy allows direct visualization and sampling of the tracheobronchial tree. A brush is applied to the surface of an endobronchial lesion, and the entrapped cells are either smeared onto a glass slide or rinsed in a collection medium for thin-layer or cell block preparation or both. If smears are made, immediate fixation (by immersion into 95% ethanol or by spray fixation) of the smears is essential to preserve morphologic detail.
The diagnostic accuracy of bronchial washing/brushing cytology is comparable to that of bronchial biopsy. Brushings with cell block preparation sometimes detect malignancy more reliably than bronchial biopsy. Accuracy improves when clinical history is provided with the specimen. The diagnostic yield also improves when several different sampling methods are used in concert.
The choice between bronchoalveolar lavage (BAL) and bronchial washing depends on the location of the airway one desires to sample. With BAL, the bronchoscope is wedged into position as far as it will go to sample the distal airways, which are flushed with sterile saline solution.
BAL is particularly useful for the diagnosis of opportunistic infections in immunocompromised patients. The specimen can be examined cytologically and a portion also submitted for microbiologic studies. The distinction between oral contamination and a real bacterial infection can be difficult, but an abundance of normal squamous cells usually indicates contamination by oral flora, whereas neutrophils imply a real infection. In immunocompromised patients, the diagnostic yield for infectious pathogens is 39%, the sensitivity 82%, and the specificity 53%. In patients with acquired immunodeficiency syndrome, BAL has a sensitivity for documenting infection comparable to that for transbronchial biopsy (86%); when used in combination with biopsy, sensitivity increases to 98%. Historically, the most common pulmonary pathogens detected by BAL in human immunodeficiency virus (HIV)–seropositive individuals were Pneumocystis carinii (78%) and bacteria (19%); the remainder were Mycobacterium tuberculosis , atypical mycobacteria, Histoplasma , and Cryptococcus . The frequency and distribution of infections has changed since the widespread use of highly active antiretroviral therapy to treat HIV. Among HIV-seropositive individuals with nonspecific cytologic results, 27% prove to have pathogens, usually bacterial or fungal, by either culture or biopsy, which emphasizes the importance of a multimodal approach to diagnosis in this setting.
BAL is also used for the diagnosis of malignancy, with a sensitivity that ranges from 29% to 69%. The sensitivity of BAL for detecting malignancy is higher for multifocal or diffuse tumors, and lower in peripheral tumors that cannot be seen bronchoscopically. Liquid-based cytology preparations yield a slightly higher sensitivity. False-positive results are occasionally encountered due to atypical type II pneumocytes in the setting of pneumonia, diffuse alveolar damage, bone marrow transplantation, and chemotherapy.
Transbronchial FNA is especially useful for pulmonary lesions hidden beneath the bronchial surface and for suspicious mediastinal lymph nodes. Transbronchial FNA eliminates the need for an addition surgical procedure in 20% of patients, at one-third the cost of mediastinoscopy. The lesion is aspirated with a retractable (Wang) needle passed through a flexible catheter that is sent down the bronchoscope.
At least a moderate number of lymphocytes must be present to ensure the adequacy of lymph node sampling and avoid a false-negative result. Ciliated respiratory epithelial cells are common contaminants because the respiratory mucosa needs to be breached to reach the target. For this reason, ciliated cells should not be taken as evidence of adequate sampling of a mediastinal lymph node. Complications from transbronchial aspiration are rare and include endobronchial bleeding, which is usually controlled by suctioning. Contraindications are coagulopathy, respiratory failure, and uncontrollable coughing.
Transbronchial FNA augments the diagnostic accuracy of bronchial washings, brushings, and endoscopic biopsy for the detection of primary pulmonary neoplasms. The sensitivity of transbronchial FNA by itself is 56% but increases to 72% when combined with bronchial brushing, washing, and biopsy. Specificity is 74%, and the positive and negative predictive values are 100% and 53% to 70%, respectively.
Transbronchial FNA is accurate in distinguishing small cell from non-small cell lung cancer. For mediastinal staging of bronchogenic carcinoma, the negative predictive value of transbronchial FNA increases from 36% to 78% when negative specimens without sufficient lymphocytes are regarded as unsatisfactory for evaluation. The most common cause of false-negative results is sampling error.
The accuracy of mediastinal staging by FNA improves with the use of ultrasound guidance. EBUS FNA is an enhanced procedure for sampling mediastinal and paratracheal lymph nodes and peribronchial lung or mediastinal lesions that is increasingly used in the United States. Its primary indication is nodal staging of non-small cell lung cancer, but it is also indicated for sarcoidosis and metastases from extrapulmonary primaries. This minimally invasive procedure is a safer alternative to cervical mediastinoscopy in selected patients. Using a bronchoscope equipped with an ultrasound probe tip, the operator performs an FNA with real-time ultrasound imaging of a lymph node or central lung mass. Sensitivity and specificity are 88% and 100%, respectively. To optimize sensitivity, a cytologist can assess the sample on site for adequacy. In the absence of lesional cells, adequacy is defined as the presence of lymphocytes (if a lymph node is being sampled) or pigmented macrophages (in the case of a lung mass). Some laboratories have developed and adopted quantitative adequacy criteria for mediastinal lymph node sampling (presence of anthracotic pigment, germinal center fragments, a minimum number of lymphocytes), but no single set of criteria has yet been universally adopted. As with transbronchial (Wang needle) FNA, ciliated respiratory epithelial cells are common contaminants and are not evidence of adequate sampling. Microscopic metallic dust from the needle ( Fig. 2.2B ) mimics anthracotic pigment. It can be recognized as a contaminant because of its haphazard distribution. The advantage of EBUS is that it increases accessibility to lower station lymph nodes: transbronchial (Wang needle) FNA can sample lymph node stations 2 to 4 and 7, and transesophageal ultrasound–guided FNA can reach stations 2 to 4 and 7 to 9, whereas EBUS can sample stations 2 to 4, 7, and 10 to 12. Thus esophageal FNA is often combined with EBUS for full accessibility.
Although the EBUS FNA procedure itself is more expensive than transbronchial (Wang) FNA, it saves downstream costs because of its greater sensitivity and the reduced need for more surgical staging, and it has largely supplanted Wang biopsy in many practices.
EBUS FNA may result in biopsy site changes, including intranodal or perinodal fibrosis and displacement of airway cartilage into lymph nodes. These artifacts, seen on subsequent lymph node excisions, should not be misconstrued as tumor-related changes.
Mediastinal lymph node sampling can also be done endoscopically by passing the needle through the esophagus. The addition of ultrasound guidance improves the accuracy of mediastinal lymph node sampling. Like bronchoscopic FNA, endoscopic FNA realizes significant cost savings and reduces the number of unnecessary thoracotomies. The advantages of endoscopic transesophageal FNA include improved image quality and the ability to sample stations 8 and 9, which are not accessible via EBUS FNA, but the primary lesion usually cannot be sampled via this route.
Endoscopic transesophageal FNA, when used in combination with transbronchial FNA, improves the diagnostic yield for mediastinal staging greatly, with a sensitivity of 93% and negative predictive value of 97%. As with transbronchial FNA, a moderate number of lymphocytes must be present to ensure the adequacy of a lymph node specimen and avoid a false-negative result.
The ease, rapidity of diagnosis, and minimal morbidity of percutaneous FNA make it an attractive alternative to surgical biopsy in the evaluation of the patient with a peripheral pulmonary mass. FNA is of greatest benefit to patients for whom it spares a more invasive surgical procedure. Surgical intervention, in fact, can be avoided in up to 50% of patients with clinically suspected lung cancer. There are some contraindications, however.
Chronic obstructive pulmonary disease
Emphysema
Uncontrollable coughing
Uncooperative patient
Bleeding diathesis (e.g., anticoagulant therapy)
Severe pulmonary hypertension
Arteriovenous malformation
Cardiac disease
Suspected echinococcal cyst
The most common complication of percutaneous FNA is a pneumothorax. A radiographically detectable pneumothorax occurs in 19% of patients ; only 4%, however, require intercostal drainage tubes. The risk of a pneumothorax increases with larger needle size and smaller target lesions and decreases if the needle path does not traverse aerated lung. Pulmonary hemorrhage occurs in 6% of patients, and transient hemoptysis occurs in 2%. Other complications are rare and include hemopericardium, hemothorax, air embolism, tumor seeding, and death.
Percutaneous FNA is usually performed by a radiologist using computed tomography or ultrasound guidance, with the latter best reserved for lesions that abut the pleura or chest wall. The needle gauge ranges from 18 to 25, and many different types of needle devices are available. Although many radiologists prefer 22-gauge Chiba needles, these require repuncture if more than one pass is needed. Another choice is the coaxial needle, with a large-bore outer needle serving as the guide for a small-bore inner needle. Once the outer needle is positioned, more than one aspiration can be performed using the inner needle.
It can be helpful if a cytotechnologist, cytopathologist, or both attend the FNA procedure to assist with specimen handling and assess its adequacy on-site. After smears are prepared, the needle is rinsed in a balanced electrolyte solution, Saccomanno’s fixative, 50% ethanol, or commercial preservative solution. The cellular suspension can be processed as a cytospin, thin-layer preparation, or cell block, and it can be apportioned for flow cytometric analysis if needed. Formalin-fixed cell blocks are ideal for histochemical and immunohistochemical stains. A decision regarding the need, if any, for special studies can be made by the cytotechnologist or cytopathologist after examination of the smears. Percutaneous FNA is a reliable and accurate way to diagnose many pulmonary neoplasms.
Sensitivity: 89%
Specificity: 96%
Positive predictive value: 98%
Negative predictive value: 70%
False-positive rate: 0.85%
False-negative rate: 8%
In a study of more than 13,000 FNA specimens from 436 institutions, the diagnostic sensitivity was 89% for the procedure itself and 99% for the pathologist’s interpretation. This difference indicates that most false-negative results are due to sampling error. The reliability of a negative FNA result is a matter of controversy, given that negative predictive values range from 50% to 91%. About 15% of false-positive diagnoses and 5% of false-negative diagnoses have a significant, permanent, or grave influence on patient outcome. For this reason, most investigators recommend a repeat aspiration or tissue biopsy when a specific benign diagnosis that accounts for the lesion cannot be made with certainty. The performance of small, cutting (“core”) biopsy is similar to that of FNA, but it is performed instead of FNA in some centers.
With regard to the management of patients with primary lung cancer, it is important to discriminate small cell from non-small cell carcinoma, and adenocarcinoma from squamous cell carcinoma. The distinction between small cell and non-small cell carcinoma is possible in more than 95% of cases and between adenocarcinoma and squamous cell carcinoma in 88% of cases diagnosed by FNA.
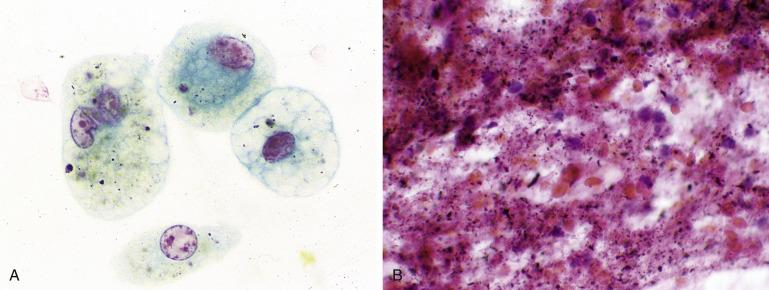
A variety of benign cells occasionally contaminate a percutaneous FNA. Such cells need to be recognized as contaminants and not misconstrued as lesional. In particular, mesothelial cells from the pleura are common, and in some cases they can be numerous ( Fig. 2.3 ). They resemble the cells of a well-differentiated adenocarcinoma (see “Adenocarcinoma”) but are identified as benign mesothelial cells by their relative flatness, cohesion, and the characteristic slit-like “windows” that separate the mesothelial cells from each other.
Mesothelial cells
Cutaneous squamous cells
Skeletal muscle
Fibroconnective tissue
Hepatocytes (trans-diaphragmatic needle path)
If the specimen consists only of one (or more) of these contaminants, it should be interpreted as insufficient for evaluation (nondiagnostic) rather than negative because there is no evidence that the lesion itself has been sampled.
Benign squamous cells from the oral cavity often contaminate sputum and bronchial cytology specimens. Inflammatory conditions of the mouth caused by trauma, candidiasis, or pemphigus can exfoliate mildly atypical squamous cells with hyperkeratinization and nuclear degeneration, usually in small numbers. Such minimal changes should not be misinterpreted as squamous cell carcinoma. More marked (but still benign) squamous cell atypias occur adjacent to cavitary fungal infections and stomas, and with almost any injury to the lung (e.g., infarction, radiation, chemotherapy, sepsis, and diffuse alveolar damage) and might result in a false-positive interpretation of squamous cell carcinoma. Another, uncommon source of false-positive results is malignant cells from head and neck cancers that contaminate sputum and bronchial specimens.
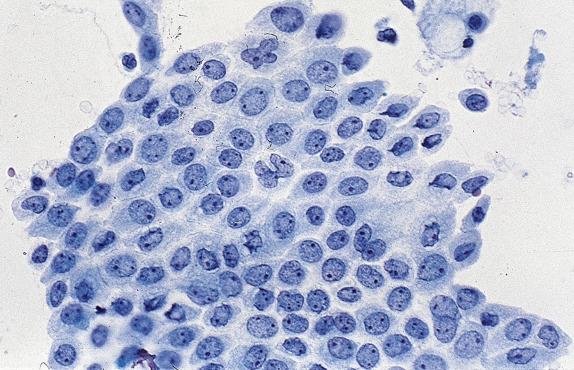
Benign, reactive bronchial cell changes occur in response to noxious stimuli such as radiation, chemotherapy, and severe inflammation. Under such conditions, ciliated columnar cells can increase their nuclear area many times over, with multinucleation, coarsely textured chromatin and large nucleoli ( Fig. 2.4A ). Large clusters of bronchial cells known as Creola bodies (named after the first patient in whom they were recognized) are commonly seen in chronic airway diseases like asthma ( Fig. 2.4B ). Bronchial cells may also undergo squamous metaplasia under conditions of chronic injury (e.g., aspiration, smoking), demonstrating an immature squamous phenotype with mild nucleomegaly and nuclear hyperchromasia.
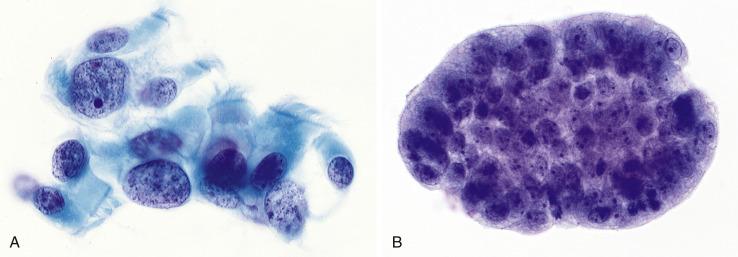
Bronchial cells with markedly reactive changes due to thoracic irradiation or cytotoxic chemotherapy mimic carcinoma ( Fig. 2.5 ). Malignancy can be excluded if the atypical cells have cilia or demonstrate a spectrum of changes (from benign to markedly atypical) rather than the two distinct cell populations typical of a malignant sample obtained bronchoscopically. Note that in sputum and FNA specimens, a helpful dual cell population (malignant cells and bronchial cells) is usually not apparent.
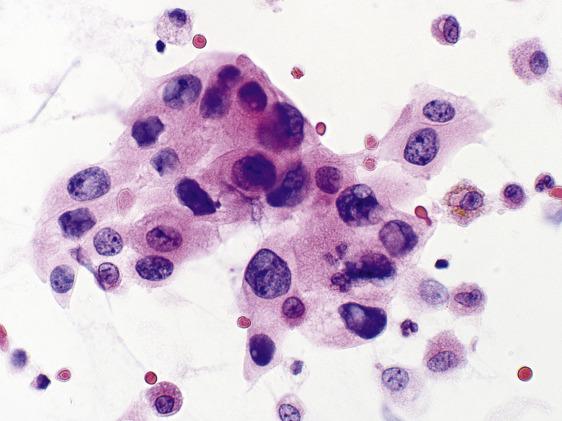
As the surface epithelium of the respiratory tract is shed during lung injury, reserve cells proliferate and are seen in bronchial washings and brushings ( Fig. 2.6 ).
Tightly packed cells
Very small cells
Smudged, dark chromatin
Nuclear molding
Scant cytoplasm
No mitoses or necrosis
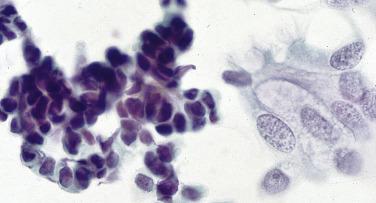
Reserve cell hyperplasia (RCH) is a mimic of small cell carcinoma but is usually easily distinguished from it. Although they can be abundant and may appear as a “second population,” the cells of RCH show greater cohesiveness than small cell carcinoma, are typically smaller (approximately the size of a red blood cell), and show no mitoses or necrosis.
Repair represents re-epithelialization of an ulcer created by trauma, radiation, burns, pulmonary infarction, or infection. Typical (and atypical) repair of the respiratory tract is very similar to that seen in the cervix and gastrointestinal tract.
Flat, cohesive sheets
Abundant cytoplasm
Enlarged, often hypochromatic nuclei
Enlarged nucleoli
Mitoses
Reparative epithelium is most commonly seen in tracheobronchial brushings and washings. The differential diagnosis of repair includes malignancy: the non-small cell lung cancers, metastases, and other, less common tumors. Malignant cells are usually less cohesive and less orderly than reparative epithelium, and they are usually more numerous. Correlation with clinical history can be helpful; a conservative approach is recommended if the findings are not conclusive and there is a history of mucosal trauma or other lung injury.
Because type II pneumocytes function as alveolar reserve cells, they proliferate after lung injury.
Pneumonia
Sepsis (diffuse alveolar damage)
Pulmonary embolus with infarction
Chemotherapeutic and immunotherapeutic drugs
Radiation therapy
Inhalant damage (e.g., oxygen toxicity)
Interstitial lung disease
When floridly hyperplastic, as in diffuse alveolar damage, the cells of type II pneumocyte hyperplasia resemble those of adenocarcinoma ( Fig. 2.7 ).
Isolated cells and three-dimensional clusters
Large nuclei
Coarse chromatin
Prominent nucleoli
Scant to abundant, sometimes vacuolated cytoplasm
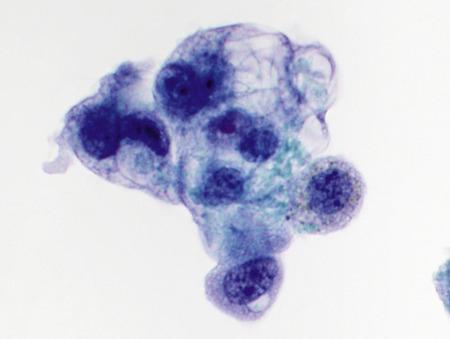
The only clue to avoiding an incorrect diagnosis of malignancy in a patient with type II pneumocyte hyperplasia may be the clinical history of respiratory distress and diffuse infiltrates in the context of an inciting stimulus such as upper respiratory infection, recent radiation, or recent administration of therapeutic drugs. Thus, in an acutely ill patient with diffuse pulmonary infiltrates, markedly atypical cells should be interpreted cautiously, particularly in the setting of an acute inflammatory background. Sequential respiratory specimens can be helpful because hyperplastic pneumocytes are not present in BAL specimens more than 1 month after the onset of acute lung injury.
Noncellular and extraneous elements in respiratory material can be inhaled, produced by the host, formed as a host response to foreign material, or introduced as laboratory contaminants.
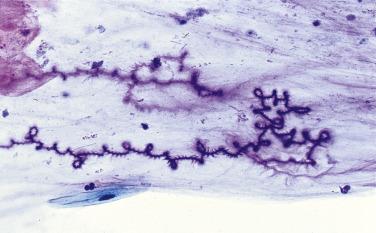
Curschmann’s spirals are coiled strands of mucus that stain purple with the Papanicolaou stain ( Fig. 2.8 ). In the past they have been associated with chronic respiratory diseases, but they are, in fact, a nonspecific finding not worth mentioning in the report.
Ferruginous bodies are mineral fibers encrusted with ferroproteins. Usually dumbbell-shaped, ranging from 5 to 200 μm in length, they stain golden-yellow to black with Papanicolaou stains ( Fig. 2.9 ). Some but not all ferruginous bodies contain a core of asbestos. So-called asbestos bodies are distinguished from other ferruginous bodies by their clubbed ends and thin, straight, lucent core. Asbestos fibers are not visible by light microscopy but are usually much more numerous than asbestos bodies. Patients with known asbestos exposure usually have high numbers of ferruginous bodies in BAL fluid.
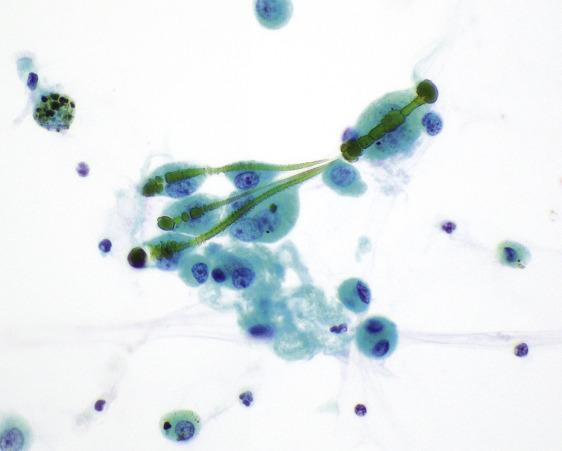
Charcot–Leyden crystals are rhomboid-shaped, orangeophilic structures derived from degenerating eosinophils in patients with severe allergic disorders like asthma or other eosinophilic conditions ( Fig. 2.10 ).
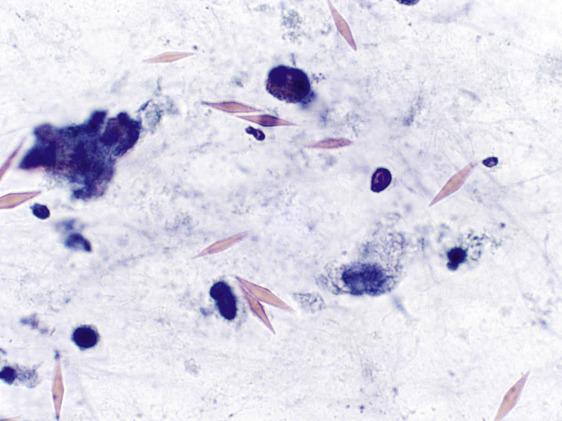
Psammoma bodies are concentrically laminated calcifications seen in malignant tumors that have papillary architecture, such as primary pulmonary adenocarcinoma, mesothelioma, or metastatic thyroid or ovarian cancer. They are also seen in benign conditions such as pulmonary tuberculosis and alveolar microlithiasis.
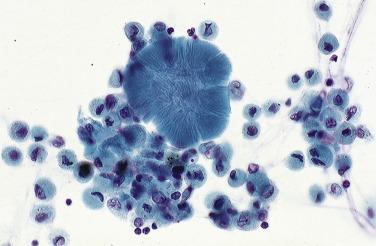
Corpora amylacea are spherical structures with circumferential and radiating lines. They measure between 30 and 200 μm and are indistinguishable from those seen in the prostate. They have no known significance but are more commonly seen in older individuals ( Fig. 2.11 ).
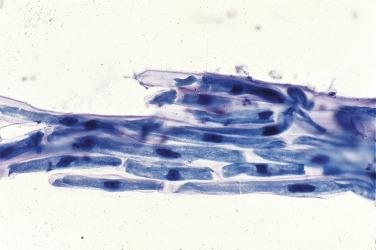
Amorphous protein in respiratory specimens may be a clue to the diagnosis of amyloidosis or alveolar proteinosis. Specimen contaminants include metallic particles (see Fig. 2.2B ), vegetable matter ( Fig. 2.12 ), pollen, and the pigmented fungus Alternaria ( Fig. 2.13A–B ).
Cytology plays an important role in diagnosing infectious diseases, particularly those in immunocompromised patients, and is being used more frequently than ever because of improved sampling methods. It is important to know that conventional inflammatory responses can be much reduced, absent, or greatly altered in patients with immune deficiencies.
Herpes simplex virus (HSV) pharyngitis, laryngotracheitis, and pneumonia most commonly affect immunocompromised patients and neonates, and HSV-1 is the most common serotype to involve the respiratory tract. Ulcerative/necrotizing infections can involve the pharynx, larynx, tracheobronchial tree, or pulmonary parenchyma, and cytopathic changes are identical to those seen in other sites: multinucleation, nuclear molding, chromatin margination, and large nuclear (Cowdry A) inclusions ( Table 2.1 ). The cytopathic changes of HSV are identical to those of herpes zoster. If the cytomorphologic changes are equivocal, the diagnosis can be confirmed by viral culture, immunohistochemistry, or in situ hybridization.
| Virus | Nuclear features | Inclusions | Other changes | |
|---|---|---|---|---|
| Herpes simplex and herpes zoster | |
Multinucleation; molding; peripheral margination of chromatin | Eosinophilic, intranuclear (Cowdry type A) | — |
| Cytomegalovirus | |
Enlargement | Large intranuclear, basophilic, with halo; small cytoplasmic, basophilic | Cytoplasmic enlargement |
| Measles virus |  |
Multinucleation | Eosinophilic, intranuclear; multiple eosinophilic intracytoplasmic | Giant cells |
| Respiratory syncytial virus | |
Multinucleation | Cytoplasmic, basophilic, with halo | Giant cells; necrosis |
| Adenovirus | |
Smudged appearance as a result of large inclusion that fills entire nucleus | Large intranuclear, basophilic | Ciliocytophthoria |
Cytomegalovirus (CMV) is one of the most common of opportunistic infections. Patients with CMV pneumonia often present with fever, dyspnea, cough, and diffuse nodular or reticular interstitial infiltrates. Viral cytopathic changes (cytomegaly and large basophilic nuclear and small basophilic cytoplasmic inclusions) are found in bronchial cells, pneumocytes, macrophages, endothelial cells, and fibroblasts ( Table 2.1 ). The diagnosis can be confirmed by viral culture, immunohistochemistry, in situ hybridization, or the polymerase chain reaction.
Measles is a highly contagious, usually self-limited disease caused by the rubeola virus. The incidence has been curtailed due to the widespread use of a vaccine. Measles pneumonia occurs as an opportunistic complication, however, in children immunocompromised due to premature birth, cystic fibrosis, malignancy, or an immunologic disorder. Infection causes a pneumonia characterized by enormous multinucleated epithelial cells (pneumocytes) with cytoplasmic and nuclear inclusions ( Table 2.1 ). Similar findings are seen with infection by the respiratory syncytial virus (RSV). The diagnosis is usually confirmed by detecting RSV antigen in BAL specimens.
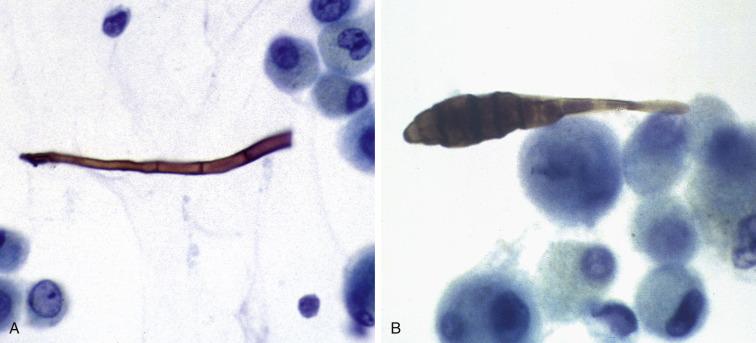
Adenovirus infection usually produces only a minor febrile illness, but adenovirus pneumonia can be severe and fatal, particularly in the immunocompromised. The virus causes two types of nuclear inclusions. One is the smudge cell, in which a large basophilic inclusion usually fills the entire nucleus and obscures chromatin detail. The other is eosinophilic inclusions that resembles the Cowdry A inclusion of HSV infection. A curious morphologic decapitation of ciliated columnar cells, called ciliocytophthoria, can be prominent. The detached cell apex, represented by only the terminal bar and cilia, without its nucleus, resembles a floating tuft of hair or eyelash ( Table 2.1 ).
Bacterial pneumonias are caused by a large number of bacteria, but most are characterized by a neutrophilic exudate. Common organisms include Streptococcus pneumoniae (pneumococcus), other Streptococci, Staphylococcus aureus , Haemophilus influenzae , Klebsiella pneumonia , Pseudomonas sp., Legionella sp., Nocardia sp., Actinomyces sp., and some anaerobic bacteria. Many but not all bacteria can be seen with routine stains as well as with the Gram stain. Cytologic examination is not usually used for the diagnosis of a bacterial pneumonia, which is typically established by correlating clinical findings with microbiologic studies.
Bacterial pneumonias often have a characteristic lobar or lobular distribution, but some pneumonias appear as round and circumscribed masses on imaging studies and thus mimic the appearance of a malignancy. In such cases, a cytologic specimen might be obtained because the working clinical diagnosis is a suspected malignancy.
Several bacteria deserve special mention. Actinomyces species are a common inhabitant of the tonsillar area and thus a common contaminant of sputum and bronchial specimens (but not FNAs). Infection by Actinomyces , however, is uncommon. Pulmonary infection occurs by aspiration of oral contents or by direct extension from subdiaphragmatic abscesses. Actinomycosis is usually a chronic infection that may result in sinus tracts. The bacteria aggregate into grossly visible sulfur granules (so-called because they look yellow on gross examination) and evoke a brisk neutrophilic response. When they appear in cytologic specimens as just an oral contaminant, Actinomyces bacteria are large blue “cotton balls” often associated with squamous cells, with no neutrophilic infiltrate, similar to what is occasionally encountered in Pap specimens (see Fig.1.23 ). A true thoracopulmonary actinomycosis should be considered if the bacteria are associated with abundant neutrophils.
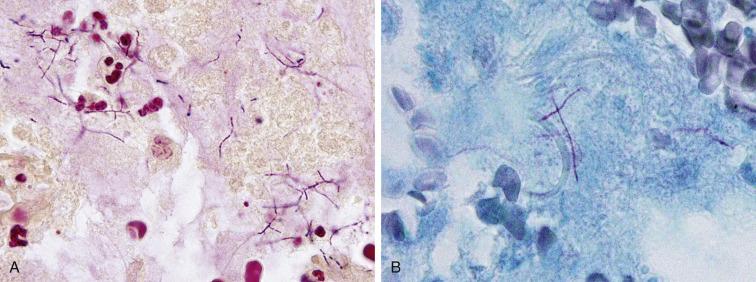
Nocardia are aerobic, filamentous bacteria that inhabit the soil and are acquired by inhalation. Nocardia asteroides accounts for 80% of Nocardial infections. Nocardia infection is most common in the setting of immunosuppression, and most patients have a subacute presentation. Cavitary nodules are seen in one-third of patients. The organisms are found among abundant neutrophils. They are thin, filamentous, and beaded, with such extensive, predominantly right-angle branching that they resemble Chinese characters. They are Gram-positive ( Fig. 2.14A ) and stain with silver stains but are only weakly acid-fast and thus require modified acid-fast stains like the Fite-Faraco for visualization. ( Fig. 2.14B ). The diagnosis is established by culture of a biopsy or BAL fluid.
Legionella pneumonia is caused by the aerobic Gram-negative bacteria Legionella sp., of which the most common is Legionella pneumophila . The organisms are seen well with silver stains like the Steiner, Warthin-Starry, or Dieterle stains. A specific identification of L. pneumophila can be made in BAL samples using immunohistochemical or immunofluorescent methods.
Infection by M. tuberculosis commonly results in granulomatous inflammation ( Fig. 2.15 ). Cytologic specimens contain aggregates of epithelioid histiocytes, lymphocytes, and Langhans’ giant cells. Necrosis may or may not be evident. Granulomas by themselves, however, are a nonspecific finding and can be seen in other conditions like fungal infections and sarcoidosis. A definitive diagnosis of tuberculosis rests on identifying the organisms with the help of histochemical stains (Ziehl-Neelsen or auramine-rhodamine), immunohistochemical stains, or by microbiologic culture. Cell block preparations are particularly useful for special stains, but rarely are more than one or just a few organisms identified (by contrast, infection by Mycobacterium avium intracellulare , as seen in immunocompromised patients, often yields innumerable acid fast organisms). A sensitive and specific assay called the Mycobacterium tuberculosis direct test is also available for the detection of M. tuberculosis and can be applied to respiratory specimens like sputum. The assay amplifies M. tuberculosis ribosomal RNA by the polymerase chain reaction and has been approved by the Food and Drug Administration (FDA) for use in both Mycobacterium smear-positive and smear-negative respiratory samples. Other highly sensitive polymerase chain reaction (PCR)–based assays and matrix-assisted laser desorption ionization-time of flight mass spectrometry for the distinction between tuberculous and nontuberculous mycobacteria are used increasingly because nontuberculous mycobacterial infections have become more frequent in developed countries.
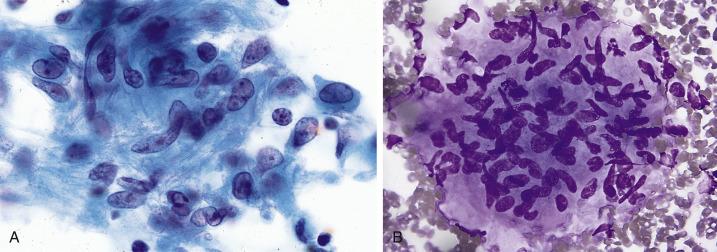
In countries where M. tuberculosis is prevalent, the yield of acid-fast bacteria among all clinically suspicious lung masses can be very high. In immunocompromised patients with tuberculosis, there may be an abundance of acid-fast organisms but few well-formed granulomas. If Romanowsky-type stains are used in such cases, the abundant acid-fast organisms can be identified as negative images.
Pulmonary fungal infections are readily diagnosed by cytology, particularly in transthoracic FNA, and should be suspected whenever there is granulomatous inflammation, necrosis, or both. Cell block material can be used for silver or periodic acid–Schiff stains. Many fungi have a characteristic microscopic appearance that enables a rapid, specific diagnosis ( Table 2.2 ).
| Disease | Organism | Demographics | Morphology | Size | Immunocompetent response | Appearance |
|---|---|---|---|---|---|---|
| Cryptococcosis | Cryptococcus neoformans | Worldwide | Yeast; variably sized; narrow-based budding; mucin capsule (immunocompetent); refractile center | 4–15 μm (diameter) | Granulomatous |  |
| Histoplasmosis | Histoplasma capsulatum | Americas, especially Ohio and Mississippi river valleys | Small budding yeast; intracellular | 1–5 μm (diameter) | Granulomatous | |
| Blastomycosis | Blastomyces dermatitidis | North America | Broad-based budding yeast; thick cell wall | 8–20 μm (diameter) | Neutrophilic/granulomatous | |
| Coccidioidomycosis | Coccidioides immitis | North American deserts | Spherules; endospores | 15–60 μm; 1–2 μm (diameter) | Granulomatous | |
| Paracoccidioidomycosis | Paracoccidioides braziliensis | Central and South America | Yeast with multiple budding (“mariner’s wheel”) | 4–40 μm (diameter) | Neutrophilic/granulomatous | |
| Sporotrichosis | Sporothrix schenckii | Worldwide | Intracellular ovoid yeast with slight halo | 2–4 μm (diameter) | Neutrophilic/granulomatous | |
| Aspergillosis | Aspergillus fumigatus; A. flavus | Worldwide | Hyphae septate; 45-degree angle branching; occasional fruiting bodies in cavities | Hyphae 10–30 μm (width) | Tissue/vascular invasion; colonization; fungus balls |  |
| Phycomycosis (zygomycosis) | Mucor; Absidia; Rhizopus; Cunninghamella | Worldwide | Hyphae variably sized, ribbonlike nonseptate; 90-degree angle branching | Hyphae 10–30 μm (width) | Tissue/vascular invasion |  |
| Candidiasis | Candida albicans; C. tropicalis; C. glabrata | Worldwide | Budding yeast forming pseudohyphae (“sausage links”) | 2–10 μm (width) | Respiratory space colonization; tissue invasion | |
Cryptococcus neoformans is an inhabitant of the soil and is found in bird droppings. It can act as both a primary and an opportunistic pathogen and may involve numerous body sites. Pulmonary invasion by this fungus is heralded clinically by a productive cough, fever, and weight loss.
Histoplasma capsulatum , another inhabitant of soil enriched by bird and bat droppings, is contracted by the inhalation of spores and more commonly affects the immunocompromised. In the United States, it occurs most commonly in the Mississippi and Ohio river valleys, but it can occur anywhere. The disease can mimic tuberculosis clinically, in that peripheral nodular lesions and mediastinal lymphadenopathy are relatively common. The organism, often present within the cytoplasm of macrophages, is so small that it may be overlooked if silver stains are not used.
Blastomyces dermatitidis inhabits wooded terrain. Although the lung is the primary target of infection, there may be distant spread to other organs, such as skin, bone, and the urinary tract.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here