Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Hypothermia remains the sole therapy for evolving HIE that is associated with a significant reduction in death or disability.
A longer duration of cooling or a lower temperature for cooling when treating hypoxic-ischemic encephalopathy is not recommended as it can be associated with harm.
Rewarming is associated with an increase in electrographic seizures that is associated with worse neurodevelopmental outcomes.
Cooling on transport is effective when performed with a device that can control core temperature.
There is little data to indicate benefit from hypothermia for hypoxic-ischemic encephalopathy among infants with a mild encephalopathy, preterm infants, or infants in low or middle-income countries.
Therapeutic hypothermia (TH) is an effective therapy for neonatal encephalopathy when the likelihood of a hypoxic-ischemic origin is high. Multiple randomized trials have demonstrated that relatively small reductions in core temperature either alone, or in combination with reduced head temperature, reduced death or disability at 18 months. The Cochrane metaanalysis indicates that TH reduced death or major neurodevelopmental disability in survivors from 61% in noncooled infants compared with 46% in infants treated with hypothermia, yielding a risk ratio of 0.75 (95% confidence interval [CI] 0.68–0.83), along with decreased death from 34% to 25% (risk ratio 0.75, 95% CI 0.64–0.88), and decreased long term disability from 24.9% to 19.2% (risk ratio 0.77, 95% CI 0.63–0.94). Neuroprotective effects of TH persisted even at 6 to 7 years. , Given the beneficial effects of hypothermia, the Committee on the Fetus and Newborn of the American Academy of Pediatrics has provided an overview of the available data and expectations for centers that provide this therapy. The importance of this therapy extends beyond the benefits provided to infants and their families; it signifies that hypoxic-ischemic brain injury is modifiable ( Fig. 7.1 ) and has accelerated testing other potential neuroprotective interventions either with or without TH.
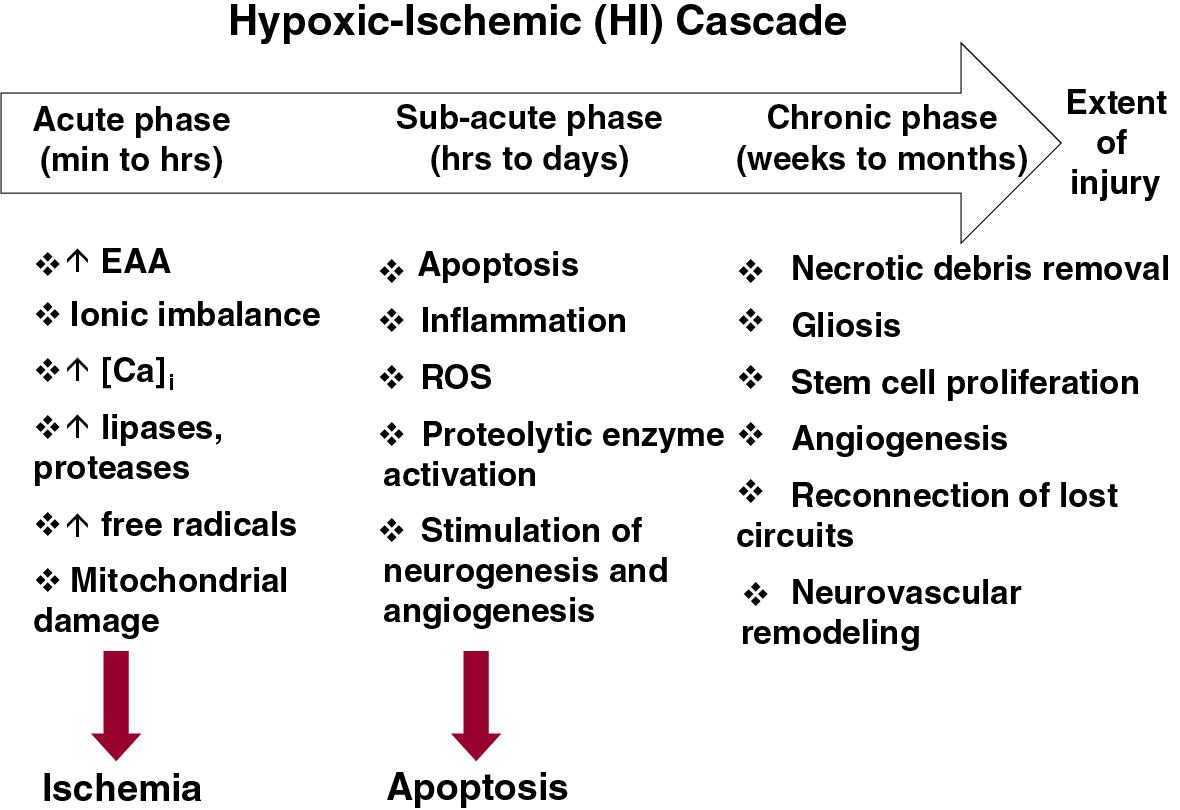
It has been almost two decades since TH has become the standard treatment to treat moderate to severe neonatal encephalopathy of hypoxic-ischemic origin, a masterpiece of translational research which remains the single effective therapy. However, questions remain regarding whether hypothermia treatment can be improved and whether it can be extended to other patient cohorts, such as preterm infants, infants with mild encephalopathy, and infants born in low- and middle-income countries. This updated review addresses the preclinical and clinical evidence behind these questions.
Hypothermia regimens have multiple components: induction, maintenance, and rewarming. Induction represents the time from initiation of cooling to reaching target temperature; maintenance represents the duration of keeping the infant at the target temperature; and rewarming represents the reestablishment of a normothermic temperature. The initial trials of hypothermia used remarkably similar cooling regimens. Specifically, the age of initiation was always less than 6 hours after birth, the extent of temperature reduction was 33.5°C for whole-body cooling and 34.5°C for head combined with body cooling, the duration of cooling was 72 hours, and the rate of rewarming was 0.5°C/hr. The similarity in hypothermia regimens facilitated metaanalyses of multiple trials to provide more accurate estimates of patient outcomes. The only major component of cooling regimens that differed among the initial trials was the mode of cooling: whole-body versus head with body cooling. Metaanalysis indicated that outcomes are similar irrespective of the mode of cooling.
There are several justifications to perform further studies of hypothermia. First, the Cochrane metaanalysis indicated that 46% of infants treated with hypothermia continue to either die or are diagnosed with moderate or severe disability. Data from the National Institute of Child Health and Human Development (NICHD) Neonatal Research Network (NRN), Optimizing Cooling Trial enrolled a more recent cohort of infants with moderate to severe hypoxic-ischemic encephalopathy (HIE) and reported that death or disability occurred in 29%. Even though clinicians and NICUs have more experience caring for infants with HIE, care practices continue to evolve and outcomes have improved over the last decade, there is still much room for improvement in outcomes. Clearly, further investigations of hypothermia are needed in that it was studied in a specific group of newborns—that is, those with a diagnosis of presumed hypoxia-ischemia presenting at less than 6 hours of age and with a gestational age of at least 36 weeks in high-income countries. These trials did not address other cohorts of newborns (preterm, mild encephalopathy, or cooling in low-resource countries). ,
This chapter addresses important gaps concerning the use of TH for HIE. Some of the gaps have been addressed, some are currently under study, and some remain to be investigated.
The depth of temperature reduction used in the first series of hypothermia trials was extrapolated from preclinical investigation and pilot studies in newborns. Recognition that small changes in brain temperature modified the extent of hypoxic-ischemic brain injury in adult animals prompted perinatal animal investigations to examine the effects of depth and duration of “modest hypothermia” at varying times after brain hypoxia-ischemia. Modest hypothermia encompassed a range of temperature reductions from as little as 2°C to as much as 5°C using newborn swine, rat pups, and fetal sheep. This range reflected concerns regarding a possible trade-off between the potential benefit of lower temperature and adverse effects of hypothermia, which increase with greater reductions in core temperature. The optimal temperature to use for hypothermic intervention in clinical trials was not known but was guided by existing animal data and the assessment of incremental reductions in core temperature in pilot human studies of head cooling combined with body cooling , and whole-body cooling. Based on this body of work, clinical trials of head cooling combined with body cooling and whole-body cooling alone were conducted at a rectal temperature of 34.5°C and an esophageal temperature of 33.5°C, respectively. ,
The optimal duration of hypothermia was also uncertain when the first series of clinical trials of hypothermia were undertaken in newborn infants. Available data were derived primarily from adult animals and indicated that increasing the duration of hypothermia reduced brain injury compared with shorter cooling intervals. Although not studied as extensively in newborn animals, 21-day-old rat pups subjected to hypoxia-ischemia had reduced brain injury when cooling was extended to 72 hours compared with 6 hours. In preparation for clinical trials, pilot studies of hypothermia after brain ischemia in fetal sheep by Gunn et al. indicated rebound epileptiform activity when cooling was stopped after 48 hours but not if cooling was continued for 72 hours. Based on these studies, clinical trials of head cooling with mild body cooling and whole-body cooling used a 72-hour cooling intervention. ,
In response to these knowledge gaps, the NICHD NRN conducted a randomized clinical trial of cooling to a lower temperature and for a longer duration (Optimizing Cooling Trial, NCT 01192776). The Optimizing Cooling Trial was a randomized 2 × 2 factorial design performed at 18 centers to determine if longer cooling (120 hours), deeper cooling (32.0°C), or both initiated before 6 hours of age are superior to cooling at 33.5°C for 72 hours among infants of at least 36 weeks’ gestation with moderate or severe HIE. The esophageal profiles of each group are plotted in Fig. 7.2 demonstrating the different interventions. The primary outcome was death or disability at 18 to 22 months adjusted for center and level of encephalopathy. The trial was closed to patient enrollment after 364 of a planned 726 infants were enrolled based on recommendations of an independent Data Safety Monitoring Committee due to a trend of higher deaths with longer and deeper cooling and futility. In-hospital mortality rates for cooling of 72 compared with 120 hours’ duration were 11% and 16%, respectively (adjusted risk ratio [aRR] 1.37, 95% CI 0.92–2.04). In-hospital mortality rates for cooling at 33.5°C compared with 32.0°C were 12% and 16%, respectively (aRR 1.24, 95% CI 0.69–2.25). Although not statistically different, the risk ratio and boundary of the 95% CI suggest that longer cooling maybe associated with an increase in mortality. Cooling for 120 hours was associated with more arrhythmias, anuria, and a longer length of hospital stay compared with 72 hours of cooling, whereas cooling to 32.0°C was associated with a higher use of inhaled nitric oxide, extracorporeal membrane oxygenation, longer use of supplemental oxygen, and a higher incidence of bradycardia compared with cooling to 33.5°C. The results at 18-month follow-up confirmed the concern for futility; specifically, death or disability occurred in 32% of infants cooled for 72 hours and 32% for infants cooled for 120 hours (aRR 0.92, 95% CI 0.68–1.25), and 32% of infants cooled to 33.5°C and 31% for infants cooled to 32.0°C (aRR 0.92, 95% CI 0.68–1.26).
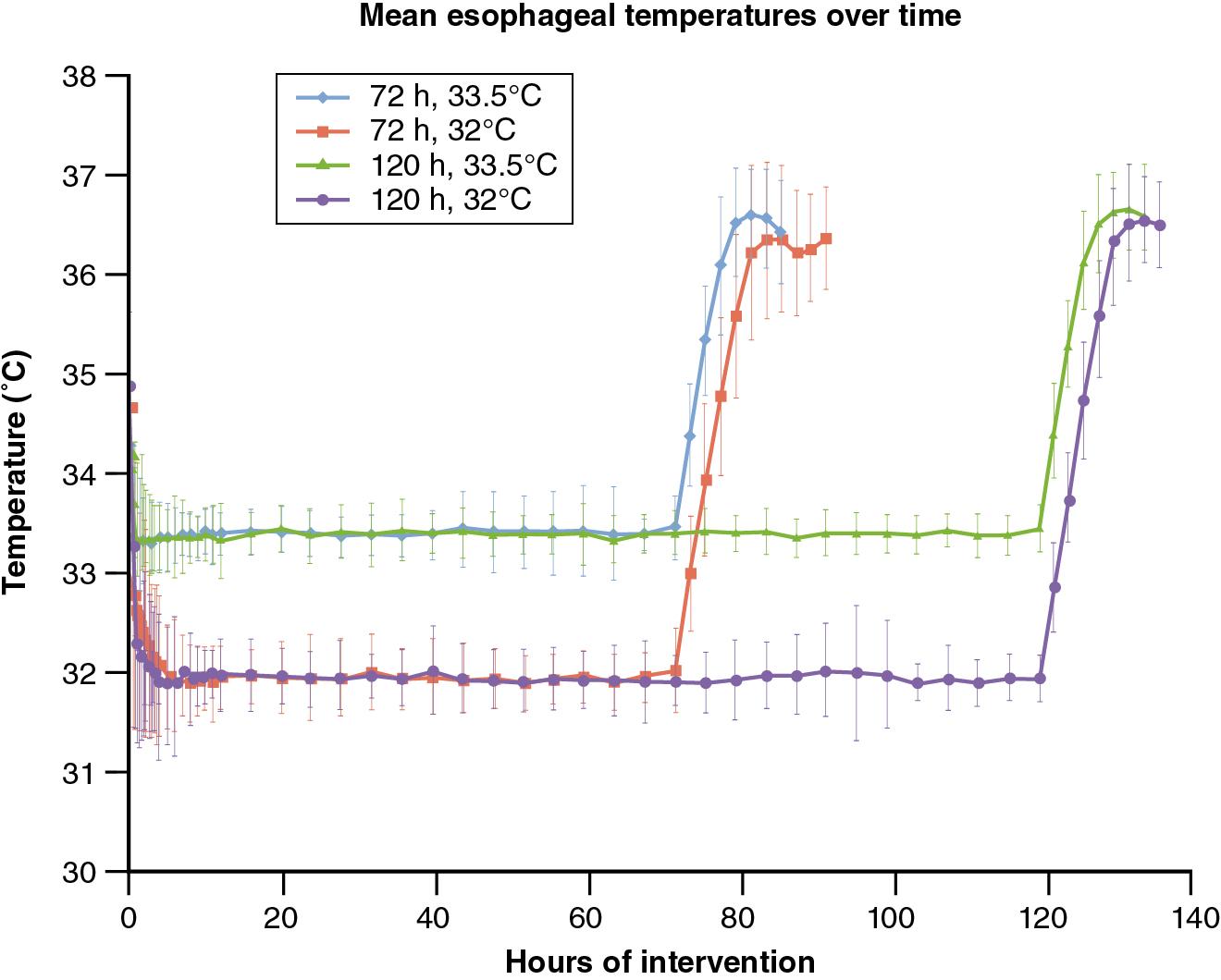
The results of this trial indicate that among infants of at least 36 weeks’ gestation with HIE, longer cooling was not superior to 72 hours of cooling, and deeper cooling was not superior to cooling to 33.5°C. The Optimizing Cooling Trial supports the continued practice of whole-body hypothermia at 33.5°C for 72 hours and drifts from this practice could be associated with increased mortality and morbidity.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here