Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Renal excretion is an important clearance pathway for many drugs and their metabolites. Impaired renal excretion, either as a result of kidney disease or the effects of co-administered drugs, can lead to accumulation of drugs and their metabolites. In many cases dosing adjustments are required to maintain optimal therapeutic effects and to avoid accumulation of drugs to toxic levels. In this chapter, the processes involved in renal drug disposition are described, and current knowledge of the role of transport proteins in renal drug secretion and reabsorption is reviewed. Special attention is given to the effect of renal drug–drug interactions and renal insufficiency on pharmacokinetics and to dosing adjustments that are required in the setting of renal dysfunction.
Keywords
renal pharmacokinetics, renal drug-drug interactions, membrane transporters, renal insufficiency, drug dosing
Numerous drugs are eliminated by the kidney. For others, polar metabolites are formed by metabolism or conjugation (usually in the liver)—that are then excreted by the kidney. Renal impairment thereby leads to the accumulation of drugs and their metabolites. For many drugs, doses must be adjusted to attain concentrations similar to those obtained in patients with normal renal function. Renal dysfunction can also affect distribution of drugs, primarily by effects on protein binding. Last, changes in renal function can affect response to drugs independent of any change in disposition.
Several general characteristics of a drug allow prediction as to whether renal dysfunction is likely to affect its disposition sufficiently to mandate changes in drug dosing ( Table 95.1 ). If a drug has a wide therapeutic index, accumulation in patients with renal insufficiency to concentrations several-fold higher than in patients with normal renal function has little, if any, consequence, and dose adjustment can be ignored. For example, a number of penicillin derivatives and many cephalosporins are primarily eliminated by the kidney but can accumulate with little risk. Toxic accumulation of these drugs can sometimes occur, but usually only with massive doses in patients with compromised excretion.
| Characteristics | Implications |
|---|---|
| Therapeutic index | If the drug has a wide therapeutic index, its accumulation poses negligible risk. |
| Protein binding | A high degree of binding (>90%) to albumin makes displacement likely in uremic patients. A high degree of binding to either albumin or α1-acid glycoprotein means little drug is available for removal by dialysis. |
| Amount of drug excreted in the urine unchanged | If ≥40% of a drug is excreted in the urine unchanged, it is highly likely to accumulate in patients with renal insufficiency. |
| Active metabolites excreted in the urine | The metabolites can accumulate, with attendant effects. |
| Volume of distribution | A small volume of distribution (that of total body water or less; that is, ≤0.7 liter/kg) means the drug may be accessible for dialytic removal if it is not highly protein bound. A large volume of distribution means little if any removal by dialysis. |
The amount of a drug or active metabolite excreted in the urine can allow predictions as to the potential for clinically important drug or metabolite accumulation in patients with renal insufficiency. In general, if about 40% or more of a drug dose is excreted in urine, dose adjustment will be needed in patients with renal insufficiency—assuming the drug in question has a sufficiently narrow therapeutic index to be of concern. An exception to this rule of thumb is drugs with the potential for undergoing a “futile cycle,” which refers to the fact that the metabolic route serves to form a reservoir for parent drug. Thus, the metabolic step is “futile” (see the section on metabolism). With such agents, little (if any) drug itself is normally excreted in the urine—yet renal insufficiency can result in its accumulation in plasma. The mechanism of this effect, and the few drugs to which it applies, are discussed in material following. It is also important to note that some drugs are metabolized to active metabolites in which case, both drug and metabolite are pharmacologically potent. Further, some drugs are pro-drugs, which are themselves inactive, but are converted to active compounds (e.g., clopidogrel or irnotecan). For these drugs, the focus of dosing adjustment should be on the active compounds.
It should be apparent from the foregoing that for drugs that have not been explicitly studied in patients with renal insufficiency one can make reasonable predictions as to the need for adjusting therapy. Although lack of quantitative guidelines makes dose adjustments tentative, a worse problem is ignoring the need to do so. When no information concerning the relevant characteristics of a drug is available, one therapeutic strategy is to use a drug of the same class—but with no dependence on the kidney for elimination. For example, if a clinician wishes to prescribe a cardio-selective beta-adrenergic antagonist to a patient with renal insufficiency one alternative is to administer atenolol in a modified dose. Another option is to administer metoprolol, which is eliminated by the liver and needs no dose adjustment in patients with renal insufficiency. The converse would apply to patients with liver disease. However, there is a growing body of literature that suggests that drug metabolism may be altered in patients with renal dysfunction, and increasingly the U.S. Food and Drug Administration is requiring studies of the effects of renal dysfunction on new drugs that are cleared by non-renal routes. Therefore, some caution should be exercised in simply switching a patient from a drug predominantly eliminated by the kidney to a drug with a predominant nonrenal elimination.
Most drugs are excreted via the kidneys, either in the form of the unchanged drug molecule or after conversion of the parent drug into polar metabolites. Mechanisms by which the kidney excretes drugs are analogous to its normal physiologic processes of glomerular filtration, active secretion, and active and passive reabsorption. Below, the processes of importance for the handling of drugs are briefly described. Effects on any of these processes, e.g., through interactions between concomitantly administered drugs or through renal disease, can mandate changes in drug dosing. The influence of altered kidney function on systemic drug disposition, and corresponding dosing strategies are discussed in more detail in subsequent sections.
The glomerulus offers no barrier to filtration of the unbound fraction of most drugs. Glomerular pores allow passage of molecules up to molecular weights of about 65,000 Daltons, and the vast majority of xenobiotics (including many biologic agents) are approximately two orders of magnitude smaller than this. Exceptions include larger proteins and dextran. Dextran is a good example of the role of molecular size; it can be administered as several different preparations, the size of which determines their ability to be filtered. Thus, dextran 1 (MW=1000) is freely filtered and is eliminated rapidly by the kidney—with a half-life of elimination of about 2 hours. In contrast, dextran 70 is too large to be filtered and is eliminated slowly by metabolism. It is detectable in plasma for 4 to 6 weeks. Dextran 40 is a mixture of both higher- and lower-molecular-weight species so that the smaller dextrans are freely filtered and eliminated quickly, with selective retention of the larger components.
For drugs freely filtered at the glomerulus, such as aminoglycoside antibiotics, renal elimination can be quite rapid. For many other drugs, binding to serum proteins restricts filtration and thus only the unbound fraction can be filtered and renal excretion, depending on the extent of binding, may be negligible. The limits to glomerular filtration of a drug, then, are usually not the glomerular barrier itself but factors that prevent filtration—predominantly binding to macromolecules too large to be filtered.
The overall renal drug clearance is determined by the relative importance of filtration, secretion of drug into the primary urine, and reabsorption back into the blood. It follows that secretion must occur if the rate of excretion is higher than that of filtration, and conversely, if the rate of excretion is lower than the filtration rate, the drug is by necessity reabsorbed.
Secretion : The classical method described for assessing drug secretion by the kidney was that of Cross and Taggart, using incubations of renal cortical slices. The sine qua non for secretion was a slice-to-medium–concentration ratio greater than unity. Numerous more sophisticated methods for characterizing secretion have since evolved, including microperfusion of isolated nephron segments, use of isolated proximal tubular cells or vesicles derived from peritubular or luminal membranes of proximal tubules, and cellular expression systems for detailed studies of drug transport by specific membrane transporters.
Drugs gain access to secretory sites via the peritubular capillary. If 20% of renal plasma flow is filtered, the remaining 80% of flow reaches sites of secretion. This process is active because an uphill concentration gradient can be generated. Moreover, depriving the proximal tubule of energy also inhibits movement of drugs from the peritubular to the tubular side of the cell. The energy for active secretion is ultimately obtained from ATP hydrolysis, either directly driving transmembrane drug flux (i.e., primary active transport), or generating concentration gradients across the cell membranes that, in turn, drive the solute flux (secondary active transport). In the latter case, energy is generated by peritubular sodium-potassium exchange via Na + ,K + -ATPase.
The efficiency of secretion is quite impressive. For example, secretion occurs for many drugs that are highly protein bound (such as furosemide). For this and other drugs, the affinity for transport exceeds that for binding. As such, binding to plasma proteins actually facilitates elimination by increasing the amount of drug in the plasma and thereby delivering it to secretory sites. That this is the case has been demonstrated in studies with analbuminemic rats. Administration of furosemide to these animals results in very low plasma concentrations of diuretic because without albumin binding the drug distributes widely outside the vascular space. Consequently, only small amounts are delivered to secretory sites and little is excreted into the urine. Administration of albumin in this setting binds furosemide, keeping it in the vascular space so that more diuretic reaches secretory sites (where more can be secreted into the urine). A similar effect has been shown to occur in humans.
Reabsorption : The kidney is capable of both active and passive reabsorption of drugs. Various membrane transporters mediate active luminal-to-abluminal flux of substrate drugs, whereas passive drug reabsorption is the consequence of concentration differences between the primary urine and the blood in the peritubular capillaries, that in turn results either from active drug secretion or, typically, from the reabsorption of water along the nephron. Passive reabsorption relies on diffusion of drugs across the lipid bilayers of the renal tubule epithelium and favors nonpolar drugs. For passive diffusion of drugs that are weak acids or bases, the nonionized form of the drug will be passively reabsorbed since it can cross the tubular membranes. Hence, for both weak acids and bases, urine pH can influence passive reabsorption, and urine pH can be manipulated to reduce passive reabsorption and enhance renal elimination. For example, in the case of patients with aspirin overdose, sodium bicarbonate is administered to alkalinize the urine. In the alkaline urine, salicylate is present predominately in the ionized form and is therefore not reabsorbed.
While energy-dependent solute transport in the kidney was demonstrated almost a century ago, the roles of transport proteins in the renal handling of drugs remain an active area of research. The human genome contains more than four hundred membrane transporters classified into the ATP-Binding Cassette (ABC) and Solute Carrier (SLC) gene families, in addition to the numerous aquaporins and ion channels that serve the same purpose of facilitating trans-membrane solute flux. Here, we limit the discussion to ABC and SLC membrane transporters that are known to transport drug molecules across the membranes of renal cells, either in the secretory or the reabsorptive direction ( Figure 95.1 ). Data on the clinical relevance of the various membrane transporters continues to emerge, and additional members are likely involved in the renal transport of some drugs. In this section we focus on the function and expression of renal drug transporters under physiological conditions, whereas the effects of renal disease is discussed in more detail below (See: Influence of Renal Disease on Drug Disposition and Response).
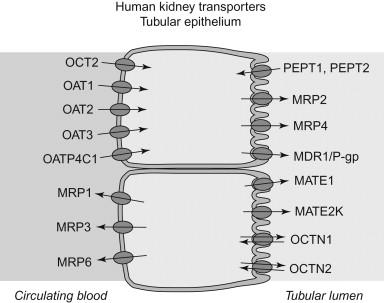
Early studies defined two separate transport systems for organic solutes in the kidneys, with broad substrate specificities towards organic anions and organic cations, respectively. During the past decades, a large number of distinct transport proteins have been identified as contributors to these transport systems, and functionally characterized with respect to their substrate specificities, cellular localization, genetic variation, and so on. The historical categorization based on substrate charge is, however, not directly mirrored in the assignment of transporters to different gene families based on similarity in amino acid sequence. While substrate specificities often are shared within subfamilies of structurally similar transporters, members of both the anion and the cation systems are found across multiple subfamilies in the ABC and SLC gene families, and some subfamilies contain both anion and cation transporting members.
ABC transporters : The human ABC gene family contains 49 distinct protein coding genes, divided into seven families (ABCA–ABCG) based on amino acid sequence similarity. All members contain a highly conserved ATP-binding region, and utilize energy from ATP hydrolysis to drive efflux of substrate molecules from the inside to the outside of cells. ABC transporters of importance for renal drug transport include P-glycoprotein (P-gp/ABCB1) and several members of the Multidrug Resistance Associated Protein (MRP/ABCC) subfamily. P-gp is by far the most well-characterized drug transporter, and has been shown to transport many structurally diverse compounds, typically of a lipophilic and uncharged or weakly basic character ( Table 95.2 ). P-gp is expressed in the luminal membrane of proximal tubule epithelium and appears to play a role in the renal clearance of digoxin.
| Transporter a | Gene Symbol | Selected Substrates |
|---|---|---|
| P-gp (MDR1) | ABCB1 | Digoxin, doxorubicin, irinotecan, loperamide, paclitaxel, ritonavir, saquinavir, vinblastine |
| MRP1 | ABCC1 | Daunorubicin, doxorubicin, etoposide, leukotriene C4, methotrexate, saquinavir |
| MRP2 (cMOAT) | ABCC2 | Methotrexate, statins, olmesartan, valsartan, glutathione and glucuronide conjugates |
| MRP3 | ABCC3 | Fexofenadine, methotrexate, glucuronide conjugates |
| MRP4 | ABCC4 | Adefovir, cyclic AMP, dehydroepiandrosterone sulphate, methotrexate, tenofovir, topotecan |
| MRP6 | ABCC6 | Leukotriene C4, BQ123 |
| OCT2 | SLC22A2 | Amantadine, amiloride, cimetidine, cisplatin, histamin, metformin, serotonin |
| OCTN1 | SLC22A4 | Carnitine, pyrilamine, quinidine, verapamil |
| OCTN2 | SLC22A5 | Carnitine, cephaloridine, quinidine, verapamil |
| OAT1 | SLC22A6 | Acyclovir, adefovir, cidofovir, ciprofloxacin, lamivudine, methotrexate, tenofovir, zalcitabine, zidovudine |
| OAT2 | SLC22A7 | Allopurinol, bumetanide, dehydroepiandrosterone sulfate, estrone-3-sulfate, 5-fluorouracil |
| OAT3 | SLC22A8 | Bumetanide, cefaclor, ceftizoxime, furosemide, NSAIDs, estrone-3-sulfate |
| OAT4 | SLC22A11 | Bumetanide, NSAIDs, tetracycline, urate, zidovudine, |
| OATP4C1 (OATP-H) | SLCO4C1 | Digoxin, estrone sulfate, methotrexate sitagliptin |
| PepT1 | SLC15A1 | Amoxicillin, captopril, cephalexin, cefadroxil, enalapril, glycylsarcosine, valacyclovir, dipeptides, tripeptides |
| PepT2 | SLC15A2 | Amoxicillin, captopril, cephalexin, cefadroxil, enalapril, glycylsarcosine, valacyclovir, dipeptides, tripeptides |
The MRP/ABCC family consists of thirteen transporters, many of which have been shown to accept drugs as substrates, in particular anionic drugs and compounds conjugated with sulfate, glucuronide and glutathione moieties ( Table 95.2 ). In the kidney proximal tubule epithelium, MRP2/ABCC2 and MRP4/ABCC4 are expressed in the luminal membrane and mediate secretion of substrates to the urine. MRP6/ABCC6 is also expressed in the kidney proximal tubules, but is instead localized to the basolateral membrane. Expression of MRP1/ABCC1 and MRP3/ABCC3 is highest in more distal parts of the nephron, including the thick ascending loop of Henle, distal tubuli and collecting duct, where their expression in the basolateral membrane suggests involvement in the reabsorption of organic anions. Based on in vivo studies in knockout mice, MRP4 appears to be important in the renal clearance of tenofovir and adefovir, two anti-viral drugs.
SLC transporters : More than 350 human membrane transporters are categorized to the SLC gene family. In contrast to the ABC family, transport by SLCs is not directly coupled to ATP hydrolysis, but is instead dependent on concentration gradients of the transported substrate or of co- or counter-transported ions or small endogenous molecules. The SLC family contains many of the transporters necessary for cellular nutrient uptake, including glucose transporters in the SLC2 and SLC5 subfamilies and amino acid transporters in the SLC1, SLC3, SLC6, SLC7, SLC36 and SLC38 subfamilies. Many of these are expressed along the nephron, where they have the important function of limiting the loss of essential nutrients to the urine. The di/tripeptide transporters PepT1/SLC15A1 and PepT2/SLC15A2 have a corresponding role in nutrient reabsorption, but also accept some peptidomimetic drugs as substrates, including β-lactam antibiotics like cephalexin and amoxicillin and angiotensin converting enzyme inhibitors like enalapril and fosinopril ( Table 95.2 ).
The SLC subfamilies with the most extensive evidence for a role in drug disposition are the organic anion (OAT) and organic cation (OCT) transporter family SLC22, the organic anion transporting polypeptide family OATP/SLCO, and the recently discovered multidrug and toxic compound extrusion family MATE/SLC47. The SLC22 family contains a mixture of structurally similar organic anion and organic cation transporters. In the kidney proximal tubule, OCT2/SLC22A2 is the primary transporter mediating cellular uptake of organic cations from the blood. Substrates are typically low molecular weight cations, and include drugs such as metformin, amiloride, procainamide, oxaliplatin and varenicline ( Table 95.2 ). Recently, MATE1/SLC47A1 and MATE2-K/SLC47A2 were identified as luminal exporters of organic cations in the proximal tubuli, and overlapping substrate and inhibitor specificities between the MATEs and OCT2 have led to the hypothesis that these form a complementary transport system for tubular secretion of small organic cations.
Renal organic anion transporters include OAT1–3/SLC22A6–8 and OAT4/SLC22A11. OAT1, 2 and 3 are expressed on the abluminal membrane of proximal tubule cells and mediate cellular uptake of anionic drugs such as para-aminohippuric acid, acyclovir, ciprofloxacin, methotrexate, ceftizoxime, and furosemide ( Table 95.2 ). OAT4 is expressed on the luminal membrane, and is involved in urate homeostasis and in the reabsorption and/or secretion of anionic drugs. In addition to the SLC22s, members of the OATP/SLCO family have been shown to transport numerous anionic drugs. In the kidney, the most prominent member is OATP4C1/SLCO4C1. In comparison to the hepatic OATPs 1B1, 1B3 and 2B1, OATP4C1 is so far much less studied. It mediates uptake from the blood into the proximal tubule cells, and substrate drugs include digoxin, estrone sulfate, methotrexate and sitagliptin.
Two other transporters in the SLC22 family have been implicated in renal drug elimination: OCTN1 (SLC22A4) and OCTN2 SLC22A5), which transports carnitine in the kidney. Mice with mutations in OCTN2 eliminate the model organic cation tetraethylammonium by renal excretion at about half the rate of their wild-type littermates.
Interindividual differences : Genetic variation in membrane transporters can result in increased or diminished drug transport. Such variation has been catalogued as part of genome-wide studies and in greater detail in gene family-focused efforts. Many commonly occurring genetic variants in important drug transporters have been characterized in cellular expression systems and some have also been shown to affect the in vivo disposition and pharmacological effect of substrate drugs. For example, torsemide renal clearance was correlated with genetic variation in OAT4/SLC22A11, and a common genetic variant (A270S) in OCT2/SLC22A2 was shown to result in changes in metformin clearance in multiple populations.
Several heritable diseases have genetic variants that result in non-functional transporters as their underlying cause. For example, Dubin-Johnson syndrome, which manifests as hyperbilirubinemia, is caused by mutations in MRP2/ABCC2 that reduces biliary excretion of bilirubin. The effects of Dubin-Johnson syndrome on renal drug handling is much less studied, but the pharmacokinetics of drugs that utilize MRP2/ABCC2 in their renal excretion may be affected in Dubin-Johnson patients.
In addition to genetic variants that cause direct functional effects by altering or truncating the amino acid sequence, mutations in regulatory genomic regions can have functional effects, by altering expression levels of the transporter. In fact, natural interindividual variation in transporter expression levels can be significant. In liver samples from 110 patients, MRP2/ABCC2 levels varied more than 300-fold, and expression level differences may thus have effects on drug disposition on par with or even greater than many coding region variants. Data on inter-individual differences in renal transporter expression is scarce, but such variation may ultimately affect the disposition of renally cleared drugs.
The kidney has the capacity to metabolize numerous drugs and proteins. The proximal nephron has high levels of glucuronyl transferases, sulfotransferases, and peptidases. The ability of the kidney to conjugate drugs via the transferases has been demonstrated unequivocally in animals, but data in man are fragmentary and less direct. Renal glucuronidation may be substantial. For example, approximately 20% of an intravenous dose of furosemide and 50% of a dose of morphine may be glucuronidated by the kidney.
When glucuronide is conjugated to a xenobiotic, the chemical bond between the two can be either an ether (e.g., phenolic) or an ester (e.g., carboxylic). The latter is called an “acyl-glucuronide.” Ether-linked glucuronides are chemically stable and are for the most part excreted in the urine. In contrast, acyl-glucuronides are unstable under physiologic conditions such that the glucuronide can deconjugate back to the parent compound. In addition, the glucuronic acid moiety can migrate to other parts of the molecule (acyl migration).
One implication of this chemical instability is analytical. Consider, for example, that one wishes to measure the amount of a drug excreted in the urine. If not just drug but its acyl-glucuronide is excreted, one must be certain that the metabolite does not deconjugate ex vivo —causing a falsely elevated measure of the parent drug. To avoid this phenomenon, samples of urine must be stabilized quickly in acidic buffer (e.g., using 75 µl of 17 M glacial acetic acid in a 20-ml urine aliquot is sufficient).
A second implication of acyl-glucuronide formation is the possibility of a futile cycle of drug metabolism ( Fig. 95.2 ). In patients with normal renal function, circulating acyl-glucuronide conjugates that are formed in the liver are readily eliminated in the urine. In patients with renal insufficiency, however, renal excretion is decreased and the conjugate accumulates in plasma—where it can spontaneously hydrolyze to reform the parent compound ( Fig. 95.2 ). This phenomenon leads to a paradox in which a drug may accumulate in patients with renal insufficiency even though negligible amounts of parent drug are eliminated in the urine in patients with normal renal function.
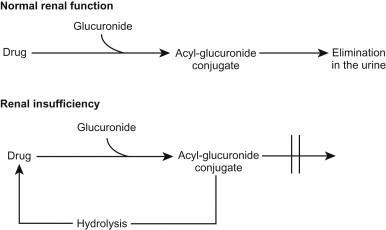
Clofibrate, diflunisal, and some of the arylpropionic NSAIDs have been shown to undergo a futile cycle. It would seem prudent to avoid drugs undergoing acyl-glucuronide conjugation in patients with renal insufficiency, or to at least use them very cautiously in such patients.
The proximal nephron also contains mixed-function cytochrome P450 oxidases (CYP), but in lower amounts than the liver. Interestingly, isoforms of CYP appear to be differentially regulated in the kidney relative to the liver. The relative contribution of renal compared to hepatic metabolism is unknown.
The kidney is able to metabolize proteins, such as insulin and other biologic agents—including interleukins, superoxide dismutase (SOD), and likely many others. In patients with normal renal function, up to 50% of insulin elimination occurs via renal metabolism. This component of overall elimination diminishes in patients with renal insufficiency and accounts at least in part for the decreased insulin requirement as a patient’s renal function deteriorates. Many biologic proteins, such as SOD, are small enough to be freely filtered by the glomerulus. They are then metabolized by the peptidases of the proximal tubule. When renal function declines, renal metabolism is decreased and the substance can accumulate.
Proximal tubule dipeptidases also metabolize imipenem. As such, if imipenem alone is administered to patients, all antibacterial effect in the urine is lost. To attain efficacy for urinary tract infections, imipenem is administered with cilastatin—which inhibits the dipeptidases, allowing sufficient amounts of unchanged imipenem in the urine to kill bacteria.
Overall, then, the kidney is metabolically active toward drugs in a variety of ways. Unfortunately, renal metabolic contributions to drug elimination in man have been inadequately explored and thus clinical implications are for the most part speculative.
In addition to the metabolic roles of the kidney described previously, the kidney excretes many drug metabolites formed in the liver. Renal insufficiency does not necessarily mean that drug metabolites will accumulate because other excretory pathways exist, such as biliary excretion. In addition, many drug metabolites presumably have no effects. On the other hand, there are numerous examples of metabolite accumulation in which the metabolites are pharmacologically active ( Table 95.3 ). Some of the metabolites listed exert pharmacologic effects similar to those of the parent compound (e.g., primidone). Others account for all of the pharmacologic activity, the parent compound having no effect (e.g., enalapril). In other examples, the metabolite has a different pharmacologic profile from the parent drug. For example, normeperidine excites the CNS and can cause seizures in contrast to the sedating effect of the parent compound meperidine.
| Drug | Active Metabolite |
|---|---|
| ANALGESICS | |
| Codeine | Morphine-6-glucuronide |
| Meperidine | Normeperidine |
| Morphine | Morphine-6-glucuronide |
| Propoxyphene | Norpropoxyphene |
| ANESTHETICS AND DRUGS USED DURING ANESTHESIA | |
| Atracurium | Laudanoside |
| Cisatracurium | Laudanoside |
| Pancuronium | 3-OH pancuronium |
| Remifentanil | Carboxylic acid metabolite |
| Vecuronium | 3-Desacetylvecuronium |
| ANTIANXIETY AGENTS, SEDATIVES, AND HYPNOTICS | |
| Buspirone | 1-(2-Pyrimidinyl)-piperazine (1-PP) |
| Midazolam | α-Hydroxymidazolam |
| ANTICOAGULANTS, ANTIFIBRINOLYTICS, AND ANTIPLATELET AGENTS | |
| Sulfinpyrazone | Thioether metabolite |
| ANTICONVULSANTS | |
| Oxcarbazepine | 10,11-Dihydro-10-hydroxycarbamazepine (HYCZ) |
| Primidone | Phenylethylmalonamide |
| Valproic acid | Not identified |
| ANTIHISTAMINES | |
| Cimetidine | Cimetidine sulfoxide |
| Ebastine | Carebastine |
| Hydroxyzine | Cetirizine |
| Ketotifen | Desmethylketotifen |
| Nizatidine | N2-monodesmethylnizatidine |
| ANTI-INFLAMMATORY AGENTS | |
| Diacerein | Rhein |
| Oxaprozin | Two hydroxylated metabolites |
| ANTIMICROBIAL AGENTS/ANTIBACTERIALS | |
| Cephalosporins | |
| Cefotaxime | Desacetylcefotaxime |
| Cefoxitin | Decarbamoylcefoxitin |
| Cephalothin | Desacetylcephalothin |
| Cephapirin | Desacetylcephapirin |
| SULFONAMIDES | |
| Sulfamethoxazole | Acetyl metabolite |
| Sulfisoxazole | Acetyl metabolite |
| URINARY BACTERIOSTATICS | |
| Nalidixic acid | 7-hydroxynalidixic acid |
| ANTINEOPLASTICS AND ANTIMETABOLITES | |
| Cyclophosphamide | 4-Hydroxycyclophosphamide |
| Aldophosphamide | |
| Ifosfamide | 4-Hydroxyifosfamide |
| Aldoifosfamide | |
| Isofosforamide | |
| Acrolein | |
| Chloracetaldehyde (oral dosing) | |
| ANTISPASTICITY AGENTS | |
| Dantrolene | Hydroxy and amino metabolites |
| CARDIOVASCULAR AGENTS | |
| ANTIARRHYTHMICS | |
| Disopyramide | Mono-N-desisopropyl-disopyramide (MND) |
| Flecainide | Meta-O-dealkylflecainide |
| Lorcainide | Norlorcainide |
| Procainamide | N-acetylprocainamide (NAPA) |
| ANTIHYPERTENSIVES | |
| Acebutolol | N-acetylacebutolol (diacetolol) |
| Captopril | Mixed disulfides with endogenous thiols |
| Delapril | 5-Hydroxyindane diacid |
| Losartan | E3174 |
| Methyldopa | Methyldopamine |
| Metoprolol | α-Hydroxymetoprolol |
| Minoxidil | Minoxidil sulfate |
| Nebivolol | β-Hydroxy metabolites |
| Nitroprusside | Thiocyanate |
| Pinacidil | Pinacidil-N-oxide |
| CARDIAC INOTROPES | |
| Digitoxin | Digoxin |
| Enoximone | Piroximone |
| Flosequinan | 7-Fluoro-1-methyl-3-methylsulfinyl-4-quinolone (flosequinoxan) |
| BLOOD LIPID-LOWERING AGENTS | |
| Clofibrate | Parachlorophenoxyisobutyric acid (CPIB) |
| Lovastatin | β-Hydroxy acid |
| Simvastatin | β-Hydroxy acid |
| DIURETICS | |
| Triamterene | Sulfuric ester of hydroxytriamterene |
| HYPOGLYCEMIC AGENTS | |
| Acetohexamide | Hydroxyhexamide |
| Tolbutamide | Hydroxytolbutamide |
| Carboxytolbutamide | |
| HYPOURICEMIC AGENTS | |
| Allopurinol | Oxipurinol |
| PSYCHOTHERAPEUTIC AGENTS | |
| Acamprosate | Acetyl-homotaurine |
| Resperidone | 9-Hydroxyresperidone |
| Tianeptine | Not characterized |
| Venlafaxine | 0-Desmethylvenlafaxine |
| SYMPATHOMIMETICS | |
| Dolasetron | R+-hydroxydolasetron |
It should be apparent that in order to safely use drugs with active metabolites in patients with renal insufficiency one must know the pharmacologic profile of the parent drug and its metabolite(s). One should try to avoid such drugs in patients with renal disease.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here