Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The initial trauma series x-rays should include a chest x-ray (CXR) and pelvic x-ray (PXR). Computed tomography (CT) of the cervical spine is essential to rule out a major fracture/dislocation.
CT of the brain will rule out most types of intracranial haemorrhage but not diffuse axonal injury (DAI), which is best diagnosed by magnetic resonance imaging (MRI).
CT of the cervical spine will rule out any bony injury but not ligamentous, disc or spinal cord injury.
MRI of the spine is required if there is suspicion of ligamentous, disc or spinal cord injury.
CT of the facial bones is essential to rule out bony facial injury.
Unconscious victims of major trauma will often require a CT panscan (whole-body CT scan) to rule out injury where an adequate physical examination cannot be made. However, clinical judgement should always be used before ordering a CT scan.
Conscious victims of major trauma should have CT scan evaluation of areas of clinical suspicion only.
Most injuries to the chest/abdomen/pelvis can be ruled out by CT scan of those areas before and after intravenous contrast.
Injuries to the thoracolumbar (TL) spine are best evaluated by sagittal and coronal reconstruction of axial CT scans of the chest/abdomen/pelvis.
If blunt cerebrovascular injury (BCVI) is suspected by the presence of relevant signs, symptoms or risk factors, a CT angiogram is the investigation of choice.
The clinical team should be mindful of the risks of radiation for both the patient and the team members. In particular, clinicians should carefully weigh the pros and cons of performing CT scans in paediatric patients.
The trauma team should take precautions to avoid being exposed unnecessarily to ionizing radiation. The clinical team should also ensure that patients are exposed to ‘As Low As Reasonably Achievable (ALARA)’ ionizing radiation.
The number of x-rays taken in the resuscitation area should be kept to a minimum. As radiation exposure decreases inversely with the square of the distance from the source, staff should position themselves at a maximal possible distance from the x-ray equipment whenever it is being used. The use of permanent lead barriers should be considered.
Ionizing radiation in x-ray and computed tomography (CT) examinations may directly or indirectly damage DNA, which may not be corrected by cellular repair mechanisms. Such damage has been associated with an increased risk of developing cancer. A large Australian study conducted over 20 years (1985 to 2005) found that overall cancer incidence was 24% greater for patients exposed to ionizing radiation from CT scans than those not exposed, and the incidence rate ratio was greater after exposure at younger ages. The cancer risk was increased for many types of solid organ cancers and haematologic malignancies. The authors suggested that future scans should be limited to situations where there is a definite clinical indication, with every scan optimized to provide a diagnostic CT image at the lowest possible radiation dose.
The radiation dose from various diagnostic imaging examinations may be calculated as an ‘effective dose’ for the purpose of comparison and quantification of risk. Effective dose, evaluated in millisieverts (mSv), refers to the radiation dose from an examination averaged over the entire body and accounts for the relative sensitivities of the different tissues exposed.
A single CT scan gives tissue doses in the range of 10 to 30 mSv. The US Food and Drug Administration estimates that CT examination with an effective dose of 10 mSv may carry a 1:2000 lifetime risk of inducing fatal cancer.
Table 3.8.1 gives typical whole-body effective doses for selected radiological examinations.
| Examination | Radiation dose (mSv) |
|---|---|
| Annual background radiation | 2.4 |
| Chest x-ray | 0.02 |
| Pelvic x-ray | 0.44 |
| Skull x-ray | 0.07 |
| Cervical spine x-ray | 0.2 |
| CT head | 1–2 |
| CT chest | 5–7 |
| CT abdomen/pelvis | 8 |
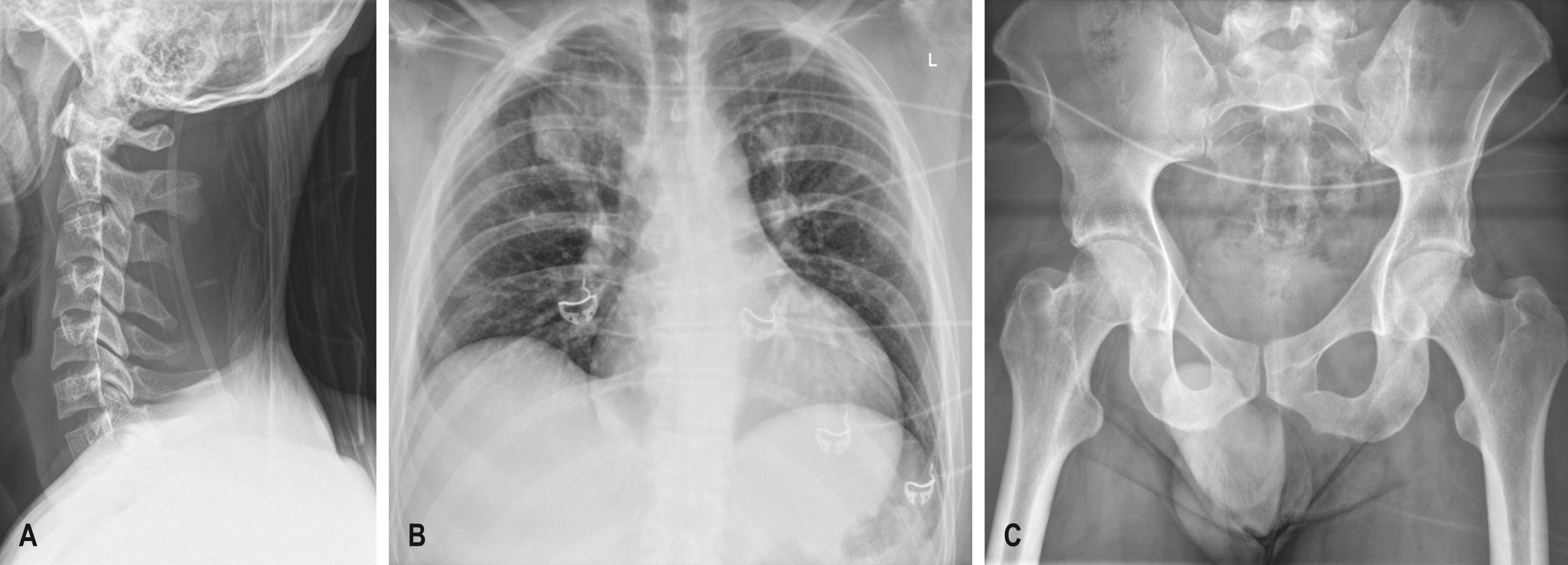
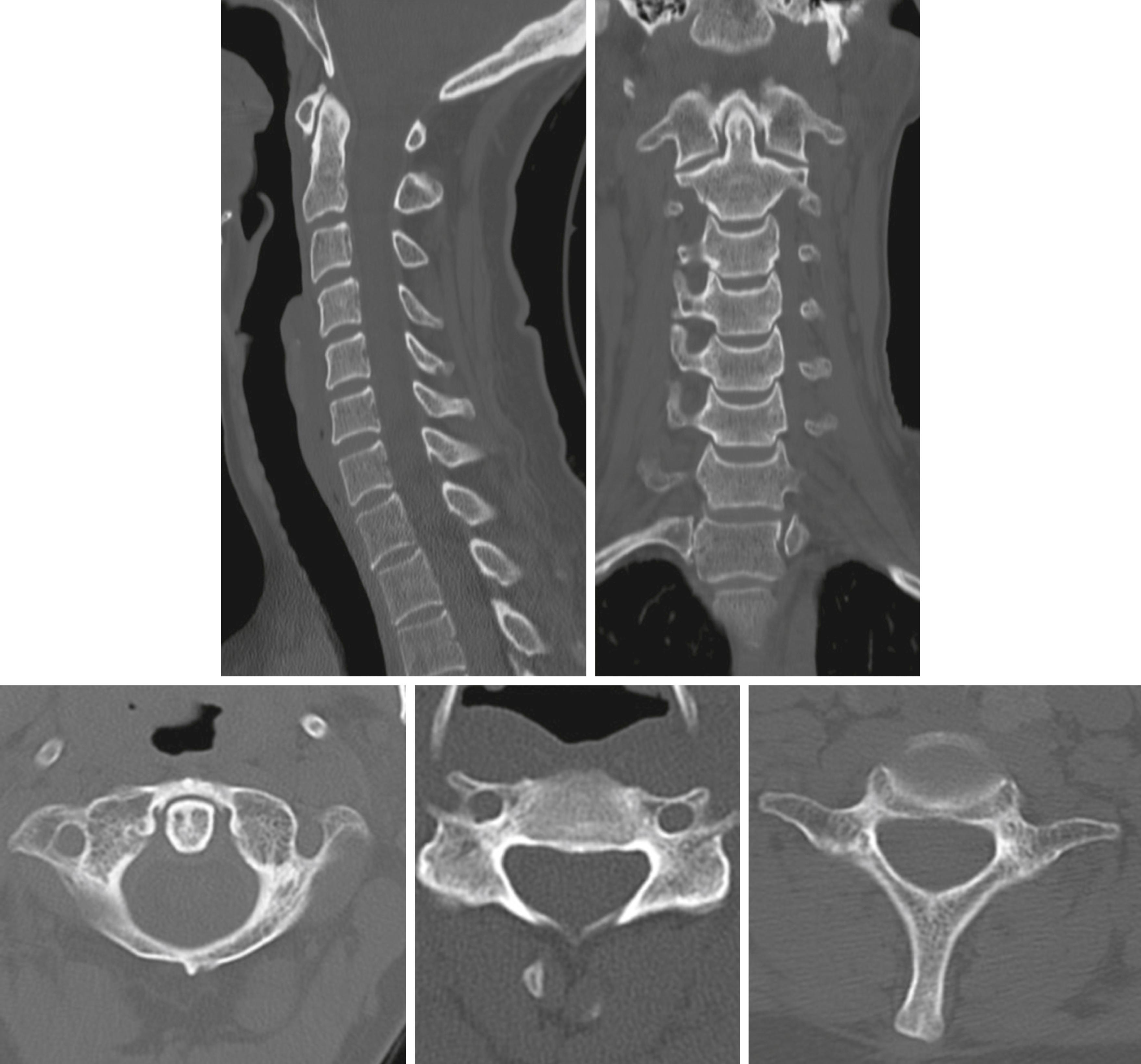
The initial trauma x-rays usually consist of a supine chest and PXR. A lateral x-ray of the cervical spine may provide limited information, and an axial CT scan of the cervical spine from occiput to T4–T5 with sagittal and coronal reconstruction will accurately rule in or out bony injury to the cervical spine ( Fig. 3.8.1 ).
A CT scan of the cervical spine should include the occipital condyles and atlanto-axial junction and extend to the T4–T5 level ( Fig. 3.8.2 ).
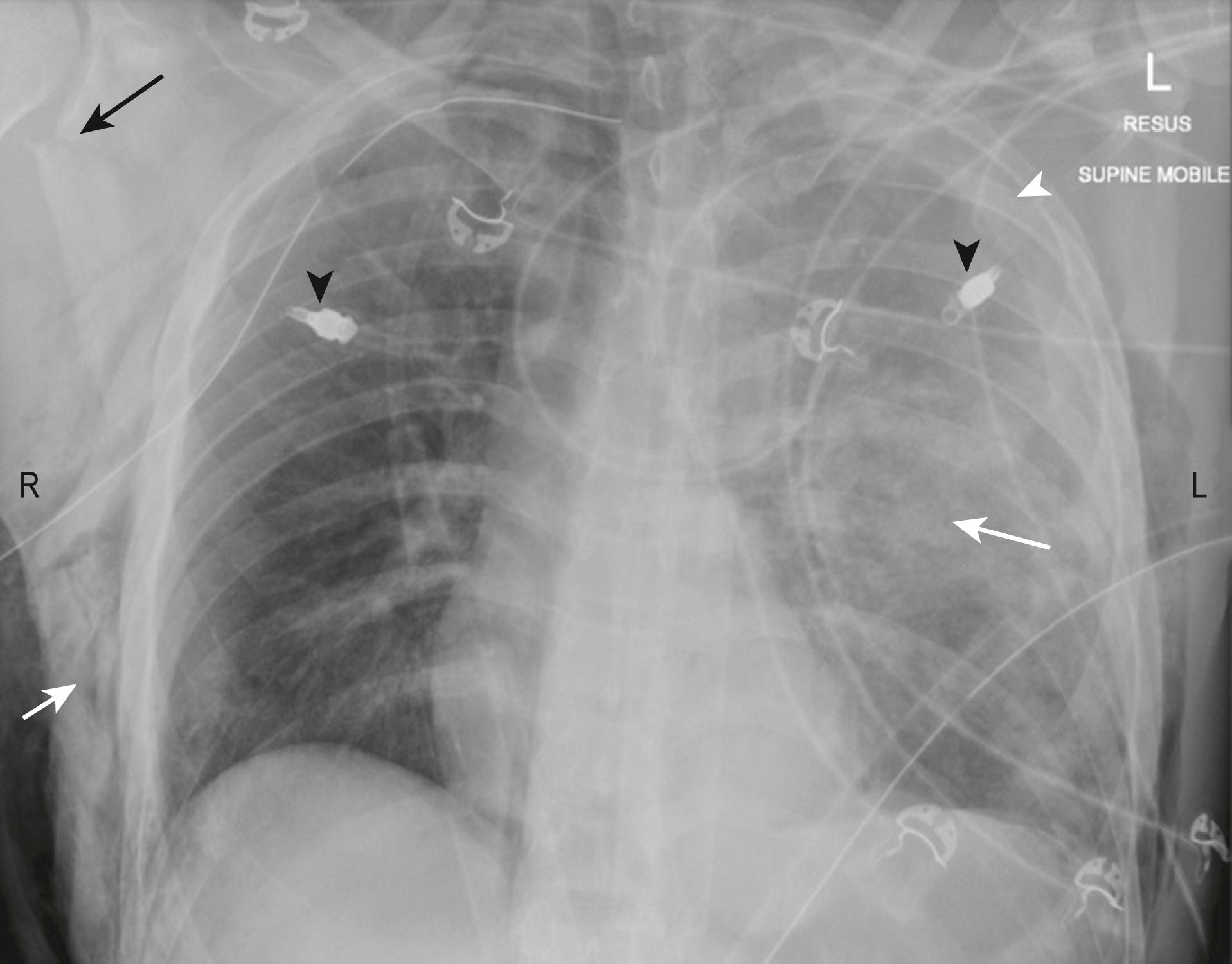
The CXR performed is usually a supine (antero-posterior [AP]) rather than an erect (postero-anterior [PA]) film owing to the inability to sit the patient up until the spine has been cleared. The CXR should include both clavicles as well as the ribs, lungs, mediastinum and diaphragm. If there is adequate penetration, the thoracic spine may be seen. The CXR will exclude significant injuries such as massive haemothorax, pneumothorax, multiple rib fractures or a widened mediastinum ( Fig. 3.8.3 )
Head trauma is responsible for up to 90% of pre-hospital trauma deaths. Some 75% of brain injuries can be classified as mild, 15% as moderate and 10% as severe. The spectrum of head injury ranges from mild concussion to diffuse axonal injury (DAI) incompatible with life; it includes all causes of intracranial haemorrhage.
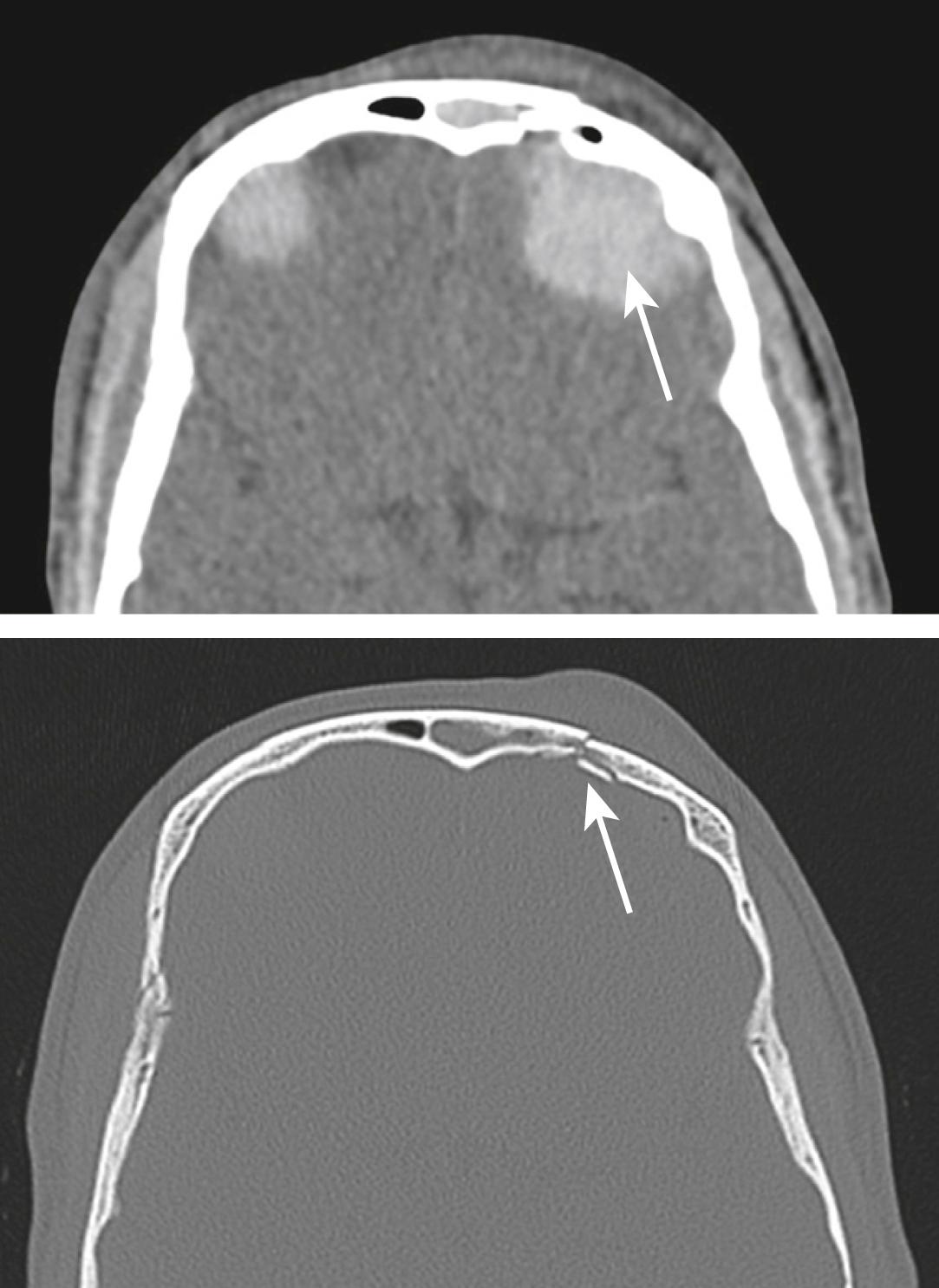
A CT brain scan is the investigation of choice for all but minor head injuries (see Table 3.8.2 for CT indications in serious head injury). A non-contrast CT brain scan with bone windows is adequate for the detection of intra-cranial haematoma, cerebral oedema with or without midline shift and skull vault fractures ( Figs 3.8.4–3.8.6 ).
| Glasgow Coma Scale (GCS) score <9 after resuscitation |
| Neurological deterioration of 2 or more GCS points |
| Drowsiness or confusion (GCS 9–13) that persists for longer than 2 h |
| Persistent headache or vomiting |
| Focal neurological signs (e.g. pupillary abnormalities or focal neurological signs) |
| Skull fracture known or suspected |
| Penetrating injury known or suspected |
| Age over 50 years with a suspicious mechanism of injury |
| Any head injury in a patient on anticoagulation therapy |
The Canadian CT Head Rule for patients with minor head injury also provides guidance for CT brain scanning in patients with minor head injury (Glasgow Coma Scale [GCS] score 13 to 15). This rule found that the presence of any of the high-risk factors ( Table 3.8.3 ) was 100% sensitive for predicting the need for neurological intervention and the presence of medium-risk factors was 97.2% sensitive for detecting clinically important brain injury.
| High risk (for neurological intervention) |
|
| Medium risk (for brain injury on computed tomography) |
|
| Minor head injury is defined as witnessed loss of consciousness, definite amnesia or witnessed disorientation in a patient with a GCS score of 13–15. |
If a compound or depressed fracture of the skull is suspected clinically, a CT brain scan should be performed. A compound depressed skull fracture is considered a neurosurgical emergency because of the increased risk of infection, such as meningitis or brain abscess.
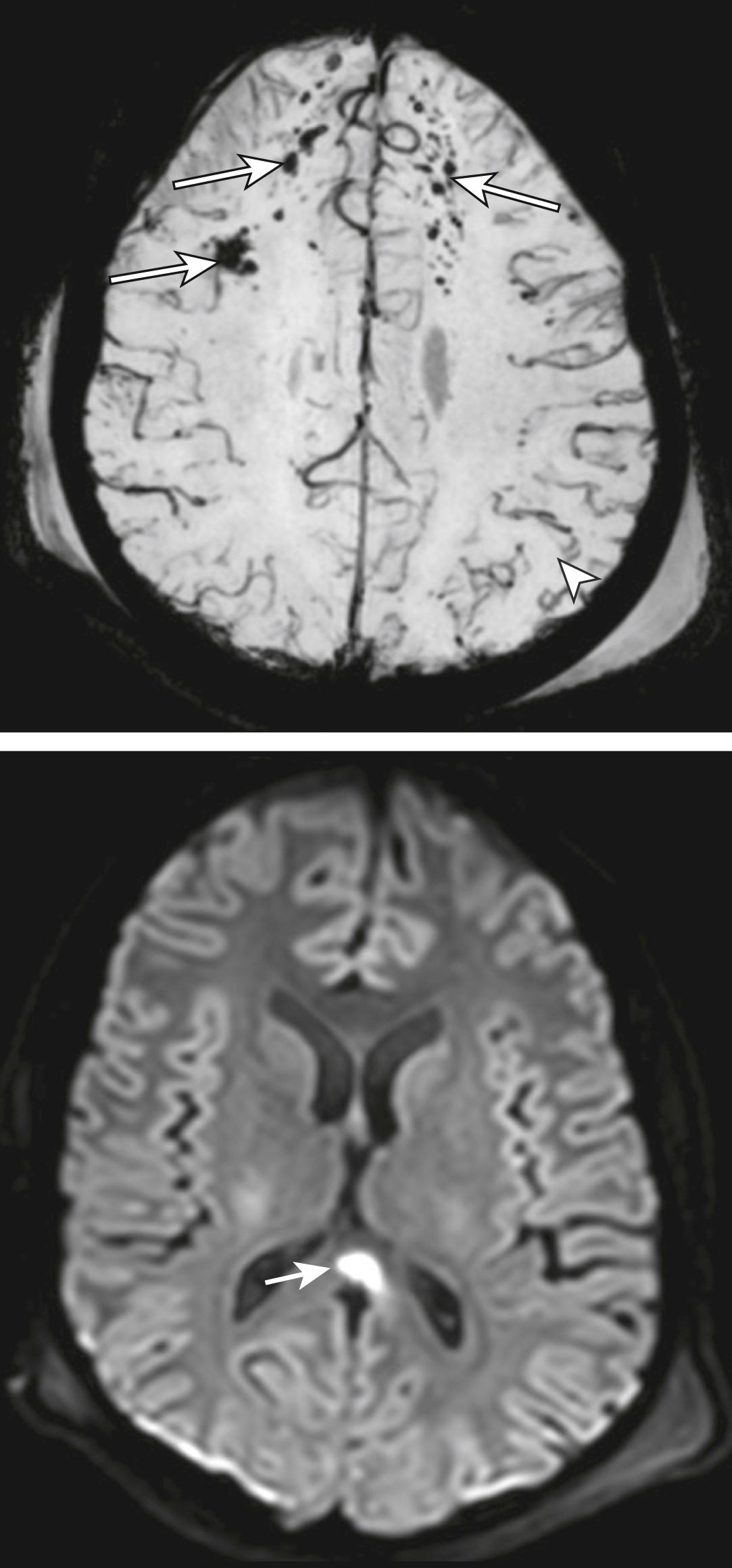
Magnetic resonance imaging (MRI) scan of the brain is the investigation of choice for the diagnosis of DAI. MRI sensitivity for the detection of contusions, shear injury, and extra-axial hematomas is higher than that of CT scan, although it is lower for fractures. It may detect white matter lesions consistent with shear injury in patients presenting with normal CT scan findings ( Fig. 3.8.7 ).
Intracranial bleeding may be classified according to location. This includes: subdural, subarachnoid, extradural, intraventricular or parenchymal bleeding. These commonly coexist in the setting of trauma.
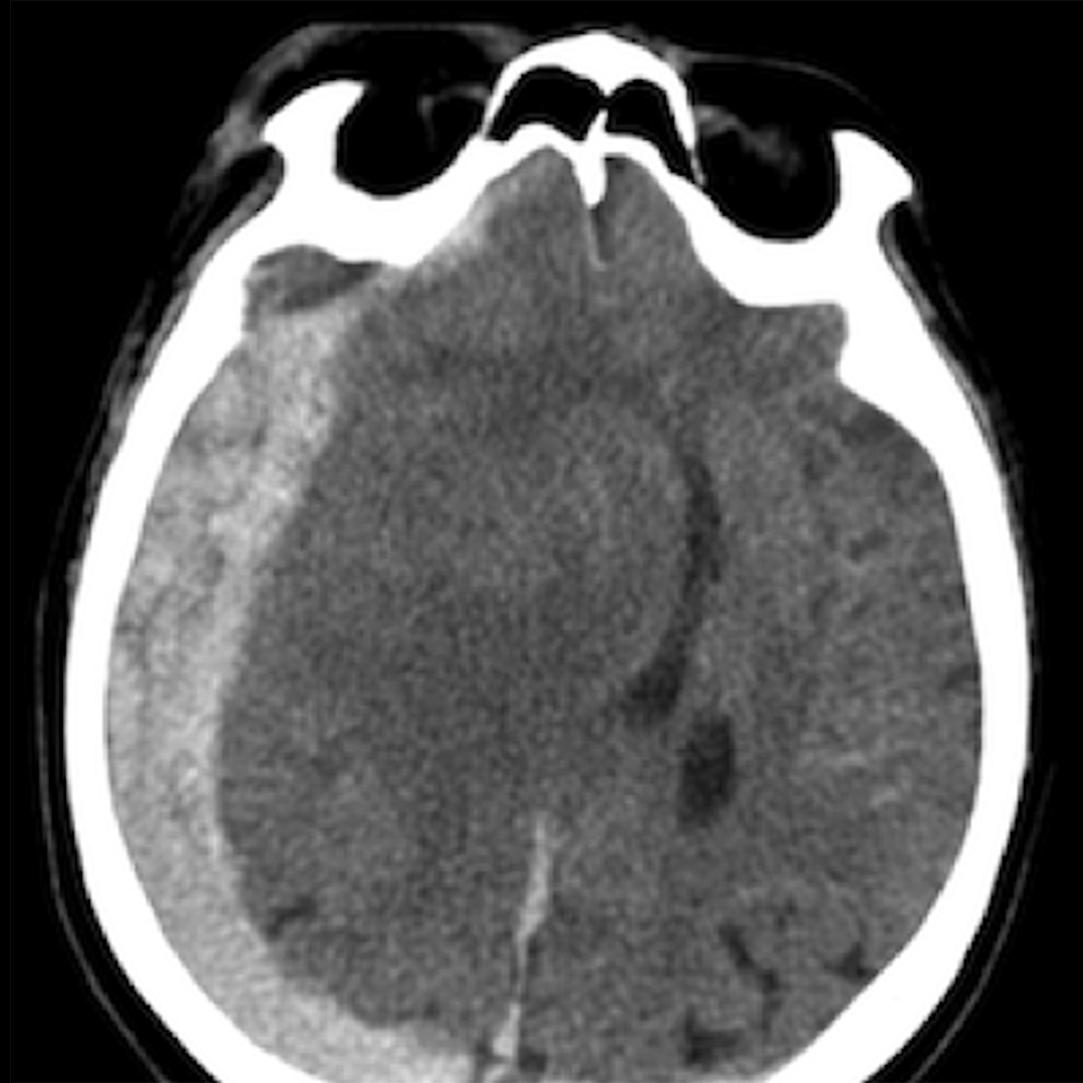
Extradural haematomas are commonly secondary to arterial bleeding due to a skull fracture with subsequent disruption of the middle meningeal artery. The haematoma is ovoid or lentiform and does not cross cranial sutures, but it may cross the midline (see Fig. 3.8.4 ).
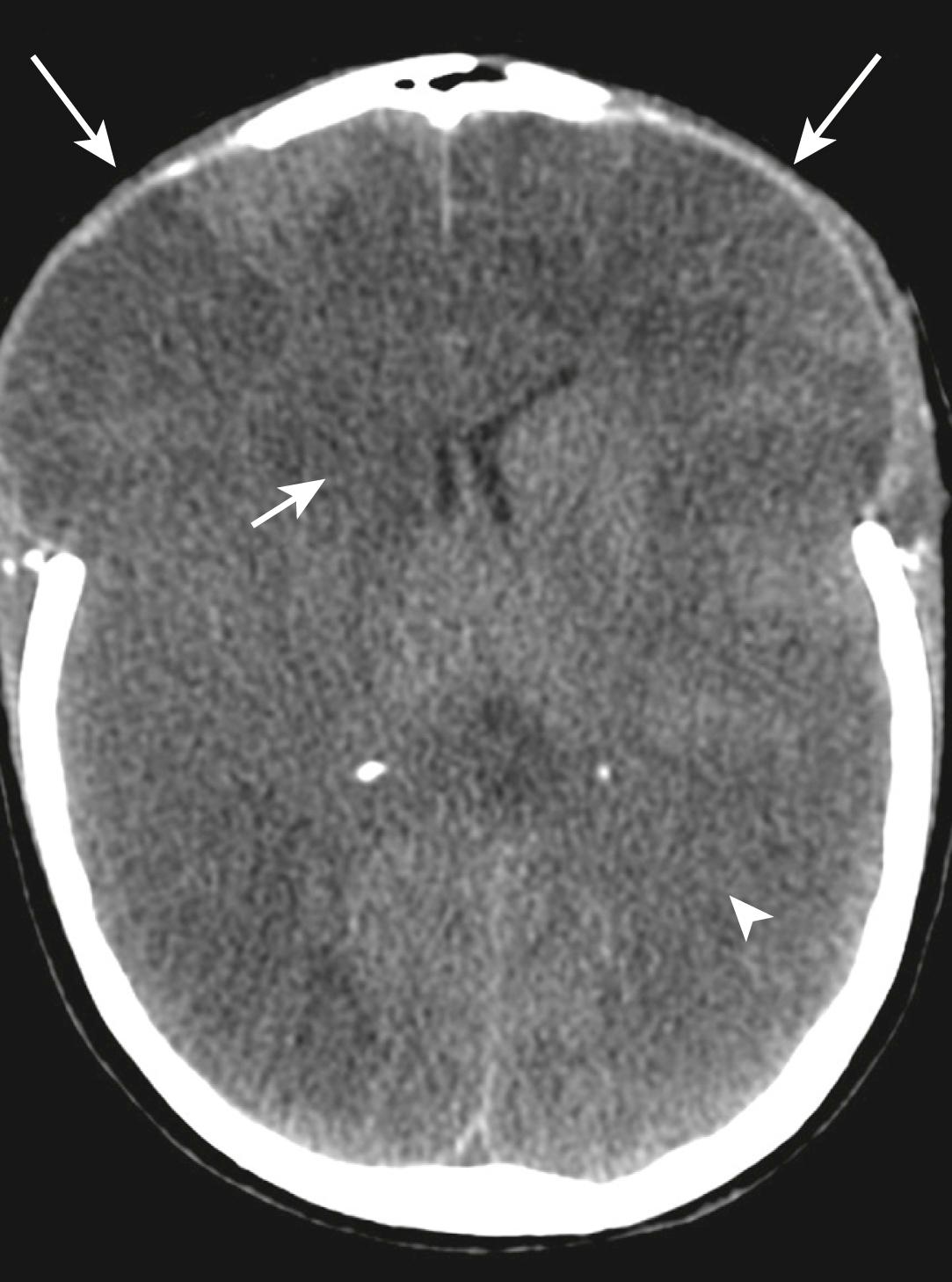
Subdural haematomas usually occur as a result of venous bleeding. These haematomas are crescentic in shape, may involve a larger area when compared with an epidural haematoma and may cross cranial sutures, but they do not cross the midline ( Fig. 3.8.8 ).
Subarachnoid haemorrhage may be due to disruption of small subarachnoid vessels or occur by direct extension from a parenchymal contusion/haematoma. Subarachnoid haemorrhage may be visualized in the sulci of the cerebral convexities or within the subarachnoid cisterns at the base of the skull ( Fig. 3.8.9 ).
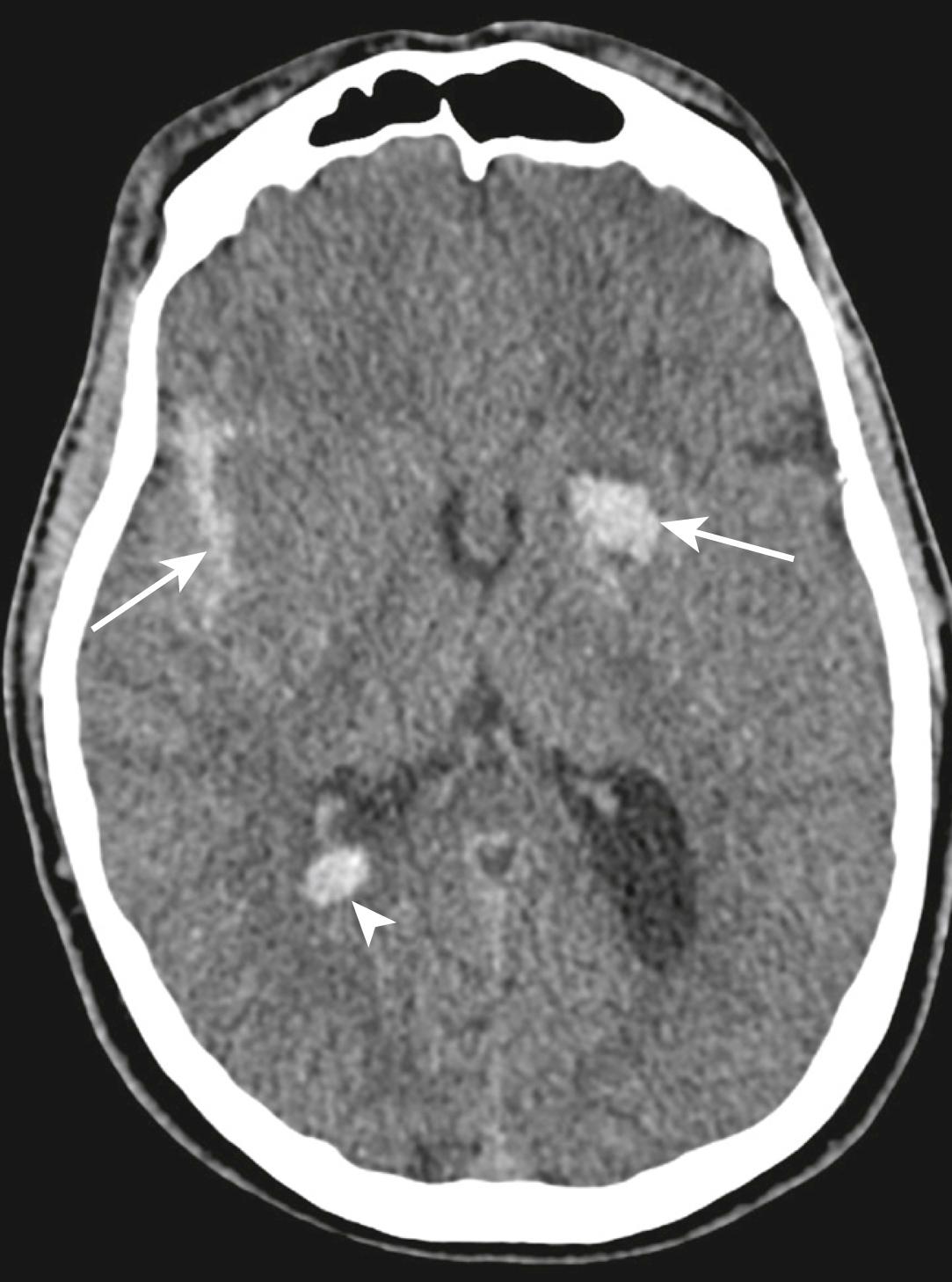
Intraventricular haemorrhage (see Fig. 3.8.9 ) may be caused by tearing of subependymal veins on the surface of the ventricles or by direct extension from a parenchymal contusion/haematoma. These blood products tend to layer dependently on a CT scan with the patient imaged in a supine position, particularly within the occipital horns of the lateral ventricles.
Cerebral contusions represent foci of bleeding within the parenchyma of the brain. These may occur within superficial grey matter/subcortical white matter due to direct contact from bony protuberances of the calvarium or base of skull. Deeper parenchymal contusions are caused by disruption of intraparenchymal blood vessels. Cerebral contusions commonly increase in size and number within the first 24 hours post-trauma due to continued bleeding (see Fig. 3.8.9 ). These haematomas also develop adjacent oedema, which may increase the associated mass effect on the remainder of the intracranial structures.
Also noteworthy is the increased presentation of patients taking anticoagulation or antiplatelet therapy who fall and suffer head injuries. The bleeding as a result of the injury may progress over 24 hours and may occur in all of the previously noted locations despite both pharmacological and neurosurgical attempts at reversal.
DAI occurs due to acceleration/deceleration forces and is a shearing-type injury to the brain. It may be initially difficult to visualize on a non-contrast CT but can be identified as tiny foci of petechial haemorrhage at the grey/white matter interface, within the corpus callosum or within the brain stem. MRI is the investigation of choice for DAI (see Fig. 3.8.7 )
Non-contrast CT is also capable of identifying areas of acute established infarction. In the setting of trauma, this may be secondary to acute vascular injury or mass effect due to cerebral oedema (see Fig. 3.8.5 ).
Blunt injury to the carotid or vertebral vessels (BCVI) occurs in about 0.1% of all trauma patients in the United States. Many of these injuries are diagnosed after the development of symptoms and signs due to central nervous system ischaemia, with neurological morbidity of up to 80% and mortality approaching 40%. However, when asymptomatic patients are screened for BCVI, the incidence rises to 1% for all admitted blunt trauma patients and up to 2.7% for those with an injury severity score (ISS) greater than 15.
The Denver Modification of Screening criteria ( Table 3.8.4 ) provide both risk factors as well as symptoms and signs for BCVI. CT angiography (CTA) is now the investigation of choice for the diagnosis of BCVI. Table 3.8.5 shows a grading scale for BCVI as proposed by Biffi et al.
| Symptoms/signs of BCVI |
|
|
|
|
|
|
| Risk factors for BCVI |
|
|
|
|
|
|
| Grade 1: intimal irregularity with <25% narrowing |
| Grade 2: dissection or intramural haematoma with >25% narrowing |
| Grade 3: pseudoaneurysm |
| Grade 4: occlusion of lumen |
| Grade 5: transection with extravasation |
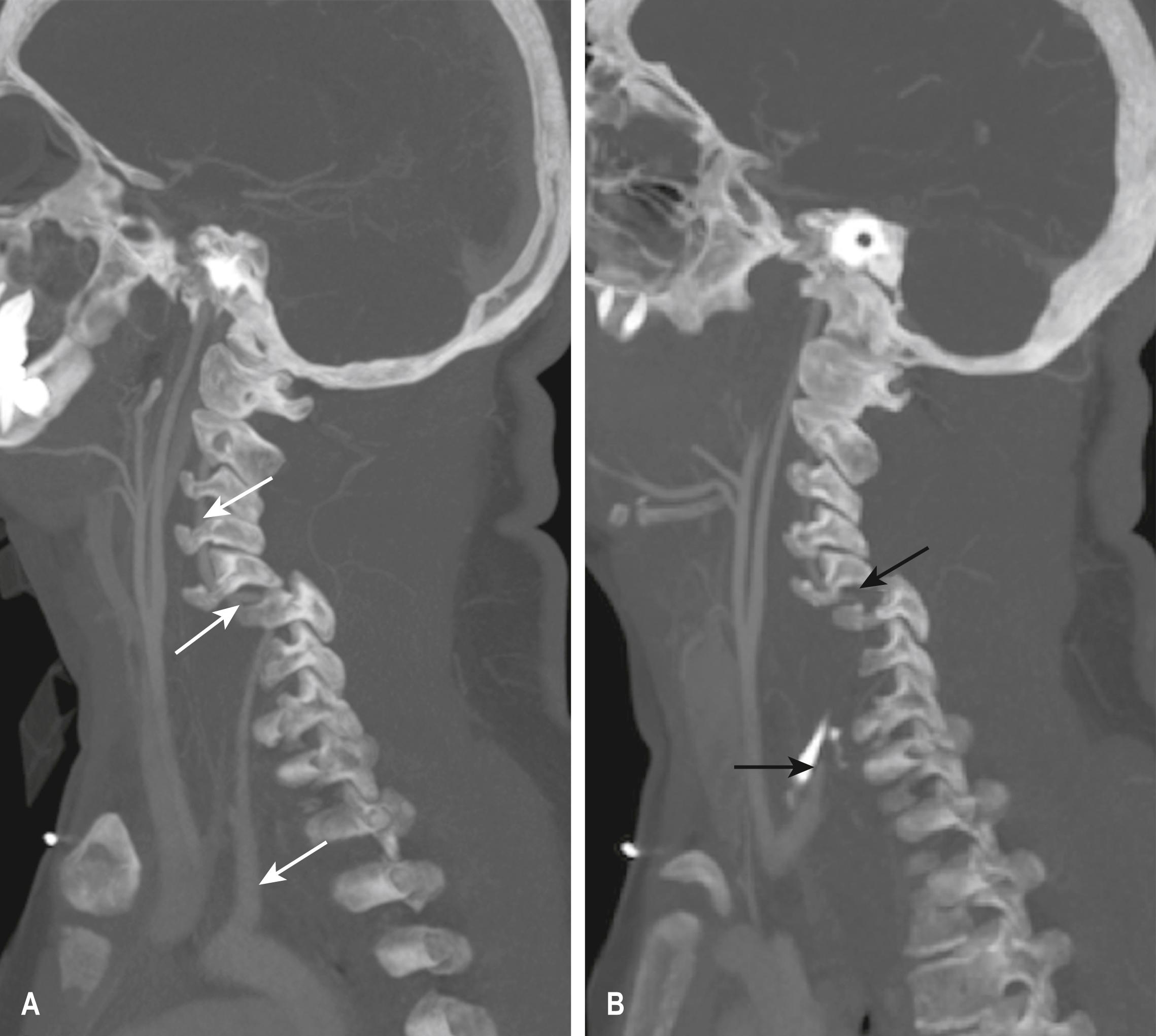
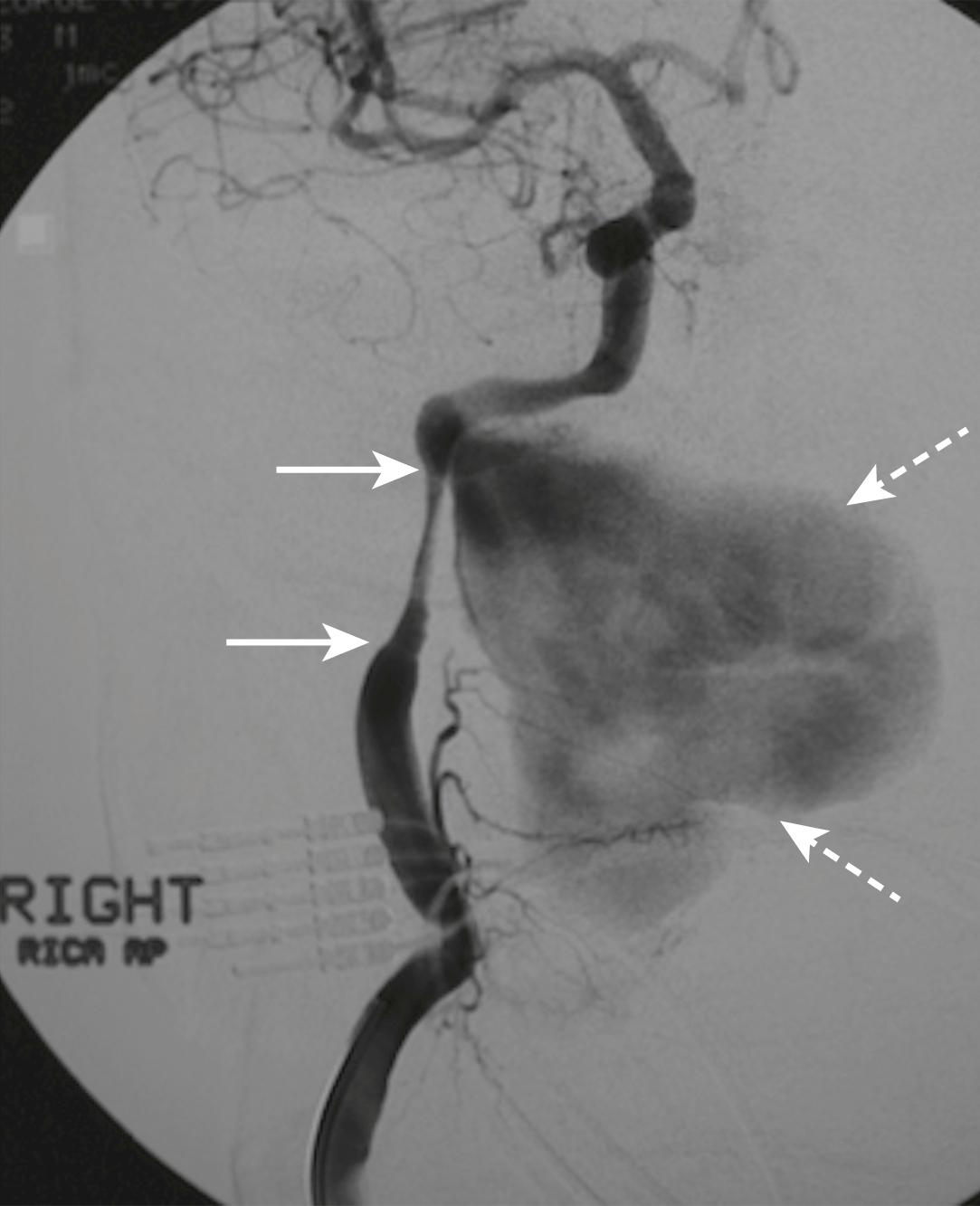
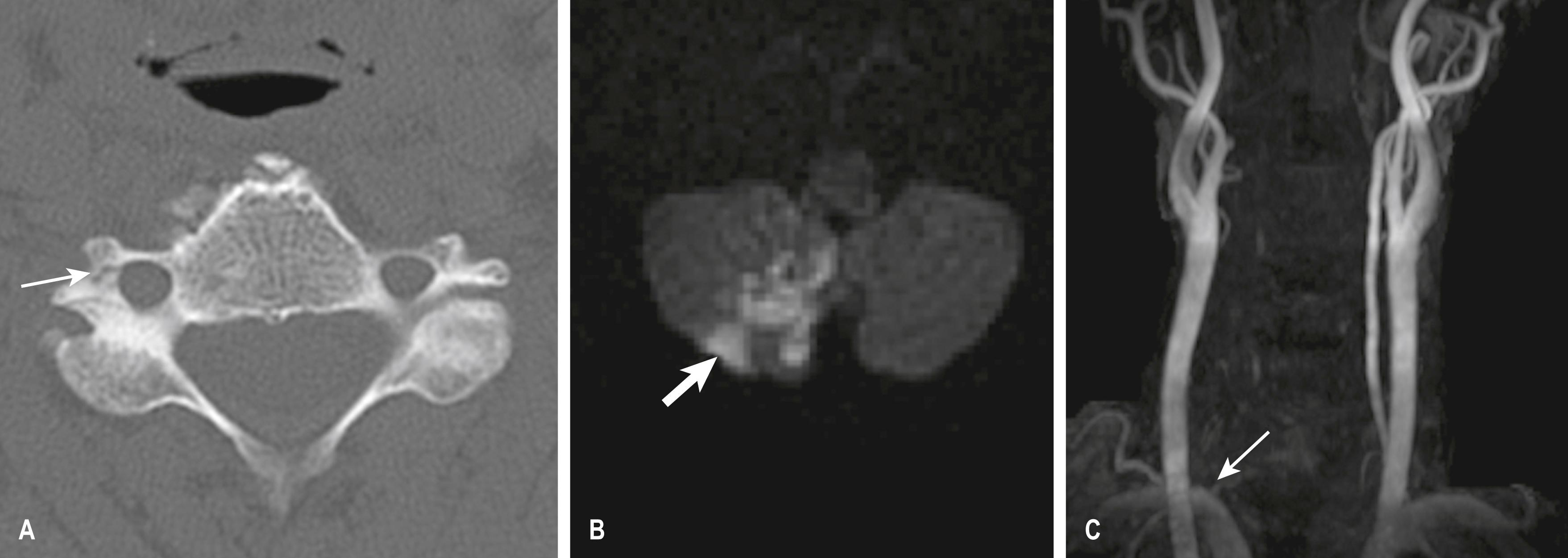
A dissection of the right vertebral artery due to blunt trauma in a patient with bilateral facet dislocation is shown in E-Fig. 3.8.1 ; E-Fig. 3.8.2 demonstrates dissection of the right internal carotid artery with a large associated pseudoaneurysm; and E-Fig. 3.8.3 shows a posterior inferior cerebellar artery infarct in a patient with a vertebral artery dissection.
Facial trauma may range from relatively minor undisplaced nasal bone fractures to the life-threatening problems of airway protection and haemorrhage associated with mid-facial (Le Fort) fractures. Underlying cerebral injury may also be associated with frontal bone fractures.
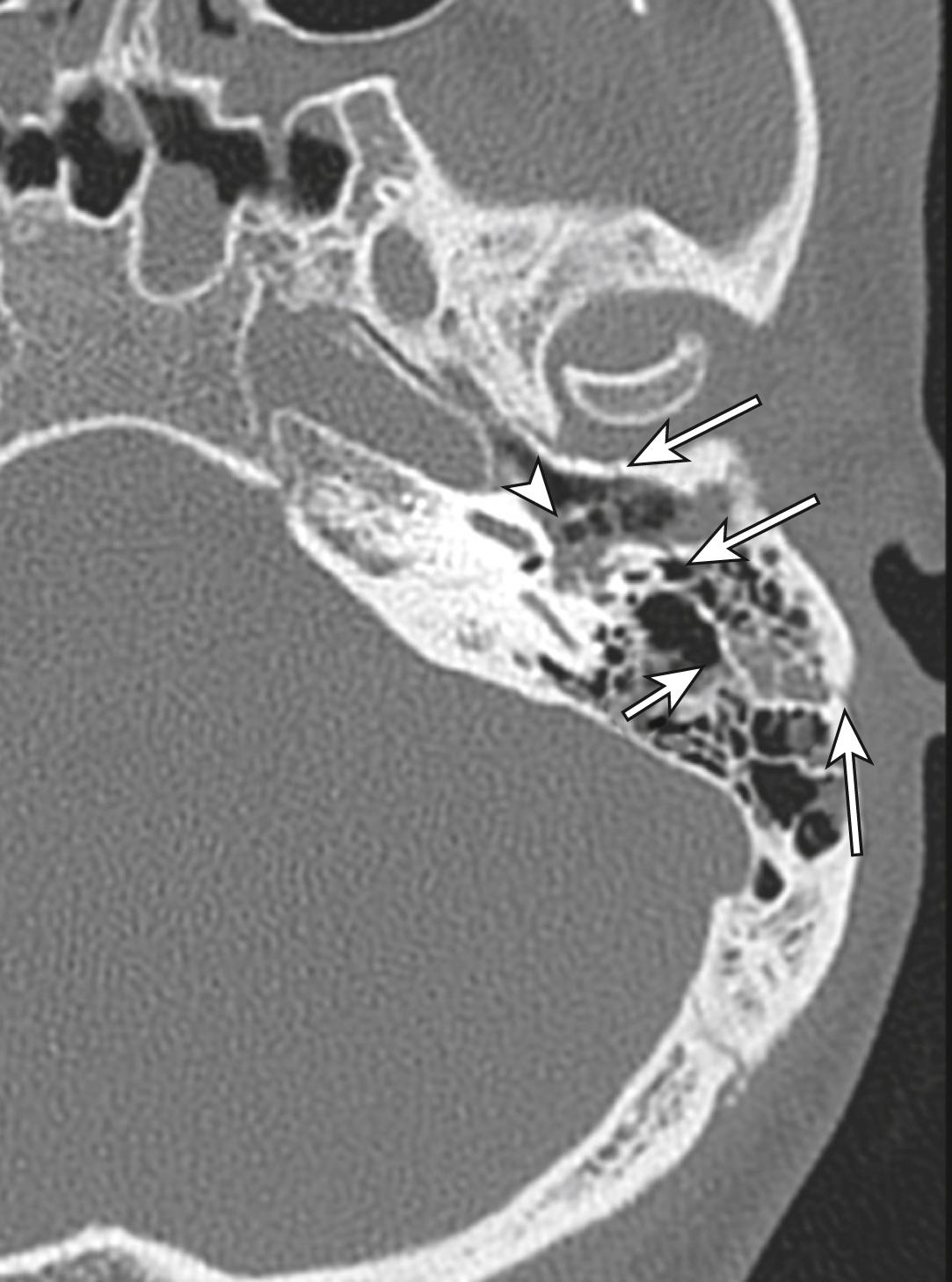
The commonest injury to the mid-face is the blowout fracture caused by a direct blow to the orbit, which results in a fracture of the orbital floor or the medial wall of the orbit in the region of the paper-thin lamina papyracea ( E-Fig. 3.8.4 ). There may be tenderness over the fractured bone associated with diplopia due to entrapment of orbital contents or (less commonly) visual disturbance due to globe or optic nerve injury. These fractures are best seen on CT scans with multi-planar reconstructions. Blowout fractures with entrapment of orbital contents require surgical elevation.
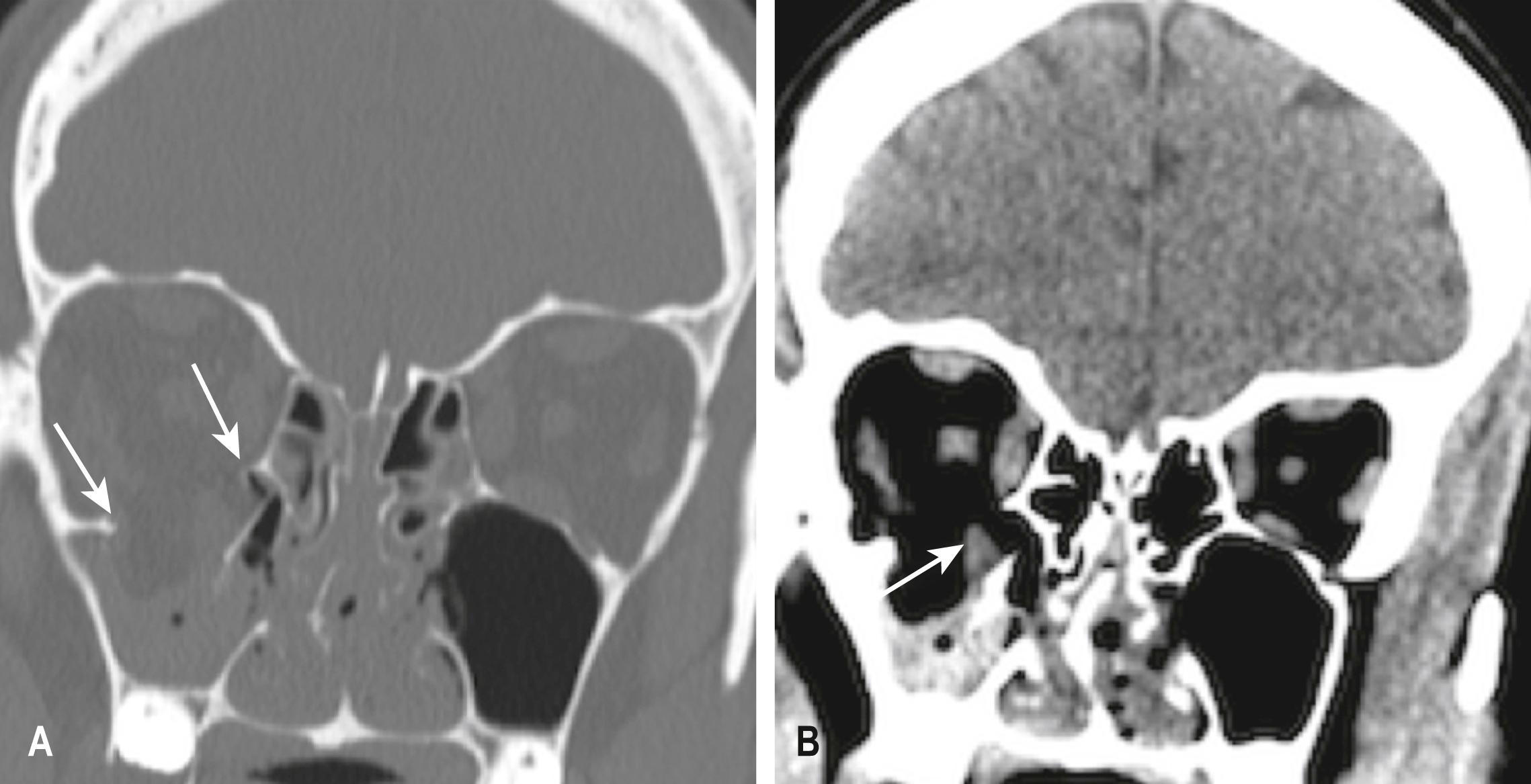
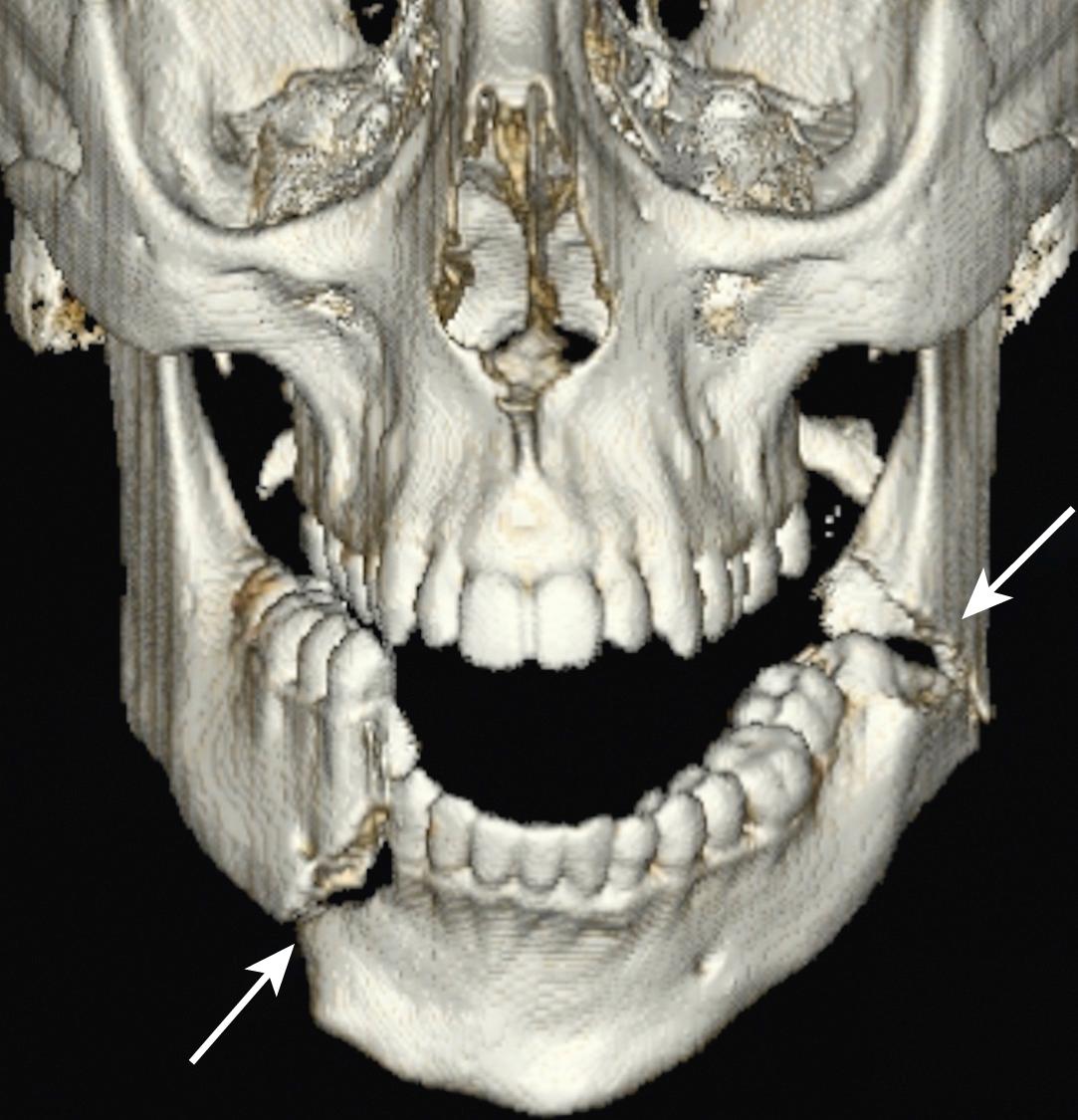
Mandibular fractures are usually obvious clinically because of pain, malocclusion and drooling. Mandibular fractures may be difficult to demonstrate on standard PA and oblique x-ray views. A panoramic view or orthopantomogram (OPG) is more useful, but CT of the mandible provides optimal demonstration of mandibular fractures, including those involving the mandibular neck, condyle and temporomandibular joint (TMJ) ( E-Fig. 3.8.5 ). Dislocation of the TMJ is also optimally diagnosed on CT ( E-Fig. 3.8.6 ).
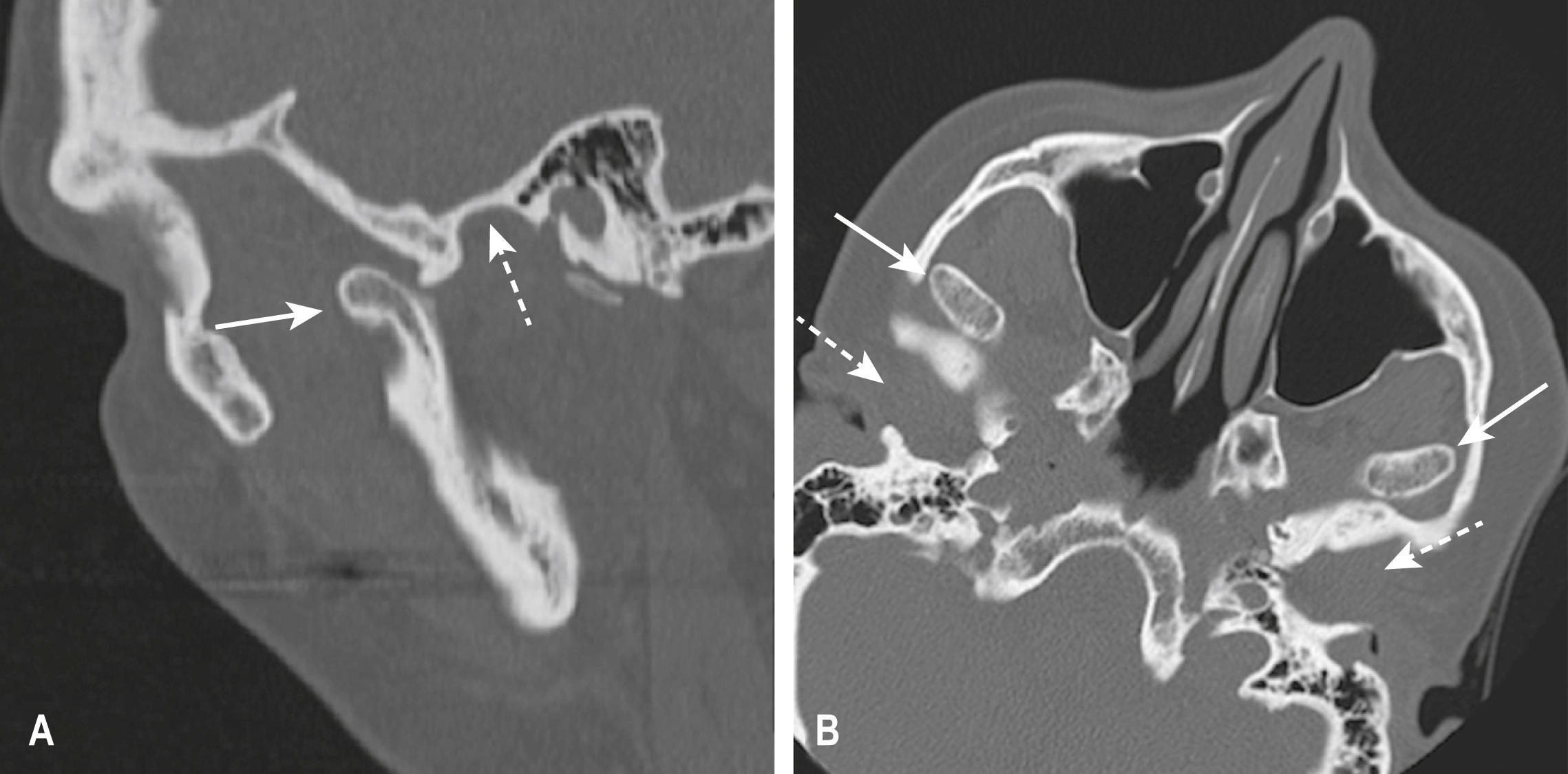
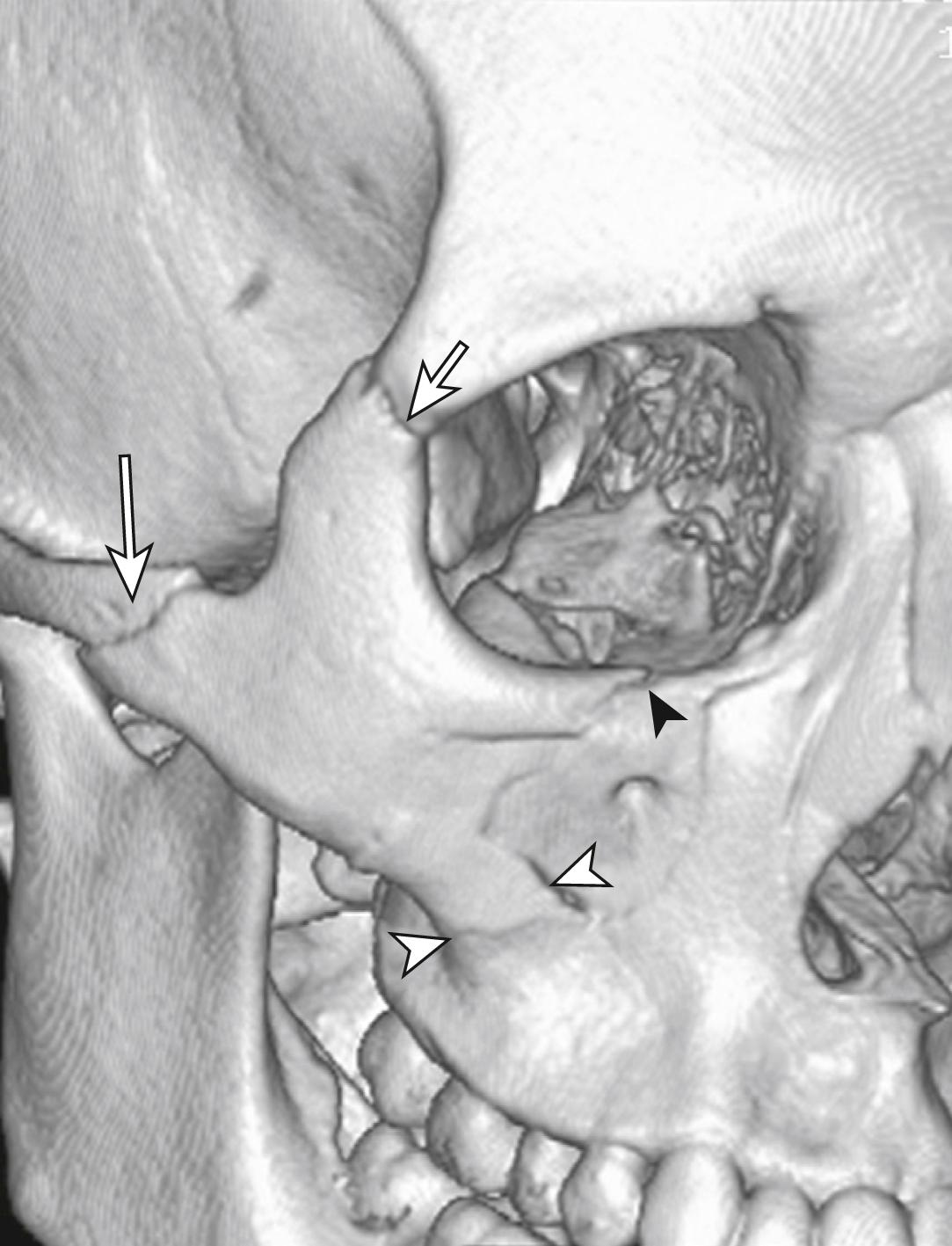
Fractures of the zygoma are classified as (1) tripod fractures and (2) isolated fractures of the zygomatic arch. The tripod fracture or zygomatico-maxillary fracture separates the malar eminence of the zygoma from its frontal, temporal and maxillary attachments. Tripod fractures are usually caused by a significant force to the body of the zygoma or malar eminence. The three fractures that constitute the tripod fracture are located in the inferior orbital margin, the lateral orbital margin or the zygomaticofrontal suture and the zygomatic arch. These fractures are best viewed on CT scans ( Fig. 3.8.10 ).
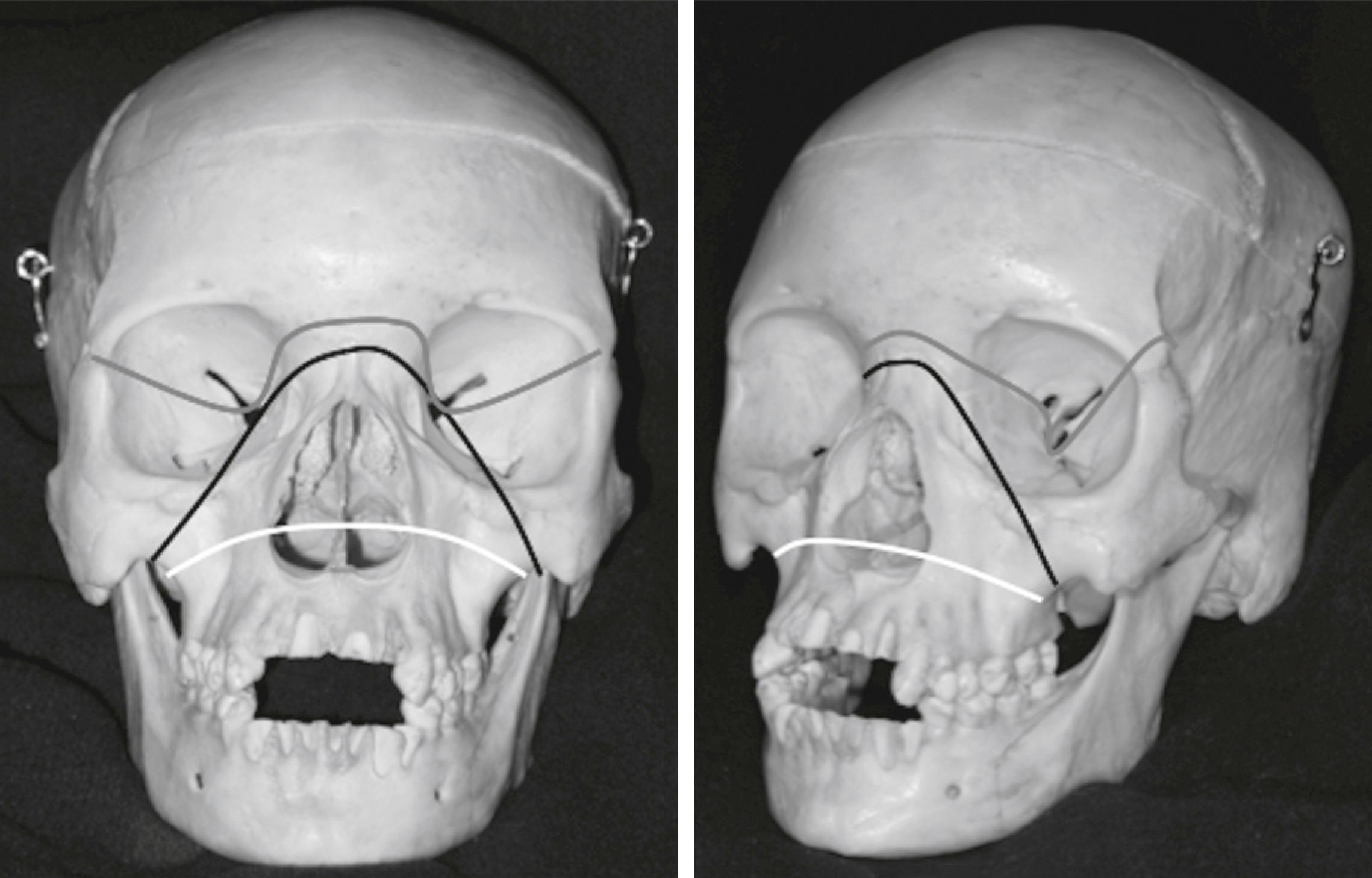
The Le Fort fractures are caused by direct trauma to the mid-face. The Le Fort 1 fracture involves the maxilla at the level of the nasal floor and will allow mobility of the palate (‘floating palate’) ( Fig. 3.8.11 ). The Le Fort 2 fracture passes through the nasal bones as well as the medial, inferior and lateral walls of the maxillary antrum. The Le Fort 3 or ‘craniofacial dysjunction/floating face’ fracture involves the nasal bones, the medial and lateral orbital walls and the zygomatic arch.
Frontal sinus fractures commonly occur as a result of direct force and are often compound, with the risk of associated intracranial infection. There may be an associated intracranial haematoma or cerebral contusion. A CT scan will best evaluate these fractures and determine the involvement of the posterior sinus wall. These fractures often require surgical exploration for debridement and repair.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here