Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Renal organogenesis is a complex interaction between gene stimulation of planned growth and complementary apoptosis, allowing appropriate development of the functioning renal organ system, vascular bed, and intertwined genital system.
The renin-angiotensin system (RAS) is critical to normal renal development through delicate interaction between the maternal–placental RAS and the developing fetal RAS, which has central hormone-specific and sex-specific configurations that help the developing vascular and renal organogenesis.
Preterm birth (<37 weeks gestation), as well as low birth weight, are associated with chronic kidney disease (CKD), and the improved survival with treatment warrants more research into long-term health optimization of these patients.
The human kidney, specifically the metanephric kidney, is an extremely complex organ with more than 25 functionally and morphologically different distinct cell types performing vitally important functions for the developing human. These actions include filtering waste products, maintaining electrolyte and fluid homeostasis, facilitating bone mineralization, regulating blood pressure, and blood composition. The nephron of the kidney performs these functions. The number of human nephrons range from 200,000 to 2,000,000 for each kidney with peak growth during the third trimester and complete nephrogenesis by 36 weeks’ gestation. Any further growth of the kidney is due to an increase in the size of the individual nephrons rather than an increase in nephron number.
Renal organogenesis is a complex and incompletely understood process. Factors affecting maternal and fetal health, including environmental factors in utero, can lead to failure of normal morphogenesis, resulting in congenital anomalies of the kidney and urinary tract. If the complement of nephrons is decreased by prematurity, low birth weight (LBW), or exposure to environmental agents causing damage to the developing renal system, adult renal disease and hypertension may occur. Many steps in the development of the human kidney are unknown but are extrapolated from the study of animal models. This chapter will review known (and postulated) molecular and cellular mechanisms of renal development, the impact of environmental factors on the developing fetal kidney, and the long-term risks of CKD and other associated adult health outcomes.
Organogenesis, or organ formation, begins with early patterning of cell groups through gene expression with transcription factors that act to determine cell fates specific to each given organ.
Transcription factors, generally ribonucleic acids (RNAs) directed by DNA information, are incorporated into larger molecular networks and ultimately, direct downstream signaling pathways. Transcription factors may have different purposes in each developing organ system. Similarly, signaling pathways are used repeatedly in each different organ’s development, and these pathways may also be used in multiple stages of differentiation in the same organ.
Signaling pathways establish epithelial morphogenesis by allowing cell polarity, bending, and folding to shape developing tissues and to allow these tissues to interact with the loose mesenchyme surrounding the epithelia through signaling. This reciprocal induction between epithelia and mesenchyme is well illustrated in kidney development, where expression of the transcription factor Wilms tumor 1 (WT1) in the metanephric mesenchyme leads to glial cell–derived neurotropic factor (GDNF) expression critical for the outgrowth of the developing epithelial ureteral bud. Reciprocally, the ureteral bud then signals back to the metanephric mesenchyme to induce formation of the renal nephron units. There is a delicate balance between the interplay between the ureteral bud and the developing mesenchymal metanephros to allow tissue differentiation into the final renal unit.
Enhanced imaging, ex vivo organ culture, and computational strategies have advanced our understanding of the physical forces inside and outside the cells in organ development. Study of different species has led to better understanding of the role of extracellular matrix in regulating the mechanical properties of the developing tissues. The extracellular matrix also provides substrata for cell migration, rotation, and elongation necessary for organ development. Most of this research is in nonhuman tissue at present, but basic understanding of generalized organogenesis is important in determining the steps critical in human organ development, with potential implications for regenerative medicine as well.
The vascular systems of all vertebrates are highly organized branched networks of arteries, veins, and capillaries. The circulatory system in combination with the hematologic system is the first functioning physiologic system to develop in embryogenesis. Development of the cardiovascular and hematologic systems must occur in tandem because simple diffusion of oxygen and nutrients would be insufficient for organ development as the embryo enlarges. Formation of the vascular system is therefore crucial for proper tissue growth and differentiation, delivery of oxygen and nutrients to the developing organism, and removal of waste.
Vasculogenesis and angiogenesis play a primary role in determining patterning of the developing embryo through the paracrine action of the endothelial cells. Blood vessels are critical for organ development, differentiation, and postnatal remodeling. There is a reciprocal relationship, with the developing organ providing signals to the endothelial cells of the developing vasculature, while the endothelial cells signal patterning instructions for organ formation. Vascular development begins shortly after gastrulation when the blood islets form in the yolk sac and angioblast precursors form in the head mesenchyme and the posterior lateral plate mesoderm. Vascular precursor cells are present in the metanephric blastema. Local environmental cues initiate differentiation of the endothelial layer in the developing kidney by forming the renal vascular tree after transplant of different extrarenal endothelial cells to the developing renal bed as studied in developing mouse kidneys. Angiogenesis generally occurs later in embryogenesis by increasing the previously laid vascular bed by sprouting, bridging, and intussusceptive growth. Ultimately, the primitive vessels branch, prune, and specialize to accommodate the probable function of each respective organ that they feed. Oxygen tension and hemodynamic forces are critical for developing the delicate patterns specific to the local vasculature. Angiogenic growth factors are necessary for vasculature development as well as for organ patterning. The most important factors responsible for angioblast differentiation and tube formation are vascular endothelial growth factors (VEGFs). VEGFs are found early in blood vessel patterning and later help modulate endothelial maintenance in normal tissues before and after birth. The endothelial cells themselves are the source of these proteins. VEGF also helps mobilize blood elements from the bone marrow through interactions with hematopoietic stem cells there. The development of the area-specific branching pattern is likely due to local factors and different local progenitor cell populations. The podocyte epithelial cells in the developing glomerular region secrete large amounts of VEGF, stimulating increased branching of the vessels forming in this area ( Fig. 74.1 ). Fibroblast growth factors (FGFs) are heparin-binding growth factors that interact with endothelial cell surface receptors. Their primary purpose is to help control branching morphogenesis of the vascular tree during organogenesis. The post-glomerular vascular bed is closely aligned to the vasa recta in the renal tubule structures and is likely regulated by angiogenic factors such as VEGF secreted by the tubules themselves. Postnatal development of the vasa recta has also been found to be mediated by VEGF. Renal vasculature development occurs through simultaneous vasculogenic and angiogenic processes. VEGF is critical in the regulation of blood vessel formation in the kidney and is also responsible for the coordinated development of the glomerulus and renal tubular development simultaneous to its regulating vasculogenesis. Animal model studies suggest that postnatal branching may be mediated by the renin-angiotensin system (RAS).
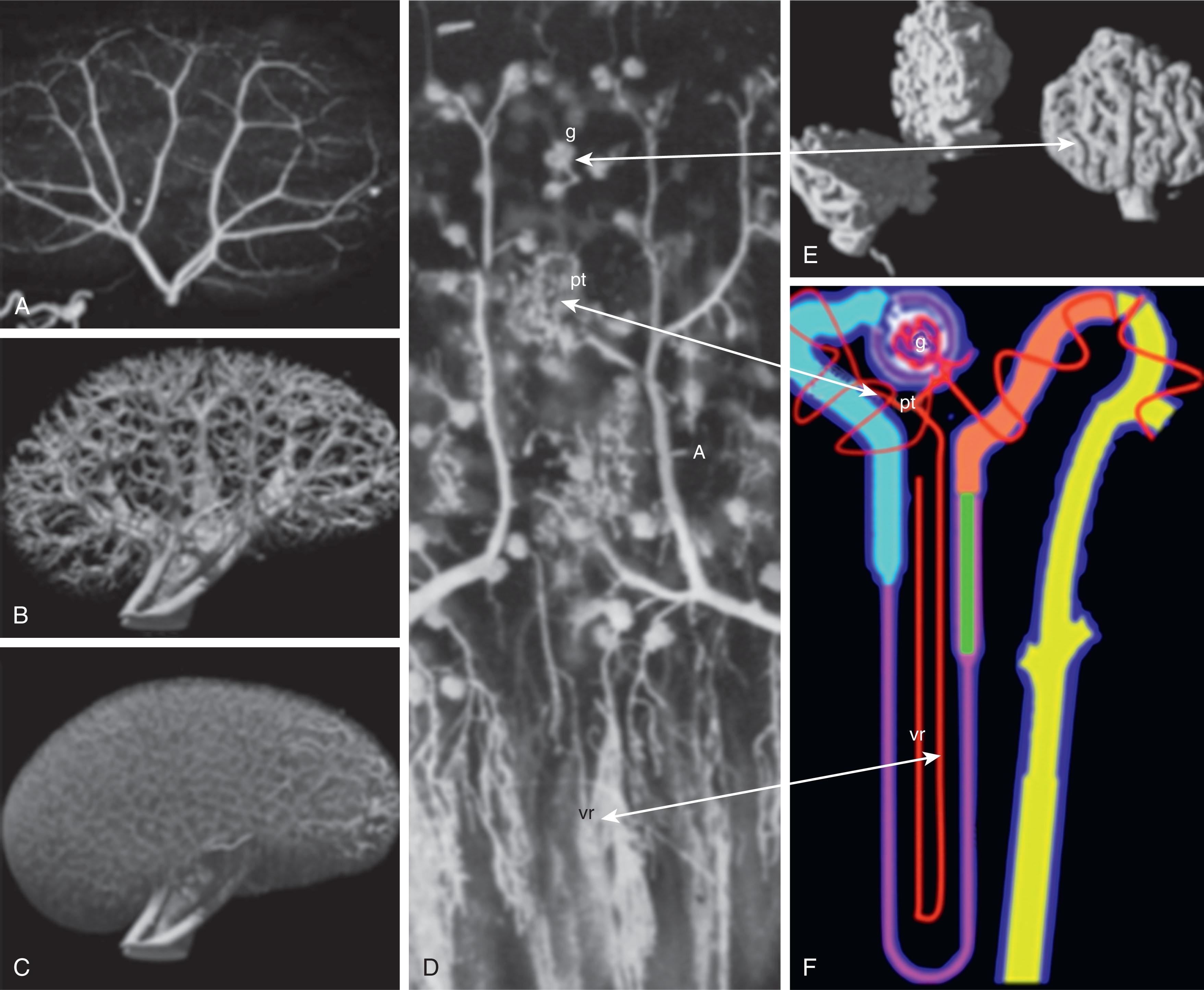
The renal vascular bed is unique compared with other organ systems. The kidneys compose less than 0.8% of the human body weight but receive 20% of the cardiac output at any given time. The volume of blood sent to the kidney is critical for clearing the body of metabolic waste, as well as maintaining fluid and ion homeostasis in the bloodstream. The renal artery enters the kidney near the central portion of the kidney or hilum, which is close to the medulla of the kidney. Although the renal artery is proximal to the medulla of the kidney, 90% of the blood it delivers is sent directly to the glomerular capillary bed in the periphery of the kidney, and only 10% is delivered to the medulla.
The arterial structure of the kidney is critical for directing blood primarily to the glomerulus. The mechanisms for this patterning during development are not well understood, but as development proceeds, the medullary portion of the arterial tree has very little branching, whereas the cortical arteries have extensive branching, especially the afferent arterioles surrounding the glomeruli.
For appropriate renal function, the glomerulus endothelium is very permeable to fluids and low molecular weight solutes. The resistance of the renal vasculature surrounding the glomerulus is very high, to allow the ultrafiltrate produced to flow through the urinary space at 125 mL/min. These high-resistance arterioles allow the amount of fluid emanating from glomerular capillaries to be about 50 times greater than the fluid outflow of other capillary systems in the body. In contrast, the peritubular capillaries have high oncotic pressure, allowing them to reabsorb solutes and fluids that would otherwise be lost by this high glomerular filtration rate (GFR). There is a complementary interplay between the renal tubules reabsorbing fluid, electrolytes, and solutes from the glomerular filtrate and the peritubular capillaries returning these substances to the systemic circulation because of this high oncotic pressure pull.
The vasa recta is an additional renal capillary bed adjacent to the renal tubules in the medulla. These vessels act by delivering oxygen and nutrients to the medulla and return electrolytes and solutes reabsorbed by the medullary renal tubules. The long-looped arrangement of the vasa recta close to the loop of Henle is critical for urine concentration by conservation of water through increased osmolarity in this region in the renal medulla. The blood is ultimately collected in the venous system at the cortical medullary region of the kidney and flows out of the kidney through the renal vein.
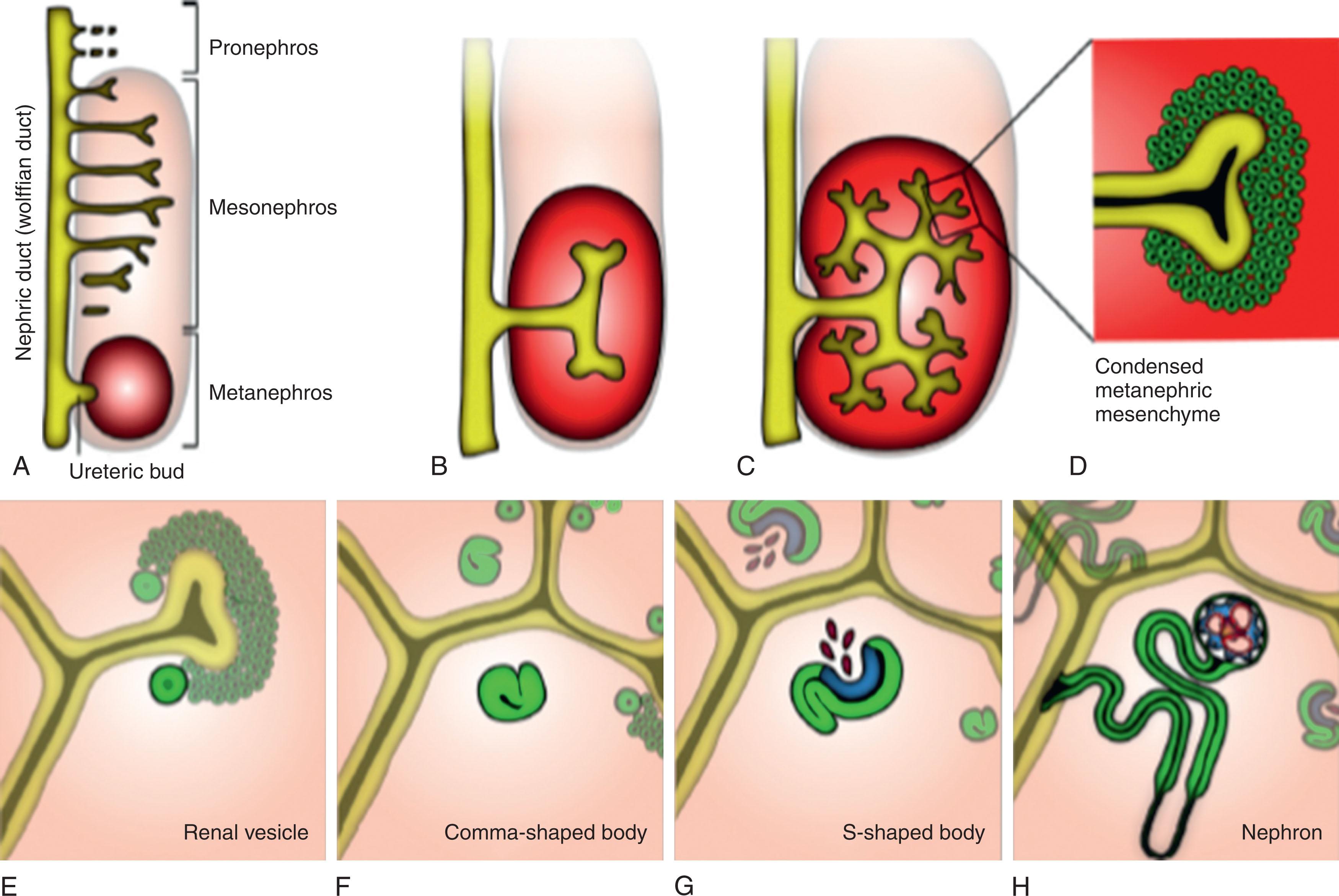
The mammalian urogenital system develops from the intermediate mesoderm. Paired epithelial nephric ducts arise dorsally and elongate caudally on the right and left sides of the embryo until they induce development of the pronephric and mesonephric duct formation from intermediate mesenchymal mesoderm. These ducts elongate until they reach and fuse with the cloaca, which is the bladder and urethral precursor. Primitive tubules develop as the pronephros and mesonephros and are transient with minimal function, and only a portion remains of the mesonephric tubules as part of the male reproductive system.
The metanephric kidney arises from the posterior nephric duct through reciprocal signaling induction between the metanephric mesenchyme and nephric duct causing the ureteral bud to form. Human metanephric differentiation starts at about 5 weeks’ gestation, and the first functioning nephrons are formed at about week 8. The ureteral bud then invades the metanephric mesenchyme to initiate the mesenchymal to epithelial differentiation giving rise to the glomerulus–nephron–collecting duct system of the mature kidney. The requirement for intermediate mesoderm appears to be regulated by bone morphogenetic protein (BMP) signaling, which is also required for maintenance of the pronephric duct gene expression to allow survival and differentiation of the developing nephric duct.
Paired box genes ( PAX2 and PAX8 ) appear to be necessary to drive nephric duct development, elongation, and maintenance. The final stage in early nephric development is the separation of the genital tract from the urinary tract. Once the ureteral bud extends from the nephric duct, the urinary tract separates the ureter from the common nephric duct through apoptosis to form the new ureterovesical junction into the cloacal structure that will be the urinary bladder. This apoptosis is most active at the caudal region and less so at the ureteral bud branching point. The apoptosis occurs through downregulation of RET (receptor tyrosine kinase) activity. RET is still needed for ureteral remodeling but at an expression level lower than that needed for generation of the ureteral bud branching off the nephric duct. Ultimate elimination of the common nephric duct is necessary for normal urinary tract function. Absence or delay in ureteral remodeling may result in ureteropelvic junction obstruction. Overactivation of apoptosis of the common nephric duct may result in vesicoureteral reflux ( Fig. 74.2 ).
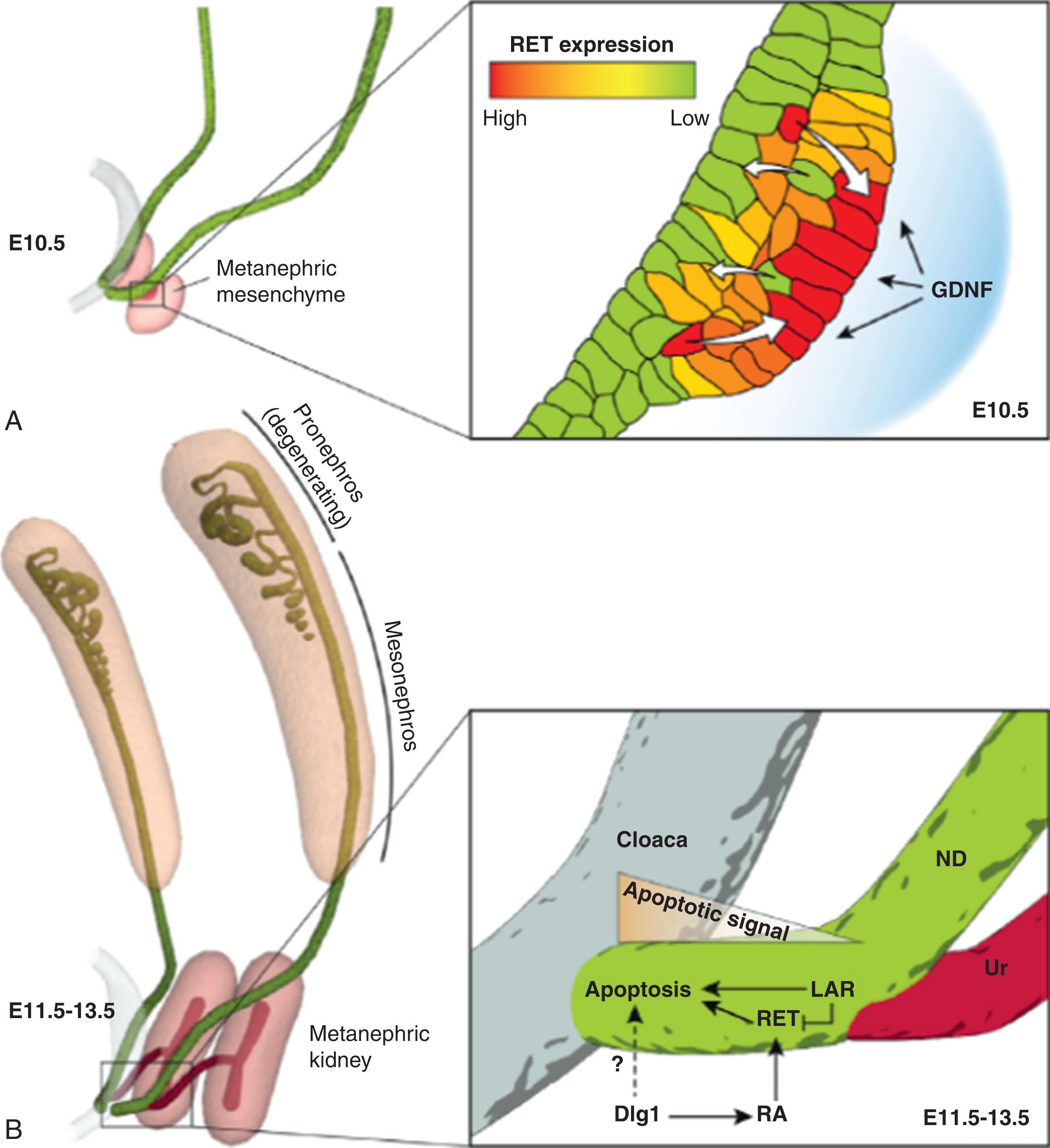
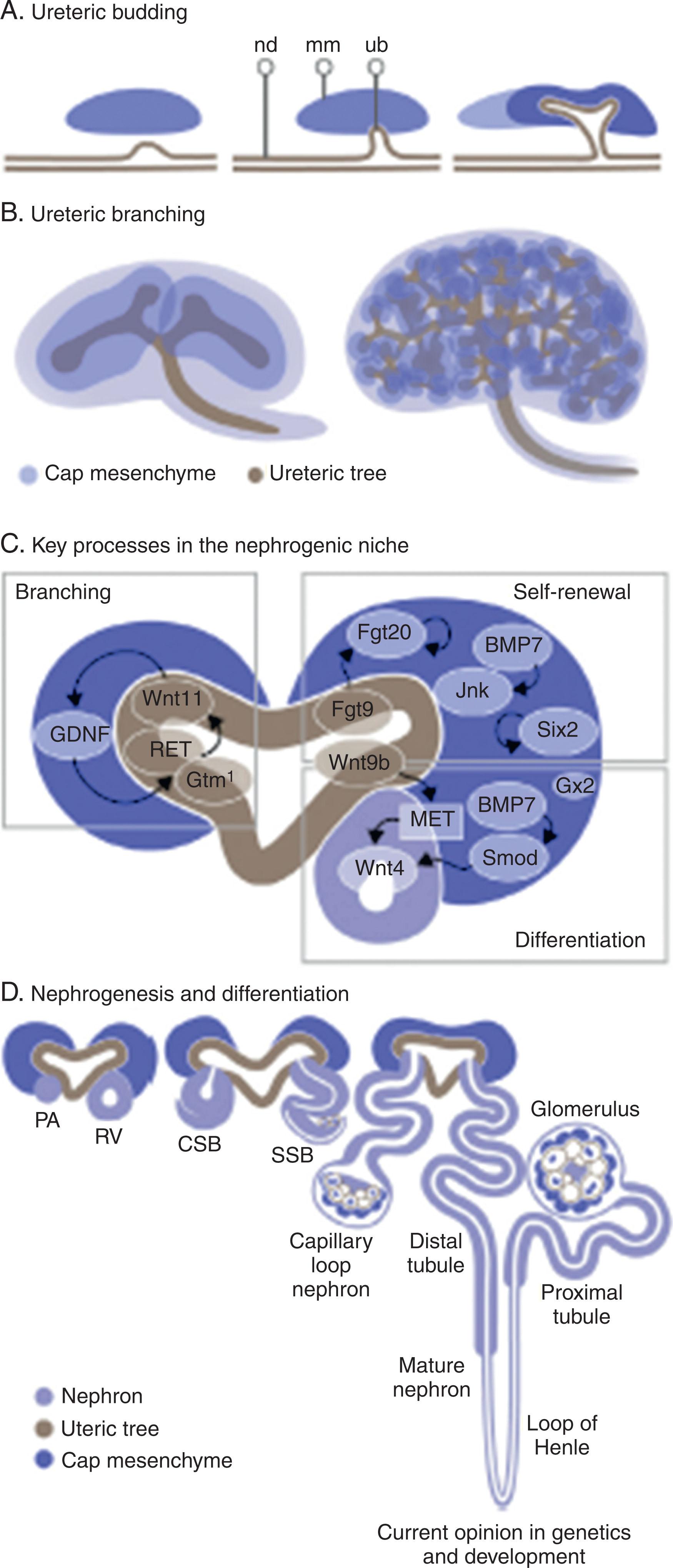
Development of the ureteral bud requires an active RET–glial cell line-derived neurotrophic factor (GDNF) pathway. GDNF is located in the metanephric mesenchyme adjacent to the nephric duct and activates ureteral bud development through interaction with RET located along the nephric duct. If RET is absent, ureteric induction fails, but the ureteral bud forms ectopically in studies where GDNF is cultured next to the nephric duct. Wnt (Wingless/integrated) pathway factors, particularly factor 11, and FGFs upregulate GDNF to help with branching of the developing ureteral nephron tree.
Once the ureteral bud has been stimulated to extend out to the developing metanephric mesenchyme, it begins to branch, forming the ureteric tree, which occurs only at the tips of ureteric tips with RET–GDNF reciprocal signaling, which upregulates RET–Wnt11, further upregulating GDNF secretion. This ureteral signal to release RET is localized to the cap mesenchyme. With continued proliferation, elongation, patterning, and segmentation, the specific functioning regions of the nephron form the recognizable adult nephron (glomerulus, proximal tubule, loop of Henle, distal tubule), which all connect back to the collecting duct ( Fig. 74.3 ).
Once the ureteral bud epithelium invades the metanephric mesenchyme at the cap mesenchyme region, this mesenchyme condenses around the ureteral bud tips under the influence of Wnt genes. The renal vesicle is the first epithelial structure that will ultimately become the future nephron and is activated by the developing ureteral bud. The next phase is the development of the comma-shaped bodies as the first condensation of the renal vesicle metanephric mesenchyme. Formation of the cleft in the comma-shaped bodies denotes the development of the S-shaped bodies, which ultimately initiate renal nephron development ( Fig. 74.4 ). The S-shaped bodies are derived from the cap mesenchyme and become the glomerular tuft once endothelial cells infiltrate this area of the developing nephron. The axis of the developing nephron is determined by the S-shaped body after it fuses to the ureteral tip. The proximal end of the S-shaped body will be the glomerulus, while the distal end will fuse to the ureteral bud branching system as the collecting duct. The S-shaped phase of development is when the nephrogenic and vasculogenic processes connect because of secretion of VEGF from the podocytes in the S-shaped body and attract migrating vascular endothelial cells to the S-shaped body cleft ( Fig. 74.5 ).
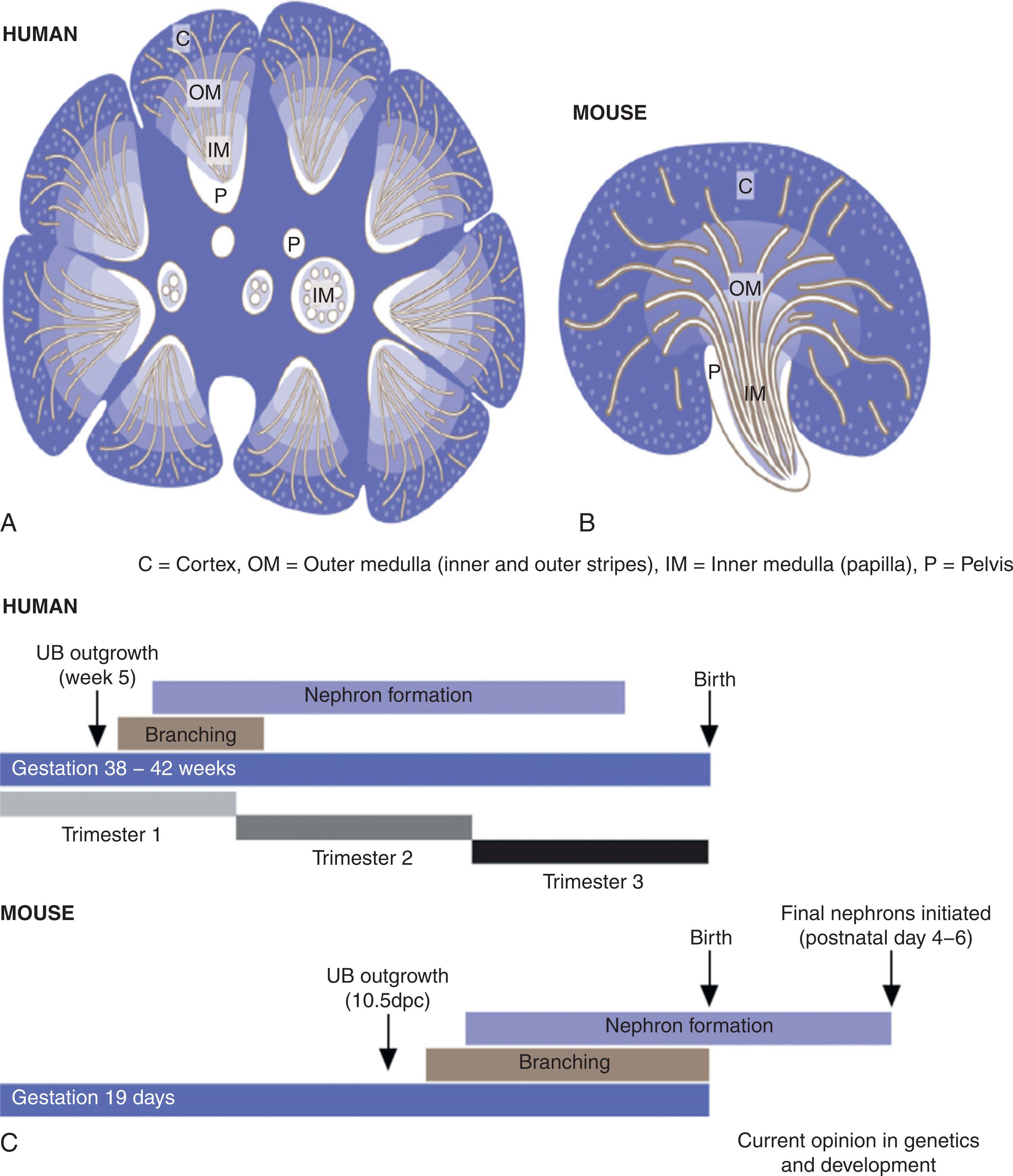
With time and continued development, the ureteral tip and cap mesenchyme decrease in size. Ureteric branching ceases in the human at around week 14 to 15 of gestation. The human kidney is multipapillate, with about 8 to 15 lobes, each having about 15 generations of collecting duct branching. The extensive patterning and segmentation that occur in nephron development are driven by anchor genes that have absolute specificity for each cell compartment where they are located in the developing embryo. The kidney alone has more than 15 distinct anatomic compartments. To date, 37 anchor genes have been defined for only six sub-compartments in the kidney. The actions of these genes are not yet well known, but the anchor genes may be responsible for initiating differentiation of the various compartments of the nephron by releasing promoters and tissue factors to the local mesenchyme to initiate formation of the functioning renal unit.
Human nephron development is compared with mouse development to extrapolate similar or speculated steps in development that may be the same (see Fig. 74.5 ). The human nephron complement may differ between individuals, particularly with lower total nephron number with variability in number based on prematurity, extreme prematurity (<28 weeks gestation), low birth weight (<2500 g), and other gestation events such as in utero exposure to maternal factors (preeclampsia, other hypertension, smoking, malnutrition, diabetes, and other maternal conditions).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here