Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The intestinal mucosa is unique among other tissues of the human body, as it exists in close proximity to an enormous number of microorganisms and their products. Such factors constitute a potential trigger for proinflammatory responses by the gut-associated lymphoid tissue (GALT) and a constant challenge to mucosal homeostasis. The latter is typically preserved by the function of a dense network of diverse, although highly interconnected, immunological checkpoints which require the continuous presence of patrolling cells of the innate and adaptive immunity. This state of “subclinical inflammation” is a hallmark of the intestinal microenvironment and, despite its name, ensures the absence of deleterious inflammatory responses during homeostasis. Nevertheless, under unfavorable genetic and/or environmental pressures, this delicate balance is occasionally broken, resulting in the development of “overt” intestinal inflammation. This is most dramatically exemplified in ulcerative colitis (UC) and Crohn's disease (CD), two major types of persisting chronic intestinal inflammatory processes, which are collectively referred to as the inflammatory bowel diseases (IBDs). A prominent characteristic of such conditions is the remarkable expansion of the cellular infiltrate within the bowel wall, which is indicative of accelerated recruitment and increased retention of immune cells within the inflamed bowel. The circulation of immunocytes to diseased gut regions is a complex process that involves a tightly coordinated sequence of adhesive interactions between those cells and the endothelial lining of local blood vessels (i.e., the postcapillary venules).
When trafficking of immune cells to the gastrointestinal (GI) tract is considered, in addition to the pathophysiological status of the target tissue (homeostatic vs acute injury vs chronic inflammation), several additional parameters should be taken into account. Included among such factors are the regional specialization along the GI tract (upper GI vs ileum vs colon), cellular diversity of the infiltrating population (lymphocytic as opposed to innate immune cell recruitment, effector vs regulatory cells), molecular components of the adhesion cascade (GI specific or universal trafficking signaling mediators), as well as the specific microenvironment associated with the particular immunological reaction (i.e., the dominant mucosal inflammatory/immunological mediators).
This chapter provides a detailed description of the basic cellular and molecular mechanisms that mediate trafficking of immune cells to the healthy and inflamed bowel. An overview of the various adhesion molecules that are involved in cell-cell interactions is presented, followed by in-depth analysis of the basic mechanisms that mediate the movement of immune cells in the various structures of GALT. As leukocyte trafficking has recently arisen as a major therapeutic target in IBD, specific emphasis is placed on those interactions that have been or are being tested as potential treatment targets for IBD.
Migration of circulating leukocytes to peripheral tissues usually occurs across the walls of postcapillary venules. Specialized endothelial cells that line these venules may be coated with chemoattractants and bear surface adhesion molecules that serve as mechanical anchors, thus facilitating the exit of leukocytes from the blood and their entrance into the tissues. This cell-cell adhesion is accomplished through homotypic or heterotypic receptor-ligand interactions. At the same time, adhesion proteins confer tissue specificity to the recruitment process, via selective patterns of expression by local vascular beds. Major classes of adhesion molecules that are involved in leukocyte recruitment include selectins and their glycoprotein ligands, as well as integrins and immunoglobulin superfamily molecules. All of these are type I transmembrane glycoproteins that span the cell membrane only once. In addition, cadherin, occludin, and claudin family of proteins have all been implicated in the adhesion cascades that take place in inflammatory sites. Table 65.1 reports several of these adhesion molecules along with their molecular function, tissue expression, and respective ligands. Myeloid cells and lymphocytes employ unique trafficking pathways, although several steps are common. Although trafficking occurs during homeostatic conditions, leukocyte recruitment is amplified in the presence of inflammation, as the original pathophysiological function of immunocytes was protection from infection. In the presence of inflammation, an orderly and well-regulated process takes place that allows for both rapid and sustained accumulation of inflammatory cells in affected tissues.
| Adhesion Molecule | Primary Tissue Expression | Major Ligands | Primary Function |
|---|---|---|---|
| P-selectin | Endothelial cells, platelets | PSGL-1, Sialylated Lewis X | Leukocyte capture and rolling, thrombosis |
| E-selectin | Endothelial cells | ESL-1, sialyated Lewis X | Leukocyte rolling and firm adhesion |
| L-selectin | Neutrophils, monocytes, most lymphocytes | CD34, GLYCAM-1, MadCAM-1 | Leukocyte rolling and homing |
| ICAM-1 | Endothelial and epithelial cells, lymphocytes, fibroblasts, and others | LFA-1, Mac-1, p150/95, fibrinogen, fibronectin (connecting segment-1) | Leukocyte adhesion and extravasation, leukocyte and endothelial cell motility, cell activation |
| ICAM-2 | Endothelial cells, lymphocytes | LFA-1 | Leukocyte adhesion and extravasation, cell activation |
| ICAM-3 | Neutrophils, monocytes, lymphocytes | LFA-1 | Leukocyte adhesion, cell activation |
| VCAM-1 | Endothelial cells | VLA-4 | Leukocyte adhesion and extravasation, cell activation |
| PECAM-1 | Endothelial cells, neutrophils, platelets, lymphocytes | PECAM-1 | Leukocyte extravasation |
| MadCAM | Endothelial cells | L-selectin, α 4 β 7 | Leukocyte homing and adhesion |
| JAM | Endothelial and epithelial cells, neutrophils, monocytes, platelets | JAM, LFA-1, Mac-1, VLA-4 | Cell-cell adhesion, regulation of polarity, cell motility |
| LFA-1 | Neutrophils, monocytes, lymphocytes, most other leukocytes | ICAM-1,2,3, JAM | Leukocyte adhesion and extravasation, cell activation, cell migration |
| Mac-1 | Neutrophils, monocytes, platelets | ICAM-1, JAM, Factor X, iC3b, fibrinogen | Leukocyte adhesion and extravasation, cell activation |
| VLA-1 | Neutrophils, lymphocytes, most other leukocytes | Collagen, laminin | Cell-matrix adhesion |
| VLA-2 | Neutrophils, lymphocytes, most other leukocytes | Collagen, laminin | Cell-matrix adhesion |
| VLA-3 | Neutrophils, lymphocytes, most other leukocytes | Fibronectin, collagen, laminin | Cell-matrix adhesion |
| VLA-4 | Neutrophils, lymphocytes, most other leukocytes | VCAM-1, fibronectin | Leukocyte rolling, adhesion, extravasation, cell-matrix adhesion |
| VLA-5 | Neutrophils, lymphocytes, most other leukocytes | Fibronectin | Cell-matrix adhesion |
| E-cadherin | Epithelial cells | E-cadherin, α E β 7 | Leukocyte adhesion |
| GPIb-IX-V | Platelets | vWF, Mac-1, thrombin, P-selectin | Platelet adhesion, thrombosis |
| GPIIa-IIIb | Platelets | vWF, fibrinogen | Platelet adhesion, thrombosis |
| vWF | Platelets | GPIIa-IIIb, collagen, P-selectin, GPIb | Platelet adhesion, thrombosis |
A multistep model of leukocyte binding to vascular endothelium ( Fig. 65.1 ) has been universally adopted to explain the sequence and time course of involvement of different adhesion molecules in the recruitment of leukocytes to inflamed tissue. The original model for leukocyte adhesion was composed of three major steps, each one of which is mediated by a specific class of proteins. The initial event is leukocyte rolling , whereby weak adhesive interactions result in rolling along the endothelial lining of blood vessels. This may occur within minutes of an inflammatory challenge and is largely mediated by selectins and their glycoprotein ligands. The second step is the exposure of leukocytes to low concentrations of chemoattractants/inflammatory mediators (mostly chemokines), bound to endothelial glycosaminoglycans, which results in leukocyte activation , rapidly leading to increased expression and/or activation of integrins on rolling leukocytes. The final event is integrin-mediated leukocyte arrest . In this phase, leukocyte integrins (e.g., CD11/CD18) bind to counter-receptors that are expressed on the surface of endothelial cells (e.g., VCAM-1), thereby allowing leukocytes to become firmly attached to the endothelium and remain stationary. The subsequent transendothelial migration of leukocytes into the interstitial compartment involves the participation of additional adhesion glycoproteins such as PECAM-1, MAdCAM-1, or JAM-A. In recent years, this three-step model has been proven oversimplistic, and new data have led to an expanded version of the leukocyte adhesion cascade, which now includes slow rolling, adhesion strengthening, intraluminal crawling, paracellular and transcellular migration, and migration through the basement membrane. In all, the actual sequence of movement from blood circulation to the extravascular space encompasses several major steps, that is, signaling and homing, tethering and rolling, arrest and adhesion, and transmigration.
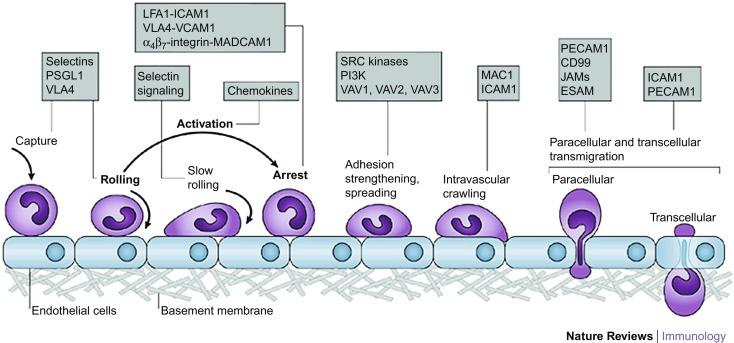
Local immune cells provide the appropriate signals to attract circulating leukocytes toward peripheral tissues, primarily through the secretion of chemokines. These are low-molecular-weight proteins, which are classified into four distinct families based on their aminoacid sequence (CC, CXC, C, and CX3C), that interact with specific chemokine G-protein-coupled receptors expressed on endothelial cells. Local concentrations of chemokines within the thymus maintain continuous circulation of lymphocytes from this maturation site to peripheral lymphoid sites. Chemokines also participate in migratory specialization via their selective tissue localization. In line with this paradigm, CCL25 is predominantly expressed in the small intestine and thymus; thus, it is believed to play an essential role in gut-specific lymphocyte trafficking. When inflammation occurs, the expression of additional “inflammatory” chemokines (CCL2, CCL3 and CCL5, CXCL1, CXCL2, and CXCL8) increases, further potentiating migration of leukocytes to the sites of inflammation.
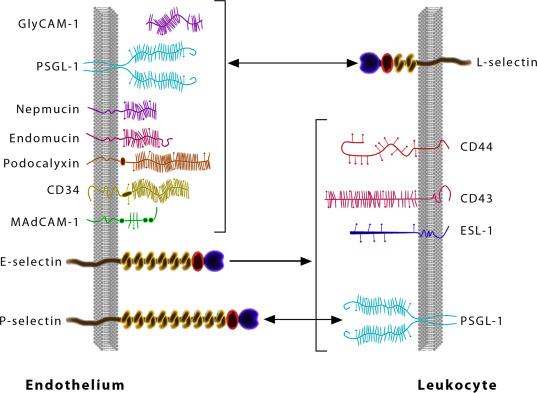
This initial step is defined by the formation of the first molecular bonds between a circulating leukocyte and the vascular endothelium that have come in close proximity. Three types of selectins mediate this process (P, E, and L-selectins) by binding to their target ligands ( Fig. 65.2 ). Additionally, L-selectins can also bind directly to the mucosal addressin cell adhesion molecule (MAdCAM)-1 integrin. Under inflammatory conditions, other mechanisms may also participate, such as secondary capture that is achieved via leukocyte-leukocyte interaction. These mechanisms are also cell specific. In particular, neutrophils start rolling as they exit capillaries. On the other hand, naive lymphocytes capture in high endothelial venules (HEVs) of peripheral lymph nodes (PLNs) and other lymphoid tissues. Finally, little is known about the capture of effector/memory lymphocytes, although selectins and CD44 may be involved. Transient bonds form and break as a result of these molecular interactions that cause white cells to stumble along endothelial cell surfaces. As the process of “rolling” of leukocytes begins, endothelial cell attachment is accomplished by the tetrasaccharides sialyl Lewis X and A receptors. In all, this transient tethering of leukocytes is the first critical step in their migration from the vasculature to inflammatory areas. A stable rolling movement follows leukocyte capture if new molecular bonds are formed before the initial molecular bonds dissociate. In this step the leukocyte is in constant contact to the vessel wall and its movement is flow driven. Again, there are cell-specific mechanisms. Naive lymphocytes utilize L-selectin, which binds to sulfated carbohydrate-containing ligands on the surface of endothelial cells. Effector T cells can arrest through the chemokine receptor CXCR3 in vitro, but the molecules involved for in vivo arrest remain unknown. Neutrophils arrest by gradually slowing down, whereas naive lymphocytes arrest immediately upon encountering the appropriate chemokine gradients.
The aforementioned interactions between selectins and their ligands facilitate adherence of leukocytes to inflamed endothelia in the presence of blood flow. In fact, L- and P-selectins require shear stress for adhesion, which strengthens the molecular bond created by selectins; hence, rolling cells are detached when flow ceases. Rolling has at least two distinct consequences for the cell: first, it facilitates stable leukocyte arrest (firm adhesion); and second, it reduces leukocyte velocity drastically (to between 1 and 100 μm/s), thus increasing the duration of exposure to the endothelial surface and to chemokines and other activating signals that are present in the local environment. This facilitates the next step of migration, during which leukocytes overcome the shear forces of the bloodstream, arrest and firmly adhere to the endothelium.
This step requires activation of the rolling lymphocyte, which is accomplished via binding of a chemokine to a heptahelical transmembrane chemokine receptor on the leukocyte surface. In flow chamber systems, this process is exceptionally rapid. Under inflammatory conditions, there are several sources for tissue chemokines. For example, endothelial cells synthesize chemokines under the influence of proinflammatory cytokines or transport chemoattractants from their abluminal surface. Additionally, mast cells and platelets also generate chemoattractants and deliver them to endothelial cells.
Firm adhesion requires binding of integrin receptors in their active conformation to their endothelial ligands. Integrins are heterodimeric proteins on cell surfaces that consist of two transmembrane glycoprotein subunits, α and β. During homeostasis, integrins are maintained in a nonactive form; nevertheless, in the presence of inflammatory mediators, integrins transition to their activated state and become capable of engaging their respective ligands (CAMs) that are present on endothelial surfaces. For example, very late antigen (VLA)-1 and lymphocyte function associated antigen (LFA)-1(CD11a/CD18) bind to intercellular cell adhesion molecule (ICAM)-1 (CD54) and ICAM-2, which are expressed on endothelial cells. Alternative pathways include the α 4 β 1 (VLA-4) and α 4 β 7 integrins, which mediate firm adhesion to their respective ligands vascular cell adhesion molecule (VCAM)-1 (CD106) and mucosal addressin cell adhesion molecule (MAdCAM)-1. Arrest enables leukocytes to firmly anchor to the mucosa. This is followed by postadhesion strengthening, which initiates the process of transmigration across the mucosal barrier, leading to the final entrance to the inflamed target tissue.
Firm attachment to the endothelium allows leukocytes to finally migrate from the intravascular space into the interstitium. This is also a complex process that develops through various steps, which may occur either sequentially or separately. Before actually crossing the walls of postcapillary venules, neutrophils and monocytes crawl inside blood vessels. This step is mediated by ICAM-1 and macrophage receptor-1 (MAC-1). Crawling helps cells to identify the best sites to transmigrate and also affects the way of exit. In fact, when crawling is disabled, transmigration is delayed and occurs preferentially through the transcellular, as opposed to the paracellular route. The paracellular migration pathway involves several adhesion molecules, mostly endothelial-expressed vascular endothelial cadherin (VE-cadherin), platelet/endothelial-cell adhesion molecule 1 (PECAM1), and junctional adhesion molecule A (JAM-A). In addition, endothelial cell-selective adhesion molecule (ESAM), ICAM2, and CD99 may also play a role in the paracellular migration pathway. Several of these molecules are involved in the transcellular route of migration. This type of migration includes the extension of membrane protrusions into endothelial cells, triggering of cytoplasmic signaling, translocation of apical ICAM1 to caveolae and F-actin-rich regions, transport to the basal plasma membrane, and the final formation of channels through which leukocytes can migrate. The final obstacles to leukocyte exit from the blood and into the interstitium are the endothelial basement membrane and pericyte sheath. This is probably accomplished through regions of low expression of matrix proteins.
These processes are affected by the function of multiple proinflammatory cytokines (TNF-α, IL-1, IL-3, IL-5), chemokines (IL-8 and MCP-1), and products of lipid metabolism (leukotriene β4 and lipopolysaccharide (LPS). These soluble mediators promote leukocyte adhesion and transmigration via various mechanisms. Certain factors induce the expression of ligands on endothelial cells, whereas others trigger integrin activation, the end product being an amplified capacity for cell-cell, ligand-receptor interaction.
Lymphocyte migration toward specific tissues and their draining lymph nodes is organized by the function of chemokines, as it is presented above. In contrast, the exodus of T cells from the lymph nodes back to the circulation (egress) is regulated by the natural bioactive lipid sphingosine-1-phosphate (S1P), which is formed upon phosphorylation of membrane-derived sphingosine by sphingosine kinase-1. There are several receptors for S1P (Sphingosine-1-Phosphate Receptors, S1P1-5), which are expressed in a cell-type specific manner within different tissues and play integral roles in lymphocyte trafficking. In particular, S1P1 is expressed on lymphocytes, in the vascular endothelium, brain, and lymph nodes. Lymphocytes migrate from the lymph node into blood following an S1P gradient that is maintained by the high levels of S1P in the blood and lymph, which far exceed those at tissues. Tissue levels of S1P are tightly controlled due to intracellular degradation by S1P lyase or dephosphorylation by S1P phosphatases. Pharmaceutical intervention via S1P receptor agonists induce internalization and degradation of S1P receptors, rendering the cells unable to follow the S1P gradient to migrate from tissues back to blood. This process that results in loss of lymphocyte receptors eventually leads to cell trapping in lymph nodes. Selective agonism of S1P1 is believed to regulate immune cell trafficking in tissues including the gut.
The selectin proteins were first discovered through the use of monoclonal antibodies that inhibited leukocyte homing (L-selectin) and leukocyte binding to endothelial cells (E-selectin). P-selectin was also found as a membrane protein within platelet granules with an unknown function, and also later identified on endothelial cells. Selectins are critically important for leukocyte-endothelial cell rolling interactions, particularly during inflammatory states. Selectin proteins consist of a short cytoplasmic domain, a transmembrane domain, a series of short consensus repeat domains (SCR domains), a single EGF like domain, and a Ca ++ dependent lectin binding domain at the amino terminus ( Fig. 65.2 ). The cytoplasmic domain can be phosphorylated on tyrosine, threonine, and serine residues; however, the importance of phosphorylation is unknown. It is believed that the SCR domains may be involved in maintaining protein structure and can possibly enhance ligand binding. Similarly, the EGF-like domain is quite homologous among all selectins and has been reported to be involved in cell adhesion. The most critical domain for selectin function is the Ca ++ -dependent lectin domain. X-ray crystallography studies have shown that Ca ++ binding induces a conformational change within this domain, which facilitates ligand binding.
The cellular distribution of selectins determines their physiological and pathophysiological roles. P-selectin is expressed by both platelets and endothelial cells, whereas E-selectin expression is only observed on endothelium. Both E- and P-selectins facilitate leukocyte rolling on the endothelial cell surface, the initial step in leukocyte recruitment from the vascular lumen to the extravascular tissue. P-selectin is also utilized for platelet adhesion. P-selectin is contained in preformed pools within either α-granules of platelets or Weibel-Palade bodies of endothelial cells. Cell activation in response to inflammatory signals leads to rapid mobilization of P-selectin to the surface of platelets or endothelial cells within a matter of minutes. In addition, the expression of both E- and P-selectins is transcriptionally induced by several inflammatory cytokines such as TNF-α, IL-1β, or bacterial LPS. Interestingly, platelet P-selectin does not affect leukocyte recruitment, whereas endothelial P-selectin is important for either leukocyte or platelet rolling on stimulated endothelium.
The role of selectins during an inflammatory response can be varied depending upon the type of inflammatory stimuli, the time course of inflammation (acute vs chronic), and the particular organ that is studied. Nonetheless, the overall interpretation of multiple studies suggests that P-selectin is important for immediate leukocyte rolling in response to inflammatory stimuli and trauma, and that the role of E-selectin may be important during longer periods of inflammation (> 4 h) and may also influence leukocyte firm adhesion. Initial reports of P-selectin knockout mice demonstrated decreased early leukocyte rolling in response to tissue injury and/or inflammation, increased leukocyte rolling velocities during longer periods of inflammation (> 1 h), and decreased leukocyte recruitment into the peritoneum over a 4-h period. It is interesting that the cytoplasmic domain of P-selectin is important for proper targeting to endothelial cell Weibel-Palade bodies in vivo and that mice containing P-selectin cytoplasmic domain deletions demonstrate many of the phenotypic features of P-selectin knockout mice.
Consistent with these observations, loss of P-selectin has been reported to be protective in several inflammatory settings, such as ischemia/reperfusion injury, peritonitis, sepsis, allergic inflammation, contact hypersensitivity, and atherosclerosis. Nevertheless, this is not a uniform association, as leukocyte adhesion in certain organ vascular beds or during specific disease conditions may be selectin independent. For example, in lung, brain, and liver inflammatory settings either P or E-selectin have been shown to be unnecessary or minimally involved in leukocyte recruitment. However, these findings may be stimulus- or injury specific, since an in vitro study using P-selectin mutant brain endothelial cells reported TNF-α and IL-1β-mediated neutrophil adhesion to be significantly influenced by P-selectin expression.
While the importance of P-selectin in inflammation has been well identified, the exact role of E-selectin has remained somewhat elusive. This may be due to different patterns of expression and modes of regulation. As mentioned earlier, P-selectin is synthesized and stored in Weibel-Palade bodies, which can be mobilized in minutes after an inflammatory response. In contrast, preformed pools of E-selectin do not exist and induction of this molecule requires transcriptional activation. However, these same signaling mechanisms also induce transcription of the P-selectin gene. Therefore, an increase in both selectin molecules can be observed simultaneously during a given inflammatory response. Therefore considerable functional overlap between the two molecules can be presumed. Detailed examination of E-selectin mutant mice has shown that this molecule is important for leukocyte slow rolling and mediates increased leukocyte transit times, thereby allowing adequate leukocyte sampling of the endothelial cell surface and facilitating interaction between these cell types. This notion is further supported by the observation that loss of E-selectin results in decreased firm adhesion of leukocytes and may be necessary for proper integration of signals involved in leukocyte arrest.
L-selectin expression is observed on most leukocytes including lymphocytes, granulocytes, and monocytes. L-selectin has been shown to be important mediator of lymphocyte homing to PLNs and Peyer's patches in response to inflammatory stimuli. Engagement of ligands, such as PNAd or MAdCAM, stimulates L-selectin mediated outside-in signaling that can enhance additional adhesion interactions such as β 2 integrin/ligand association. Importantly, L-selectin is proteolytically cleaved from the leukocyte surface following activation, which has become a useful indicator in specific immune states.
The immunoglobulin superfamily proteins share a similar structural motif, in that they all contain Ig-like extracellular domains that are responsible for cell adhesion. Members of this family are intercellular adhesion molecule-1, 2, and 3 (ICAM-1-3), vascular cell adhesion molecule (VCAM), platelet endothelial cell adhesion molecule (PECAM), mucosal adressin cell adhesion molecule (MAdCAM), and junctional adhesion molecule (JAM) ( Fig. 65.3 ). These molecules are important for normal immune surveillance, leukocyte adhesion, and leukocyte emigration.
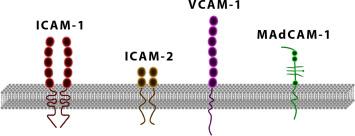
ICAM-1 was originally discovered through studies using blocking monoclonal antibodies against the integrin LFA-1 (CD11a/CD18). ICAM-1 also binds to integrins Mac-1 (CD11b/CD18) and p150/95 (CD11c/CD18). ICAM-1 is constitutively expressed at low levels and is transcriptionally induced by several inflammatory mediators. ICAM-1-ligand interactions are involved in leukocyte firm adhesion and emigration through engagement with the endothelium and are also involved in leukocyte-leukocyte aggregation and activation. These cellular events are facilitated by ICAM-1 mediated outside-in signaling through integrin binding. Cross-linking of ICAM-1 has been shown to stimulate many endothelial cell signal transduction cascades, such as MAP kinase, src kinase, and NOX4 pathways. Recent work has also revealed that ICAM-1-signaling-dependent responses regulate endothelial cell redox status by increasing intracellular glutathione levels and that this controls cellular activation and inflammatory responses.
Inhibition or genetic deficiency of ICAM-1 protects mice against ischemia-reperfusion injury in several organs, including the heart, kidney, and brain. ICAM-1 is also important during the progression of chronic inflammatory diseases, inasmuch its genetic loss ameliorates experimentally induced colitis, SLE-associated vasculitis and glomerulonephritis, and reduces susceptibility to collagen induced arthritis.
Given the diverse functions of ICAM-1 during immune responses, its precise role in mediating leukocyte-endothelial interactions has been examined. Interestingly, loss of ICAM-1 specifically influences the adhesion of certain leukocyte types in a stimulus-dependent manner. For example, in the absence of ICAM-1 signaling eosinophil adhesion in mesenteric venules in response to ragweed allergen challenge is decreased. However, TNF-α stimulation of neutrophil adhesion in ICAM-1 mutant mice has revealed that this protein is either not required for adhesion or is redundant with other adhesion molecules. Thus, ICAM-1 appears to be required for chemoattractant-mediated adhesion in resting venules, but not in TNF-α stimulated venules. In vitro studies with endothelial cells from ICAM-1-deficient mice showed that it is important for monocyte firm adhesion and T-cell emigration across endothelial monolayers. In addition, ICAM-1 was shown to be necessary for optimal selectin-mediated rolling, as leukocyte rolling velocities on TNF-α-stimulated vessels and endothelium were faster compared to wild-type controls. A previously unappreciated step of the leukocyte recruitment cascade involving intraluminal crawling after firm adhesion but before transmigration has been reported. ICAM-1 interaction with Mac-1 counter ligand appears to govern intraluminal neutrophil crawling on the endothelial cell surface perpendicular to the direction of blood flow thus allowing the leukocyte to locate cell-cell junction regions for transmigration. These findings highlight a complex role for ICAM-1 in regulating endothelial responses leukocyte-endothelial interactions during inflammation.
ICAM-2 was primarily described on endothelial cells. The structures of ICAM-1 and 2 are similar in that they consist of a cytoplasmic domain, a transmembrane domain, and Ig-like regions. However, they differ in that ICAM-1 contains 5 Ig-like domains compared to only 2 for ICAM-2. ICAM-2 is constitutively expressed on the cell surface and, unlike ICAM-1, it is not inducible by inflammatory mediators. This suggests a role for ICAM-2 in normal immune surveillance. Gene-targeted loss of ICAM-2 does not alter lymphocyte homing or development of leukocytes but it reduces eosinophil transmigration across endothelial monolayers and prolongs allergic eosinophil accumulation in certain organs.
ICAM-3 is expressed on all resting leukocytes and, similar to ICAM-1, contains five Ig domains. ICAM-3 is important for leukocyte aggregation and activation through its interaction with the counter-ligand LFA-1.
Vascular cell adhesion molecule (VCAM-1) is a cytokine-inducible surface molecule that mediates adhesion of various leukocyte types, such as lymphocytes, monocytes, and eosinophils. VCAM-1 was identified through direct expression techniques using a cell-cell adhesion-based screening procedure with cytokine-induced cDNA genes. Candidate genes were transfected into epithelial cells and examined for adhesion to lymphocytic cell lines. Since its original characterization, VCAM-1 has been shown to participate in both acute and chronic inflammatory responses, being primarily expressed by endothelial cells. VCAM-1 binds α 4 β 1 (VLA-4) and α 4 β 7 integrin and participates in leukocyte firm adhesion and migration. VCAM-1 has also recently been reported to bind the α 9 β 1 integrin of neutrophils and facilitate neutrophil migration across endothelial cells.
Platelet endothelial cell adhesion molecule (PECAM-1) was originally identified through the serological characterization of surface proteins of vascular endothelium and contains six Ig-like domains, a transmembrane domain, and a cytoplasmic tail that can be differentially phosphorylated and palmitoylated. Modification of the cytoplasmic tail facilitates outside-in signaling, which can influence endothelial cell function (e.g., angiogenesis or cell survival) and leukocyte recruitment. PECAM-1 preferentially localizes at intercellular junctions of endothelial cells, suggesting a role in leukocyte emigration. Consistent with this hypothesis, blocking antibodies against endothelial PECAM-1 prevents leukocyte emigration across endothelial monolayers. PECAM-1 expression was also shown on several other cell types, including platelets, monocytes, neutrophils, natural killer cells, and certain T-cell subsets, which may facilitate adhesion and transmigration through homotypic binding of endothelial PECAM-1.
MAdCAM-1 was identified through monoclonal antibody screening studies that showed discrete staining of HEVs of mucosal lymph nodes (Peyer's patches). Additional studies of MAdCAM-1 expression identified constitutive expression in tissues involved in mucosal immunity as well as inducible expression by proinflammatory mediators (e.g., TNF-α). As suggested by its tissue distribution, MAdCAM-1 mediates lymphocyte homing by directing adhesion and emigration across HEVs of mucosal tissue. MAdCAM-1 contains three extracellular Ig-like domains with a mucin-like region between domains 2 and 3. Primary ligands for MAdCAM-1 include L-selectin and α 4 β 7 integrin, which bind to Ig domain 1. Importantly, several reports have documented an important role for MAdCAM-1 in mediating chronic inflammatory states involving gastrointestinal (GI) tissues such as ulcerative colitis, Crohn's disease, and experimental colitis models. Ongoing trials are assessing the efficacy of its blockade in UC, while Phase 2 studies in CD did not show efficacy.
The most recently reported immunoglobulin adhesion molecule is JAM. Three different JAMs (JAM-A, B, and C) have been described. JAM expression is observed on endothelial and epithelial cells, leukocytes, and platelets and consists of two extracellular Ig-like domains and a cytoplasmic tail that differentially associates with multiple signaling and cytoskeletal-associated proteins. The original identification of JAM-A revealed junctional localization in endothelial cells that participated in monocyte transmigration. Studies demonstrated that JAM proteins localize to tight junctions in both epithelial and endothelial cells and that members of the JAM subfamily bind either homotypic (i.e., other JAMs) heterotypic ligands. The latter include integrins LFA-1 (α L β 2 ), Mac-1 (α M β 2 ), VLA-4 (α 4 β 1 ), and α V β 3, emphasizing the importance of these molecules in regulating leukocyte recruitment. Studies have shown that JAM-integrin interactions control leukocyte infiltration during inflammation. However, JAM may also regulate intestinal permeability and inflammatory responses through homotypic interactions. Recent work by Laukoetter et al. revealed complex roles of JAM-A in controlling intestinal inflammation. Interestingly, genetic deficiency of JAM-A did not alter GI epithelial architecture but did increase leukocyte infiltration and lymphoid aggregates concomitant with increased mucosal permeability. Surprisingly, these animals showed attenuated DSS induced colitis and increased epithelial cell proliferation. Together, these results imply that adhesion molecules often influence additional GI functions besides leukocyte recruitment and inflammation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here