Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Embryogenesis results from the divergent proliferation, migration, survival, and differentiation of cells derived from a single cell, the fertilized egg. Regulatory signals, applied to successive generations of cells, establish the architecture of the early embryo. The same processes are recapitulated during the subsequent development of limbs, organs, and craniofacial structures. Consequently, disruption of these reiterative mechanisms distorts seemingly unrelated structures, as observed in human malformation syndromes. Conversely, the same mechanisms guide tissue regeneration following injury. The elucidation of embryonic regulatory mechanisms is therefore of therapeutic importance.
This chapter introduces representative modes of cellular regulation in the context of early embryonic events. The outcome of these mechanisms is modulated expression of proteins that initiate critical developmental events. Protein transcription is controlled by promoters upstream of the coding region and enhancers that lie at a distance from the gene. Promoters and enhancers contain DNA motifs that bind specific transcription factors that then modulate gene transcription. Transcription factors typically bind multiple genes, some of which encode additional transcriptional regulators. Besides its expression, the activity of a transcription factor depends on its posttranslational modifications, its translocation to the nucleus, and the presence or activation of cofactors. The same variables also determine whether that factor increases or decreases the activity of a particular gene. The genes that can be activated at any moment are dependent on whether the chromosomes on which they reside are open and active or whether they are condensed and inaccessible. The state of a cell’s chromatin is governed by enhancers and by epigenetic modifications that change during development. Gene expression during embryogenesis is therefore subject to regulation by multiple mechanisms.
Regulatory processes may be broadly divided into autonomous and conditional mechanisms. Autonomous mechanisms are internal to the cell, meaning that the regulated behaviors will continue if the cell is moved to a different environment. An example occurs during the implantation of female embryos, when the dividing cells within the morula are segregated into the blastocyst inner cell mass, which becomes the embryo, and the trophoblast, which becomes the placenta ( Fig. 4.1 ). This segregation is determined by the parental origin of the active X chromosome within each cell. Balanced gene expression requires that one of the two X chromosomes within each cell be silenced. This inactivation occurs randomly, and the inactivated X chromosome becomes the Barr body visible in somatic cells. Blastocyst cells containing paternally imprinted active X chromosomes form the trophoblast, while maternally dominated cells become the inner cell mass.
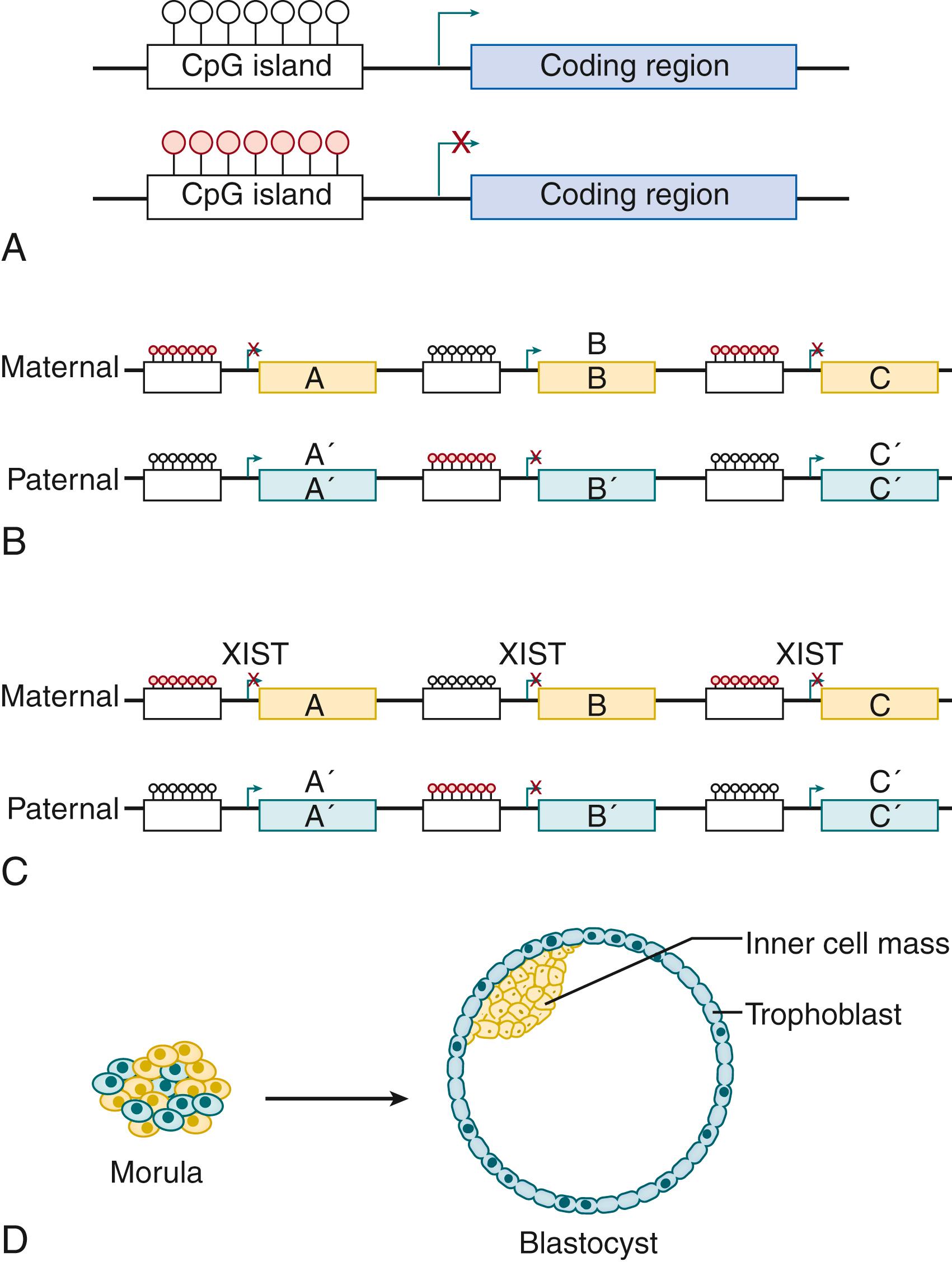
The genes expressed from the active X chromosome differ by parental source. This distinction is conferred by DNA methylation, the covalent addition of methyl groups to cytosine bases within gene promoters. Methylated cytosines are typically located next to guanosines, resulting in the methylation of both DNA strands at diagonally adjacent cytosines. Regulated genes contain clusters of these cytosine/guanosine dinucleotides (so-called CpG islands ), and their methylation usually reduces gene transcription. The methylation of maternally derived chromosomes differs from those contributed by the sperm, and these patterns can be transferred to daughter cells upon replication. The persistence of paternal methylation also differs from that of the mother; sperm DNA is demethylated within hours of fertilization, whereas the methylation of maternal chromosomes persists into the early morula. The balance between maternally and paternally imprinted chromosomes is critical; a zygote with no maternal DNA (resulting from fertilization of an egg with no nucleus) will form a hydatidiform mole, a dysplastic and occasionally invasive placenta with little or no fetal tissue.
Parental imprinting also contributes to later development, as demonstrated by the expression of UBE3A, a component of the ubiquitin pathway. The maternal allele of UBE3A is almost exclusively expressed in the developing hippocampus and cerebellum, and maternal mutations in UBE3A underlie Angelman syndrome, a disorder characterized by developmental disability, ataxia, and seizures. , In contrast, paternal mutations in UBE3A result in Prader-Willi syndrome. Although these children also exhibit developmental delay, they are differentiated by hypotonia, obsessive-compulsive behaviors, and insatiable hunger.
In contrast to autonomous regulation, conditional regulation is imposed on cells by environmental factors. The predominance of conditional regulation was demonstrated by Hans Spemann and Hilde Mangold in 1923, who found that implantation of the anterior dorsal lip from one newt embryo into the ventral mesoderm of another induced the formation of a complete second body axis, including a second head. Because most of this material was derived from recipient tissue, it was apparent that the anterior lip, named the organizer by Spemann, induced ventral tissue to undergo dorsal differentiation, which is necessary for neural, heart, kidney, and somite as well as head formation.
In mammals, one of the earliest examples of conditional regulation arises during formation of the bilayer germ disk, during which the inner cell mass differentiates into the epiblast, the pluripotent precursor of the fetal tissues, and the hypoblast, a transient extraembryonic tissue. In some mammals, fibroblast growth factor (FGF)-4 is secreted by primordial epiblast cells to induce the differentiation of as yet uncommitted cells of the inner cell mass into hypoblast ( Fig. 4.2A ). FGFs comprise a large family of soluble peptides that are secreted into the extracellular environment and bind transmembrane receptors containing tyrosine kinases.
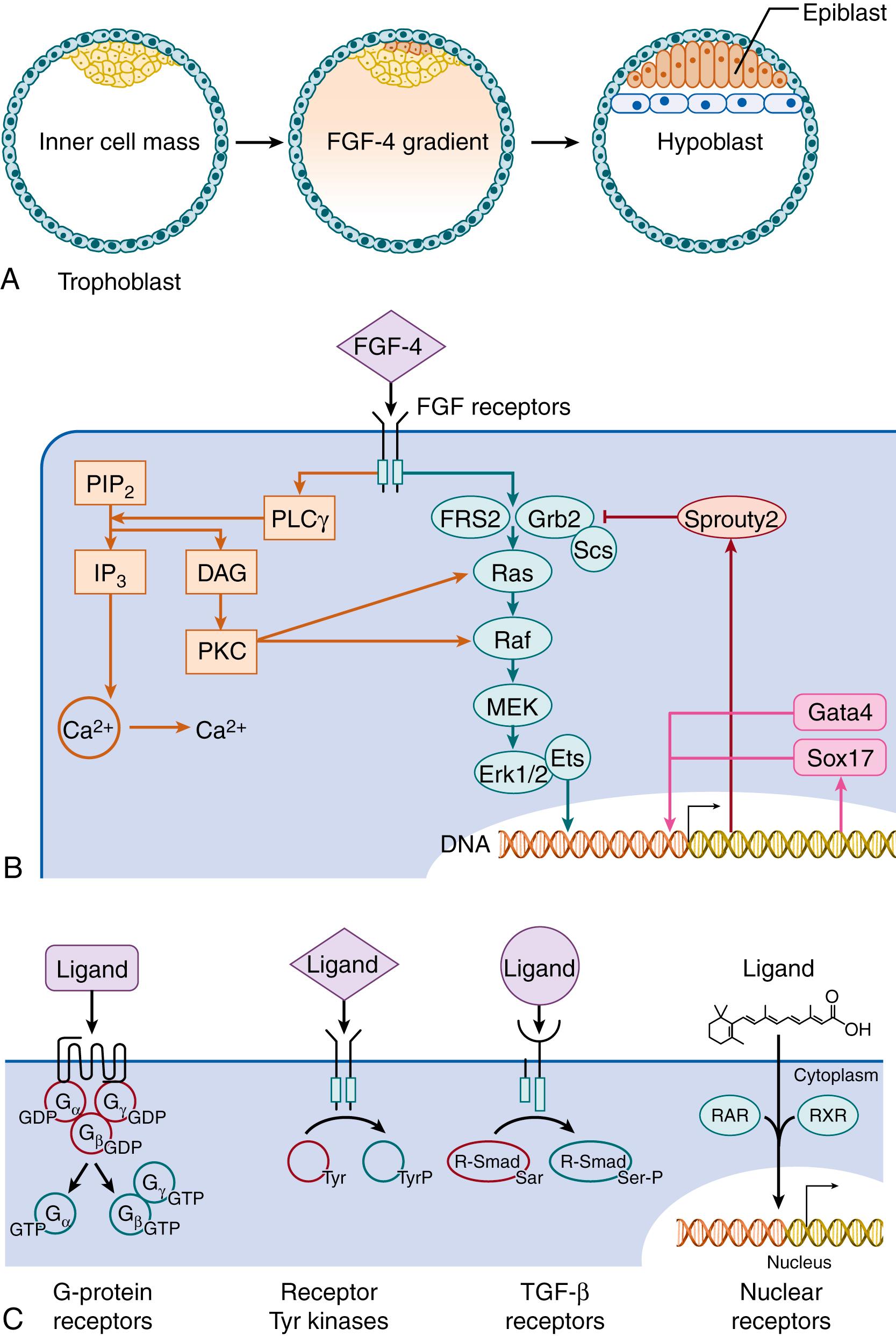
The mechanism by which FGF-4 signals are transmitted to the nucleus is representative of most receptor tyrosine kinases (see Fig. 4.2B ). Upon binding ligand, FGF receptors dimerize and activate one another. The activated receptors phosphorylate intracellular substrates including FGF receptor substrate (FRS) 2 and Grb2, which then bind the GTP-exchange protein Sos. The resulting heterotrimer translocates to the plasma membrane to activate Ras, which, in turn, initiates the sequential activation of Raf, MEK, the mitogen-activation protein (MAP) kinases Erk1 and Erk2, and, ultimately, Ets family transcription factors that then enter the nucleus to activate the promoters of numerous genes. Each step in this process represents a node at which the FGF-4 signal may be amplified or attenuated, and the entire process from receptor to transcription factor constitutes a signaling pathway. The FGF pathway also incorporates a negative feedback mechanism; Sprouty2 is a cytosolic protein that, upon phosphorylation by FGF receptors, interrupts Erk activation by inhibiting the association of FRS2 with Grb2. Negative feedback stabilizes signaling and is integral to most biologic and human-engineered control systems.
The activity of the FGF signaling pathways typically peaks within a few minutes and is terminated within an hour by receptor aggregation, internalization, and destruction. However, the resulting transcriptional events may induce sustained changes in the embryo by inducing or interrupting the expression of transcription factors that sustain their own expression. Cells fated to epiblast express the self-sustaining transcription factors Nanog and Oct4, whereas those destined to become hypoblast express Gata4 and Sox17 instead. ,
The many molecules that regulate embryogenesis can be categorized on the basis of their receptors. Besides the fibroblast, epidermal, and insulin-like growth factors that signal through receptor tyrosine kinases, important mediators include members of the transforming growth factor (TGF)-β superfamily that signal through receptor serine/threonine kinases; Wnt and Hedgehog, which signal through G-protein coupled 7-transmembrane domain receptors, and lipid-soluble factors such as retinoic acid and steroids that cross the plasma membrane, complex with nuclear receptors and enter the nucleus to bind DNA directly (see Fig. 4.2C ).
Although each receptor type is associated with a canonical pathway, multiple signaling pathways are initiated by each receptor. Besides the ERK pathway, FGF receptors also modulate intracellular calcium by activating phospholipase C gamma (PLCγ), an enzyme that hydrolyzes phosphatidylinositol 4, 5-bisphosphate (PIP 2 ) into inositol 1,4,5 triphosphate (IP 3 ). IP 3 receptors embedded in the endoplasmic reticulum release calcium into the cytosol, where it regulates cellular activities as diverse as muscle contraction, nuclear breakdown, and egg fertilization. On the other hand, most intracellular pathways are also activated by multiple receptors; for example, calcium fluxes are also induced by ion channels and G-protein receptors, and Erk signaling is also initiated by receptor serine-threonine kinases. Cell signaling is therefore best described as a network of mutually regulating parallel processes that modulate cell behaviors in a time-dependent and recursive manner.
Signaling proteins can sustain or suppress the activity of their own expression, respectively conferring positive or negative feedback on their own signaling. The autoregulation of TGF-β family signaling peptides contribute prominently to embryonic morphogenesis. Epiblast cells produce Nodal, a member of the TGF-β superfamily that also includes activin and the bone morphogenetic proteins (BMPs). Like other TGF-β family proteins, Nodal is secreted as an inactive precursor into the extracellular space, processed into a mature active dimer by the extracellular enzymes furin and PACE4, and binds complexes of type I and type II cell surface serine-threonine kinase receptors to stimulate targeted cells ( Fig. 4.3A ).
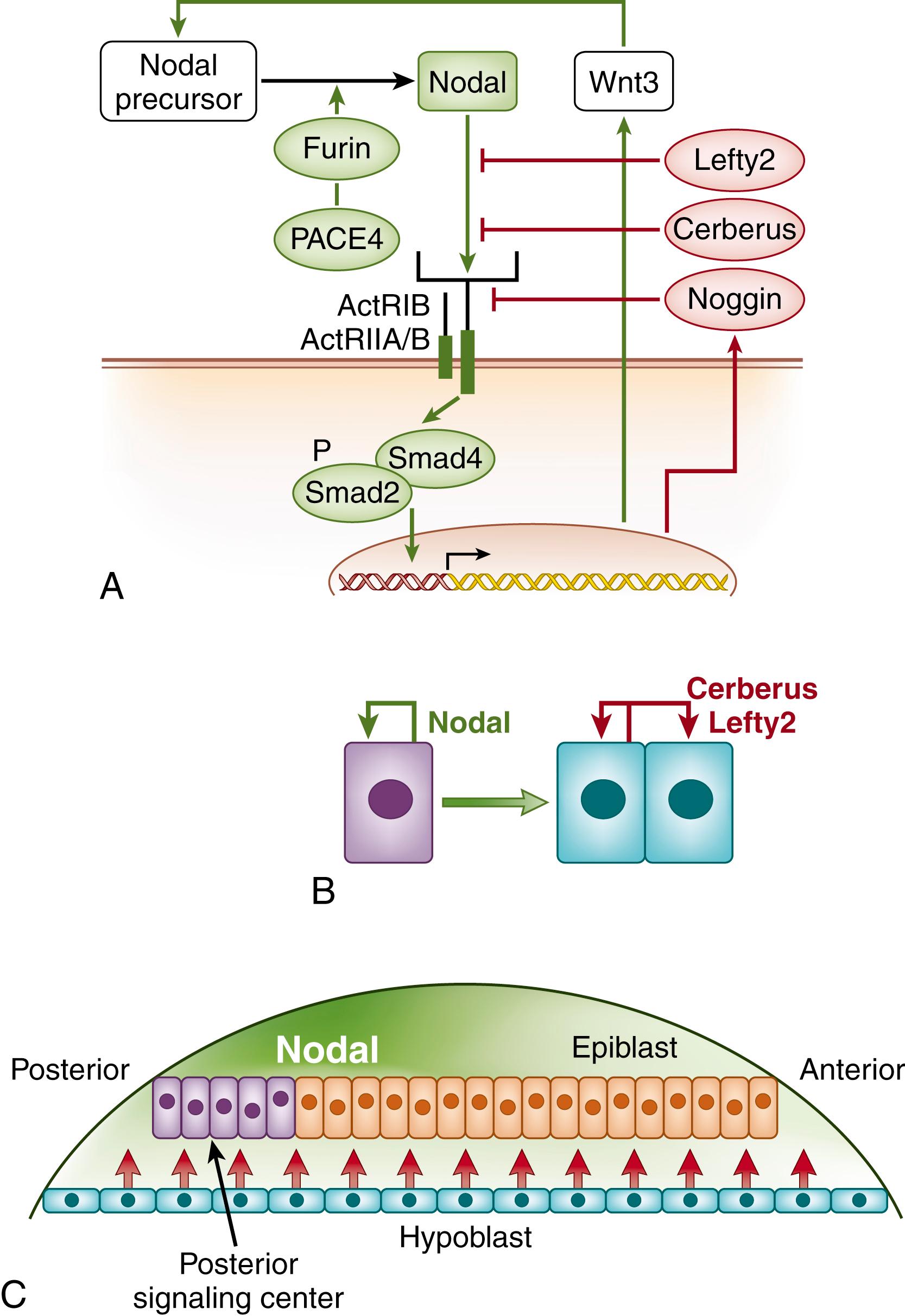
Upon ligand binding, TGF-β receptor complexes activate cytoplasmic Smad proteins. Of the eight mammalian SMAD proteins, five are receptor activated. One, Smad4, is a cofactor for receptor-activated Smads, and two, Smad6 and Smad7, inhibit Smad signaling. Nodal stimulation causes Smad2 to become phosphorylated and bind Smad4. A cofactor that confers specificity to the complex is also recruited; one such cofactor is the forkhead-family transcription factor FoxH1, which targets the complex to the promoter of Mix2 , a homeodomain transcription factor (discussed later). Similarly, Smad3 partners with FoxO1, FoxO2, and FoxO3 to up-regulate the cyclin-dependent kinase inhibitor p21Cip1. Nodal thus modulates the transcription of multiple genes that pattern the embryo and slow cell proliferation.
Among the proteins induced by Nodal is Nodal itself. Nodal up-regulates Wnt3, which, in turn, maintains Nodal expression in the posterior part of the epiblast (see Fig. 4.3B ). Nodal also induces the expression of the Nodal inhibitors Cerberus and Lefty2 in the hypoblast. Together, Nodal autoinduction and autoantagonism restrict its expression to a region termed the posterior signaling center.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here