Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Acute renal ischemia occurs because of a transient sudden drop in the total or regional blood flow to the kidney. Acutely diminished renal perfusion results in acute kidney injury. Impaired blood flow to both kidneys or to a solitary functional kidney can occur due to generalized hypoperfusion in cases of hypovolemia or to decreased cardiac output in acute cardiac decompensation, leading to acute renal failure. Acute unilateral renal artery occlusion occurs usually as a result of trauma or arterial embolism leading to acute renal ischemia ( Table 31.1 ). Patients usually present with acute onset of loin pain, vomiting, and hematuria.
|
The pathophysiology of ischemic acute renal failure is complex. Renal occlusive disease leads to cortical hypoxia associated with microvascular rarefication, inflammatory injury, and fibrosis. The degree of ischemia and the cascade of pathophysiological changes depend to a great extent on the coexistence of renal artery stenosis and intrarenal small vessel pathology.
The precise role of revascularization and the optimal timing of intervention in patients with acute renal occlusion are not clearly defined. The goals of revascularization include the preservation of renal function and the amelioration of renovascular hypertension. Salvage of viable parenchyma requires revascularization before ischemia has progressed to infarction. The relief of renovascular hypertension is a secondary goal in the acutely ischemic kidney and may be accomplished by either revascularization or nephrectomy. The choice between these two alternatives requires an assessment of the quantity of the remaining functional renal tissue.
The most important inquiry is the optimal elapsed time to renal revascularization before irreversible kidney damage occurs. Considering data from surgical series of patients undergoing partial nephrectomy, this time window is really limited. The human kidney tolerates complete ischemia for about 60–90 minutes at normothermia. The decline in renal function is time dependent. After 2 hours of acute ischemia, minimal renal function can be salvaged. However, there is an interindividual variability of renal function preservation as the degree of ischemia in an acutely occluded renal artery depends on the presence of collaterals. Progression to occlusion of a chronic renal artery stenosis due to atherosclerosis rarely leads to acute renal failure due to ischemia. Variable tolerance to ischemia is also seen with a partially occluded renal artery. Hence the effect of revascularization in cases of acute renal ischemia is mainly time dependent, but can be influenced by other parameters as well. Factors that might predict recovery of renal function and potential renal salvage are presented in Table 31.2 .
|
Revascularization methods for acute renal ischemia include percutaneous transluminal angioplasty with or without stenting, intraarterial thrombolysis, and surgical revascularization. Endovascular revascularization techniques are valuable minimally invasive alternatives that may provide major benefits in selected patients.
Traditionally, surgery has been the treatment of choice for renal artery revascularization in the setting of acute renal ischemia. With the advent of minimally invasive treatments, endovascular approaches are increasingly used as attractive alternatives. The main indications ( Table 31.1 .) for treatment are described in the sections that follow.
Renal artery embolism is an infrequent but important cause of acute renal ischemia. It is detected in only 1% of acute kidney injury cases. Diagnosis is difficult and the condition may go unnoticed, especially in unilateral renal artery thromboembolism. It should be suspected in patients presenting with an acute onset of renal colic, hematuria, and comorbidities associated with embolism (e.g., atrial fibrillation).
The main causes of renal artery embolic disease are blood or cholesterol clots. The atheroemboli typically occlude the medium-sized arterioles (150–200 μm in diameter) and glomerular capillaries, and in such cases revascularization has no actual role. Systemic thromboemboli commonly affect the renal artery and its major branches. In 94% of patients thromboemboli originate from the heart and are typically seen in patients with atrial fibrillation. In a study of more than 600 patients with atrial fibrillation and peripheral arterial thromboembolism, the reported incidence of renal thromboembolism was 2%.
Other significant cardiac disorders, including ischemic heart disease, cardiomyopathy, and valvular disease, are also predisposing factors for systemic thromboembolism.
Endovascular techniques are increasingly used, including thrombolysis, aspiration thrombectomy, percutaneous transluminal angioplasty, and stenting for residual thrombus. Prompt and immediate treatment within 90 minutes is crucial to prevent irreversible kidney injury. Nonetheless, few case reports have been published regarding partially occluded renal arteries in which revascularization was beneficial beyond this time limit.
The incidence of renal vascular lesions after blunt abdominal trauma is really low, around 0.08%. Nevertheless, this incidence has been increasing over the past few decades, possibly because of the liberal use of the computed tomography scan in the management of abdominal trauma.
Renal vascular trauma results commonly from renal artery stretching during deceleration injuries or compression of the right renal artery on lumbar vertebrae, leading to renal artery dissection and/or partial or complete occlusion. Complete renal artery avulsion may also occur, albeit rarely.
Renal vascular lesions after trauma are usually associated with other injuries, whereas renal ischemia after trauma may lead to renal hypertension and progressive loss of renal function.
Treatment options include conservative measures with anticoagulation therapy, especially in unilateral kidney involvement, or revascularization by either surgical or endovascular means. Early revascularization is clearly important in bilateral kidney involvement or in the presence of a solitary functioning kidney.
Nonoperative management of unilateral injuries in hemodynamically stable patients can be successful with a 6% risk for developing renovascular hypertension. 17
Nephrectomy is also performed especially if associated with major parenchymal.
Surgical revascularization options include open surgical thrombectomy, repair of the artery, and bypass graft surgery but with poor outcomes in long-term kidney function salvage.
Prolonged ischemia and delayed revascularization have been correlated with poor outcomes, whereas incomplete renal artery occlusion at the time of revascularization and postprocedure antiplatelet therapy are associated with significantly better clinical outcomes.
Endovascular techniques, including angioplasty, with or without stenting are the mainstay of treatment of renal vascular injuries. Renal artery thrombolysis may occasionally be suitable for some trauma patients with no evidence of significant hemorrhage locally or elsewhere. Unstable patients who must undergo laparotomy for other injuries should be managed surgically.
The incidence of iatrogenic renal vascular injuries has increased because of the growing number of interventional procedures. Vascular injury may result in vascular rupture and bleeding. Iatrogenic dissection with or without thrombosis of the renal artery and its branches may also occur.
Flow-limiting dissection requires renal artery stenting for restoration of the renal blood flow. Similarly, thrombosis should be treated aggressively by thrombolysis, aspiration thrombectomy, and mechanical displacement of the dissection flap by stenting. Surgical revascularization may be necessary to avoid kidney loss.
Stent fractures or suboptimal stent deployment with residual stenosis or thrombosis also require secondary interventions to prevent renal artery ischemia.
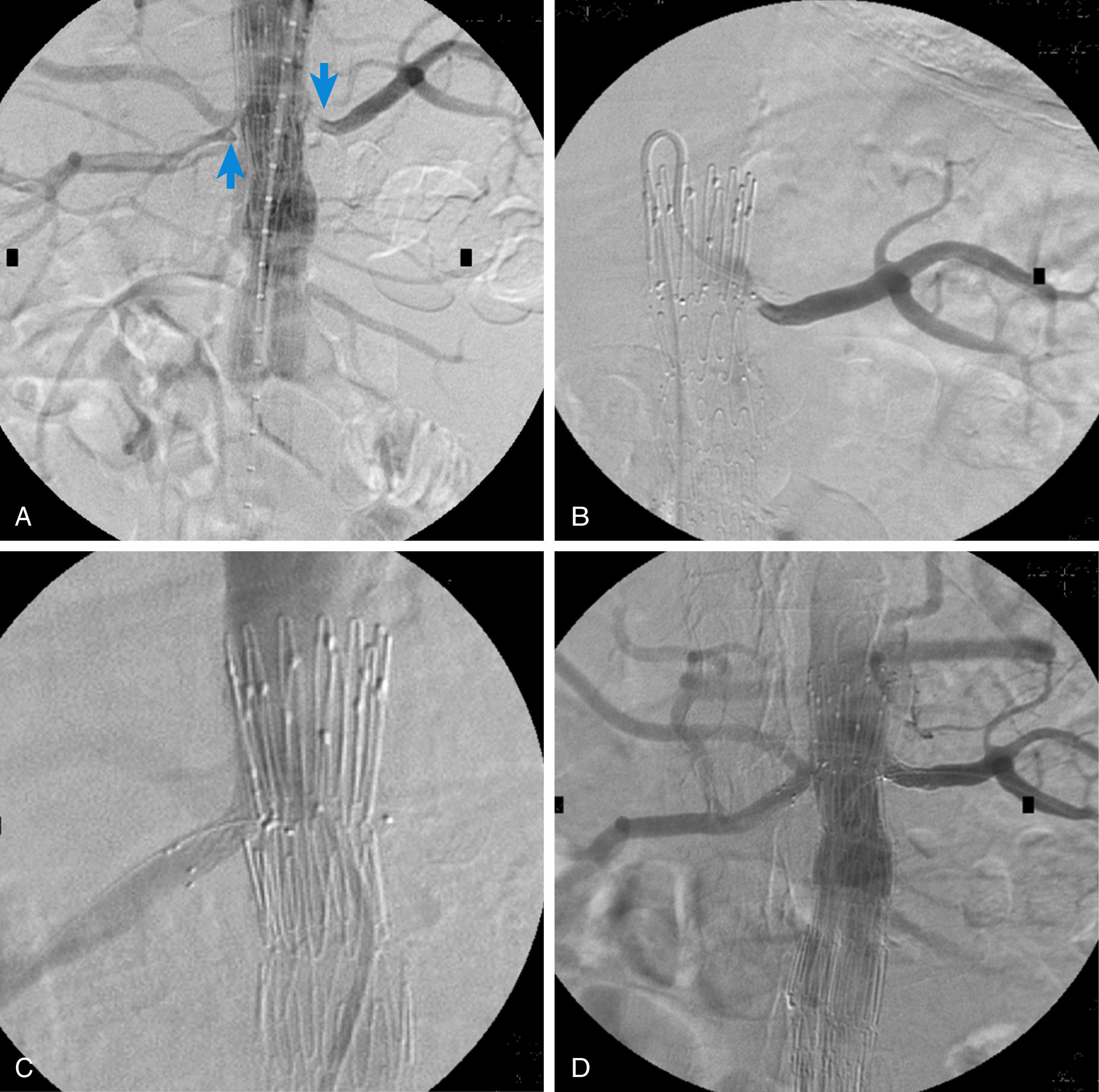
Moreover, proximal malposition of an endograft during endovascular abdominal aneurysm repair (EVAR) may result in renal artery coverage and renal ischemia, requiring immediate management with endovascular stenting if this is technically feasible. Moreover, the expanded use of endovascular stent grafts for abdominal aortic aneurysm repair has introduced a new indication for renal artery stenting to protect renal perfusion when aortic endografts are placed across the origin of the renal arteries during fenestrated EVAR, branched EVAR, or chimney aortic EVAR procedures ( Fig. 31.1 ). Moreover, renal artery angioplasty and stenting are required in the case of renal artery stenosis or occlusion after these complex aortic endografting procedures.
The progression of atherosclerotic renal artery stenosis to occlusion may be clinically silent or may result in deteriorating control of hypertension or renal function.
Acute obstruction of an atherosclerotic renal artery may occur due to thrombus formation on a preexisting atherosclerotic plaque or hemorrhage within the plaque. Acute thrombosis can also been seen in the postoperative setting of hypotension and decreased blood flow across a renal artery stenosis. The abrupt onset of oliguria in an elderly hypertensive patient with atherosclerosis without another identifiable cause should raise the suspicion of sudden progression of renal artery disease to critical stenosis or thrombosis. Patients may also suffer flash pulmonary edema at the initial presentation due to fluid overload. As a result, in a hypertensive patient presenting with abrupt renal failure, exacerbation of hypertension, and/or pulmonary edema, global renal ischemia should be suspected. The degree of renal ischemia varies because a network of capsular collaterals may maintain a subcritical blood flow in the disease-free hilar branches sustaining renal parenchymal survival despite the critical reduction in renal excretory function ( Fig. 31.2 ). Morris et al. showed that a pressure of 20 mmHg is sufficient to maintain the viability of renal tissue but is inadequate to maintain the excretory function of renal tubules.
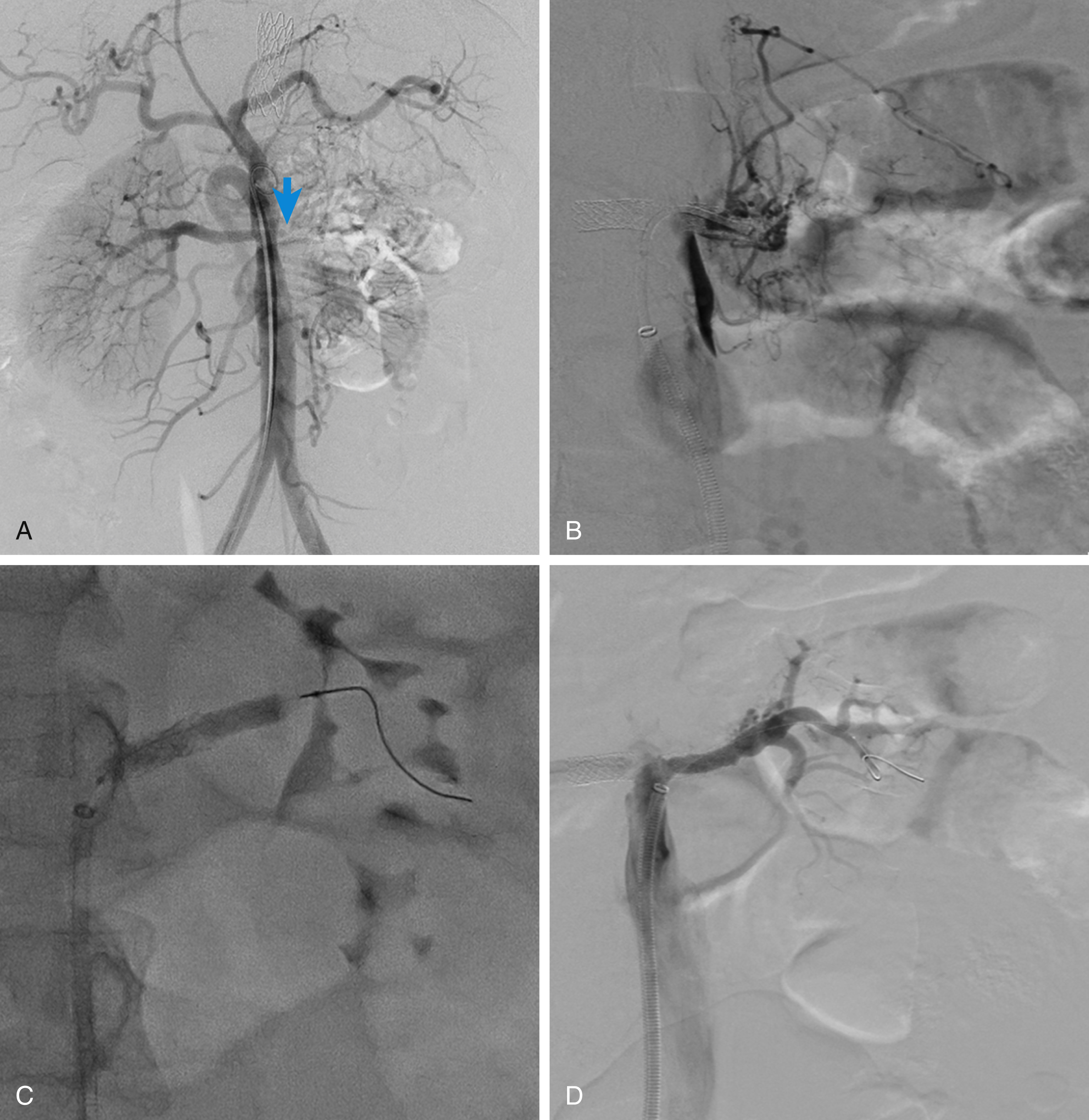
Once obstructive uropathy and other causes of oliguric renal failure are excluded, renal artery revascularization is indicated in selected patients. Kidney size above 9 cm and a patent renal artery beyond the main stem occlusion are the most reliable predictors of kidney salvagability.
The use of angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers plus diuretics can also be a cause of acute renal failure, which is precipitated by a reduction in blood pressure below a “critical perfusion pressure.” If this occurs, it strongly suggests severe intrarenal ischemia from arteriolar nephrosclerosis and/or a severe preexisting main renal artery stenosis. A subgroup of these patients may also benefit from renal artery revascularization.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here