Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter was prepared through the financial support of grant HL 56470 (R.J. Martin and Y.S. Prakash), HL138402 (P.M. MacFarlane), and HL 133459 (T.M. Raffay) from the National Institutes of Health.
Significant progress has been made in elucidating the fetal and neonatal development of lower airway structures and regulation of their function. During early development, airway smooth muscle differentiates from the mesenchyme of the primordial lung and envelops the emerging bronchial tree. Airway smooth muscle at this early stage provides phasic rhythmic contractility that is thought to propel lung fluid distally and enhance lung development. Neural structures emerge in parallel to airway muscle, and their functional roles are rapidly integrated such that during postnatal life, tonic, rather than phasic, contractile, and relaxant functions dominate.
Although it is well accepted that the potential effect of the lower airway on prenatal and postnatal lung function is considerable, our understanding of the link between neurotransmitter-specific networks involved in the regulation of airway function is incomplete. Furthermore, compared with healthy and diseased adult airways, little attention has been paid to the diverse neural mechanisms that regulate airway caliber in the perinatal period. This subject has gained considerable interest because of the injurious effects of increased inspired oxygen and positive pressure ventilation on neonatal airway function. It is therefore important to gain a greater understanding of the normal maturational changes exhibited by airway smooth muscle contractile and relaxant mechanisms superimposed on the immature structural elements that compose the airways.
Most studies on neural control of the airways during development have focused on the regulation of smooth muscle, even though many of the same principles apply to control of mucus production and blood flow in the airways. In general, at any given age, neural control of airway functions ( Fig. 61.1 ) involves integrated networks along the neural axis that funnel information to tracheobronchopulmonary effector units via airway-related vagal preganglionic neurons (AVPNs) in the medulla oblongata. The AVPNs are the final common pathway from the brain to the airways and transmit excitatory signals to the intrinsic tracheobronchial ganglia that are part of the network for feedback control.
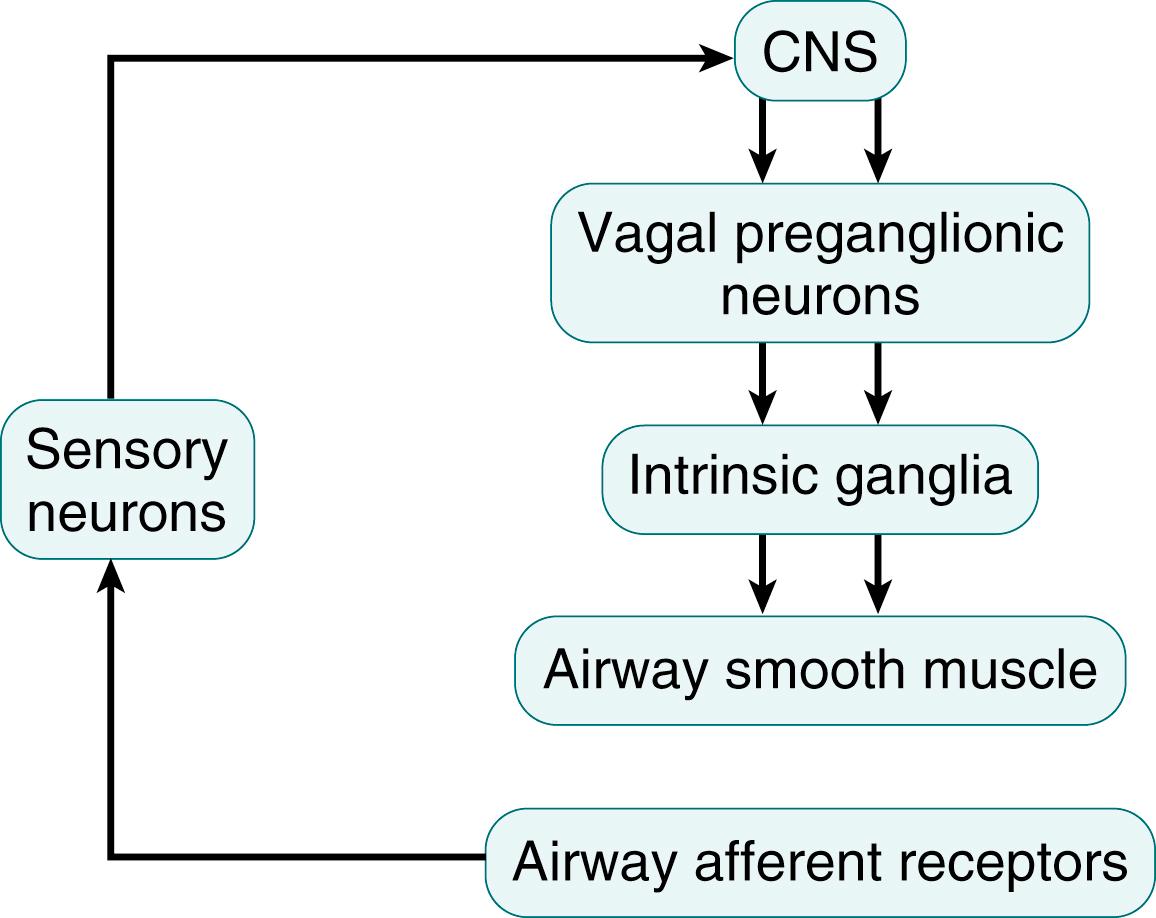
The AVPNs, innervating airways from the extrathoracic trachea to the most distal bronchiole, arise mainly within the brain stem from the rostral nucleus ambiguus and to a lesser degree from the rostral portion of the dorsal motor nucleus of the vagus nerve. These cholinergic cells (see Fig. 61.1 ), which express vasoactive intestinal peptide and brain-derived neurotrophic factor (BDNF), transmit cholinergic signals to intrinsic tracheobronchial ganglia. The individual innervation of postganglionic neurons from the vagus nerve gives rise to postganglionic fibers that regulate the functions of specific effector targets. In the postnatal period, neuronal innervation is already well developed, and choline acetyltransferase, a specific marker for cholinergic traits that synthesizes acetylcholine, appears in vagal preganglionic neurons, postganglionic neurons, and postganglionic fibers.
Signals transmitted through preganglionic nerves are relayed, filtered, integrated, and modulated by intrinsic ganglionic neurons before reaching their airway neuroeffector sites through postganglionic axons. This structural organization could explain the strong effects of a relatively small number of vagal efferent fibers on coordinated reflex changes in airway smooth muscle tone, submucosal gland secretion, and blood flow along the tracheobronchial tree. Postnatally, cholinergic innervation is the principal tonic input to airway smooth muscle bundles and may serve to protect the compliant immature airways from collapsing during expiration-induced compressive narrowing ( Fig. 61.2 ).
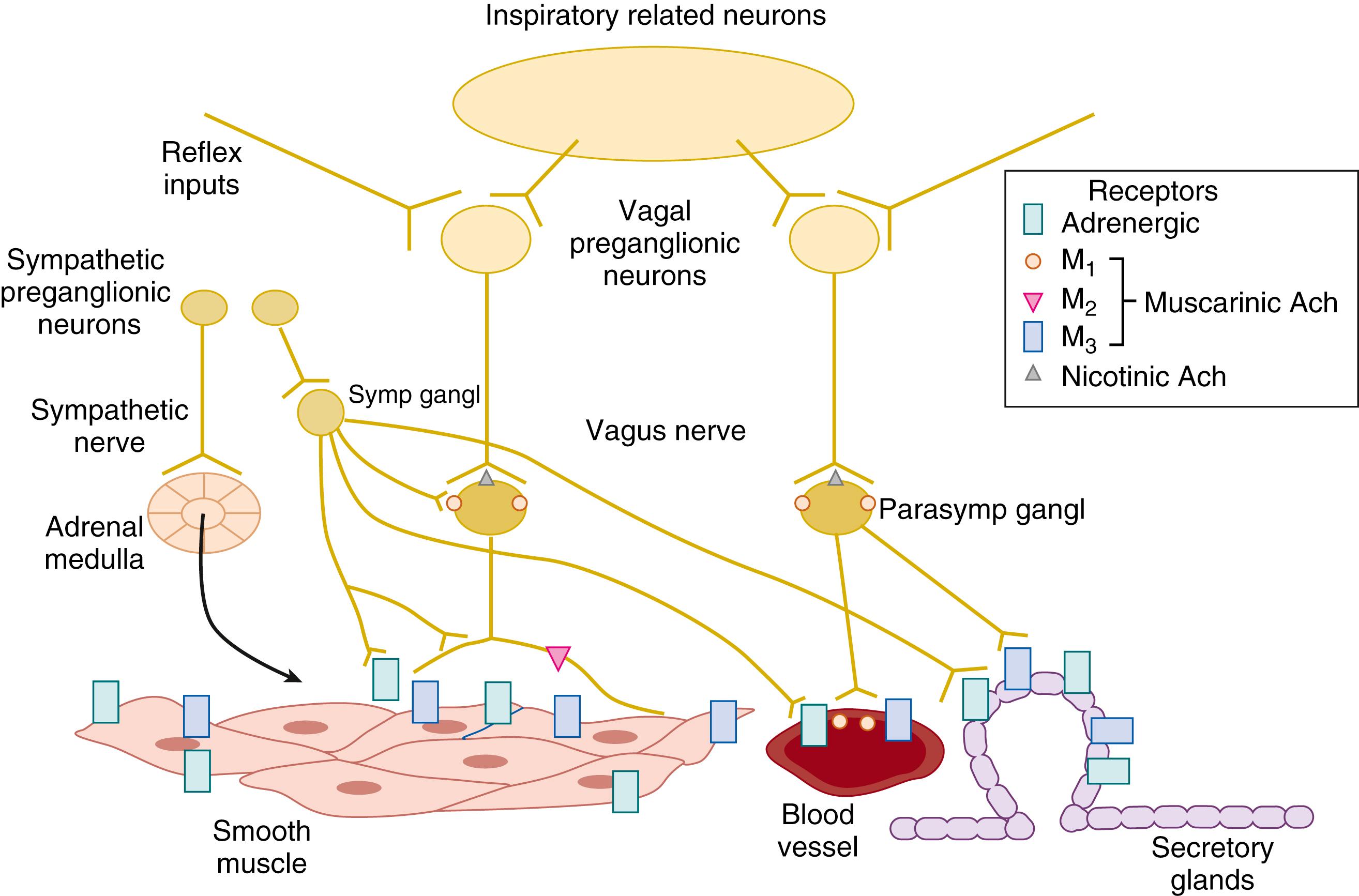
Muscarinic receptors mediate the responsiveness of airway smooth muscle to acetylcholine during early development and adult life. Studies in the developing airways and porcine lung from birth to adulthood reveal maturational changes in muscarinic receptor subtypes (M 1 , M 2 , M 3 ) that may explain pharmacologic changes during development. These muscarinic receptor subtypes, coupled to the family of G proteins, mediate airway contractile responses and their modulation. M 1 receptors are largely present on neuronal tissue and ganglia, and the selective M 1 receptor antagonist pirenzepine reduces the contractile response to vagal stimulation in newborn animals. ,
M 2 receptors are located on prejunctional postganglionic cholinergic fibers in airway smooth muscle in some species and exhibit an autoinhibitory action whereby the quantal release of acetylcholine in response to nerve stimulation is reduced by feedback inhibition. Sinus node M 2 receptors also mediate the bradycardia seen with vagal stimulation. Selective blockade or down-regulation of M 2 receptors may enhance vagally-mediated bronchoconstrictor responses and cause a reduction in the bradycardia response. M 2 autoinhibitory actions may be reduced or absent in the newborn because blockade of M 2 receptors does not enhance bronchoconstrictor responses to vagal stimulation. The potential role of lung injury and infection in modulating physiologic responses coupled to M 2 receptors in the newborn remains to be explored.
M 3 receptors are present on smooth muscle and mucous glands and airway epithelial cells where they initiate the events leading to smooth muscle contraction, airway narrowing, and mucus secretion. In the newborn, the density of M 3 receptors has been reported to be similar to that in the adult; however, they do not appear to be tightly coupled to G-protein signal transduction mechanisms that lead to smooth muscle contraction. In vivo studies show that M 3 receptor antagonism decreases bronchoconstrictor responses to vagal stimulation in the newborn that at low doses does not affect the bradycardic response. Among the many unanswered questions in the newborn are the extent of receptor subtype differentiation in human neonates, investigation of G-protein signal transduction, the role of M 2 autoinhibitory receptors in modulating airway tone, and the possible effect of inflammatory lung disease on muscarinic receptor regulation and its pharmacologic manipulation.
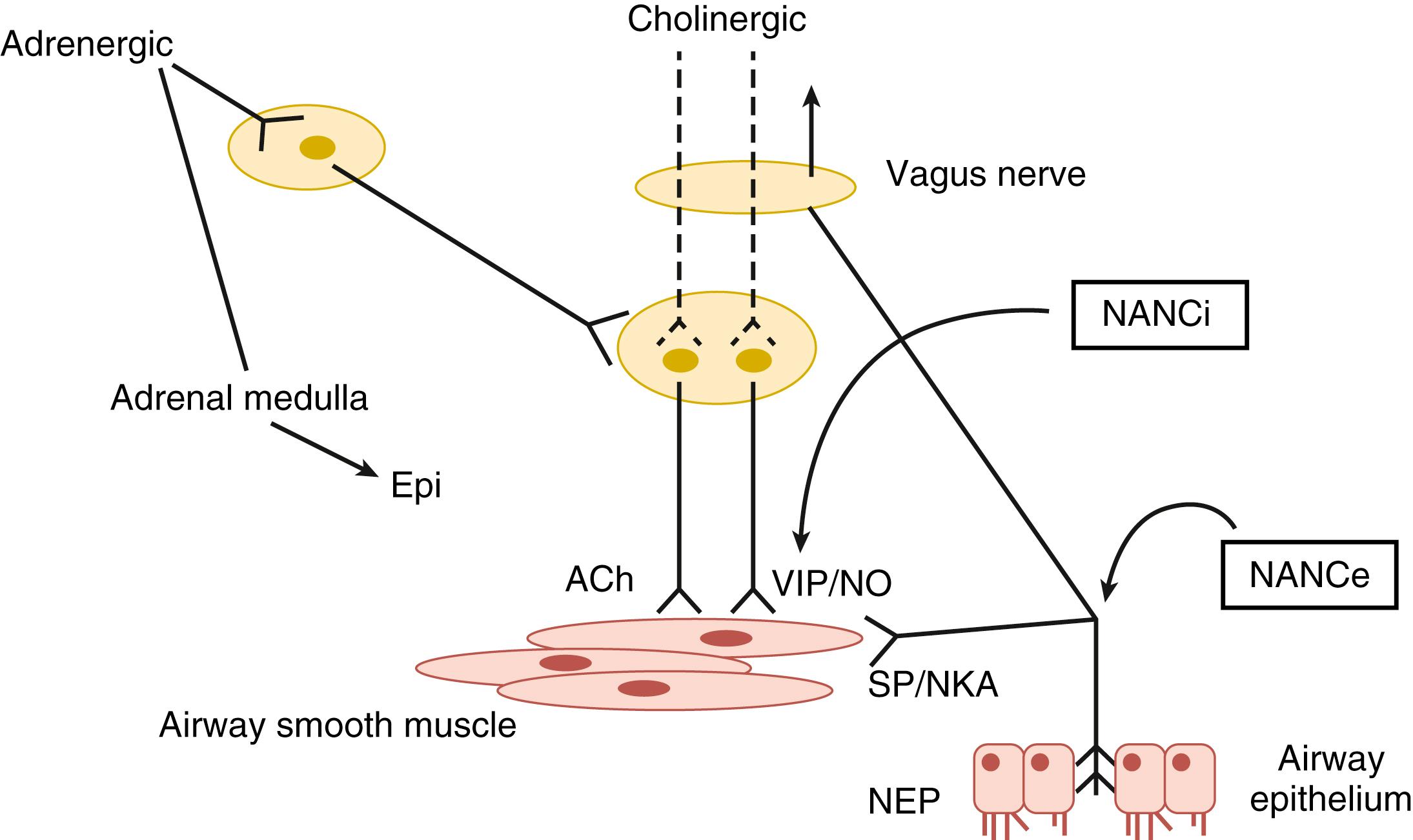
The bronchopulmonary sensory receptors are specialized units for detecting changes in chemical, mechanical, or thermal stimuli. They originate from the bipolar airway vagal afferent neurons that are located in the nodose and jugular ganglia and participate in reflex events. Sensory fibers affect the function of lower airway effector units via a local network that includes axon reflex responses. Endogenous substance P released from sensory nerve endings (i.e., C fibers that are mainly nonmyelinated nerve terminals) facilitates synaptic transmission in airway postganglionic neurons, suggesting the presence of control mechanisms within the tracheobronchial system, which also reflexly influence activity of AVPNs.
Sensory neural fibers from the airways also enter the brain stem through the solitary tract and make synapses with the second-order nucleus tractus solitarius neurons that project to the AVPNs. Excitatory signals arise from bronchopulmonary rapidly adapting lightly myelinated receptor afferents or nonmyelinated C-fiber terminals via release of glutamate, which activates α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors expressed by these nucleus tractus solitarius neurons. The processed information is then transmitted to AVPNs via the glutamate-AMPA signaling pathway and from the AVPNs to the bronchopulmonary effector system through acetylcholine release. Maturational data in ferrets indicate a decrease in the number of efferent AVPNs during the second postnatal week. It is possible that early postnatal injury may modify this normal remodeling.
Extrinsic sympathetic innervation of airway smooth muscle is highly species-specific, and in airway smooth muscle in humans, direct sympathetic innervation appears to be lacking ( Fig. 61.3 ). Nevertheless, circulating catecholamines activate airway adrenoreceptors to exert specific actions that affect smooth muscle contractile function. β-Adrenergic responses in airway smooth muscle are composed of two inhibitory actions. First, relaxation of airway smooth muscle mediated by airway β 2 -receptors that are coupled to the stimulatory G protein and adenylate cyclase; and second, inhibition of acetylcholine release from postganglionic vagal axons through prejunctional α 2 -adrenergic and β 1 receptors in some species. Activation of β-adrenergic receptors is the pharmacologic basis for neonatal bronchodilator therapy. The airway relaxant response to β-adrenoreceptor stimulation actually appears to decrease with advancing maturation, and several mechanisms, including greater muscarinic antagonism of β-receptor responses and attenuated expression of M 2 muscarinic receptors, have been proposed.
The second category of adrenergic responses is attributed to α-adrenoceptors of which both α 1 and α 2 subtypes play a role. Data indicate that in adult humans, α-adrenergic contractile responses of airway smooth muscle are weak or absent, although this may not hold true for the newborn. A potential role for α-adrenergic receptors in the control of airway smooth muscle in newborn infants with bronchopulmonary dysplasia (BPD) is supported by observations in preterm infants with chronic lung disease in whom ophthalmic application of the α 1 -adrenergic agonist phenylephrine resulted in an increase in total pulmonary resistance and a decrease in compliance. The deterioration in lung mechanics was attributed to α 1 -receptor-mediated contraction of airway smooth muscle.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here