Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The authors and editors acknowledge the contributions of the authors of this chapter in the 7th edition of the Textbook of Pediatric Rheumatology, Drs. Rubén Burgos-Vargas and Janitzia Vázquez-Mellado.
This chapter is dedicated to the memory of Elia Ayoub, MD.
Reactive arthritis (ReA) is a term for a group of diverse inflammatory arthropathies in which the joint and extraarticular manifestations are caused by a preceding extraarticular infection with specific bacteria. ReA is sometimes included under the umbrella term spondyloarthritis , which refers to a group of heterogenous diseases characterized by arthritis, enthesitis, acute anterior uveitis, and HLA-B27 positivity. ReA accounts for less than 2% of spondyloarthritis cases. ReA can be triggered by enteric, genitourinary, or pharyngeal infection. The two forms of ReA after streptococcal pharyngitis are called rheumatic fever , and poststreptococcal reactive arthritis (PSRA) , and will be discussed separately from ReA from enteric and genitourinary infections.
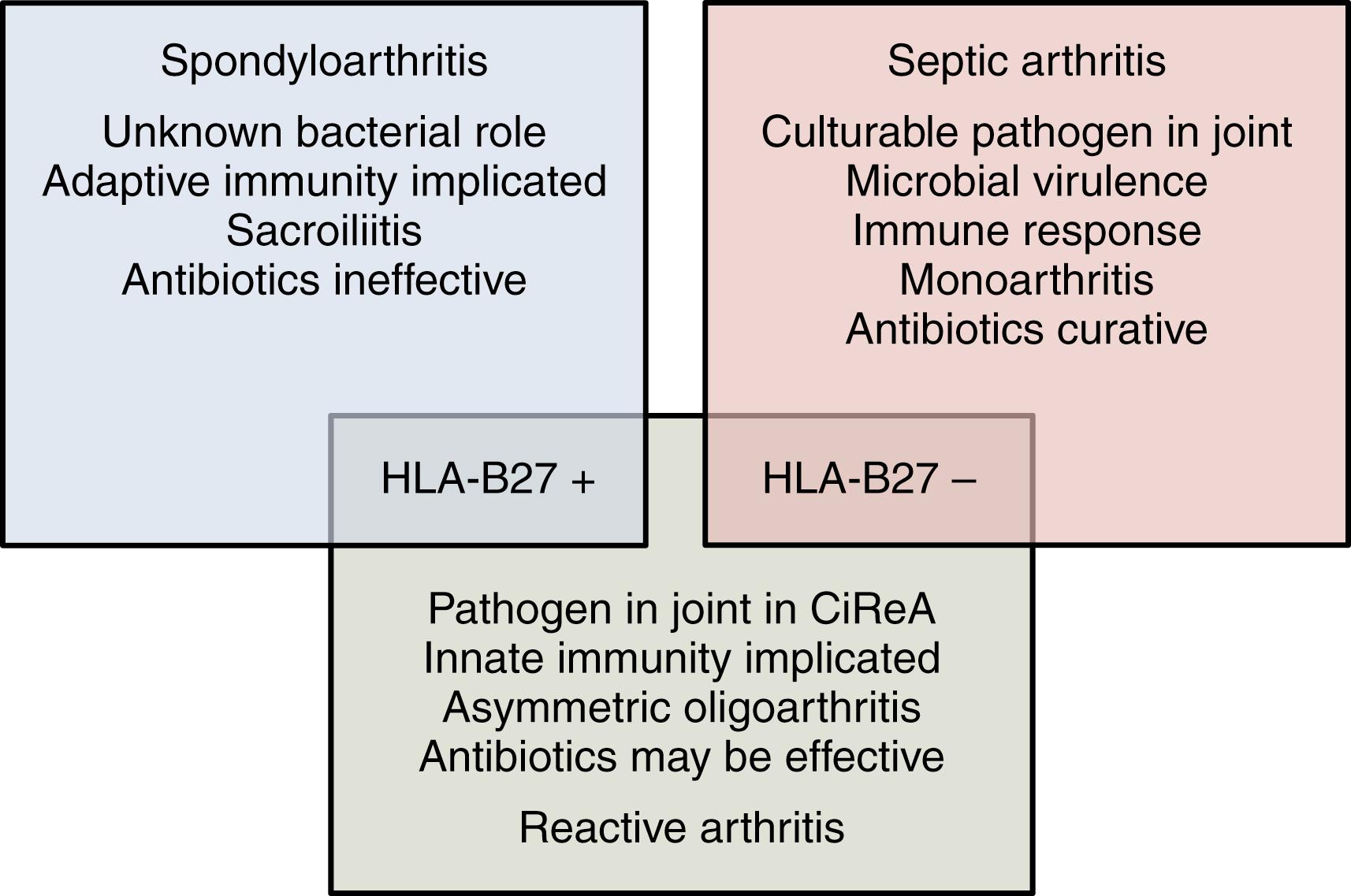
The most common arthritogenic enteric and genitourinary bacteria are Yersinia , Salmonella , Shigella , Campylobacter , and Chlamydia trachomatis . , Less common triggers of ReA include Chlamydia pneumoniae , Mycoplasma pneumoniae , and Clostridium difficile . The hypothesized relationship between ReA, spondyloarthritis (SpA), and septic arthritis according to Gracey and Inman is shown in Fig. 46.1 . The triad of arthritis, conjunctivitis, and urethritis (or cervicitis) was previously called Reiter syndrome but this eponym has been deleted from contemporary literature.
There are no validated diagnostic criteria for ReA, but criteria proposed in Berlin in 1995 by the Third International Workshop on Reactive Arthritis are shown in Box 46.1 . Laboratory tests for documenting preceding infection are shown in Box 46.2 . Preceding bacterial infection is most frequently documented serologically, but its actual value is controversial.
Predominantly lower limb, asymmetrical oligoarthritis
Plus
If there is a clear history of diarrhea or urethritis within the preceding 4 weeks, laboratory confirmation is desirable but not essential.
Where no clear clinical infection is identified, laboratory confirmation of infection is essential.
Patients with other known causes of monoarthritis or oligoarthritis (such as other defined spondyloarthropathies, septic arthritis, crystal arthritis, Lyme disease, and streptococcal reactive arthritis) should be excluded.
Routine
Culture of stool and urethra
Serology: antibodies against specific arthritogenic bacteria
Research studies
Urethral swab for detection of chlamydial DNA by polymerase chain reaction (PCR) ∗
∗ A potential diagnostic test.
† Research tools, not suitable for routine diagnostic use.
Synovial fluid or synovial membrane biopsy for detection of bacterial DNA by PCR ∗
Immunofluorescence microscopy for detection of bacteria in synovial biopsy specimen †
Stimulation of synovial fluid lymphocytes with antigens from arthritogenic bacteria †
The prevalence of ReA in adults worldwide is 1 per 1000 people. In a systematic review, the incidence of ReA after Campylobacter, Salmonella, and Shigella infections were 9, 12, and 12 per 1000 cases, respectively. Four to eight percent of cases of C. trachomatis and 1% to 2% of cases of C. difficile infection are followed by ReA. The relative frequency of ReA among patients in pediatric rheumatology clinics in the United States, , the United Kingdom, and Canada ranges between 4.1% and 8.6%. This wide variation may be attributable to differences in diagnostic and classification criteria used in each study, as well as HLA-B27 prevalence in the various populations. Most cases of ReA occur in boys between the ages of 8 and 12 years, but sex and age distribution vary according to the causative organism. Enteric infections are responsible for ReA at all ages, but ReA after genital infections with Chlamydia occurs more frequently during adolescence and in adults. In an Italian study of children with Yersinia -triggered ReA, most cases occurred between the ages of 3 and 7 years and there was a slight predominance of girls.
Although ReA predominantly occurs in individuals who are human leukocyte antigen (HLA)-B27 positive, a significant proportion of patients are HLA-B27 negative. The frequency of HLA-B27 in children with ReA varies widely; in children with mild forms of Yersinia-, Campylobacter -, Chlamydia-, and Mycoplasma pneumoniae – related ReA, the frequency of HLA-B27 is similar to that of the population.
Other associations have been reported in specific populations. An association with the tumor necrosis factor (TNF) c1 allele independent of HLA-B27 was reported in a predominantly adult Finnish population with ReA. In a similar population, TAP2J, a polymorphism of transporters associated with antigen processing (TAP2), was more frequent in HLA-B27–positive patients with ReA. An association between the Toll-like receptor 2 and ReA was described after an outbreak of Salmonella enteritidis in Canada. Single nucleotide polymorphisms in the interferon (IFN)-γ gene (rs2430561 and rs1861493) appear to predispose the Dutch to ReA, and solute carrier family 11 member A1 gene polymorphisms are increased in the Chinese. In contrast, the chemokine receptor 5 (CCR5)-delta 32 mutation does not play a role in the susceptibility to ReA in patients with C. trachomatis infection.
Because the prevalence of HLA-B27 is increased in patients with ReA, HLA-B27 has long been thought to play a role in pathogenesis. Three things differentiate HLA-B27 from other major histocompatibility complex (MHC) class I molecules: (1) peptide binding specificity, (2) tendency to form heavy chain homodimers, and (3) propensity to misfold. Each of these features has been hypothesized to play a role in the pathophysiology of ReA. In the arthritogenic peptide hypothesis, the HLA-B27 molecule binds a unique bacterial or self-antigenic peptide, which is then presented to cytotoxic (CD8 + ) T cells. , However, in rat models of disease, CD8 T cells are not required for development of disease , ; this has been shown through antibody depletion and chemically induced mutations that eliminate CD8 protein expression. Additionally, to date no arthrogenic peptide has been discovered. In the second hypothesis, HLA-B27 forms homodimers that are recognized by the KIR receptor on CD4 + cells that then trigger an inflammatory response. In the third hypothesis, misfolded HLA-B27 in the endoplasmic reticulum causes stress and triggers the unfolded protein response (UPR). ,
The UPR aims to restore normal cell function by reducing or stopping protein translation, degrading misfolded proteins, and increasing folding capacity. If the UPR is prolonged, apoptosis occurs. Overactivation of the UPR is not unique to ReA—it is also implicated in the pathogenesis of inflammatory bowel disease, lupus, SpA, and multiple sclerosis. The UPR, driven by HLA-B27 misfolding, is also associated with excessive interleukin (IL)-23 production 33a and, to a lesser extent, IL-12 production. IL-23 then stimulates T helper (Th)17 cells to produce IL-17.
HLA-B27 also modulates the production of cytokines and influences both bacterial invasion of cells and killing of bacteria. As a result, intracellular survival of arthritogenic bacteria is prolonged. Arthritogenic bacteria invade the gut mucosa and replicate within polymorphonuclear cells and macrophages. Studies in murine fibroblasts transfected with HLA-B27, not replicated in human cells, indicated the expression of this antigen-inhibited cell invasion by arthritogenic bacteria. Persistence of the organism within B27 cells was prolonged. ,
Because not all patients with ReA are HLA-B27 positive, the role of microbial factors has also been explored. Various bacterial components (including lipopolysaccharide, DNA, and RNA) have been identified in both synovial fluid cells and synovial membranes of patients with ReA. Chlamydia, a microorganism with both intracellular and extracellular life cycles, may turn into a nonreplicative, nonculturable, but viable state within the cell. , In this sense, it is now possible to consider that Chlamydia ReA may actually be a form of septic arthritis. , The antibody response against arthritogenic bacteria in ReA lasts longer than in infected patients who do not develop arthritis. Additional findings suggest a role for heat shock proteins , and bacterial peptidoglycan in the pathogenesis of ReA.
The course and severity of ReA vary considerably. Symptoms of infection usually precede the onset of arthritis, enthesitis, or extraarticular disease by 1 to 4 weeks. After an active period of weeks to months, the arthritis subsides and the patient then enters a sustained remission or a phase of recurrent disease activity, which may evolve into enthesitis-related arthritis or SpA, including ankylosing spondylitis.
An appreciation of the characteristics of the enteric or genitourinary infections that trigger ReA could aid in the identification of the bacteria involved in the pathogenesis of the disease.
A period of high fever, with or without watery diarrhea, and cramping abdominal pain lasting 48 to 72 hours may be followed in 7 to 21 days by the sudden onset of nonmigratory oligoarthritis (knees and ankles) lasting from several weeks to 3 or 4 months. Diagnosis requires a history, the presence of agglutinins to Shigella flexneri serotype 2 or 2a, and an attempt to isolate the organism from the stool. Because of the long interval between the diarrhea and the joint complaints, blood cultures are positive in less than 4% of patients.
Enteric infection Salmonella typhimurium or S. enteritidis may be mild, but the onset of arthritis is usually accompanied by low-grade fever. Because Salmonella infection can also result in osteomyelitis and septic arthritis, it is important to make certain that the synovial fluid is sterile. The erythrocyte sedimentation rate (ESR) is usually elevated, and the leukopenia that may accompany the acute infection is generally followed by leukocytosis. Stool cultures are usually positive, even late in the disease course, but seroconversion to Salmonella H and O antigens occurs in only 50% of patients.
Yersinia enterocolitica causes gastroenteritis in young children and a syndrome of abdominal pain similar to that of appendicitis in older children. Contact with the organism is through infected drinking water or milk. In one study of children hospitalized because of Yersinia infection, 35% had arthritis lasting 3 to 22 months (average, 6.5 months). Of those with arthritis, 85% had HLA-B27. Yersinia can occasionally cause septic arthritis.
In an epidemic outbreak of Campylobacter jejuni enteritis in Finland, 2.6% of patients—all adults—developed oligoarthritis or polyarthritis 4 days to 4 weeks after infection. Synovial fluid cultures were negative, and 33% of the patients with arthritis were positive for HLA-B27.
Genitourinary tract infection with C. trachomatis is often asymptomatic but may cause dysuria, frequency, and a urethral or vaginal discharge. ReA may also be related to upper respiratory tract infections with Chlamydia pneumoniae. Artamonov et al. found evidence of nasopharyngeal infection in 45 of 52 children with ReA. Although the prevalence of HLA-B27 was higher than that in the control population (relative risk, 2.5), it was much lower than that in those who developed ReA after intestinal infection. Chlamydia infection is increasingly recognized in teenagers, and particularly in young adults.
The arthritis of ReA is usually acute and painful and the joints are often warm and red. It is typically oligoarticular (<five joints) and may be additive or migratory. Some children show only slight to moderate joint pain and swelling over several weeks. In other children, arthralgias appear before the onset of arthritis for a variable amount of time. The initial episode of arthritis usually affects the knees or ankles. The pattern of arthritis in the metatarsophalangeal (MTP) joints and the proximal and distal interphalangeal (IP) joints of the feet may be that of a dactylitis and may involve two or three joints in one or more digits in combination with tenosynovitis and bursitis. Arthritis of the small joints of the hands caused by Yersinia and Salmonella has also been described in ReA . , , , Enthesitis may occur alone or with arthritis, tenosynovitis, or bursitis ( Figs. 46.2 and 46.3 ).
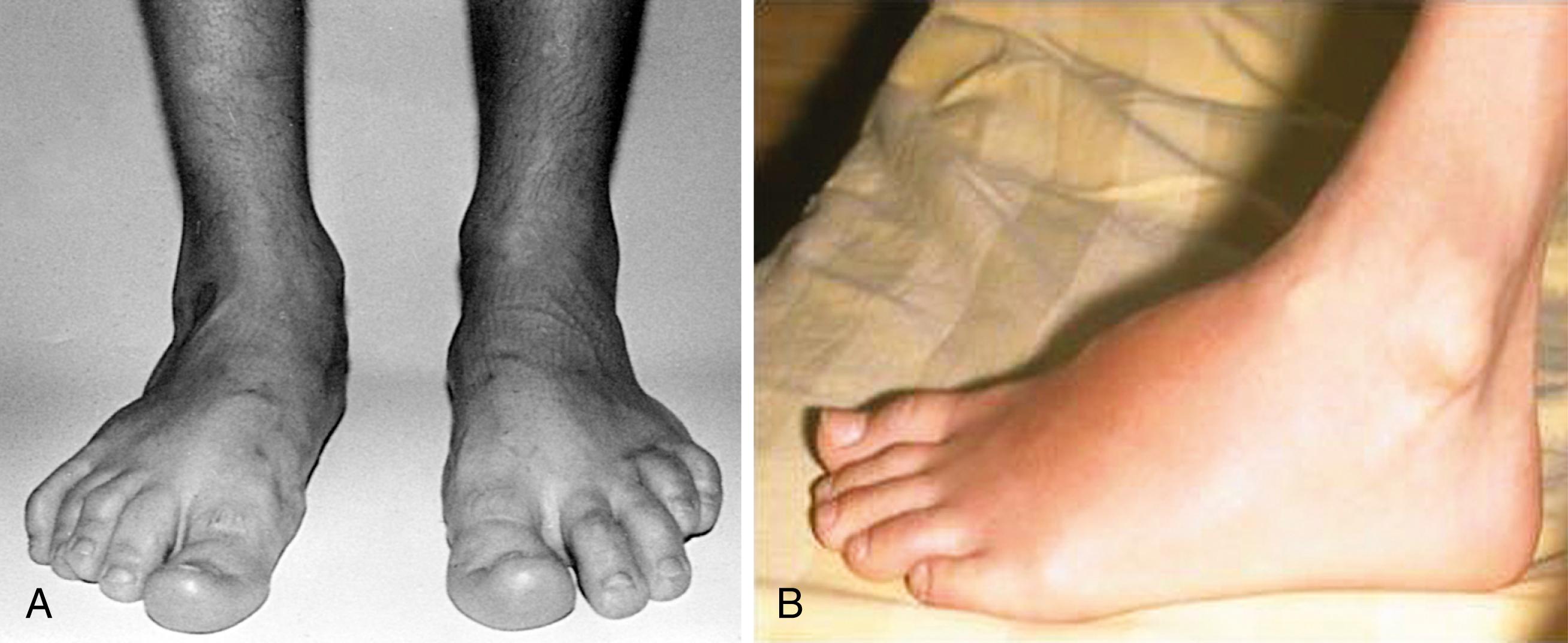
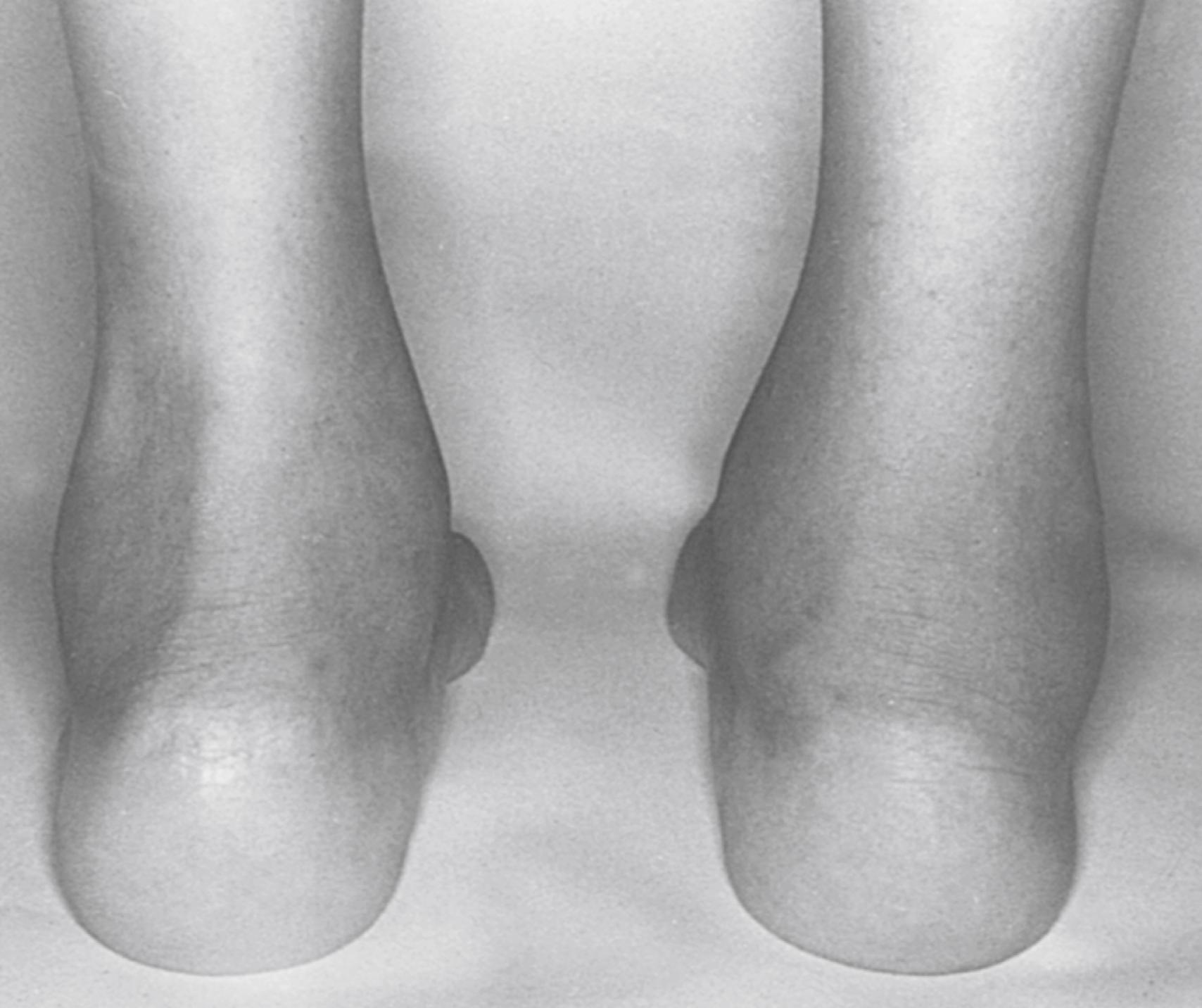
The synovial fluid effusion is usually marked, but proliferative synovitis is uncommon. In addition to involvement of peripheral joints and entheses, there may be inflammation of joints of the axial skeleton resulting in spinal and sacroiliac pain, stiffness, and reduced mobility of the lumbar and cervical spine.
Apart from the infection itself, children with ReA may have fever, weight loss, fatigue, and muscle weakness during active periods of disease. Myalgia, arthralgia, and stiffness sometimes accompany these symptoms. Myocarditis and pericarditis have been described in children with S. enteritidis -triggered ReA.
Painless, shallow ulcers of the oral mucosa and palate are common and often asymptomatic. Aphthous stomatitis occurs in some patients. Urethritis and cervicitis are rare manifestations, occurring more frequently in adolescents with sexually acquired ReA caused by Chlamydia. These conditions are often mild, and girls tend to have no symptoms; they are detected only because of the presence of sterile pyuria. Diarrhea occurs in association with bacterial infection but may also be part of a generalized episode of mucositis.
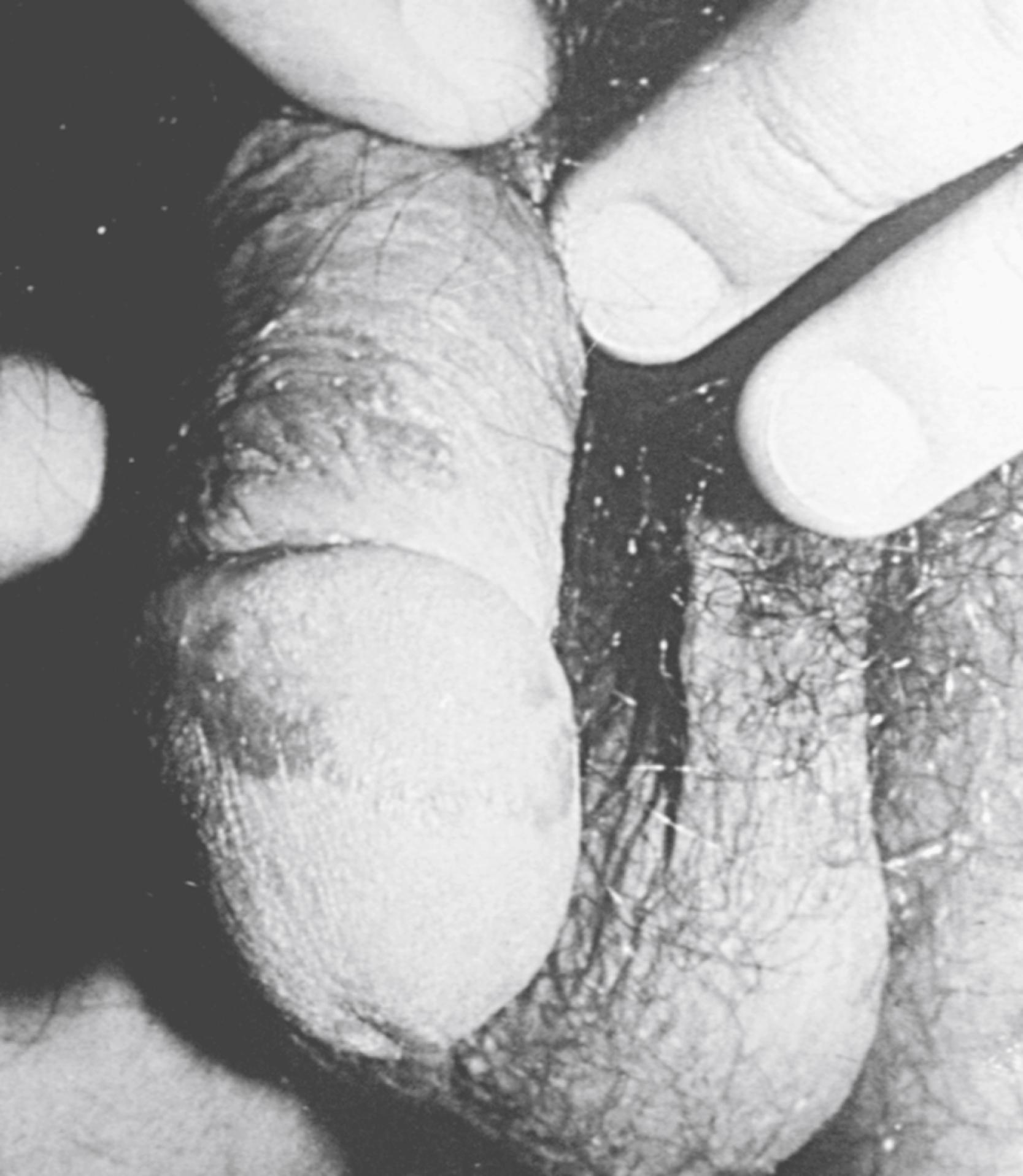
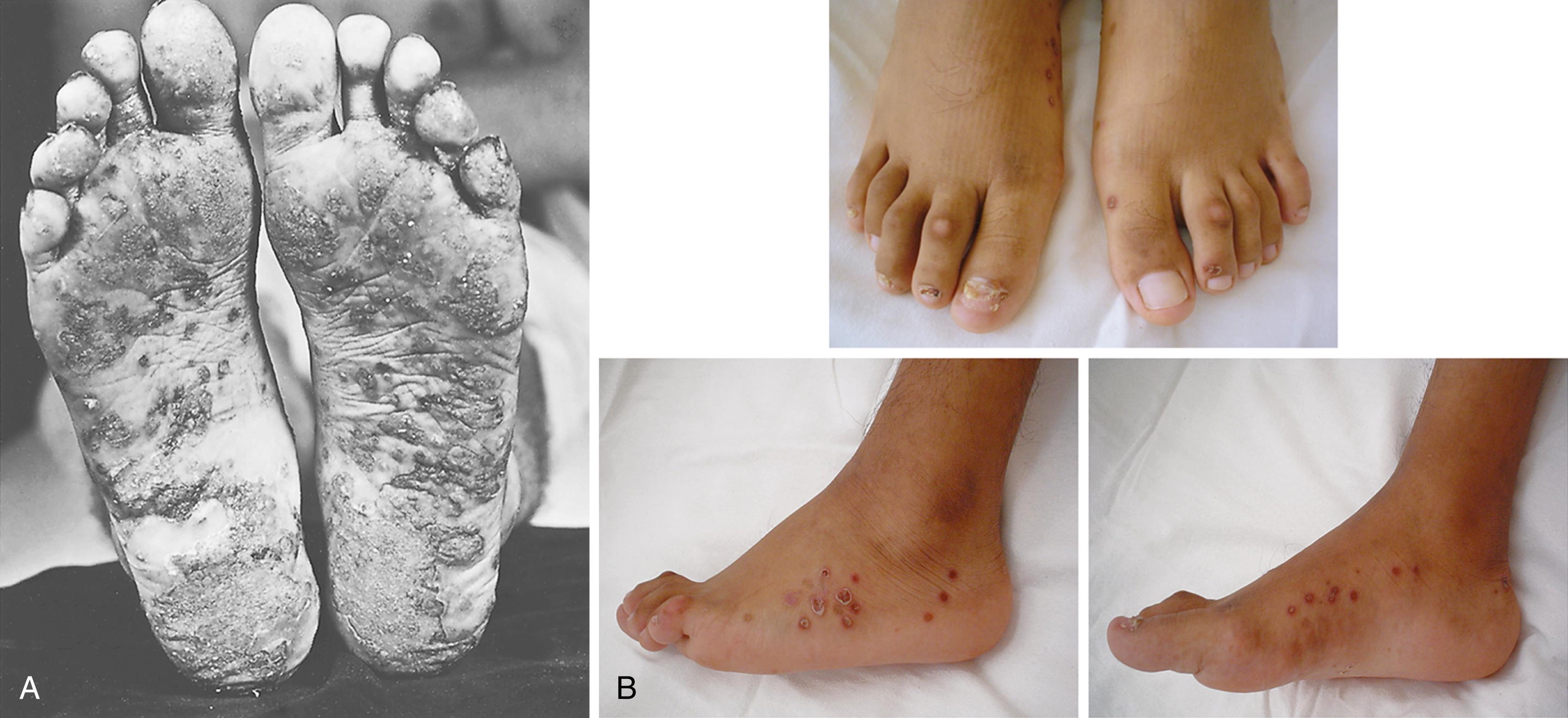
Skin lesions in ReA include erythema nodosum in some children with Yersinia -triggered ReA, circinate balanitis ( Fig. 46.4 ), and keratoderma blennorrhagicum ( Fig. 46.5 ), with or without conjunctivitis or urethritis. , , Keratoderma may be clinically and histologically indistinguishable from psoriasis. Mucocutaneous involvement in ReA tends to parallel disease activity in the peripheral joints.
Conjunctivitis occurs in about two-thirds of children at onset. In Yersinia -triggered ReA, conjunctivitis may be purulent and severe. , Acute iridocyclitis in these cases is characterized by flare and cells in the anterior chamber, small keratic precipitates, cells in the vitreous, and occasionally fibrinous exudates, posterior synechiae, and macular edema in a unilateral or bilateral pattern. Acute anterior uveitis has also been described in ReA triggered by S. typhimurium . Although there are few studies of the visual prognosis in children with ReA, the frequency of patients with permanent ocular sequelae appears to be low.
In the early inflammatory phase, there may be anemia, mild leukocytosis, neutrophilia, thrombocytosis, and hypergammaglobulinemia. The ESR and C-reactive protein (CRP) are often elevated and tend to correlate with disease activity. In patients with severe disease—particularly in those with polyarthritis and polyenthesitis, fever, weight loss, fatigue, mucositis, or dermatitis—laboratory abnormalities may be quite abnormal. Autoantibodies (e.g., rheumatoid factor, antinuclear antibodies) are usually absent. HLA-B27 positivity is found in 50% to 80% of patients. Synovial fluid analysis typically shows a pleocytosis with 10 to 50,000 white blood cells per high powered field and neutrophil predominance; culture may help distinguish ReA from septic arthritis. ,
With the exception of epidemics and some isolated reports, the clinical and laboratory confirmations of infection as a trigger in children with ReA are seldom made. When available, cultures obtained at the time of the infection may be helpful. Salmonella, Yersinia, Shigella, Campylobacter, and C. difficile may be isolated from the gut during an episode of diarrhea, or Chlamydia may be cultured from the urethra, but negative results do not exclude the diagnosis of infection-related arthritis. Because Salmonella and Chlamydia may also be present in asymptomatic carriers, these organisms can occasionally be cultured from patients who have arthritis not directly related to these organisms. Nonetheless, it is important to bear in mind that viable but nonculturable Chlamydia might be found in the joints of patients with arthritis. Electron microscopy, immunochemistry, and DNA studies might help in identifying extracellular elemental and intracellular replicative bodies and other constituents of Chlamydia. ,
More frequently, ReA is diagnosed in the appropriate clinical setting because of the presence of high titers of serum antibodies against arthritogenic bacterial antigens. , Hemagglutination tests are useful in documenting recent infections with Salmonella or Yersinia. , , , , Both the sensitivity and the specificity of circulating immunoglobulin (Ig)A and IgM antibodies to Salmonella, Yersinia, and Campylobacter detected by enzyme-linked immunoassay are acceptable, but results must be compared with those in the control population. C. difficile infection can be confirmed with polymerase chain reaction and C. trachomatis can be confirmed using nucleic acid amplification of a urethral swab. IgG antibodies are useful if levels change significantly; a rising titer of IgA antibodies may be noted. Overall, the use of these types of tests and their interpretation are still somewhat controversial.
Radiographic abnormalities early in the disease consist only of nonspecific soft tissue swelling, juxtaarticular osteopenia, and (less frequently) slight periosteal irregularities at tendon attachments. The occurrence of subchondral cysts, erosions, and sometimes extensive destruction of joints, such as the hips, proximal and distal IPs of the hands and feet, and, less commonly, joints of the wrist, may occur. Ultrasonographic studies may delineate synovial sheath and tendon thickening and the accumulation of synovial fluid within the tendon sheath and bursae. Short tau inversion recovery (STIR) and T1 (with or with gadolinium or T2 fat-suppressed magnetic resonance imaging [MRI]) sequences may show bone edema, which is interpreted as inflammation, as well as synovitis, tenosynovitis, and bursitis. Various entheses, especially those at the insertion of the plantar fascia to the calcaneus, may show erosions, marked bony proliferation, and spur formation. These abnormalities may also be apparent in the navicular bone, greater trochanter, and ischium. Symptomatic and radiographic involvement of the sacroiliac joint and spine are rare in children with ReA, but MRI of the sacroiliac joints may reveal acute and chronic changes (see Chapter 9 ).
Differentiation of ReA from other types of arthritis is often difficult ( Box 46.3 ). ReA can typically be distinguished from juvenile idiopathic arthritis (JIA) because it is usually more painful and associated with erythema of the overlying skin. Specific clinical features, such as rash, subcutaneous nodules, and lymphadenopathy, help differentiate diseases such as Kawasaki disease and Lyme disease from ReA. Septic arthritis is also a painful arthritis associated with overlying erythema of the joint that can be confused with ReA. Septic arthritis may be distinguished from ReA by the presence of fever and positive culture from synovial fluid; ReA can affect more than 1 joint, whereas septic arthritis affects only single joints in immunocompetent hosts. Early JIA and the arthritis of inflammatory bowel disease must also be considered. Laboratory test abnormalities, such as a positive synovial fluid culture or elevated antistreptolysin O titers suggest septic arthritis or rheumatic fever. The presence of elevated inflammatory indexes helps exclude orthopedic conditions.
Presumed viral arthritis (including transient synovitis of the hip)
Poststreptococcal arthritis (including rheumatic fever)
Lyme disease
Septic arthritis, tuberculosis, gonococcal arthropathy
Juvenile idiopathic arthritis
Arthritis associated with Crohn disease and ulcerative colitis
Synovitis, acne, pustulosis, hyperostosis, and osteomyelitis (SAPHO) syndrome
Behçet disease
Kawasaki disease
Legg–Calvé–Perthes disease, Osgood–Schlatter disease
“Growing pains”
Idiopathic pain syndromes (fibromyalgia, reflex sympathetic dystrophy)
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here