Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The term “rare coagulation factor deficiency” refers to disorders of thrombin and/or fibrin formation caused by mutations in a single gene, other than those for von Willebrand factor (see Chapter 133 ), factor VIII, or factor IX (see Chapter 134 ), that reduce plasma activity of one or more coagulation proteins. The most common inherited deficiencies that cause defects in plasma coagulation are those for factor VIII (hemophilia A) and factor IX (hemophilia B), with frequencies of 1 in 10,000 and 1 in 30,000 male births respectively (see Chapter 134 ). In comparison, severe deficiency of fibrinogen, the protease zymogens prothrombin or factors VII, X, or XI, the cofactor factor V, or the transaminase factor XIII are estimated to occur in one in 500,000 to 2 million individuals ( Table 135.1 ). These conditions are inherited primarily as autosomal recessive conditions, implying carrier frequencies (∼1 in 1000 persons) that are 10-fold higher than the carrier frequencies for alleles causing X-linked hemophilia A or B. The rarity of these disorders is therefore due to their recessive nature and not low allele frequency. This is important to keep in mind because partial (heterozygous) deficiencies of these proteins are relatively common and may contribute to bleeding symptoms. As with any recessive trait, incidences are up to 10-fold higher in areas where consanguinity is common.
| Protein | Factor Plasma Half-Life (h) | Vitamin K Dependent Modification | Factor Level in Pregnancy | Incidence of Severe Inherited Deficiency | Autosomal Inheritance Pattern of Deficiency | Other Names for Deficiency State | Bleeding Diathesis in Severe Deficiency | Screening Tests in Deficiency | |
|---|---|---|---|---|---|---|---|---|---|
| PT | PTT | ||||||||
| Fibrinogen | 72–120 | No | ↑ | ∼1:1 × 10 6 | Recessive or Dominant | Afibrinogenemia | Severe | ↑ | ↑ |
| Prothrombin | 60–100 | Yes | ↔ | ∼1:2 × 10 6 | Recessive | Hypoprothrombinemia | Severe | ↑ | ↑ |
| Factor V | 12–14 | No | ↔ | ∼1:1 × 10 6 | Recessive | Parahemophilia | Moderate to Severe | ↑ | ↑ |
| Factor VII | 3–4 | Yes | ↑ | ∼1:5 × 10 5 | Recessive | Serum prothrombin conversion accelerator deficiency | Moderate to Severe | ↑ | ↔ |
| Factor X | 20–40 | Yes | ↑ | ∼1:1 × 10 6 | Recessive | Stuart-Prower factor deficiency | Severe | ↑ ↔ | ↑ ↔ |
| Factor XI | 45–52 | No | Inconsistent | ∼1:1 × 10 6 | Recessive or Dominant | Hemophilia C, plasma thromboplastin antecedent deficiency | Asymptomatic to Moderate | ↔ | ↑ |
| Factor XII | 60 | No | ↑ | Unknown | Recessive | Hageman trait | Asymptomatic | ↔ | ↑ |
| Prekallikrein | Not known | No | ↔ | Unknown | Recessive | Fletcher trait | Asymptomatic | ↔ | ↑ |
| High molecular weight kininogen | 170 | No | ↔ | Unknown | Recessive | Flaujeac trait, Williams trait, Fitzgerald trait | Asymptomatic | ↔ | ↑ |
| Factor XIII | 150 | No | ↓ | 1:2 × 10 6 | Recessive | Fibrin stabilizing factor deficiency | Severe | ↔ | ↔ |
| ↑ Increase in coagulation factor; ↓ decrease; ↔ no change. | |||||||||
Fig. 135.1A shows a scheme reflecting current understanding of the major enzyme reactions involved in thrombin generation and fibrin formation. Factor VIIa binds to tissue factor in the wall of a damaged blood vessel, forming a complex that activates factor X to Xa, which converts prothrombin to thrombin in the presence of factor Va. Mice lacking prothrombin, factor VII, factor X, or factor V die in utero or soon after birth, demonstrating the importance of these reactions. Thrombin converts fibrinogen to fibrin. Factor IX is also activated by factor VIIa/tissue factor and, with factor VIIIa, sustains thrombin generation by activating factor X. In some situations, factor IX activation by factor XIa is required. The older model shown in Fig. 135.1B shows the order of reactions during coagulation in the prothrombin time (PT) and activated partial thromboplastin time (aPTT) assays (see Chapter 127 ). Here factor XI activation requires factor XII, prekallikrein, and high molecular weight kininogen. However, deficiencies of these proteins do not result in abnormal bleeding, indicating that other mechanisms exist for factor XI activation. In Fig. 135.1A thrombin activates factor XI. Finally, factor XIII is activated by thrombin and cross-links fibrin monomers within a fibrin polymer, increasing the tensile strength of fibrin (see Fig. 135.1A ).
Rare coagulation factor deficiencies represent 3% to 5% of inherited plasma coagulation factor deficiencies. This chapter contains separate sections describing inherited and acquired deficiency states for each factor. A number from the Online Mendelian Inheritance in Man (OMIM) database is given in the title for each section on an inherited deficiency. Updated lists of mutations associated with factor deficiencies can be found at several websites such as http://www.clotbase.bicnirrh.res.in and http://www.hgmd.org . The rarity and clinical heterogeneity of the inherited disorders described in this chapter, combined with limited availability of testing, present challenges for establishing evidence-based treatment guidelines. Common symptoms include bleeding from mucosal surfaces, heavy menstrual bleeding, and hemorrhage with invasive procedures and childbirth. Bleeding involving the central nervous system or umbilical cord stump are most frequent in severe deficiencies of fibrinogen, factors X, and factor XIII, while gastrointestinal bleeding is a particular problem in factor X deficiency. Hemarthroses can occur with afibrinogenemia and severe deficiency of factors II or X. The World Federation of Hemophilia (WHF, www.wfh.org ) and the International Rare Bleeding Disorder Database (RBDD, www.rbdd.org ) collect information for the rare coagulation factor deficiencies ( Fig. 135.2 ). The European Network of Rare Bleeding Disorders (EN-RBD) has subclassified these conditions based on clinical features to facilitate development of evidence-based diagnostic and treatment strategies ( Table 135.2 ). They noted strong associations between bleeding severity and plasma factor levels in fibrinogen, factor X, and factor XIII deficiencies, weaker associations for factor V or factor VII deficiency, and very weak association in factor XI deficiency.
| Disorder | EN-RBD Severity Category | Clinical Correlation With Factor Level | ||
|---|---|---|---|---|
| Severe | Moderate | Mild | ||
| Fibrinogen deficiency | Undetectable | 0.1–1.0 gm/dL | >1.0 g/dL | Strong |
| Prothrombin deficiency | Undetectable | ≤0.1 IU/mL (≤10%) | >0.1 IU/mL (>10%) | Strong |
| Factor V deficiency | Undetectable | <0.1 IU/mL (<10%) | ≥0.1 IU/mL (≥10%) | Weak |
| Factor VII deficiency | <0.1 IU/mL (<10%) | 0.1–0.2 IU/mL (10%–20%) | >0.2 IU/mL (>20%) | Weak |
| Factor X deficiency | <0.1 IU/mL (<10%) | 0.1–0.4 IU/mL (10%–40%) | >0.4 IU/mL (>40%) | Strong |
| Factor XI deficiency | — | — | — | Very weak |
| Factor XIII deficiency | Undetectable | <0.3 IU/mL (≤30%) | ≥0.3 IU/mL (>30%) | Strong |
| Combined factor V and VIII deficiency | <0.2 IU/mL (<20%) | 0.2–0.4 IU/mL (20%–40%) | >0.4 IU/mL (>40%) | Weak |
| Vitamin K-dependent factor deficiencies | — | — | — | Weak |
Table 135.3 summarizes treatment recommendations. Several therapeutic advances could improve treatment of rare bleeding disorders soon. Single factor concentrates are now available for fibrinogen and factors VII, X, XI, and XIII, although availability for some is limited. A new factor V concentrate awaits clinical testing. Most of these preparations are derived from human plasma; recombinant versions are currently available only for factor VIIa and the A domain of factor XIII. Strategies for enhancing thrombin generation and activity such as siRNAs to reduce plasma antithrombin, the bispecific factor IX/factor X antibody emicizumab, and the anti-tissue factor pathway inhibitor antibody concizumab have shown promise in patients with hemophilia (see Chapter 134 ), and may turn out to be useful in some rare coagulation factor deficiencies.
| Protein | Blood Product Sources | Concentrate (Manufacturer) | Minimum Plasma Level Required for Hemostasis | Minimum Plasma Level Required for Surgery | Dose of Preferred Treatment for Surgery or Bleeding | Dosing Frequency for Surgery or Bleeding | Prophylaxis for Severe Deficiency | Pregnancy: Suggested Plasma Levels for Invasive Procedures and Delivery |
|---|---|---|---|---|---|---|---|---|
| Fibrinogen | CRYO FFP | >50 mg/dL | 100 mg/dLuntil healing complete |
|
Q 24–72 h depending on consumption | Keep level 50 mg/dL for recurrent bleeders | Maintain at 100 mg/dL throughout pregnancy | |
| Prothrombin | FFP | PCC a | ∼5% | 30% | 20–30 factor IX units/kg | Q 48–72 h | 40 factor IX units/kg Q 5–6 days | 20%–30% |
| Factor V | FFP a platelets | None | ∼10% | 25% | 20 mL FFP/kg then 5–10 mL/kg q12 h | Q 12–24 h | Not usually required | 15%–25% |
| Factor VII | FFP | 5%–10% | 15%–25% | 15–30 μg factor VIIa/kg | Q 2–12 h | 20–30 μg/kg 2–3 times per week | 10%–20% | |
| Factor X | FFP |
|
∼10% | 25%–40% |
|
Q 24 h |
|
10%–20% |
| Factor XI | FFP a |
|
15% | 30%–40% |
|
Q 1–2 days | Not required | May withhold treatment unless bleeding. 20%–40% if bleeding occurs |
| Factor XIII | CRYO FFP |
|
1%–2% | 20%–50% | 20–30 factor XIII units/kg | Single dose may be sufficient | 40 factor XIII units/kg q 28 days | 250 units/week through week 22, then 500 units/week with a 1000 unit bolus during labor |
a Preferred treatment for inherited deficiency
b Assuming baseline fibrinogen is not known.
c FDA approved for the treatment of adults and adolescents (12 years old and older) with hemophilia A or B with inhibitors, but not currently approved for treatment of patients with inherited factor VII deficiency.
d Plasma concentration of factors can vary significantly, however 15 to 20 mL/kg should result in an increase of 10% to 20% factor XI activity. We recommend assessment of factor activity prior to surgical procedures and in the setting of significant bleeding if feasible.
Fibrinogen was first purified from plasma in the late 19th century. Fibrinogen and fibrin were designated factor I and Ia , respectively, by the International Committee for Nomenclature of Blood Clotting. Inherited absence of fibrinogen ( afibrinogenemia ) was described in 1920 and has an estimated incidence of 1 in 1 million people (see Table 135.1 ). Partial fibrinogen deficiency is called hypofibrinogenemia . Fibrinogen is synthesized in hepatocytes as a 340,000-Dalton protein composed of two trimers, each containing an Aα, Bβ, and γ chain ( Fig. 135.3 ) that are encoded by separate genes ( FGA, FGB , and FGG ) within a 50-kilobase span on chromosome 4. Thrombin converts fibrinogen to fibrin by cleaving fibrinopeptides A and B from the Aα and Bβ chains respectively (see Chapter 121, Chapter 125 ). Fibrinogen also binds glycoprotein IIb/IIIa, facilitating platelet aggregation. Fibrinogen in platelet α-granules is taken up from plasma by a glycoprotein IIb/IIIa-dependent mechanism. Between 8% and 15% of plasma fibrinogen contains at least one γ chain that is a product of an alternatively spliced mRNA called γ′-fibrinogen. γ′-fibrinogen modulates thrombin and factor XIII activity and influences clot architecture.
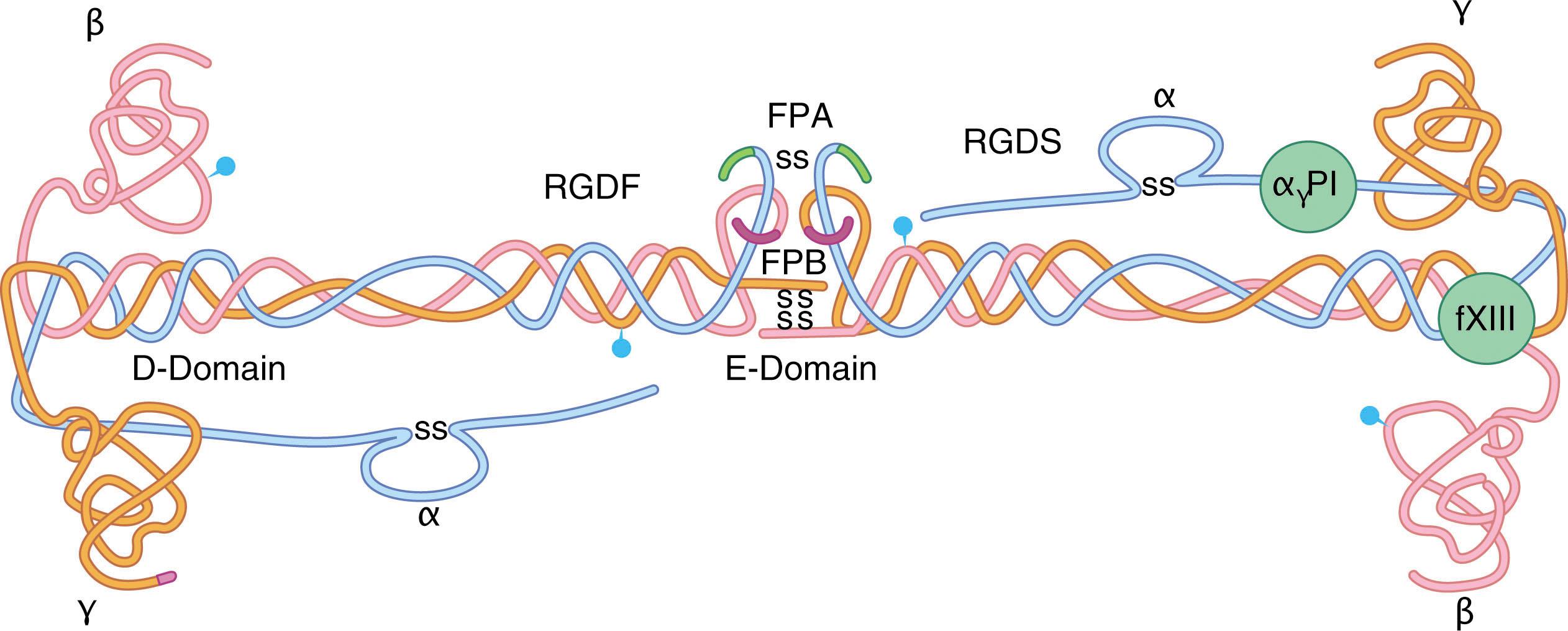
The normal plasma fibrinogen concentration is 1.5 to 4.0 g/L (150 to 400 mg/dL). Afibrinogenemic patients have levels less than 0.1 g/L as determined by clotting and immunoreactive assays, due to homozygosity or compound heterozygosity for fibrinogen gene mutations. Hypofibrinogenemia is caused by heterozygosity for such mutations. The first mutation causing fibrinogen deficiency was reported in 1999. Over 200 FGA, FGB , or FGG deletions, frameshifts, and point mutations have been identified in afibrinogenemic and hypofibrinogenemic patients’ ( https://site.geht.org/base-de-donnees-fibrinogene ). FGA is most affected. Missense mutations are more prevalent in FGB and FGG , and cluster in the polypeptide C-termini, affecting D-domain formation (see Fig. 135.3 ) and interfering with secretion. In afibrinogenemia, fibrinogen is not secreted due either to a lack of synthesis of a fibrinogen chain (Aα, Bβ, or γ) or a defect that prevents secretion. Fibrinogen polypeptides that are not secreted are usually degraded in hepatocytes. However, a few FGG mutations such as Gly284Arg (fibrinogen Brescia), Arg375Trp (fibrinogen Aguadilla), and Thr314Pro (fibrinogen Al DuPont) lead to mutant γ-chain accumulation of hepatocytes in the endoplasmic reticulum (ER) of hepatocytes and cause hepatic dysfunction and cirrhosis in a process like that associated with α 1 -antitrypsin deficiency (ER storage disease).
Most hypofibrinogenemic patients are asymptomatic, but some bleed excessively with trauma or surgery, particularly with fibrinogen levels less than 0.5 g/L. Spontaneous bleeding is rare with levels greater than 0.7 g/L and bleeding with surgery is unusual with levels greater than 1.0 g/L. Most (85%) afibrinogenemic patients bleed from mucosal surfaces. Heavy menstrual bleeding and bleeding from the skin, gastrointestinal tract, and genitourinary tract are common. Hemarthroses and muscle hematomas were frequent in one series (54% and 72%, respectively), but were less so (25%) in another study. Hemarthrosis-related arthropathy is less pronounced than in hemophilia A or B. Intracranial bleeding occurs in up to 10% of patients, providing justification for prophylaxis. Intracranial and umbilical cord stump bleeds are common in infancy. Afibrinogenemic patients may be prone to spontaneous splenic rupture. Pregnancy loss, usually in the first trimester, is common in afibrinogenemic women in the absence of factor replacement. Fibrinogen-deficient mice also have difficulty sustaining pregnancy, confirming the importance of maternal fibrinogen to fetal viability. Pre- and postpartum bleeding are common, and intraabdominal bleeds from ruptured corpus luteal cysts have been reported.
Curiously, arterial and venous thrombosis can occur in afibrinogenemic patients. While some events are likely precipitated by known risk factors or by fibrinogen replacement, no identifiable cause is evident in many instances. By binding thrombin, fibrinogen and fibrin down regulate thrombin activity (a property previously referred to as “antithrombin I” activity), which may explain why thrombosis occurs.
The PT and aPTT assays use fibrin formation as an endpoint and will be infinitely prolonged in afibrinogenemia. In hypofibrinogenemia, the PT and aPTT may be normal because they are relatively insensitive to low fibrinogen levels. The von Clauss method is commonly used for quantifying the plasma fibrinogen concentration and is based on measuring time to clot formation after addition of a standard amount of thrombin (see Chapter 127 ). The assay is not reliable at low fibrinogen levels (<50 mg/dL) and may give falsely low readings with fibrinogen variants that polymerize slowly (dysfibrinogenemias), in situations where high levels of substances that interfere with fibrin polymerization are present (paraproteins, fibrin degradation products), or if the sialic acid content of fibrinogen is high (newborns, liver disease). Thus, it is important to demonstrate absence of immunoreactive fibrinogen to confirm a diagnosis of afibrinogenemia. In hypofibrinogenemia, functional and antigenic fibrinogen levels are proportionately decreased. A functional level that is low relative to the antigen level indicates dysfibrinogenemia. Results for the template bleeding time and standard platelet aggregometry are usually abnormal in afibrinogenemia. However, aperture closure times in the PFA-100 platelet function screen are often normal, as this test depends on von Willebrand factor and not fibrinogen to support platelet aggregation. Afibrinogenemic patients have low erythrocyte sedimentation rates and develop little induration with skin tests for delayed hypersensitivity because of the absence of fibrin deposition.
Fresh frozen plasma (FFP) contains fibrinogen, but large volumes are required to correct significant fibrinogen deficiency. Cryoprecipitate is used most often in the United States to treat low fibrinogen levels (see Table 135.3 ). Each unit of cryoprecipitate has ∼ 300 mg of fibrinogen. The plasma volume of a patient with a normal hematocrit is ∼40 mL/kg body mass. Administering cryoprecipitate to an afibrinogenemic patient at 1 unit/5 kg of body mass increases plasma fibrinogen to ∼1 g/L. The fibrinogen concentrate RiaSTAP (CSL Behring, marketed as Haemocomplettan in some European countries) was approved by the United States Food and Drug Administration (FDA) in 2009 for treating bleeding and for prophylaxis in severe fibrinogen deficiency. In 2017 the FDA approved another fibrinogen concentrate, Fibryga (Octapharma), for treating acute bleeding in adults and adolescents with inherited fibrinogen deficiency. RiaSTAP and Fibryga have undergone viral inactivation. In 15 patients given RiaSTAP (70 mg/kg), the median plasma fibrinogen concentration 1-hour post-infusion was 1.3 g/L (half-life of 77.1 hours). Pharmacokinetic parameters for Fibryga are similar, but clearance appears to be slower. In patients with inherited fibrinogen deficiency, RiaSTAP was effective in 26 of 26 bleeding episodes, 10 of 11 surgical procedures, and all 90 prophylactic administrations. Subsequent studies involving trauma, cardiothoracic surgery, and obstetrical hemorrhage confirmed its ability to improve coagulation and reduce blood loss. In a randomized trial, Fibryga was non-inferior to cryoprecipitate in terms of the number of transfused blood components, leading to expansion of its approval to include treatment of acquired fibrinogen deficiency in 15 European countries. Low-molecular-weight heparin may be administered with fibrinogen concentrate to reduce the risk of thrombosis. The required dose of concentrate can be determined using the following formula:
For significant bleeding, the United Kingdom Haemophilia Centre Doctors Organization guidelines recommend that the plasma fibrinogen concentration be maintained at 1.0 g/L until hemostasis is achieved and above 0.5 g/L until wound healing is complete. A similar strategy makes sense for managing surgical patients. A review of replacement therapy and outcomes for 50 patients with inherited fibrinogen deficiency generally agrees with these recommendations. A fibrinogen concentration of 0.5 to 1.0 g/L was adequate for the prevention or treatment of bleeding in nonsurgical or obstetrical settings, while 1.0 to 2.0 g/L was effective for the prevention of bleeding during surgery. This review reported thrombotic episodes (related or unrelated to replacement) in 30% of patients. As the half-life of transfused fibrinogen is ∼3 days, dosing every 2 to days is usually adequate in the absence of consumption. Increased dosing frequency may be needed for massive hemorrhage, major surgery, or advanced pregnancy, and monitoring of fibrinogen levels is recommended to facilitate dose adjustments. The use of thromboelastometry to monitor fibrinogen concentrate dosing is popular in surgical settings.
Long-term prophylaxis may be useful for preventing initial bleeding in young patients or for preventing recurrence, particularly after central nervous system hemorrhage. Administering concentrate at 20 to 30 mg/kg every 7 to 14 days to maintain plasma levels of ∼0.5 g/L is recommended. Prophylactic fibrinogen administration is required to maintain pregnancies in afibrinogenemic women and to reduce postpartum hemorrhage. Therapy should be initiated early in pregnancy because fetal loss in the first trimester is common. One study suggested that initiating therapy before conception is beneficial. Although some authors recommend maintaining fibrinogen levels above 0.5 g/L during pregnancy and the peripartum period, others suggest a higher level (1.0 g/L) based on reports of fetal loss with levels near 0.5 g/L.
The increased use of fibrinogen concentrates has prompted concerns about adverse events such as thromboembolism, hypersensitivity reactions, and viral transmission. An analysis of 27 years of pharmacovigilance data identified 21 cases of suspected viral transmission (1 per 124,300), 28 cases of thromboembolism (1 per 93,300), and 20 cases of hypersensitivity (1 per 130,600 doses). These data and recent experience from multiple centers suggest an acceptable safety profile.
Antifibrinolytic therapy with ε-amino caproic acid is an effective substitute for blood products for management of some mucosal bleeds and dental extractions (see box on Adjuncts to Factor Replacement in Inherited Factor Deficiencies ). There remain concerns that such therapy increases the risk of thrombosis, particularly in patients with a history of thrombosis and also during pregnancy, surgery, or immobilization. However, the therapy is well tolerated by most patients. Fibrin sealant may be useful for tooth extraction and estrogen/progesterone therapy may be useful for controlling heavy menstrual bleeding.
The antifibrinolytic agents ε-amino caproic acid and tranexamic acid can be effective adjuncts to factor replacement when treating inherited or acquired bleeding disorders and are useful alternatives to replacement therapy for mild bleeding or minor procedures. These drugs inhibit clot dissolution by interfering with plasminogen activation and plasmin activity and are particularly effective when bleeding involves tissues with high fibrinolytic activity such as the oral and nasal cavity. They are also useful for treating menorrhagia, limiting blood loss with surgery, controlling epistaxis, and reducing some types of gastrointestinal bleeding. A typical loading dose of ε-amino caproic acid is 100 mg/kg (4 g maximum) followed by 50 mg/kg (2 g maximum) every 6 h. If bleeding subsequently occurs, the dose can be increased, but should not exceed 24 g in 24 h. ε-amino caproic acid is available in oral and intravenous formulations. Identical dosing for either preparation may be used due to excellent bioavailability. Tranexamic acid may be administered at doses up to 1300 mg every 6 h.
For dental extraction in factor VIII or IX deficiency, many centers utilize a single 50%–100% dose of factor concentrate followed by 7 days of ε-amino caproic acid and it is reasonable to use this approach for patients with some rare bleeding disorders. Patients with factor XI deficiency do well with antifibrinolytic agents alone for tooth extraction (see box on Managing Factor XI Deficient Patients ). Prolonged therapy with anti-fibrinolytics should be undertaken with caution in patients who are not mobile, who have a history of thrombosis, or who have significant urogenital bleeding. These drugs interfere with urokinase-mediated fibrinolysis in the genitourinary tract and can lead to thrombotic occlusion of the ureter. Antifibrinolytic agents in combination with activated prothrombin complex concentrates or recombinant factor VIIa have been used in some patients, but this should be done with caution because of the potential for increasing thrombotic risk. Patients may develop nausea or vertigo with high doses of ε-amino caproic doses and, in rare cases, rhabdomyolysis.
Recombinant factor VIIa may stop or prevent hemorrhage in rare bleeding disorders. The mechanism by which this agent contributes to hemostasis is not completely understood, particularly when used in patients with deficiencies of common pathway factors (prothrombin and factors V and X). For patients with rare bleeding disorders and an antibody inhibitor directed against the missing factor, factor VIIa may be the treatment of choice. Doses range from 15 to 120 μg/kg every 2–6 h depending upon the severity of bleeding. Because of the potential for thromboembolism with factor VIIa, we recommend frequent re-evaluation and reduction to the lowest effective dose. Doses of 90 μg/kg every 2–3 h have been used in patients with inherited factor V deficiency or combined deficiencies of factors V and VIII, with acquired factor X deficiency associated with amyloidosis, and with antibody-mediated acquired prothrombin deficiency associated with lupus anticoagulants. When compared with FFP, the volume of infused material is smaller with factor VIIa, and the risks for complications such as of fever, urticaria, transfusion-related acute lung injury (TRALI), and anaphylaxis are lower. Caution must be exercised when using factor VIIa in older patients with cardiovascular disease as arterial thrombosis can result, particularly when therapy is combined with anti-fibrinolytic therapy or prothrombin complex concentrate.
In dysfibrinogenemia , structural variants of fibrinogen circulate in plasma. Cases in which the dysfunctional protein is present at low concentration are referred to as hypodysfibrinogenemia . The first family with dysfibrinogenemia (15 amino acid insertion after Gln350 in the γ chain [fibrinogen Paris I]) was described in 1964. The true incidence of inherited dysfibrinogenemia is unknown, because the majority of affected individuals are asymptomatic.
Inherited dysfibrinogenemias are mostly autosomal dominant traits due to missense mutations in a fibrinogen gene ( https://site.geht.org/base-de-donnees-fibrinogene/ ). Amino acid substitutions that affect fibrinopeptide release, fibrin polymerization or cross-linking, or fibrin degradation are described. The diagnosis is established by a measurement of fibrinogen using a rate-based clotting assay that is low relative to immunoreactive fibrinogen. The functional defects most often reported are influenced by the assays available in clinical laboratories and are unlikely to represent the full spectrum of mutations causing fibrinogen dysfunction. Variants easily detected in clinical laboratories typically have defects in fibrinopeptide release ( FGA -Arg16His and FGA -Arg16Cys [fibrinogens Bicêtre and Metz]) or polymerize slowly ( FGG -Ser434Asn [fibrinogen Caracas II], FGG -Arg275Cys and FGG -Arg275His). Indeed, ∼45% of mutations in the dysfibrinogenemia database involve substitutions at FGA -Arg16 or FGG -Arg275, reflecting the ease with which such variants are detected by commonly used assays. Hypodysfibrinogenemia may be caused by single mutations that interfere with both fibrinogen assembly/secretion and function, or compound heterozygosity for reduced synthesis of one allele (usually a null mutation in FGA or FGB ) and a dysfunctional second allele. As with dysfibrinogenemia, missense mutations are most common in hypodysfibrinogenemia.
Most individuals with dysfibrinogenemia are asymptomatic. In a multicenter study of 101 patients with inherited dysfibrinogenemia (with genotypes), 58% were identified incidentally because of an abnormal coagulation test. Over a mean follow-up of 8.8 years, the incidences of major bleeding and thrombosis after diagnosis were 2.5 and 18.7 per 1000 patient years, respectively, with estimated cumulative incidences of 19.2% and 30.1% at 50 years of age. There was no clear association between symptoms and fibrinogen level, fibrinogen functional abnormalities, or gene mutations, findings consistent with older observations that common substitutions such as FGA -Arg16His and FGA -Arg16Cys occur in asymptomatic individuals as well as in patients with bleeding or thrombosis.
Bleeding symptoms tend to be mild, with epistaxis, easy bruising, and heavy menstrual bleeding being common. More serious bleeding events including soft tissue hematomas, hemarthroses, postoperative hemorrhage, and bleeding during and after pregnancy occur, but are rarer. Major bleeding seems to occur primarily between the ages of 20 and 40 years, partly due to hemostatic challenges related to childbirth. Abnormal wound healing and spontaneous abortion have been reported.
Thrombosis primarily involves the venous circulation, although arterial events can occur. The mean ages for venous and arterial events in one study (34 and 49 years) were lower than for the general population. There is a high prevalence of VTE at the time of diagnosis, with an incidence during follow-up like that for carriers of the factor V Leiden polymorphism. Mutations clustering at the C-terminus of the Aα chain and near the thrombin cleavage site on the Bβ chain are associated with venous thrombosis as are abnormalities in fibrin polymerization, cross-linking, clot structure, and susceptibility to fibrinolysis. The Dusart syndrome caused by FGA -Arg554Cys (fibrinogen Paris V) was associated with venous thrombosis and sudden death from pulmonary embolism in adolescents and young adults in several families. Dysfibrinogenemia was reported in 5 of 33 patients with chronic thromboembolic pulmonary hypertension, with the FGB -Pro235Leu substitution found in three unrelated patients. Dysfibrinogenemias can alter fibrin polymer structure and its susceptibility to lysis, suggesting that the variants interfere with clot dissolution. Despite these associations, a review of 2376 patients with venous thrombosis found dysfibrinogenemia in less than 1%. Therefore, testing for abnormal fibrinogen in patients with venous thrombosis is not widely recommended (see Chapter 138 ).
As discussed in the section on inherited fibrinogen deficiency, maternal fibrinogen is required to maintain pregnancy. Obstetrical and postpartum complications such as spontaneous miscarriage, placental abruption, and postpartum hemorrhage occur in women with inherited dysfibrinogenemia, particularly those with a past history or family history of such complications. There are few guidelines for management of pregnant women with dysfibrinogenemia.
A group of mutations in the C-terminus of the fibrinogen Aα chain is associated with autosomal dominant hereditary amyloidosis. The amyloid deposits contain fragments of the variant fibrinogen. The kidneys are affected first, but visceral and nerve involvement may occur. Renal grafts become involved with amyloid, and liver transplantation may be a better treatment option. The allele for one responsible mutation, FGA -Glu526Val, is relatively common and may account for 5% of patients with apparent sporadic amyloid.
Dysfibrinogenemia is usually diagnosed because of abnormalities on routine coagulation tests such as the PT or aPTT. The thrombin time is often used to screen for dysfibrinogenemia, although its sensitivity is not established. The thrombin time involves measuring the time to clot formation after addition of a relatively small amount of thrombin to plasma (see Chapter 127 ). Specificity for dysfibrinogenemia is low because heparin, thrombin inhibitors (argatroban, dabigatran, hirudin), elevated levels of fibrin degradation products, paraproteins, and low fibrinogen levels all prolong the thrombin time. The reptilase time has been used as an alternative screen and is useful in combination with the thrombin time (see Chapter 127 ). Clot formation is induced with an enzyme from the venom of Bothrops jararaca or Bothrops atrox that cleaves fibrinopeptide A (but not fibrinopeptide B) from fibrinogen, and which is not sensitive to heparin or direct thrombin inhibitors. The plasma concentration of fibrinogen as determined by the von Clauss method (see “Inherited Fibrinogen Deficiency”) may be falsely low in some forms of dysfibrinogenemia. Levels of immunoreactive fibrinogen are usually normal but are decreased in hypodysfibrinogenemia. Fibrin degradation products may appear to be elevated because variant fibrinogens may be incompletely incorporated into the clot, leading to the false impression that disseminated intravascular coagulation (DIC) is present (see Chapter 137 ).
Most dysfibrinogenemic patients are asymptomatic. When symptoms occur, there is little correlation with coagulation assay abnormalities, which renders it difficult to make general therapeutic recommendations. The patient’s personal and family history can help to guide therapy. Active bleeding can be treated with replacement therapy as in afibrinogenemia and such treatment may be indicated in some patients before invasive procedures. In general, patients with thrombosis and dysfibrinogenemia should be treated in the same manner as other patients with thrombosis. There are no data on which to formulate recommendations as to duration of anticoagulant therapy; thus, past history, family history, coexisting conditions, and the nature (unprovoked or provoked by pregnancy or surgery) and severity of the thrombotic event are taken into consideration (see Chapter 140, Chapter 141 ). As with any thrombotic event, the risk for bleeding associated with prolonged anticoagulant therapy needs to be considered. Recurrent spontaneous abortions have been associated with dysfibrinogenemia in several families and pregnancies have been carried to term using replacement therapy. While some investigators recommend fibrinogen replacement early in pregnancy, as in afibrinogenemic patients, the prothrombotic nature of the peripartum period may dictate against this approach in certain patients.
Acquired hypofibrinogenemia occurs in DIC and primary fibrinolysis (see Chapter 137 ). Fibrinogen levels are usually normal or increased in liver disease, but levels less than 1 g/L may be seen in cirrhosis with hepatic failure or fulminant hepatic necrosis and indicate a poor prognosis. Patients receiving l -asparaginase for hematologic malignancies may develop severe hypofibrinogenemia (<0.2 g/L), while other coagulation factors are normal or only slightly reduced.
Acquired dysfibrinogenemia is common in liver disease, with 80% to 90% of patients with cirrhosis or liver failure showing some evidence of fibrinogen dysfunction. Increased sialic acid content like that seen with neonatal fibrinogen (see Chapter 148 ) decreases the rate of fibrin polymerization in the von Clauss assay, reducing the apparent fibrinogen concentration. Increased sialic acid content probably does not contribute significantly to abnormal hemostasis. Similarly, monoclonal paraproteins in patients with multiple myeloma may interfere with fibrin polymerization but usually do not cause abnormal hemostasis. Acquired dysfibrinogenemia also has been associated with malignancies and bone marrow transplantation.
Alloantibodies to fibrinogen have been described in persons with afibrinogenemia after exposure to fibrinogen containing products, including fibrin sealant and fibrinogen concentrates. While clinically significant in some persons, the frequency of this occurrence is rare, likely because many afibrinogenemic patients have trace amounts of circulating fibrinogen. Anti-fibrinogen antibodies were reported in only two afibrinogenemic patients after receiving fibrinogen concentrate and the incidence of antibody development may be lower than in patients receiving cryoprecipitate.
Autoantibodies against fibrinogen have been described in various clinical scenarios, including pregnancy, malignancy, and autoimmune disorders. Like inherited fibrinogen abnormalities, the antibodies can result in clinically silent laboratory changes or be associated with hemostatic and/or thrombotic abnormalities.
More than a century ago, Morawitz proposed that insoluble fibrin is formed from fibrinogen through the activity of fibrin ferment or thrombin . Thrombin was generated from a precursor, prothrombin, by thrombokinase (probably factor Xa). Prothrombin and thrombin are designated factors II and IIa . Total prothrombin deficiency is probably not compatible with life. Complete absence of the protein has not been observed in a human, and prothrombin-deficient mice succumb to bleeding in utero or shortly after birth. Severe deficiency of plasma prothrombin activity caused by reduced plasma prothrombin antigen ( hypoprothrombinemia ) or circulating dysfunctional prothrombin ( dysprothrombinemia ) affects ∼1 in 2 million people (see Table 135.1 ). However, prothrombin deficiency is the third most common coagulation factor disorder in Puerto Rico (carrier frequency ∼1 in 700), accounting for 6% of inherited bleeding disorders other than von Willebrand disease at the University of Puerto Rico Hemophilia Center. In the North American Rare Bleeding Disorder Registry, 62% of patients with prothrombin deficiency were of Latin American origin, possibly reflecting the prevalence of the Arg457Gln variant prothrombin Puerto Rico I. Globally almost 70% of patients with prothrombin deficiency have Latin or Hispanic ancestry.
Prothrombin is a 72,000 Da protein that is converted to thrombin by factor Xa in complex with factor Va on phospholipid surfaces (see Fig. 135.1A ). Thrombin catalyzes multiple procoagulant events including cleavage of fibrinopeptides A and B from fibrinogen (see Fig. 135.3 ), activation of factors V, VIII, XI, and XIII, and cleavage of protease activated receptors on a variety of cells including platelets (see Chapter 121, Chapter 125 ). Thrombin binds to thrombomodulin on endothelial cells, downregulating coagulation by activating protein C and attenuating fibrinolysis by activating thrombin-activatable fibrinolysis inhibitor (TAFI).
Prothrombin deficiency is an autosomal recessive disorder that manifests as hypoprothrombinemia (reduced activity and antigen; cross-reactive material negative [CRM−] deficiency), dysprothrombinemia (activity reduced relative to antigen; CRM+ deficiency), or a combination of both. Plasma prothrombin activity is typically 1% to 10% of normal in hypoprothrombinemia and 1% to 20% in dysprothrombinemia. Heterozygotes for either condition have 40% to 60% of normal activity. About 50 prothrombin gene ( F2 ) mutations have been described in patients with prothrombin deficiency, three-quarters of which are missense mutations. In dysprothrombinemia, missense mutations typically cause defects in prothrombin conversion to thrombin (e.g., pArg457Gln [prothrombin Puerto Rico I] and pArg271Cys [prothrombin Madrid]), or result in functionally defective thrombin (e.g., Arg418Trp [prothrombin Tokushima]). A subset of recently identified dysprothrombinemias, caused by missense mutations in F2 exon 14, alter thrombin regulation. Substitution of prothrombin Arg596 with leucine (prothrombin Yukuhasi), glutamine (prothrombin Belgrade), or tryptophan (prothrombin Padua 2) interferes with thrombin-antithrombin complex formation, resulting in increased circulating thrombin and a prothrombotic state that has been referred to as “antithrombin resistance.” In contrast to other dysprothrombinemias, these CRM+ variants do not cause excessive bleeding despite the apparent low plasma prothrombin activity. Prothrombin deficiency can be inherited in combination with deficiencies of other vitamin K–dependent proteins (see “Combined Deficiency of Vitamin K–Dependent Proteins”).
Severe hypoprothrombinemia is inevitably associated with bleeding that may be life threatening. A committee of the International Society on Thrombosis and Haemostasis (ISTH) proposed classifying prothrombin deficiency as severe (<5% of normal activity), moderate (5% to 10% activity) or mild (>10% activity). This system has been adopted in the EN-RBD survey (see Table 135.2 ). Central nervous system hemorrhage was reported in 8% to 12% of prothrombin-deficient patients, and in 20% with prothrombin levels less than 1% of normal activity. Soft tissue hematomas, bruising (60%), and hemarthroses (42%) are common. The bleeding diathesis can present at circumcision in neonates, with trauma or surgery, or as easy bruising, epistaxis, heavy menstrual bleeding, or gastrointestinal hemorrhage. Heterozygotes occasionally have excessive bleeding with surgery or tooth extraction, but most are asymptomatic. Bleeding tends to be less severe in dysprothrombinemia and some variants are particularly mild. Homozygosity for the Arg67His variant causes a significant reduction in plasma prothrombin activity (<20% of normal) but relatively few symptoms.
In the PT and aPTT assays, prothrombin is converted to thrombin by factor Xa in complex with factor Va on phospholipid surfaces (see Fig. 135.1B ), and severe prothrombin deficiency will prolong the clotting time in both tests (see Chapter 127 ). Some PT and aPTT reagents are relatively insensitive to reductions in prothrombin, and mild deficiencies may be missed. The diagnosis is usually confirmed, and the degree of deficiency established, by a modified PT assay using prothrombin-deficient plasma. A prothrombin antigen level is needed to distinguish between hypoprothrombinemia (activity and antigen are equivalent) and dysprothrombinemia (activity is less than antigen). Prothrombin deficiency must be distinguished from deficiencies of fibrinogen, factor V, or factor X, which also prolong the PT and aPTT (see Chapter 127 ).
Prothrombin complex concentrates (PCCs) (see Table 135.3 ) are the preferred treatment for prothrombin deficiency. PCCs come in four-factor forms containing prothrombin, factors VII, IX and X (e.g., Kcentra), and three-factor forms (prothrombin, and factors IX and X, e.g., Profilnine). Four-factor concentrates often include heparin, antithrombin, and/or proteins C and protein S to reduce thrombotic risk. PCCs are derived from human plasma and undergo viral inactivation. They were originally approved for use in factor IX deficiency, and their activities are still standardized in factor IX units. Prothrombin activity in PCCs is comparable to, or higher than, the factor IX activity. Hemostatic levels of prothrombin are estimated to be 20% to 40% of normal for major surgery or trauma, but 10% to 15% may be adequate for milder challenges. A dose of 20 to 30 factor IX units/kg usually produces plasma prothrombin levels of 20% to 30% of normal in a severely deficient patient. The half-life of prothrombin is ∼3 days and dosing every 2 to 3 days can maintain adequate levels until healing is complete. Alternatively, one fourth of the loading dose each day should keep the level therapeutic (>10%). Prophylactic infusion of PCC every 5 to 6 days prevented spontaneous bleeding in one severely deficient patient. PCC administration has been associated with thrombosis, although a meta-analysis of studies using PCCs to reverse the effect of warfarin suggests the risk is less than 2%. Similar rates of thrombosis have been reported when PCCs are used to reverse direct oral anticoagulants. It is reasonable to maintain the factor VII, IX, and X levels at less than 150% of normal to reduce risk. Dental procedures or minor bleeding may respond to antifibrinolytic therapy. Prothrombin deficiency may also be treated with FFP for bleeding episodes or surgical intervention (15 to 20 mL/kg loading dose followed by 3 mL/kg/day). Because of the long half-life, additional doses may not always be needed. Cryoprecipitate is not a source of prothrombin and plasma prothrombin levels do not increase after infusion or inhalation of desmopressin (DDAVP).
Acquired prothrombin deficiency occurs with warfarin therapy, poisoning with rodenticides such as brodifacoum, vitamin K deficiency, liver disease, and DIC. In these situations, other coagulation factors are also reduced. Cephalosporins, particularly those with N-methyl-thiotetrazole side chains, can also decrease prothrombin levels.
There are no reports of neutralizing antibodies forming after replacement therapy in inherited prothrombin deficiency, consistent with patients having at least trace amounts of circulating prothrombin. Phospholipid-dependent anti-prothrombin antibodies are common in patients with lupus anticoagulants and/or the antiphospholipid syndrome (see Chapter 139 ). They do not compromise hemostasis. More rarely, patients with a lupus anticoagulant or systemic lupus erythematosus develop antibodies that enhance prothrombin clearance, leading to true deficiency. This acquired hypoprothrombinemia occurs primarily in children under 10 years of age, usually in association with lupus anticoagulants after a viral illness. The episodes typically resolve spontaneously, but excessive bleeding can occur. Intravenous immunoglobulin has been effective in reducing prothrombin clearance in this population. In contrast to lupus anticoagulants, which prevent correction of prolonged clotting times when patient plasma is mixed with normal plasma, the abnormal clotting time caused by acquired hypoprothrombinemia does correct.
Treatment of antibody-mediated acquired prothrombin deficiency associated with lupus anticoagulants in patients with autoimmune diseases often requires immune suppression, because the condition does not resolve spontaneously. Steroids are effective in most patients, although relapse is common during weaning or after stopping treatment. Treatment with azathioprine or cyclophosphamide has successfully eradicated the antibody. Rituximab also is effective and is likely the preferred therapy. In a rare case of quinidine-induced lupus anticoagulant with a concomitant antiprothrombin antibody, cessation of the drug led to spontaneous resolution of acquired prothrombin deficiency, but not the lupus anticoagulant. The low prothrombin activity in these patients may protect them from thrombosis related to their lupus anticoagulant, as suggested by reports of thrombosis after successful eradication of the antiprothrombin antibody.
In 1943, Quick reported that a labile plasma factor distinct from prothrombin was required for coagulation. At the same time, Owren noted that a patient with a lifelong bleeding diathesis lacked a plasma factor that, unlike prothrombin, did not adsorb onto aluminum hydroxide. Owren’s patient was deficient in the labile factor described by Quick, which is now called factor V . Moderate to severe inherited factor V deficiency (1% to 10% of normal plasma level) occurs in 1 in 1 million persons (see Table 135.1 ). Full-length factor V is the 330,000-Dalton precursor of the cofactor factor Va , which facilitates prothrombin activation by factor Xa on phospholipid surfaces (see Fig. 135.1 ). During coagulation, the B-domain of factor V is removed by thrombin or factor Xa to produce factor Va ( Fig. 135.4 ). An ∼250,000 Da form of factor V lacking 703 amino acids from the B-domain is a minor constituent of normal plasma (see Fig. 135.4 ). This truncated form, referred to as factor V-short, is the product of an alternatively spliced mRNA. Eighty percent of factor V in blood is in plasma, with the remainder in platelet α-granules. In humans, platelet factor V is primarily of plasma origin. Megakaryocytes take up the protein in a process requiring low-density lipoprotein receptor-related protein-1 (LRP-1) and convert it to a partially activated form (see Chapter 123, Chapter 124 ).
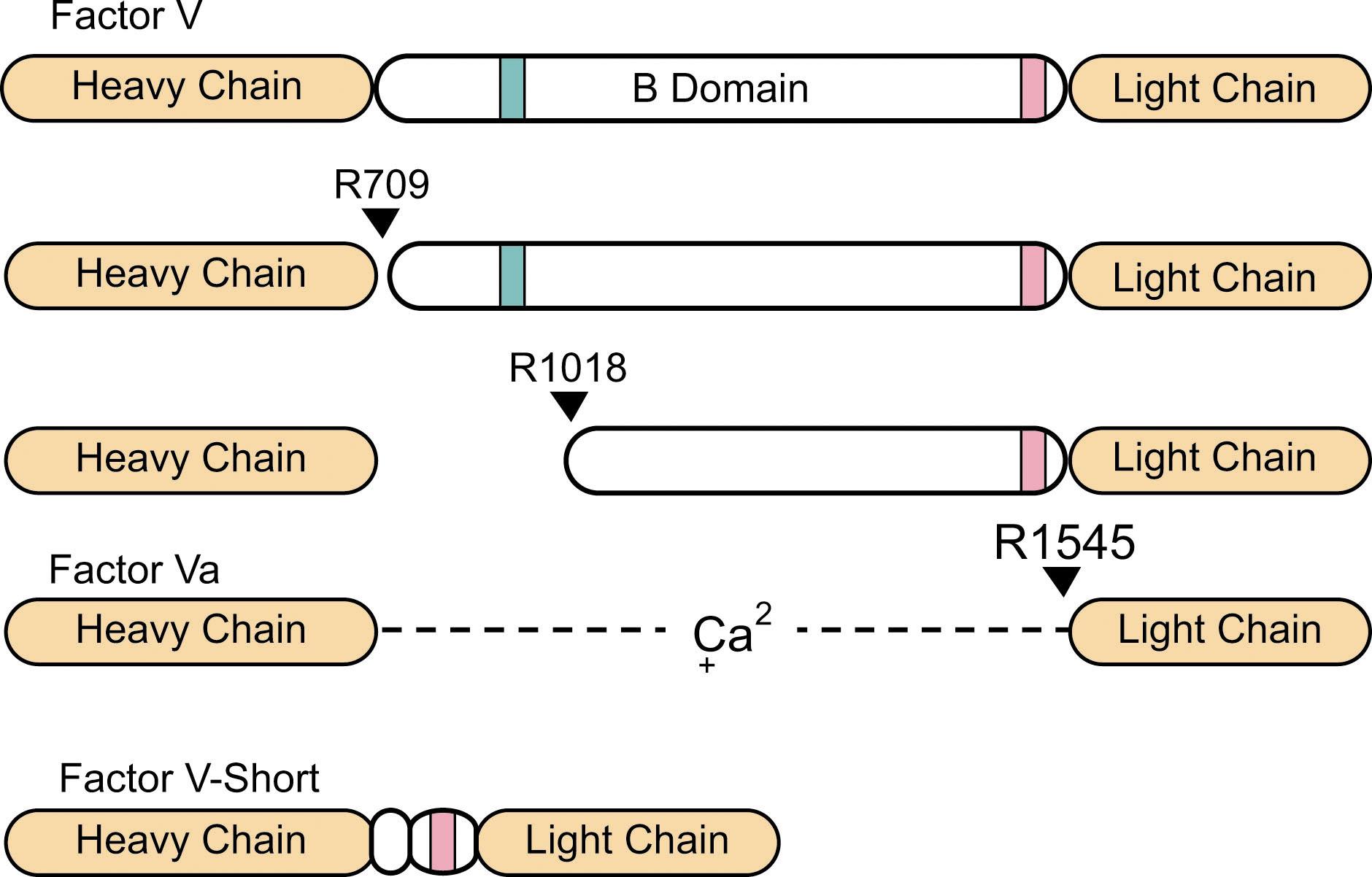
Severe factor V deficiency is an autosomal recessive trait with undetectable (<1% of normal) plasma factor V. In moderate deficiency the level is between 1 and 10% of normal (see Table 135.2 ). More than 150 factor V gene ( F5 ) mutations have been described in factor V deficient patients. Frameshifts and splice variants are common and are distributed throughout the gene. Nonsense mutations show a predilection for the B domain while missense mutations cluster in the A2 and C2 domains, resulting in polypeptides that are degraded within the cell and low levels of plasma factor V antigen (CRM− deficiency). Only two F5 mutations have been reported in which plasma antigen exceeds activity (CRM+ mutations) The Ala221Val (factor V New Brunswick) and His147Arg substitutions appear to reduce factor Va activity by affecting protein stability. A few patients with abnormalities specific to platelet factor V have been described (see Chapter 123 ). In the Quebec platelet disorder, an autosomal dominant condition with low platelet factor V and normal plasma factor V levels, excessive proteolysis of α-granule proteins is caused by overexpression of urokinase. Platelet factor V activity is also reduced in Factor V New York, but here the platelet factor V protein appears normal and the mechanism for the disorder is unknown. Combined factor V and factor VIII deficiency is caused by mutations in proteins required for secretion of both proteins (see “Combined Factor V and Factor VIII Deficiency”).
The autosomal dominant East Texas bleeding disorder is caused by an A2440G substitution in F5 exon 13 (factor V East Texas), that increases mRNA encoding factor V-short (see Fig. 135.4 ), leading to increased plasma levels of this truncated form of the protein. In this disorder plasma levels of tissue factor pathway inhibitor (TFPI), an important coagulation regulator, are 10-fold higher than normal. The B-domain of full-length factor V contains basic and acid regions that likely bind to each other (see Fig. 135.4 ). In factor V-short, the basic region is missing, leaving the acidic region free to bind TFPI. It is thought that TFPI-mediated inhibition of factor Xa and the factor VIIa-tissue factor complex contribute to symptoms in East Texas bleeding disorder. A similar bleeding disorder was observed in a Dutch family with a C2588G substitution in F5 exon 13, leading to loss of 632 B domain amino acids (factor V Amsterdam). These patients also have markedly elevated TFPI levels.
Symptoms in patients with severe factor V deficiency are variable and the correlation between bleeding and plasma factor V levels is relatively weak (see Table 135.2 ). Significant, and even life-threatening bleeding occurs, but the frequency tends to be less than in patients lacking factor VIII or factor IX. Several factors may contribute to the variability. Some patients with mild bleeding have sufficient platelet factor V to support thrombin generation. This implies that their factor V is unstable in plasma but can still be taken up by platelets. Patients with the most severe bleeding may lack plasma and platelet factor V. In one such individual treated with plasma, infused factor V was taken up by megakaryocytes and was detectable in platelets 2 weeks after it was no longer detectable in plasma. Many patients with factor V deficiency have reduced plasma TFPI levels that may partly offset the need for factor V. Mucosal bleeding is the primary abnormality in inherited factor V deficiency, with 60% of patients experiencing epistaxis, heavy menstrual bleeding, or oral bleeding. Hematomas and hemarthroses occur in up to 25% of patients, but debilitating arthropathy is infrequent. Severe bleeding in the central nervous system or gastrointestinal tract occurs, but is rare. Postpartum hemorrhage is common and there may be an increased incidence of miscarriage. Trauma, surgery, and dental extraction are associated with a high bleeding risk in untreated patients. Bleeding with surgery involving the urogenital tract, nose, or mouth may be particularly problematic because of high local fibrinolytic activity. Mild factor V deficiency (>10% of normal level) is not usually associated with excessive bleeding, although 10% to 15% of patients report some symptoms.
Thrombosis has been reported in factor V deficient patients. In most cases, the deficiency may not protect the patient. Indeed, except for deficiency of prothrombin or factor X, thrombosis has been reported in all severe coagulation factor deficiency states. However, the situation with factor V may be more complex. Mild to moderate factor V deficiency can occur in patients with the procoagulant polymorphism factor V Arg506Gln (factor V Leiden). If Arg506Gln and a mutation causing factor V deficiency are on opposite alleles (in- trans ), most plasma factor V will have the Gln506 substitution. This is referred to as pseudo-homozygosity for activated protein C resistance, and it may substantially increase the risk of thrombosis beyond that typical for carriers of Arg506Gln (see Chapter 138 ). These patients usually do not bleed excessively, despite reduced plasma factor V antigen.
Factor V is required for normal prothrombin activation by factor Xa (see Fig. 135.1B ). Therefore, factor V deficiency prolongs the PT and aPTT. The diagnosis and severity are usually established with a modified PT assay using factor V-deficient plasma (see Chapter 127 ). With severe deficiency, the template bleeding time may be prolonged, primarily in patients with concomitant reductions in platelet factor V. Patients with factor V deficiency should be tested for factor VIII deficiency so that the combined deficiency disorder is not missed (see “Combined Factor V and Factor VIII Deficiency”). In patients with East Texas bleeding disorder or factor V Amsterdam, the prolonged PT and aPTT are due to the inhibitory effects of TFPI and the PT and aPTT will not completely correct on mixing with normal plasma. The plasma TFPI level will be elevated, and factor V activity is usually normal.
FFP is the mainstay for treating bleeding and preparing for surgery in factor V deficient patients (see Table 135.3 ). The minimum factor V level required for hemostasis is 10% to 15% of normal. This can be achieved in patients with less than 1% plasma factor V activity by administering 15 to 20 mL/kg FFP, followed by 5 mL/kg every 12 hours. A plasma level of 25% is recommended for major surgery. Estimates of the factor V plasma half-life vary, but 12 to 14 hours is assumed for replacement purposes. Infusing 20 mL/kg FFP over 3 to 4 hours before surgery, followed by 5 to 10 mL/kg every 12 hours is usually adequate. Infusions should be continued for 7 to 10 days to permit wound healing. Fluid overload from large volumes of FFP may be a problem in some patients. Plasma exchange has been used successfully to prepare factor V deficient patients at risk for fluid overload for surgery. Mucosal bleeding from the nose and mouth may respond to antifibrinolytic agents and superficial lacerations usually respond to local pressure. A factor V concentrate (manufactured by Kedrion) prepared from cryo-supernatant corrects clotting abnormalities in factor V deficient plasma in vitro, but it has not been extensively tested in patients. Cryoprecipitate is not a source of factor V and plasma levels do not respond to administration of DDAVP. Platelets contain factor V and may be particularly useful in patients with severe bleeding or with factor V inhibitors (see “Acquired Factor V Deficiency”). However, platelet transfusions are not recommended as routine first-line treatment because of the possibility of developing antiplatelet alloantibodies (see Chapter 123 ).
Heavy menstrual bleeding is common in factor V-deficient women. Symptoms can be managed with antifibrinolytic therapy (ε-amino caproic acid 50 to 60 mg/kg every 4 to 6 hours, or tranexamic acid 15 mg/kg every 6 to 8 hours), oral contraceptives, levonorgestrel-releasing intrauterine devices, replacement therapy, or surgical intervention (endometrial ablation or hysterectomy). Replacement should be adjusted to maintain a factor V level of 10% to 15% of normal. Factor V-deficient women may have significant bleeding with childbirth and should be treated in a similar manner.
Plasma factor V levels may be reduced along with other coagulation factors in patients with liver disease or DIC (see Chapter 137 ). Alloantibody inhibitors to factor V have been reported in a few severely deficient patients after replacement therapy and in patients treated with older topical bovine thrombin preparations that also contained bovine factor V. The latter situation occurs less frequently with newer recombinant human thrombin preparations. Acquired factor V deficiency caused by factor V autoantibodies has been reported in patients after surgery, blood transfusion, or therapy with β-lactam or aminoglycoside antibiotics, and in patients with cancer, myeloproliferative disorders, and autoimmune diseases. Rare patients with factor V deficiency in the setting of amyloidosis have been reported. Bleeding in patients with antibodies to factor V may be severe, although some patients are asymptomatic. When acquired factor V deficiency responds poorly to FFP infusion, antibodies that neutralize factor V activity may not be identifiable. Instead, it is likely that non-neutralizing antibodies that enhance the clearance of infused factor V are present.
Neutralizing factor V inhibitors cause prolongation of the PT and aPTT that does not correct when patient plasma is mixed with normal plasma. Factor V inhibitor titers are established using the Bethesda method used for inhibitors to factors VIII and IX (see Chapter 127 ). Unlike inhibitors to factor VIII, which typically take 1 to 2 hours to fully inactivate their target in a mixing study with normal plasma, factor V inhibitors inhibit factor V almost immediately. The thrombin time should be normal, except when the inhibitor is induced by bovine thrombin, where antibodies against thrombin may also be present.
Most factor V inhibitors are transient. Steroids, immunosuppressive therapies, and exchange transfusions have been used in a few patients. Platelet transfusion may be useful. Platelet factor V may either be protected from inhibition or be sufficiently different in structure from its plasma counterpart to not cross-react with antibodies directed against factor V. The effectiveness of recombinant factor VIIa (rfVIIa) and PCCs have not been clearly established. While the defect caused by factor V deficiency would hypothetically render therapy with factor VIIa ineffective, this agent has been used with apparent success in at least two patients with severe factor V deficiency requiring surgery.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here