Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The kidney is the principal organ of homeostasis. The kidney’s filtration of blood, and modification of that filtrate by epithelial transport, produces urine to accomplish the following functions:
Control of the composition of body fluids, the concentration of electrolytes, and the excretion of metabolic waste products and foreign substances.
Control of body fluid volume and osmolality.
Regulation of the acidity of the blood.
The kidney also performs hormonal and metabolic functions, including:
Production and secretion of erythropoietin (EPO)
Activation of vitamin D
Gluconeogenesis (the synthesis of glucose from amino acids and other noncarbohydrate precursors, which also occurs in liver and muscle tissue)
In this chapter, we focus on the initial step in urine formation, the production of a plasma ultrafiltrate by the process of glomerular filtration.
The kidneys are retroperitoneal organs, lying alongside the vertebral column at the level of the T12-L3 vertebrae (see Fast Fact Box 17.1 ).
Each adult kidney weighs between 115 and 170 g, and is approximately the size of the human fist.
Fig. 17.1 shows the gross anatomic features of the human kidney and urinary system.
The medial side of the kidney has an indentation, the hilum, where the renal artery and nerves enter the kidney and the renal veins exit.
The funnel-shaped renal pelvis also exits at the hilum.
The ureter, forming a contractile conduit that pushes the urine from the kidney to the bladder.

The kidney can be divided into two regions:
Outer region called the cortex
Inner region called the medulla
The medulla forms 10 to 18 cone-shaped structures, the renal pyramids.
The apex of each pyramid, called the papilla, projects into the urinary collection space.
Urine expressed from each papilla is collected by a minor calyx, the smallest branch of the urinary collection system → coalesce to form major calyces → form the renal pelvis.
In between the renal pyramids are renal columns, also called columns of Bertin, that are considered to be extensions of renal cortical tissue.
The kidney is one of the most well-perfused tissues in the human body, receiving 20% of 25% of total cardiac output (~1.25 L/min). The disproportionately large amount of blood flow the kidney receives allows it to excrete large quantities of nitrogenous waste products (see Clinical correlation Box 17.1 ).
It is important that the kidney has the ability to regulate renal blood flow and glomerular filtration rate independently. In some disorders of reduced blood volume or increased osmolarity, the kidney has the ability to compensate through mechanisms that reduce renal blood flow.
The renal vascular system is illustrated in Fig. 17.2 .

The renal artery enters the kidney at the hilum → interlobar arteries → arcuate arteries → interlobular arteries → afferent arterioles → glomerular capillaries → coalesce to form the efferent arterioles → peritubular capillaries, a capillary network surrounding the renal tubules.
Each glomerulus is a discrete functioning unit connected to its own afferent and efferent arteriole.
A subset of the peritubular capillaries called the vasa recta supplies the tubules in the medulla.
As blood exits the peritubular capillaries, it enters the venous system, which follows approximately the same course as the arteries (see Fast Fact Box 17.2 ).
The renal circulation has two sets of capillary beds in sequence: glomerular and peritubular. Blood exiting the glomerular capillaries remains in the arterial system and passes through the peritubular capillaries before entering the venous system. The kidney is one of a few organs with this unusual circulatory anatomy (another is the pituitary gland).
Why does the renal circulation have such an unusual design? Each of the kidney’s two capillary beds has a specialized function:
The glomerular capillaries = filter large volumes
The peritubular capillaries = reabsorb fluid and solute
Flow regulation through each of these capillary beds is critical to kidney function.
The functional unit of urine formation in the kidney is the nephron ( Fig. 17.3 ). Each kidney contains approximately 1 million nephrons (range of approximately 600,000–1.2 million) (see Clinical Correlation Box 17.2 ).

The kidney cannot regenerate nephrons. When nephrons are lost from disease or by normal aging, the kidneys compensate with adaptive changes in the remaining nephrons. This is the basis for living donor kidney transplants, as the donor can survive with only one kidney.
The glomerulus, the initial part of the nephron, filters plasma into the urinary space of a surrounding pouch called Bowman’s capsule (the parietal epithelium of the glomerulus and its basement membrane) → The filtrate then flows into the proximal convoluted tubule → medullary proximal straight tubule (Pars recta) and the loop of Henle, a hairpin-shaped structure divided into the → thin descending limb, the thin ascending limb, and the thick ascending limb → continues to the distal tubule. A specialized segment of the distal tubule, at a point where the tubule passes between the afferent and efferent arterioles, makes up the macula densa → distal tubules from several nephrons empty into a cortical collecting duct → then become medullary collecting ducts in the outer medulla. The medullary collecting duct descends to the tip of the renal papilla (inner medulla), emptying into the collecting system.
Nephrons can be broadly divided into two groups:
Superficial cortical nephrons have glomeruli in the outer regions of the cortex and short loops of Henle, which lack a thin ascending limb and barely dip into the medulla.
Their peritubular capillaries form extensive networks surrounding the tubules, allowing for an efficient exchange of substances and water between the tubules and the circulation.
Juxtamedullary nephrons have glomeruli near the medulla, and their loops of Henle travel deep into the medulla.
Note that all glomeruli are in the cortex. Juxtamedullary nephrons have vasa recta. These long, thin peritubular capillaries travel alongside the loops of Henle into the medulla and then loop back toward the cortex.
The vasa recta play an important role in the concentration of urine and in nature, mammals that must excrete concentrated urine have the longest nephron loops.
During development, the glomerular capillaries push into the closed end of the proximal tubule, like a fist pressing into a balloon ( Fig. 17.4 ).
This invagination forms Bowman’s capsule (the punched-in “balloon”), made up of two epithelial layers: the inner visceral layer tightly enveloping the “fist” of glomerular capillaries, and the outer parietal layer.
The space between these two, which remains connected to the lumen of the proximal tubule, forms the urinary space, or Bowman’s space. The visceral epithelial cells, or podocytes, tightly envelope the capillaries, adhering to them with foot processes. The histology of the glomerulus is illustrated in Fig. 17.5 .


Fig. 17.6 is a closer view of the layers that substances must cross in traveling from the blood into Bowman’s space. These layers make up the filtration barrier.
The first layer is the capillary endothelium. The endothelium has many holes, or fenestrations, that make it highly permeable to water and also allow small molecules, including many plasma proteins, to pass freely.
The second layer is basement membrane containing collagen type IV and negatively charged proteoglycans. The basement membrane provides a charge barrier for the negatively charged plasma proteins.
The third layer is the visceral epithelium. In the visceral epithelium, small spaces between the podocytes, called filtration slits, are bridged by a thin diaphragm. The filtrate passes through the filtration slits and flows around the foot processes into Bowman’s space.

As shown in Fig. 17.5 , the region where the distal tubule passes between the afferent and efferent arterioles contains a set of structures, the macula densa and the juxtaglomerular cells, known together as the juxtaglomerular apparatus.
The epithelial cells of the distal tubule in this region form the macula densa.
The juxtaglomerular cells are modified smooth muscle cells in the arteriolar walls adjacent to the macula densa. They secrete the enzyme renin, stored in intracytoplasmic granules.
The important role these structures play in the auto-regulation of renal blood flow (RBF) will be discussed later in this chapter.
Three distinct processes determine the amount of a substance excreted in the urine ( Fig. 17.7 ).
Glomerular filtration generates a cell-free and protein-free ultrafiltrate by glomerular filtration which continues through Bowman’s space
Tubular reabsorption from the tubule lumen back into the peritubular capillaries
Tubular secretion from the peritubular capillaries into the tubule lumen

The processes are related in the following mass balance equation:
Glomerular filtration requires substances traveling from the glomerular capillaries into the urinary space to traverse the filtration barrier, composed (sequentially) of a fenestrated capillary endothelium, the negatively charged basement membrane, and the filtration slits between podocytes ( Fig. 17.8 ). The driving force for filtration is the mechanical energy from contraction of the heart. This force is greatest during systole, and there is a pressure wave in the afferent and efferent arterioles. All the energy expended by the kidney after filtration is due to electrochemical gradients for specific solutes, flow along the peritubular capillaries and the tubular lumen, with the contribution of metabolic energy in the form of ATP.
Freely filtered into ultrafiltrate:
Water
Small molecular-weight solutes, whether positively or negatively charged (e.g., sodium, chloride, bicarbonate, and small peptides and proteins) are freely filtered ( Fig 17.9 ).

Excluded from ultrafiltrate:
Small molecules bound to proteins in plasma (e.g., calcium bound to albumin)
Large negatively charged macromolecules (e.g., albumin)
Large positively charged macromolecules (e.g., immunoglobulins) (see Clinical Correlation Box 17.3 ).

In some renal diseases, the glomerular basement membrane is damaged and loses its negative charge. The consequences of this injury and loss of surface area are predictable. The filtration barrier becomes more permeable to the negatively charged plasma proteins, and large amounts of protein (mostly albumin) are lost in the urine where it can be measured clinically, a condition called proteinuria . Extreme losses of albumin from the plasma can lead to a shift in fluid from the blood vessels to the extracellular spaces because of decreased oncotic pressure, leading to edema. Severely low albumin resulting from heavy proteinuria is one of the causes of edema in the condition called nephrotic syndrome (see Ch. 19 ).
Glomerular filtration rate (GFR) is the volume of plasma that is filtered in the glomeruli per unit time. It represents only a fraction of blood flow through the glomerular capillaries (~20%) as the majority of substances are not filtered. A normal value is 125 mL/min, or 180 L/day. It is interesting to note by analogy that 20-25% of cardiac output goes to the kidney. Just as only a fraction of blood from the heart goes to the kidneys, so only a fraction of blood flow to the kidney gets filtered.
Note that because renal arterial flow and venous flow are so much greater than the urine flow rate, we presume that the arterial and venous flow rates are equal. The renal artery plasma flow rate is ~800 mL/min, and the urine flow rate is ~1 mL/min, so the renal venous flow rate will be ~799 mL/min. We presume that 800 mL/min = 799 mL/min for ease of calculation.
At 125 mL/min this tremendous filtration rate is the first step in a process that allows the kidneys to regulate the composition and volume of body fluids in the subsequent segments of the renal tubule.
GFR acts as an indicator of kidney function as a whole in clinical settings. Estimating a patient’s GFR has several important clinical applications, from assessing the degree of damage to the kidneys to determining appropriate doses of medications (because many medications are excreted in the urine).
We can measure GFR by using substances that are freely filtered in the glomerulus but are neither secreted nor reabsorbed in the tubules. “Freely filtered” means that filtered plasma holds the same concentration of a substance as unfiltered plasma. Inulin (different from insulin) is one such substance, which is found in dahlia roots and the Jerusalem artichoke. Not all solutes are freely filtered. Factors that may affect the filtration rate of a solute include the molecular weight, the charge, and whether the solute binds to other macromolecules in the plasma.
Because inulin is neither secreted nor reabsorbed, all the inulin that is filtered by the glomerulus is excreted in the urine ( Fig. 17.10 ). Broadly, filtered solute is equal to GFR multiplied by the plasma solute concentration. This is known as the filtration rate of the solute, in units of mg/min. Likewise, the excreted solute is equal to the urine flow rate (
) multiplied by the urine solute concentration. This is known as the excretion or elimination rate of the solute in the urine, in units of mg/min. Note that the filtration rate of a solute is different than GFR, which is the filtration rate of water. We can state this concept as a mass balance equation:
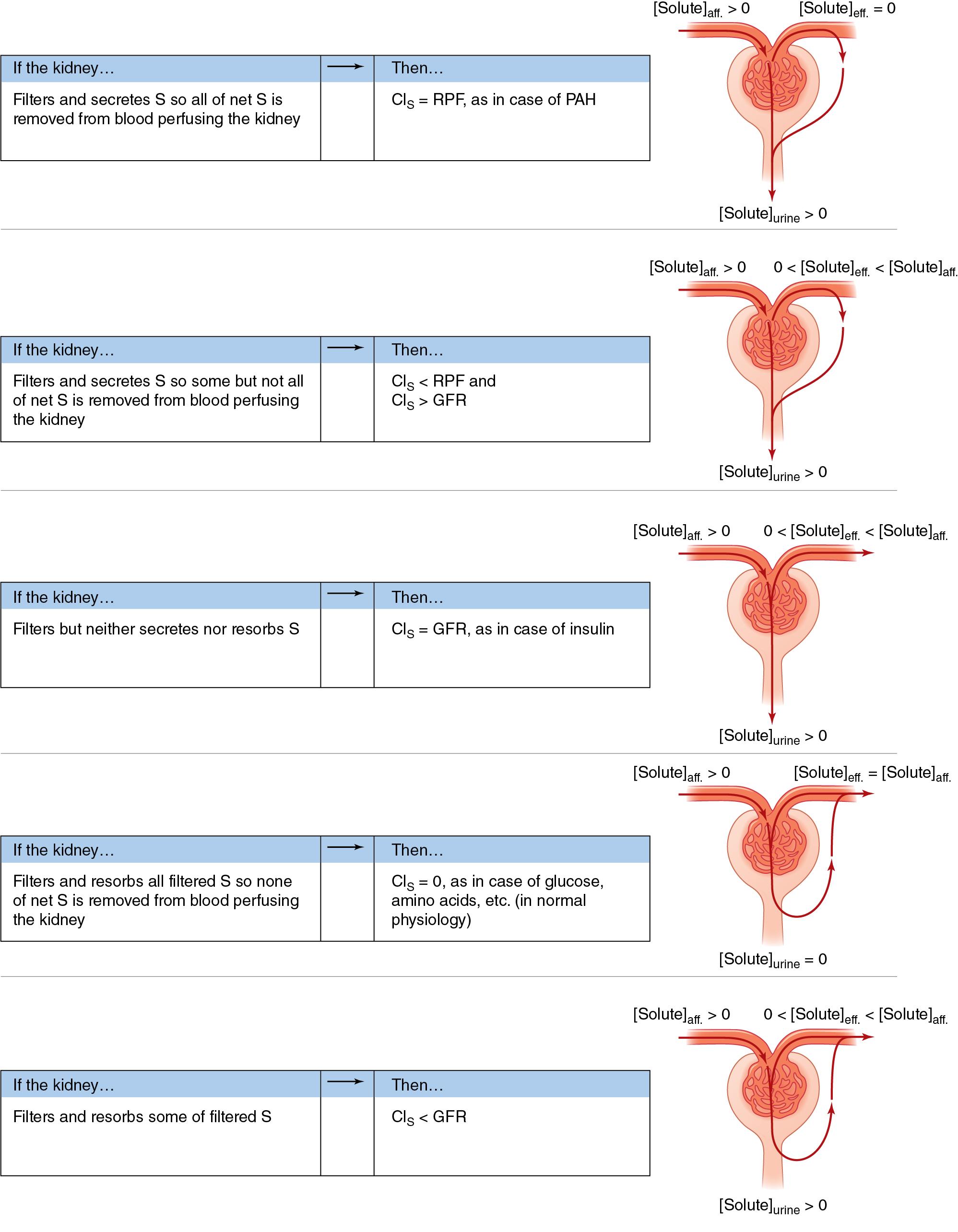
Recall that the mass of a solute equals the concentration of the solute times the volume of the solution. Thus the amount of inulin filtered per minute equals the volume of plasma filtered per minute—the GFR—times the plasma inulin concentration, [inulin] P . Similarly, the amount of excreted inulin equals the urine flow volume per minute (
) times the concentration of inulin in the urine, [inulin] U . It is critical to be consistent with units. Choose the units and do a dimensional analysis of all calculations.
We can then rewrite the mass balance equation as:
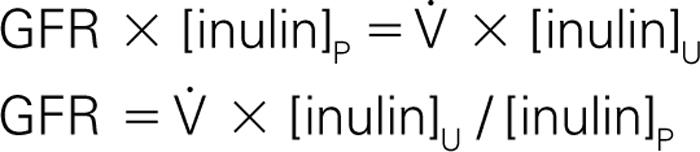
Thus if we infuse inulin into a patient so that it reaches a steady-state plasma concentration, we can measure the volume of urine and the inulin concentrations in the plasma and urine to obtain an estimate of the patient’s GFR (see Clinical Correlation Box 17.4 ). We can measure the inulin concentration in peripheral venous blood and use that value for renal arterial concentration because none of the structures that the venous blood will pass through on its way to the kidney has any appreciable ability to eliminate water or solutes from the plasma.
When physicians want to estimate glomerular filtration rate (GFR), they use a common clinical technique for estimating GFR which entails measuring levels of creatinine, a product of creatine phosphate hydrolysis in skeletal muscle. It enters the plasma from muscle at a relatively fixed rate determined by the muscle mass of the individual. In the kidney, creatinine is filtered by the glomerulus and is secreted to a small extent but not reabsorbed. Thus measuring plasma and urine creatinine levels and urine volume per time (a standard is 24 hours) allows us to estimate GFR.
The filtration faction, or the fraction of renal plasma flow (RPF) filtered through the glomerulus, can be calculated using the GFR and RPF, the volume of plasma that enters the kidney per minute. This is different from GFR, the number of milliliters per minute that is filtered.
Normally, this fraction is approximately 20%. In other words, of the plasma entering the kidney, 20% is filtered through the glomerular capillaries and the other 80% exits the glomeruli via the efferent arterioles and continues on to the peritubular capillaries.
Measuring RPF requires a substance that is completely eliminated from the plasma entering the kidney, and thus is completely excreted in the urine. Because no substance is completely filtered (as not all plasma water is filtered) a substance that is completely eliminated must require a secretory mechanism to eliminate in excess of filtration. Thus even if a solute has a small molecular weight, no inhibition of filtration due to charge, and no appreciable binding to other plasma macromolecules, in other words it is freely filtered, that filtration rate will still not equal RPF. You never filter all of your plasma.
A commonly used substance that approximates these characteristics at low concentrations is paraaminohippurate (PAH). Thus, for low concentrations of PAH (<<Km), the clearance is equal to RPF, as the venous concentration of PAH will be 0.
Using a similar calculation as that to determine GFR by inulin, the mass of PAH entering the kidney per minute equals the plasma volume entering the kidney per minute (the RPF) times the concentration of PAH in the plasma, [PAH] P . The mass of PAH excreted in the urine per minute equals the urine flow rate (V in units such as mL/min) times the concentration of PAH in the urine, [PAH] U . Thus:
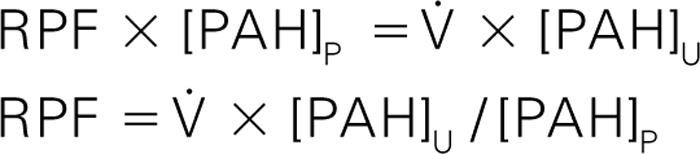
This equation lets us estimate RPF by using an infusion of PAH and measuring the urine output and the concentrations of PAH in the plasma and urine. Other methods that do not require complete elimination of the solute into the urine can be used to measure RPF, (see Genetics Box 17.1 , Development Box 17.1 and Pharmacology Box 17.1 ).
A dramatic alteration in renal structure occurs in patients with autosomal dominant polycystic kidney disease, with the progressive formation of numerous fluid filled cysts in both kidneys. Most such patients have a mutation in either the PKD1 gene or the PKD2 gene. How the products of these genes (polycystin-1 and -2, respective) lead to cyst formation is incompletely understood.
In autosomal dominant polycystic kidney disease, as multiple cysts deriving from various nephron segments grow and coalesce, renal function progressively declines. Affected patients can manifest with hematuria, flank pain from renal hemorrhage or kidney stone formation, or proteinuria. As the glomerular filtration rate declines, generally around age 50 years in PKD1-driven disease and in patients in their 70s in PKD2-driven disease, hypertension and other manifestations of renal failure ensue. Some patients also develop cysts in other organs (thyroid, liver, spleen.)
Treatment of autosomal dominant polycystic kidney disease is largely symptomatic and supportive. In addition to counseling patients to adopt a low sodium diet, hypertension can be treated with angiotensin converting enzyme inhibitors and angiotensin receptor blockers. Tolvaptan, a vasopressin receptor antagonist, decreases the rate of loss of glomerular filtration rate. Octreotide, a long acting version of somatostatin may decrease the rate of fluid accumulation in cysts.
RBF (RBF) can be derived from RPF.
Recall that hematocrit (HCT) equals the percentage of blood volume occupied by red blood cells (e.g., an HCT of 40% indicates that a fraction of 0.4 of total blood volume is made up of red blood cell mass).
Everything else is the plasma; thus, (1 – HCT) represents the fraction of blood volume occupied by plasma. In the example of a HCT of 40%, the fraction of blood that is plasma is 0.6.
Consequently,
If we measure the RPF, we calculate RBF. By determining the RBF and with knowledge of the cardiac output, we can assess what percentage of the cardiac output is directed into the kidneys.
Clearance refers to the volume of incoming plasma from which all substance is removed and excreted into the urine per minute.
A solute that is neither secreted nor reabsorbed, such as inulin, has a clearance that actually corresponds to a real value of plasma volume filtered.
A solute that is secreted extensively, such as PAH, never actually reaches the renal vein. Although the solute is fully cleared from plasma, the total renal plasma volume never left the circulation.
Note that the value for the clearance of any specific substance S is a calculated number used to describe the way the kidney handles substance S. The kidney may leave some of S in the blood, but we still say there was clearance of S. The clearance of S is a calculated theoretical number answering this question:
Given the amount of S excreted by the kidney (amount filtered + amount secreted – amount reabsorbed), what volume of incoming plasma per minute would have to be totally cleared of S to surrender that amount of excreted S?
It may help to imagine that the plasma flowing through the kidney comes in two volume compartments ( Fig. 17.11 ):
The first compartment contains the S that will not be excreted after filtration, secretion, and reabsorption
The second compartment contains the S that will be excreted after filtration, secretion, and reabsorption. This imagined volume containing the S that is destined for excretion is the volume that is “cleared” of S.

Then imagine that the two compartments coalesce upon leaving the kidney in the veins, after being worked on by filtration, secretion, and reabsorption. Imagine that the S from the nonexcreted compartment diffuses into the cleared compartment. That is how we can have a clearance for S without removing all of S from the RPF.
We can derive a general formula for clearance by beginning with a mass balance equation for hypothetical substance S.
As before, [S] P and [S] U represent the concentrations of S in the plasma and urine, and V represents the volume of urine produced per minute.
Now, we will denote the clearance of S as Cl S , the volume of plasma per minute from which S has been removed and excreted into the urine. Using the concept of mass balance, we write:
Mass cleared from the plasma/time = Mass found in the urine/time
Because mass/time equals concentration times volume/time, we find that:
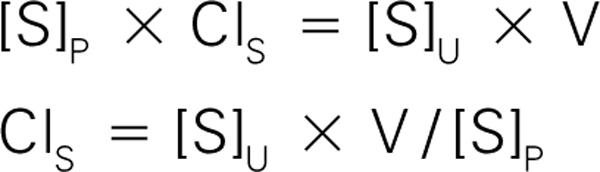
Looking back to our earlier equations, we verify that the clearance of inulin equals the GFR and the clearance of PAH equals the RPF. We can also verify that the units of clearance are mL/min (the units for concentration cancel out and V, the urine flow rate or volume of urine per minute, can be measured in mL/min). These units are in accordance with our definition of clearance as the volume of plasma being cleared of a substance per minute.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here