Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The editors wish to thank David P. Carlton for his excellent work on this chapter in the fifth edition. It has been republished here essentially unchanged.
Before birth, the lung is filled with liquid secreted by the epithelia lining the potential air spaces of the fetal lung. The amount of liquid retained in the fetal lung is similar to the functional residual capacity of the postnatal lung, and maintenance of this dynamic template is an important factor in fetal lung growth. Under normal circumstances, secretion of liquid into the potential airspaces slows as birth approaches, ultimately switching to frank liquid absorption during labor and after birth. Liquid is removed from the lung lumen by a combination of mechanical drainage and liquid absorption across the lung epithelium ( Fig. 59.1 ). The liquid absorption mechanism is augmented by an increase in epinephrine associated with labor and delivery. This epinephrine responsiveness is absent in the very immature fetal lung and is triggered in the last half of gestation by thyroid and steroid hormones. The increase in oxygen tension associated with the onset of air breathing also contributes to the absorptive response of the epithelium. Because the absorptive mechanism of the fetal lung is maturationally regulated, premature infants and term infants who are “immature for gestational age” may not clear fluid from their airspaces in an effective manner.
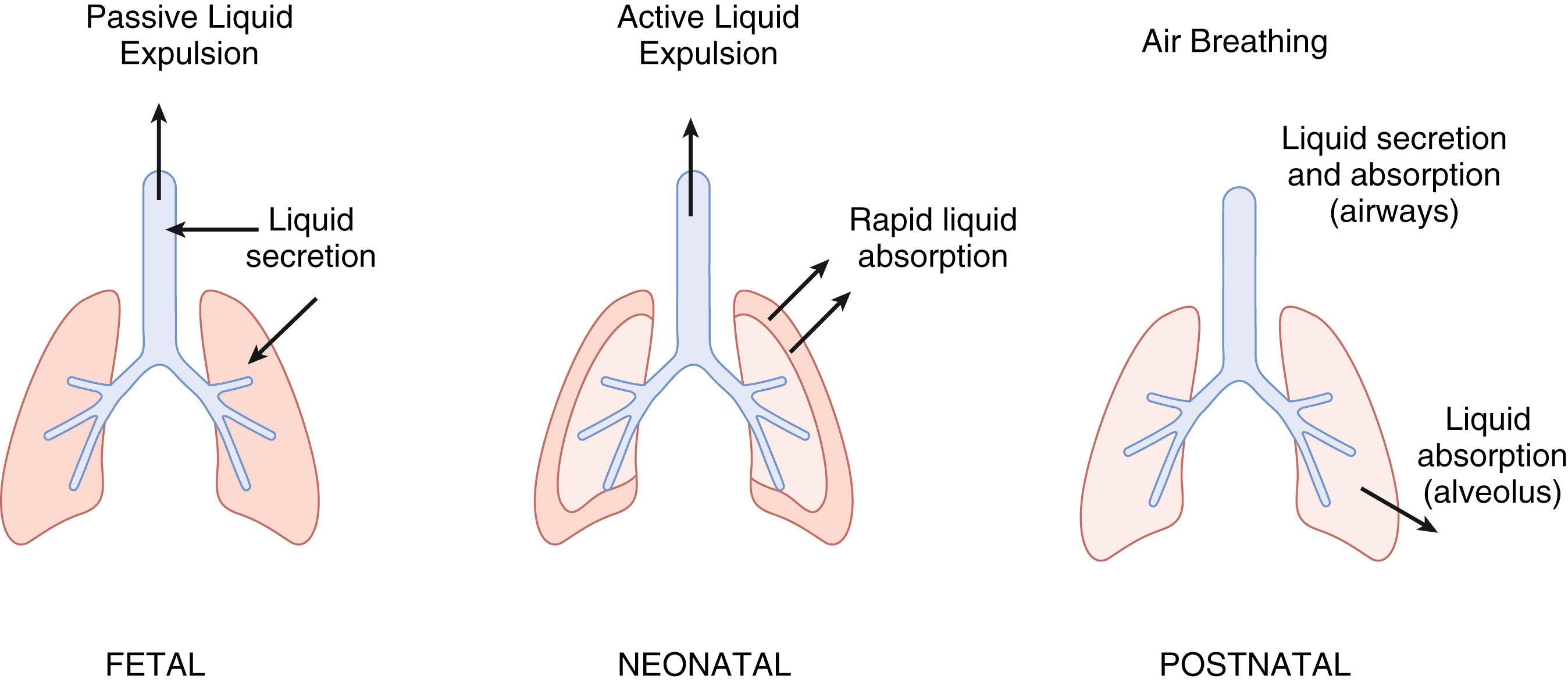
Initial observations about the nature of fetal lung liquid centered around the intuitive assumption that the liquid was amniotic fluid aspirated into the lung. However, the observation that tracheal ligation allowed progressive lung distention made this proposal moot. Later, experiments in fetal sheep showed not only that the electrolyte composition of the lung liquid and amniotic fluid were distinct, but also that the chloride (Cl) concentration of fetal lung liquid was higher than that in plasma, suggesting that there might be an active secretory component to the formation of fetal lung liquid. ,
Fetal lung liquid is generated by active secretion across the lung epithelium. The rate of liquid secretion has been measured in fetuses of several mammalian species, but most confidently in the fetal lamb. The secretion rate increases in fetal sheep from approximately 1.5 mL/kg/h at around midgestation to 5 mL/kg/h later in gestation. , After its secretion, the liquid exits the lung into the pharynx where it either contributes to the amniotic fluid or is swallowed and contributes to normal fetal gastrointestinal contents. The lung liquid generates a pressure of approximately 2 mm Hg relative to that measured in amniotic fluid, largely due to restriction to flow through the larnyx. In the more mature fetal lamb, the lung volume has been found to be similar to that of the functional residual capacity of the postnatal lung, approximately 30 mL/kg body weight. This filling of the lung with liquid results in a sort of “dynamic template,” around which the lung architecture develops. If the expected fetal lung volume is diminished experimentally, the lung becomes hypoplastic, and if the volume is made to increase, the lung becomes, by some measures, hyperplastic.
Fetal breathing results in small and irregular movements of liquid (∼0.5 mL) in the fetal airways. If fetal breathing is altered neurologically, the lung becomes hypoplastic, presumably as a result of alterations in fetal lung volume.
Peristaltic contractions of the developing airways cause changes in local lung liquid flow and pressure that may also be important in lung development. Striking contractions of embryonic airways were first observed in chick embryos in the 1920s. Studies on various animal models demonstrate that these contractions cause peristalsis of the airway similar to that seen in the gut. These peristaltic waves propagate distal movement of intraluminal liquid and cause expansion of the endbuds. Smooth muscle contractions were inhibited by the calcium (Ca 2+ ) channel blocker nifedipine. However, it is not clear whether the observed lung hypoplasia after nifedipine administration was a result of the inhibition of peristalsis or whether liquid secretion was influenced by this intervention.
Given that ligation of fetal airways promotes lung growth distal to that point, surgical obstruction of the fetal airway has evolved as a technique for prevention or correction of potential lung hypoplasia, particularly with lung anomalies associated with congenital diaphragmatic hernia. However, early enthusiasm for fetal surgery has been tempered by poor results. The traditional hypothesis that lung hypoplasia results from compression by herniating abdominal viscera has been challenged because pulmonary anomalies have been observed before herniation of abdominal contents into the chest. , The role of ion transport in the pathogenesis lung hypoplasia is not known.
At the time of birth, at least in the term newborn, approximately 100 mL of lung liquid must be removed. This is achieved as a result of a series of mechanical and ion transport events.
Experiments in fetal animals demonstrate that both lung liquid volume and secretion decrease near delivery. , , , The decrease in lung liquid volume is likely a result not only of a decrease in transepithelial fluid movement, but also from the exit of fluid from the lung.
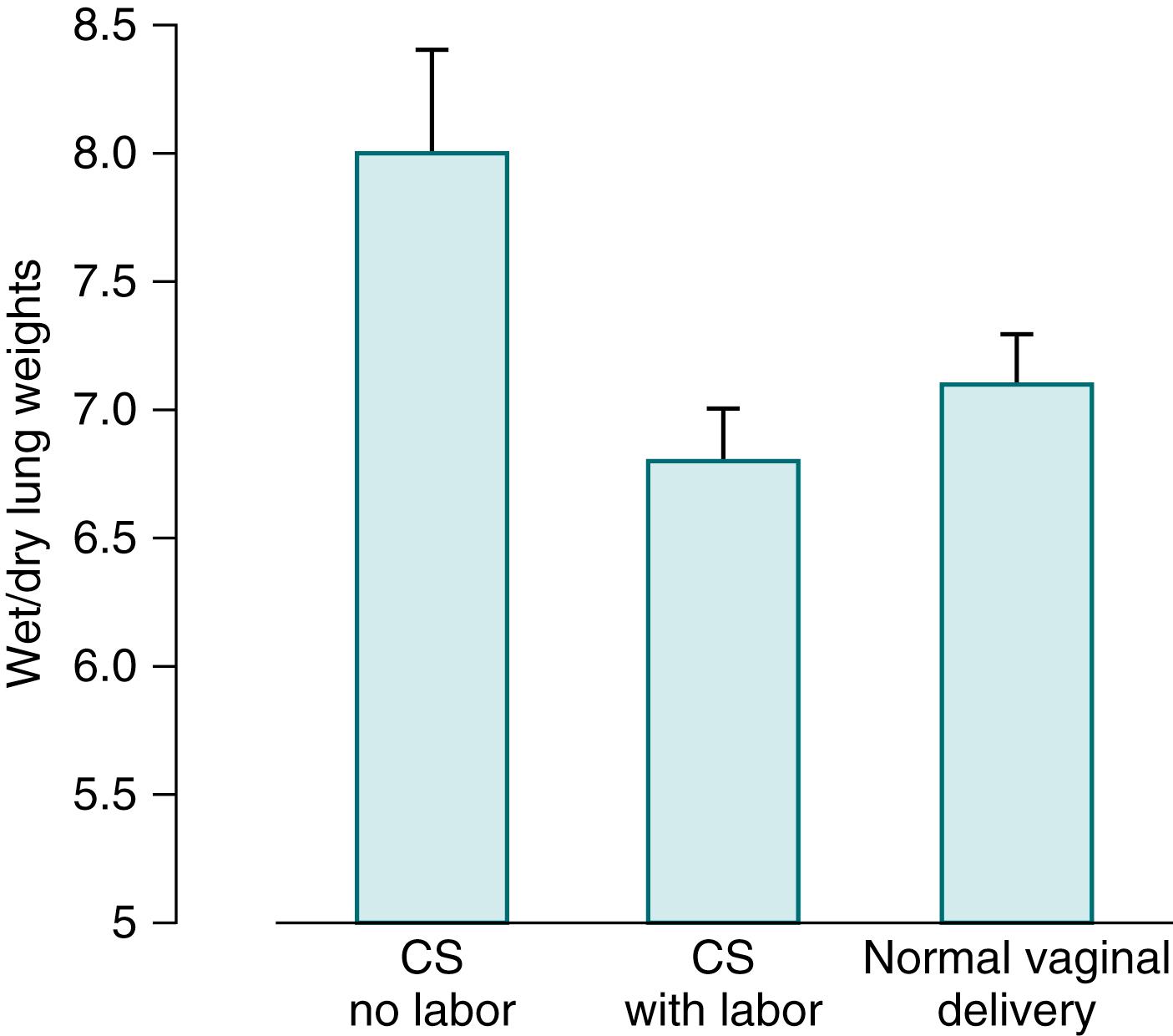
Several lines of evidence suggest that delivery by cesarean section (CS) slows lung liquid clearance, particularly when it occurs before the beginning of labor. , Rabbits that are born at term, either vaginally or by CS after the onset of labor, have less water in their lungs than do rabbits that are delivered by CS without prior labor ( Fig. 59.2 ). This observation is one of several that challenge the intuitive appeal of the “thoracic compression” during vaginal birth as critical for lung liquid removal.
Once labor begins, at least some portion of lung liquid begins to move across the pulmonary epithelium in a direction the reverse of that before labor. Active transcellular Na + absorption is the mechanism that drives liquid out of the lumen into the interstitial space. The excess interstitial liquid then moves into the pulmonary circulation and lung lymphatics for return to the central circulation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here