Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Characterization of the structure and function of red blood cell (RBC) membrane proteins and their genes ( Fig. 46.1 ) has led to considerable advances in our understanding of the molecular pathology of membrane-associated disorders, including the definition and characterization of mutations of membrane proteins as a well-defined cause of hereditary hemolytic disease. Likewise, knowledge of the molecular mechanisms underlying changes in RBC deformability, structural integrity, and shape has advanced. RBC shape abnormalities often provide a clue to the pathobiology and diagnosis of the underlying disorder. This chapter categorizes RBC membrane disorders according to the following morphologic and clinical phenotypes: (1) hereditary spherocytosis (HS); (2) hereditary elliptocytosis (HE), hereditary pyropoikilocytosis (HPP), and related disorders; (3) Southeast Asian ovalocytosis (SAO); (4) hereditary and acquired acanthocytosis; and (5) hereditary and acquired stomatocytosis ( Tables 46.1 and 46.2 ).
| Gene | Disorder | Comment |
|---|---|---|
| α-Spectrin | HS, HE, HPP, NIHF | Location of mutation determines clinical phenotype. α-Spectrin mutations are most common cause of typical HE |
| Ankyrin | HS | Most common cause of typical dominant HS |
| Band 3 | HS, SAO, NIHF | In HS “pincer-like” spherocytes on smear presplenectomy. SAO erythrocytes have transverse ridge or longitudinal slit |
| β-Spectrin | HS, HE, HPP, NIHF | Location of mutation determines clinical phenotype. In HS, acanthrocytic spherocytes on smear presplenectomy |
| Protein 4.2 | HS | Common in Japanese HS |
| Protein 4.1 | HE | |
| GPC | HE | Concomitant protein 4.1 deficiency is basis of HE in GPC defects |
| Shape | Pathobiology | Diagnosis |
|---|---|---|
| Microspherocytes |
|
|
| Elliptocytes |
|
|
| Poikilocytes/fragments |
|
|
| Schistocytes, fragmented red cells | Red cells “torn” by mechanic trauma (fibrin strands, turbulent flow) | “Microangiopathic” hemolytic anemia associated with disseminated intravascular coagulation, thrombotic thrombocytopenic purpura, vasculitis, heart valve prostheses |
| Acanthocytes |
|
|
| Echinocytes |
|
|
| Stomatocytes |
|
|
| Target cells |
|
|
For a better understanding of the pathobiology of membrane disorders, membrane protein-protein and protein-lipid interactions are classified into two categories, vertical and horizontal interactions. Vertical interactions, which are perpendicular to the plane of the membrane, stabilize the lipid bilayer. These interactions include spectrin-ankyrin–band 3 interactions, spectrin-protein 4.1R–junctional complex proteins linkage, spectrin-ankyrin–Rh multiprotein complex linkage, and the weak interactions between the skeletal proteins and the negatively charged lipids of the inner half of the membrane lipid bilayer. Horizontal interactions, which are parallel to the plane of the membrane, support the structural integrity of erythrocytes after their exposure to shear stress. Horizontal interactions involve the spectrin heterodimer association site, where spectrin heterodimers assemble into tetramers, the principal building blocks of the membrane skeleton, and the contacts of the distal ends of spectrin heterodimers with actin and protein 4.1 R within the junctional complex. Although interactions between proteins of the erythrocyte membrane are significantly more complex than can be classified by horizontal and vertical interactions, the model serves as a useful starting place for understanding erythrocyte membrane protein interactions, particularly in reference to membrane-related disorders.
According to the vertical/horizontal model, HS is considered a disorder of vertical interactions. Although the primary molecular defects in HS are heterogeneous (including deficiencies or dysfunctions of α- and β-spectrin, ankyrin, band 3, and protein 4.2), one common feature of HS RBCs is a weakening of the vertical contacts between the skeleton and the overlying lipid bilayer membrane together with its integral proteins. Consequently, the lipid bilayer membrane is destabilized, leading to the release of bilayer lipids from the cells in the form of skeleton-free lipid vesicles. This lipid loss, in turn, results in membrane surface area deficiency and spherocytosis.
In most patients with HE and the related disorder HPP (see Hereditary Elliptocytosis and Related Disorders), the principal lesion involves horizontal membrane-protein associations, primarily spectrin dimer-dimer interactions. In a subset of HE patients with a deficiency or a dysfunction of protein 4.1 R or glycophorin C (GPC), the horizontal defect resides in the junctional complex, where the distal ends of spectrin tetramers connect to actin, in conjunction with protein 4.1 R. In patients with severely dysfunctional spectrin mutations, the weakened spectrin dimer-dimer self-association disrupts the skeletal lattice, leading to a marked skeletal instability and cell fragments. In patients with mildly dysfunctional spectrins, the RBC shape is that of biconcave elliptocytes. It is speculated that elliptocytes are permanently deformed cells because the weakened horizontal interactions facilitate a shear stress-induced rearrangement of skeletal proteins, precluding recovery of the normal biconcave shape. This hypothesis is not applicable to all forms of elliptocytosis. For example, in SAO, the elliptocytic/ovalocytic cells containing mutant band 3 protein are rigid and “hyperstable” rather than unstable.
The mechanism of acanthocytosis and stomatocytosis associated with defects of membrane proteins is much less clear. Most forms of acanthocytosis are associated with either acquired or inherited abnormalities of membrane lipids (e.g., acanthocytosis in end-stage liver disease or abetalipoproteinemia). In rare cases with acanthocytosis, membrane protein abnormalities have been detected, but the associated mechanisms leading to acanthocyte formation are unknown. These abnormalities occur in the McLeod phenotype, the chorea-acanthocytosis syndrome, and other rare disorders. In acanthocytosis erythrocytes, agents that interact with the lipids of the inner lipid bilayer leaflet normalize the shape. These studies suggest that the shape abnormalities reflect an asymmetry in the distribution of membrane lipids between the two halves of the RBC lipid bilayer as predicted by the bilayer couple hypothesis. According to the bilayer hypothesis, the shape of the RBC reflects the ratio of the surface areas of the two hemileaflets of the bilayer. The preferential expansion of the outer leaflet leads to RBC crenation (echinocytosis or acanthocytosis), whereas expansion of the inner lipid bilayer produces a cup shape (stomatocytosis) and surface invaginations.
The typical features of HS include a dominantly inherited hemolytic anemia of mild to moderate severity, spherocytosis on the peripheral blood film, and a favorable response to splenectomy. The clinical spectrum of HS is variable and includes both mild and asymptomatic forms, as well as severe forms that appear in infancy. The previously reported HS prevalence in Western populations of 1 in 4000 persons is an underestimation because milder forms of HS might be asymptomatic, suggesting a prevalence of 1 in 2000 individuals. HS has been reported worldwide, particularly in Northern European and Japanese populations, but its prevalence in other ethnic groups is unknown.
Two major factors are involved in HS pathophysiology: (1) an intrinsic RBC defect and (2) an intact spleen that selectively retains and damages abnormal HS erythrocytes. An inherited deficiency or dysfunction of proteins of the erythrocyte membrane leads to a multistep process of accelerated HS RBC destruction. Destabilization of the lipid bilayer facilitates a release of lipids from the membrane, leading to surface area deficiency and the formation of poorly deformable spherocytes that are selectively retained and damaged in the spleen.
The molecular basis of HS is heterogeneous. Based on densitometric quantitation of membrane proteins separated by polyacrylamide gel electrophoresis, HS can be divided into the following subsets: (1) isolated deficiency of spectrin, (2) combined deficiencies of spectrin and ankyrin, (3) deficiency of band 3 protein, (4) deficiency of protein 4.2, and (5) no abnormality identified.
The reported mutations of isolated spectrin deficiency include defects of both α- and β-spectrin. Mutations of the β-spectrin gene have been identified in a number of patients with dominantly inherited HS associated with spectrin deficiency. A few cases have been associated with de novo β-spectrin gene mutations. With a few exceptions, these mutations are private and may be associated with decreased β-spectrin messenger ribonucleic acid (mRNA) accumulation. Mutations in the highly conserved region of β-spectrin involved in the interaction with protein 4.1 R likely lead to dysfunctional binding to protein 4.1 R and thereby the linkage of spectrin to actin.
In nondominantly inherited HS associated with isolated spectrin deficiency, the defect involves α-spectrin. In normal erythroid cells, α-spectrin is synthesized in large excess of β-spectrin. Thus, patients with one normal and one defective α-spectrin allele are asymptomatic because α-spectrin production remains in excess of β-spectrin synthesis, allowing normal amounts of spectrin heterodimers to be assembled on the membrane. Patients who are homozygotes or compound heterozygotes for α-spectrin defects suffer from moderate to severe HS.
The biochemical phenotype of combined spectrin and ankyrin deficiency is the most common abnormality found in the erythrocytes of HS patients. Ankyrin represents the principal binding site for spectrin on the membrane; thus, it is not surprising that ankyrin deficiency is accompanied by a proportional decrease in spectrin assembly on the membrane despite normal spectrin synthesis. Similar to HS associated with β-spectrin mutations, most ankyrin defects are private point mutations associated with decreased mRNA accumulation. In some cases, mutations of the ankyrin promoter leading to decreased ankyrin expression have been found. Approximately 15% to 20% of ankyrin gene mutations reported are de novo mutations.
A number of patients with atypical HS associated with karyotypic abnormalities involving deletions or translocations of the ankyrin gene locus on chromosome 8p have been described. Ankyrin deletions may be part of a contiguous gene syndrome with manifestations of spherocytosis, mental retardation, typical facies, and hypogonadism.
Deficiency of band 3 protein is found in a subset of HS patients who present with a phenotype of a mild to moderate dominantly inherited HS. Most, if not all, of these patients also have concomitant protein 4.2 deficiency. Numerous band 3 mutations associated with HS have been reported, spread throughout both the cytoplasmic and the membrane-spanning domains.
A number of band 3 mutations clustered in the membrane-spanning domain that replace highly conserved arginines have been described. These arginines, which are all located at the cytoplasmic end of a predicted transmembrane helix, exhibit defective cellular trafficking from the endoplasmic reticulum to the plasma membrane.
Alleles have been identified that influence band 3 expression and that, when inherited in trans to a band 3 mutation, aggravate band 3 deficiency and worsen the clinical severity of the disease.
Recessively inherited HS caused by mutations in protein 4.2 is relatively common in Japan. In these cases, an almost total absence of protein 4.2 from the erythrocyte membranes of homozygous patients is detected. Protein 4.2–deficient erythrocytes can also have a decreased content of ankyrin and band 3. Protein 4.2 deficiency also occurs in association with band 3 mutations, probably as a result of abnormal binding of protein 4.2 to the cytoplasmic domain of band 3.
A detailed listing of HS mutations is available in mutation databases maintained by the Human Gene Mutation Database (HGMD, http://www.hgmd.org ).
Hereditary spherocytes are intrinsically unstable, releasing lipids under a variety of in vitro conditions, including adenosine triphosphate (ATP) depletion or exposure of cells to shear stress. The loss of membrane material occurs through the release of vesicles containing integral proteins devoid of spectrin. During in vitro incubation, the loss of membrane material is sufficient to augment the surface area deficiency, as evidenced by increased osmotic fragility of the cells after incubation. It is assumed but not proved that a similar process takes place in vivo.
The molecular basis of HS is heterogeneous; thus, it is likely that surface area deficiency is a consequence of several distinct molecular mechanisms whose common denominator is either a weakening of the vertical connections between the skeleton and the lipid bilayer membrane or a weakening of the stabilizing effect of transmembrane proteins on adjacent lipid molecules of the plasma membrane. Hypothetic pathways that can lead to surface area deficiency are depicted in Fig. 46.2 . In patients with isolated spectrin deficiency or a combined deficiency of spectrin and ankyrin, the loss of RBC surface may be caused by an uncoupling of the lipid bilayer membrane from the underlying skeleton. In normal RBCs, the skeleton forms a nearly monomolecular submembrane layer occupying more than one-half of the inner surface of the membrane. Consequently, spectrin deficiency leads to a decreased density of this network. As a result, areas of the lipid bilayer membrane that are not directly supported by the skeleton are susceptible to release from the cells in the form of microvesicles.

In HS associated with a deficiency of band 3 protein, two hypothetic pathways may lead to a loss of surface area. One mechanism may involve a loss of band 3 protein from the cells. Because band 3 protein spans the lipid bilayer membrane many times, it is likely that a substantial amount of “boundary” lipids are released together with the band 3 protein, thus leading to surface area deficiency. Another possible mechanism may involve a formation of band 3-free domains in the membrane, followed by the formation of membrane blebs, which are subsequently released from the cells as microvesicles. Such a hypothesis is based on the observation that aggregation of intramembrane particles (composed principally of band 3) in ghosts leads to the formation of particle-depleted domains from which membrane lipids bleb off as microvesicles. Additional evidence supporting the latter model comes from the band 3 knock-out mouse model and from human, cow, and zebrafish cases of complete band 3 deficiency. Erythrocytes lacking band 3 spontaneously shed membrane vesicles, leading to spherocytosis and hemolysis.
HS RBCs, particularly those collected from the spleen, are somewhat dehydrated and abnormally permeable to monovalent cations, presumably as a consequence of the underlying membrane defect. The cellular dehydration may be caused by activation of pathways causing a selective loss of potassium and water or a hyperactive Na + /K + pump.
The importance of the spleen in the pathophysiology of hemolysis in HS was appreciated in the original description of the disease and has been substantiated by subsequent studies. HS cells are selectively destroyed in the spleen because of their poor deformability and because of the unique anatomy of the splenic vasculature that acts as a microcirculation filter.
The poor RBC deformability is principally a consequence of a decreased cell surface/cell volume ratio resulting from the loss of surface material. Normal discocytes have an excess surface, allowing them to deform and pass through narrow microcirculation openings. In contrast, HS RBCs lack this extra surface, and their poor deformability may be further impaired by cellular dehydration.
The principal sites of RBC entrapment in the spleen are fenestrations in the wall of splenic sinuses, where blood from the splenic cords of the red pulp enters the venous circulation. In rat spleen, the length and width of these fenestrations, 2 to 3 µm and 0.2 to 0.5 µm, respectively, are approximately half the RBC diameter. Electron micrographs show that very few HS RBCs traverse these slits. Consequently, the nondeformable spherocytes accumulate in the red pulp, which becomes grossly engorged.
Once trapped in the spleen, HS erythrocytes undergo additional damage or conditioning with further loss of surface area and an increase in cell density, as is evident in cells removed from the spleen at splenectomy. Some of these conditioned RBCs reenter the systemic circulation, as revealed by the “tail” of the osmotic fragility (OF) curve, indicating the presence of a subpopulation of cells with a markedly reduced surface area. After splenectomy, this RBC population disappears.
Contributions to conditioning may include a relatively low pH in the spleen as well as in the sequestered RBCs that may further compromise the poor HS RBC deformability and contact of RBCs with macrophages that may inflict additional damage on the RBC membrane. The conditioning effect of the spleen appears to represent a cumulative injury. The average residence time of HS RBCs in the splenic cords is between 10 and 100 minutes compared with 30 to 40 seconds for normal RBCs, and only 1% to 10% of blood entering the spleen is temporarily sequestered in the congested cords, whereas the remaining 90% of blood flow is rapidly shunted into the venous circulation.
The HS genes are assigned to several chromosomes, including chromosome 1 (α-spectrin), chromosome 8 (ankyrin), chromosome 14 (β-spectrin), chromosome 15 (protein 4.2), and chromosome 17 (band 3). In approximately two-thirds of HS patients, inheritance is autosomal dominant. In the remaining patients, inheritance is nondominant. In many of these patients, HS is caused by a de novo mutation, which is inherited in an autosomal recessive fashion in subsequent generations. Recessively inherited HS cases manifesting with severe hemolytic anemia have been reported. The majority of the affected patients were found to be severely deficient in RBC spectrin, associated with α-spectrin defects. The remaining cases characterized by a recessive inheritance pattern are caused by a defect in protein 4.2, a deficiency that is associated with relatively mild hemolysis.
Only a few cases of homozygous or “double dominant” HS have been reported. These patients have a severe hemolytic anemia, whereas their mostly consanguineous parents have a mild to moderate form of the disease or are asymptomatic.
Although the clinical severity of HS is highly variable among different kindred, in general, it is relatively uniform within a given family, in which HS is typically inherited as an autosomal dominant disorder. However, HS kindred have been described in which there was great variability in the clinical severity of affected family members. Several explanations might account for these observations, including variable penetrance of the genetic defect, a de novo mutation, presence of a mild recessive HS in the kindred, presence of a modifier allele that influences the expression of a membrane protein, or a tissue-specific mosaicism of the defect.
The typical HS patient is relatively asymptomatic. As noted in the earliest descriptions of HS, mild jaundice can be the only symptom of the disease. Anemia is usually mild to moderate but may be absent because of compensatory bone marrow hyperplasia manifest by reticulocytosis. Splenomegaly gradually develops in most patients, with the spleen occasionally reaching large dimensions.
In some families, anemia is absent, the reticulocyte count is normal or only minimally elevated, laboratory evidence of hemolysis is minimal or absent, and the changes in RBC shape can be mild, escaping detection on the peripheral blood film. The presence of HS is detected only by laboratory testing or during evaluation of a relative with a more symptomatic form of the disease. Some patients are first diagnosed during transient viral infections such as infectious mononucleosis or parvovirus infection, during pregnancy, or even in the 7th to 9th decades of life as the bone marrow’s ability to compensate for hemolysis wanes.
The relatively uncommon patients with nondominant forms of HS can present with a severe life-threatening hemolysis early in life. Some patients can be transfusion dependent during early infancy and childhood. The underlying molecular defects include severe spectrin or band 3 deficiency.
In most HS cases, the clinical manifestations are confined to the erythroid lineage, probably because many of the nonerythroid counterparts of the RBC membrane proteins (e.g., spectrin and ankyrin) are encoded by separate genes or because some proteins (e.g., protein 4.1 R, β-spectrin, ankyrin) are subject to tissue-specific alternative splicing. However, several HS kindred have been reported with a cosegregating neurologic or muscular abnormality, such as a degenerative disorder of the spinal cord, cardiomyopathy, or mental retardation. The observation that both erythrocyte ankyrin and β-spectrin are also expressed in muscles and the brain, particularly the cerebellum, and spinal cord raises the possibility that these HS patients may have a defect in one of these proteins. This hypothesis is further supported by studies of nb/nb mice, a mouse model of HS caused by an ankyrin mutation. These mice develop a neurologic syndrome with a progression that coincides with the loss of ankyrin from the Purkinje cells of the cerebellum.
Mutations of band 3 without HS have been described in patients with distal renal tubular acidosis. With a few rare exceptions, most patients with heterozygous mutations of band 3 and HS have normal renal acidification.
Most HS patients have mild to moderate anemia or no anemia at all, reflecting the fact that the hemolytic rate can be very mild and that the hemolysis is fully compensated for by increased RBC production, as evidenced by reticulocytosis. Some patients, however, particularly those with nondominantly inherited HS, are severely anemic, with hemoglobin concentrations as low as 4 to 6 g/dL.
Despite the increased percentage of reticulocytes with a larger volume than mature RBCs, the mean corpuscular volume (MCV) of HS RBCs is often low normal or even slightly decreased, and the mean corpuscular hemoglobin concentration (MCHC) is usually increased (>35 g/dL), together reflecting mild cellular dehydration.
The finding of an MCHC greater than 35.4 g/dL combined with an RBC distribution width (RDW) <14% has been found to be an excellent screening test for HS. Another screening method measures MCV by light scattering and provides a histogram of hyperdense erythrocytes (MCHC > 40 g/dL) claimed to identify nearly all HS patients. These hyperdense erythrocytes can be detected with newer laser-based blood counters or using aperture impedance analysis available in many clinical laboratories.
Evidence of accelerated RBC destruction, as indicated by increased lactate dehydrogenase and unconjugated bilirubin levels and by decreased haptoglobin, as well as by reticulocytosis, is present in typical HS patients. However, these abnormalities can be absent in individuals with a mild form of the disease.
In a typical case of HS, spherocytes are readily identified by their characteristic shape on the peripheral blood film ( Fig. 46.3 ). They lack central pallor, their mean cell diameter is decreased, and they appear more intensely hemoglobinized, reflecting both altered RBC geometry and increased cell density. In a three-dimensional view, some spherocytes have a spherostomatocytic shape that is occasionally appreciated on the peripheral blood film. In mild forms of the disease, the peripheral blood smear can appear normal because the loss of surface area can be too small to be appreciated by blood smear evaluation; the cells appear as “fat” disks rather than as true spherocytes.

Additional morphologic features have been described in some HS patients (see Fig. 46.3 ). A subset of HS patients whose RBCs are deficient in band 3 protein have some pincer-like RBCs on the peripheral blood film, a finding that is both sensitive and specific for this HS subset. These pincer-like cells disappear after splenectomy. Surface spiculations or acanthocytic spherocytes have been described in cases of HS associated with defects in β-spectrin. Frequent sphero-ovalocytes and stomatocytes have been reported in Japanese patients with protein 4.2 deficiency.
Both OF testing and eosin-5′-maleimide (EMA) binding are used in evaluating HS patients.
The OF test ( Fig. 46.4 ) measures the in vitro lysis of RBCs suspended in solutions of decreasing osmolarity. The normal RBC membrane is unstretchable and is virtually freely permeable to water. Thus, the cell behaves as a nearly perfect osmometer in that it increases its volume in hypotonic solutions progressively until a “critical hemolytic volume” is reached. At this point, the RBC membrane ruptures, and hemoglobin escapes into the supernatant solution. As a result of the loss of membrane and the ensuing surface area deficiency, the critical hemolytic volume of spherocytes is considerably lower than that of normal RBCs. Consequently, these cells hemolyze more than normal RBCs when suspended in hypotonic sodium chloride solutions. However, a finding of increased osmotic fragility is not unique to HS and is also present in other conditions associated with spherocytosis on the peripheral blood film, such as autoimmune hemolytic anemia.
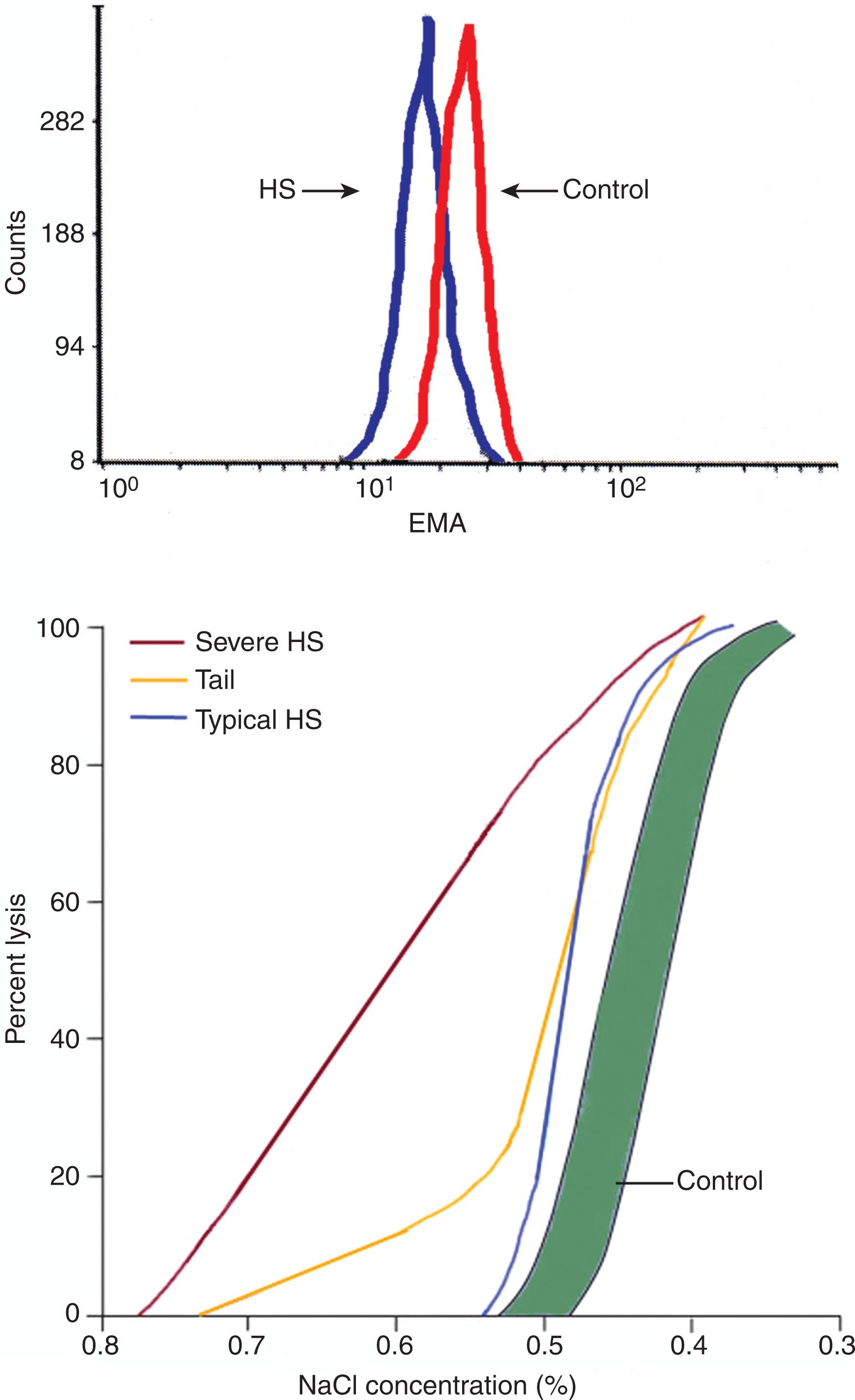
The OF curve often reveals uniformly increased osmotic fragility. A “tail” of the OF curve can be present in nonsplenectomized HS patients, indicating a subpopulation of particularly fragile RBCs conditioned by splenic stasis. This subpopulation of cells disappears after splenectomy. In patients with mild HS, osmotic fragility can be normal and abnormalities can be found only after incubation that further augments the loss of surface area; however, the sensitivity of the incubated OF test can be outweighed by a loss of its specificity. The relative contributions of cell dehydration and surface area deficiency can be accurately determined by osmotic gradient ektacytometry, available in specialized laboratories.
OF testing is unreliable in patients who have small numbers of spherocytes, including those who have been recently transfused, and it is abnormal in other conditions where spherocytes are present.
The binding of EMA to band 3 and Rh-related proteins with a 1 : 1 stoichiometry in the erythrocyte membrane is the basis for the EMA binding test. After binding of fluorescently labeled EMA to erythrocyte membranes, the relative amount of fluorescence, reflecting the amount of EMA binding, is analyzed by flow cytometry. The intensity of EMA binding is decreased in HS erythrocytes (see Fig. 46.4 ). Although defects of band 3 protein are only found in ~25% of typical HS patients, decreased EMA fluorescence is also observed in HS erythrocytes with primary defects in ankyrin and spectrin, thought to be caused by the transmission of long-range effects of varying protein defects across the membrane, influencing EMA binding. EMA binding has high sensitivity and specificity. In laboratories with the ability to perform fluorescence-activated cell sorting-based studies, it is simple and rapidly performed, even on samples after shipment or storage.
Like osmotic fragility, EMA binding struggles in the diagnosis of mild HS where results may be normal or indeterminate. In addition, normal EMA binding has been reported in some cases of spectrin-deficient HS. Other erythrocyte abnormalities such as defects of erythrocyte hydration and variants of dyserythropoietic anemia can also yield abnormal results.
RBC autohemolysis, the spontaneous hemolysis of RBCs incubated under sterile conditions without glucose, was previously advocated as a sensitive test for the detection of HS. This test is being used less frequently and is probably no more sensitive than the incubated OF test. Other tests described in the literature such as the glycerol lysis test, the pink test, hypertonic cryohemolysis, and the skeleton gelation test are infrequently performed in diagnostic laboratories in the United States. The former two tests, which use glycerol to retard the osmotic swelling of RBCs, are preferred by some laboratories because they are easy to perform and can be adapted to microsamples. Cryohemolysis testing in particular remains popular in Europe.
Because the most common finding in erythrocytes of patients with HS is a deficiency of one or more of the membrane proteins, molecular studies often include sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) solubilized RBC membrane proteins followed by densitometric quantitation. The results are expressed as ratios of individual red cell membrane proteins to band 3. This technique reveals abnormalities in approximately 70% to 80% of patients, defining the distinct biochemical phenotypes discussed previously. Direct quantitation of membrane proteins by radioimmunoassay is superior to densitometric quantitation and permits accurate measurement of the copy number of the individual proteins per RBC.
Application of molecular genetic analyses including DNA sequencing and other molecular studies complement clinical and laboratory screening and provide definitive diagnosis in most cases. Mutation detection in the major erythrocyte membrane protein genes is now available commercially. Gene-based studies are of use in diagnosing difficult cases and in cases in which a molecular diagnosis is desired. Some practitioners perform genetic diagnostic studies prior to splenectomy to ensure the correct diagnosis. Molecular analyses have potential pitfalls. In some cases, variants of unknown significance are detected, making genetic diagnosis uncertain. Mutations not detected by a study of coding regions and splice junctions may be causative, such as in distant regulatory elements, deep intronic splicing mutations, and intragenic deletions. In these cases, diagnosis is assigned based on clinical, laboratory, and biochemical findings.
Bilirubin stones are found in approximately 50% of patients with HS, often even in those with a very mild form of the disease. Gallstones have occasionally been detected during infancy, but they are most likely to occur in older children and young adults. The coinheritance of Gilbert syndrome increases the risk for gallstones in HS patients. Because of the high incidence of gallstones, HS patients should be periodically examined by ultrasonography for the presence of gallstones, beginning in childhood.
True hemolytic crises are relatively rare and only occasionally reported in association with infections. Aplastic crises during viral infections are largely attributable to infection by parvovirus B19. This infection (erythema infectiosum, fifth disease) manifests with fever, chills, lethargy, malaise, nausea, vomiting, abdominal pain with occasional diarrhea, respiratory symptoms, muscle and joint pains, and a maculopapular rash on the face (slapped cheek appearance), trunk, and extremities. The virus selectively infects erythroid precursors and inhibits their growth. The ensuing anemia, often profound, can be the first manifestation of HS. Multiple family members with undiagnosed HS who are infected with parvovirus have developed aplastic crises at the same time. Infection with parvovirus is a particular danger to susceptible pregnant women because it can infect the fetus, leading to fetal anemia, hydrops fetalis, and fetal demise.
Rarely, at least in developed countries, patients present with megaloblastic crises caused by folate deficiency. This typically occurs in patients with increased folate demands, such as those recovering from an aplastic crisis, pregnant women, and older adults. Megaloblastic crisis in pregnancy has been reported as the first manifestation of HS. Folate supplementation is recommended for patients with moderate to severe HS.
In patients with more severe forms of HS, other complications include gout, leg ulcers, or chronic dermatitis of the legs that heal after splenectomy. Symptoms of expanded erythroid space, including paravertebral or renal pelvic masses of extramedullary hematopoiesis, which can mimic an underlying neoplasm, may occur. Several cases of hemochromatosis in HS patients have been reported. In some, iron overload resulted from repeated transfusions; in others, the patients had two genetic defects, one involving HS and the other involving a hemochromatosis carrier state. Other rare complications include thrombosis, pulmonary hypertension, spinocerebellar degenerative syndromes, movement disorders, myopathy, and hypertrophic cardiomyopathy.
More than a dozen cases of HS and hematologic malignancy, including myeloproliferative disorders, multiple myeloma, and leukemia, have been reported. It is unknown if long-standing hematopoietic stress predisposes to the development of these secondary disorders or if they occurred randomly.
Because of the relatively asymptomatic presentation of HS, this diagnosis should be considered during an evaluation for unexplained splenomegaly, unconjugated hyperbilirubinemia of unknown cause, gallstones at a young age, severe anemia during pregnancy, or transient anemia during acute infections. The diagnosis of HS can be missed in mild forms of the disease because spherocytosis might not be apparent on the peripheral blood film. Autoimmune hemolytic anemia should be ruled out by negative results of a Coombs test.
More typical forms of HS, characterized by relatively uniform spherocytosis with increased MCHC, are usually easily distinguished from other disorders manifesting with spherocytosis, such as immune hemolytic anemias and unstable hemoglobins. In some patients, the spherostomatocytes in the rare Rh-null syndrome and the intermediate syndromes of hereditary stomatocytosis can be confused with HS RBCs.
Spherocytosis is transiently improved, and both the osmotic fragility and hemolysis are normalized in patients with obstructive jaundice. This is because of an expansion of RBC surface area that follows an increased uptake of phospholipids and cholesterol from the abnormal plasma lipoproteins. In normal RBCs, this leads to target cell formation; in HS, spherocytes are transformed to discocytes. Spherocytosis and the increased osmotic fragility of HS red cells are likewise improved by iron deficiency, but the RBC life span remains shortened. In addition, the coexistence of β-thalassemia trait and HS partially corrects the HS phenotype.
Splenectomy is curative in almost all patients with typical forms of HS because RBC survival is normalized, and anemia and hyperbilirubinemia are corrected. Spherocytosis and the increase in osmotic fragility persist, but the tail of the OF curve, indicating the presence of a subpopulation of cells conditioned by the spleen, disappears. In patients with severe, nondominantly inherited HS, splenectomy produces a dramatic clinical improvement, but hemolysis is only partially corrected.
Several weeks to months before splenectomy, patients should be immunized with polyvalent vaccine against pneumococcus as well as vaccines against Haemophilus influenzae type b and meningococcus.
Splenectomy is a permanently curative treatment in most cases of HS. Thus, for years, splenectomy was recommended for all HS patients regardless of the severity of anemia, gallbladder disease, or other symptoms. However, increasing concerns regarding overwhelming postsplenectomy infection (OPSI), the emergence of penicillin-resistant pneumococci, and increased risk for cardiovascular diseases have tempered these recommendations. When considering splenectomy, health care providers, the patient, and the patient’s family should review and consider the risks and benefits. Individual factors that may pose additional risk, such as the distance from medical care in case of febrile illness and residence in or travel to areas where parasitic diseases such as malaria or babesiosis occur, should be considered. Expert opinions vary on indications for splenectomy. There are no studies to guide practice. However, because the risk for OPSI is highest in infancy and childhood, most agree it is best to avoid total splenectomy in early childhood.
Anemia ameliorated
Risk for hemolysis-associated gallbladder disease eliminated
Risk for aplastic crisis eliminated
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here