Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Owing to increasing state and federal regulations, direct patient access to publicly available information about the quality of care, as well as pressures from payers for cost-efficiency, the delivery of care in the cardiac catheterization laboratory is being examined closely.
Efforts to improve the quality of medical care in the United States started more than 150 years ago, and new initiatives such as public reporting were incorporated into the Patient Protection and Affordable Care Act of 2010.
Donabedian’s triad of three domains—structure, process, and outcomes—provides a conceptual framework for evaluating the quality of medical care. Catheterization laboratory personnel should understand the existing structural elements, processes, and outcomes.
Each catheterization laboratory should develop a framework of quality to ensure that the right patient gets the right procedure with the right execution and the right outcome on a daily basis.
The framework should include the specific elements and activities that constitute the building blocks of quality.
Public reporting increases transparency and is part of the proposed mechanisms to tie reimbursement to the quality of care. An increasing focus on how to improve public reporting is necessary in order to decrease unintended consequences—such as avoiding treatment of high risk patients—and increase public understanding of the results.
Quality is everyone’s responsibility. W. Edwards Deming
Interventional cardiology has grown rapidly since its inception more than 30 years ago. Invasive cardiovascular procedures have become the cornerstone for the evaluation and management of many cardiovascular diseases, especially coronary artery disease. Since the start of interventional cardiology, there have been efforts to measure and evaluate the quality of care.
Considerable attention has been focused on the cardiac catheterization laboratory because these procedures are widely used, easily identified with claims data, expensive, and associated with important complications. Attention continues to intensify because of increasing state and federal regulations, requests for public reporting of hospital and physician data, consumer pressures for care of the highest quality, and pressure from payers for cost-efficient care and the appropriate use of diagnostic cardiac catheterization and percutaneous coronary interventions (PCIs). There is also growing awareness that the quality of health care is compromised by preventable medical errors, the absence of evidence-based standards in many areas, lack of emphasis on disease prevention, patients’ inadequate personal responsibility for maintaining their health, and disparities in health care delivery related to race, gender, income, and insurance status.
Numerous government and private agencies in the United States have been involved in the developing measurements of quality and disseminating data ( Table 69.1 ). Report cards on cardiac operations and PCIs for hospitals and individual physicians exist in some states. Interest in these reports continue to increase as data have emerged showing that patients fail to receive up to 45% of the tests and treatments recommended by evidence-based guidelines. In an attempt to promote quality and improve outcomes, pay-for-performance programs, in which providers receive financial incentives for achieving certain benchmark goals in patient care, were developed. With payment reform and Centers for Medicare and Medicaid Services (CMS) demonstration projects such as bundled payment care initiatives, there will be increasing focus on the efficient delivery of percutaneous procedures as part of a complete episode of care over a specified time period (e.g., 30 to 90 days).
| Organization | Mission, Goals, and Focus | Website |
|---|---|---|
| Agency for Healthcare Research and Quality (AHRQ) | Lead federal agency charged with improving the quality, safety, efficiency, and effectiveness of health care for all Americans | www.ahrq.gov |
| National Guideline Clearinghouse (NGC) | An initiative of AHRQ that is a public resource for evidence-based clinical practice guidelines | www.guideline.gov |
| Centers for Medicare and Medicaid Services (CMS) | Host for Hospital Compare, which reports the process of care, risk-adjusted outcomes, and patient satisfaction measures for all hospitals in the United States | www.medicare.gov/hospitalcompare |
| CMS | Host for Physician Compare, which helps consumers find and choose physicians and other health care professionals enrolled in Medicare and will report several provider metrics in the future | www.medicare.gov/physiciancompare |
| Department of Health and Human Services (DHHS) | Provides links to hundreds of sites on the internet that contain reliable health care information and links to many government and nongovernment sources of information on health care quality | www.healthfinder.gov |
| The Joint Commission | Provides accreditation to hospital and other health care facilities; provides quality care and hospital quality measures for public reporting through the ORYX reporting program | www.jointcommission.org |
| National Committee for Quality Assurance (NCQA) | A private, 501(c)(3) not-for-profit organization dedicated to improving health care quality; operates the Healthcare Effectiveness Data and Information Set (HEDIS), a tool used by more than 90% of America’s health plans to measure performance on important dimensions of care and service | www.ncqa.org |
| The American Health Quality Association (AHQA) | An educational not-for-profit national membership association dedicated to health care quality through community-based, independent quality evaluation and improvement programs | www.ahqa.org |
| National Quality Forum (NQF) | Sets national priorities and goals for performance improvement and endorses national consensus standards for measuring and publicly reporting on performance | www.qualityforum.org |
| American Medical Association (AMA) | AMA-sponsored Physician Consortium for Performance Improvement (PCPI) enhances quality of care and patient safety through development, testing, and maintenance of evidence-based clinical performance measures and measurement resources for physicians | www.ama-assn.org |
| American College of Cardiology Foundation (ACCF) | In collaboration with other professional organizations, develops clinical practice guidelines, expert consensus documents, and other quality programs, including Guidelines Applied in Practice (GAP) to assist with guideline application in clinical practice and Hospital to Home (H2H), an effort to improve the transition from inpatient to outpatient status for those with cardiovascular disease | www.cardiosource.org |
| American Heart Association (AHA) | In collaboration with other professional organizations, develops clinical practice guidelines, expert consensus documents, and other quality programs, including Get With the Guidelines, a hospital-based quality improvement program to ensure consistent treatment according to evidence-based guidelines, and Mission:Lifeline, a national, community-based initiative to improve systems of care for patients with ST-elevation myocardial infarction | www.my.americanheart.org |
| The Leapfrog Group | A voluntary program organized by large employers to promote big leaps in health care safety, quality, and customer value | www.leapfroggroup.org |
Although there are no formal national standards with which to judge the quality of cardiac catheterization laboratories, some states have used clinical practice guidelines and expert consensus documents published by the American College of Cardiology Foundation (ACCF), the American Heart Association (AHA), and the Society for Cardiovascular Angiography and Interventions (SCAI) to develop standards of quality. Although these documents were never intended to serve as state or national standards, they have become the de facto basis for licensure regulations imposed by state health departments in an attempt to improve quality. To understand the current status of efforts to improve quality in the cardiac catheterization laboratory, it is helpful to examine some of the major events that comprise the history of efforts to improve the quality of health care in the United States.
Despite concerns about the state of American medicine, there have been profound improvements during the past 150 years. The American Medical Association (AMA) was founded in 1847, in part to address the disorganized and poor quality of health care in the United States. As a precursor of future efforts, the American College of Surgeons established the Hospital Standardization Program in 1917, promoting five basic patient care standards and surveying health care organizations to determine their acceptability for accreditation. In 1952, several other organizations collaborated with the American College of Surgeons to form the Joint Commission on Accreditation of Hospitals, now called The Joint Commission.
A structure for organizing quality standards in health care was proposed by Avedis Donabedian in 1966 with the publication of an article that provided a broad definition of quality. It recommended evaluations in three areas: structure, process, and outcome. This format was widely adapted and is still in use today.
During this early period, quality was often assessed by random chart audits or outcome-oriented chart surveys to evaluate metrics such as the use of blood products in surgical cases. Audit requirements were later minimized in favor of hospital-wide quality assurance programs designed to detect care that was thought to be outside acceptable standards. Physician profiles reflecting the number of procedures performed, indications, and complications were compared with grouped data from similar physicians to identify outliers in the hope that they could be persuaded to change their practice habits by colleagues, the hospital, or other agencies.
About 1990, a technique developed primarily for industry, called continuous quality improvement (CQI), was advocated by The Joint Commission. Compared with quality assurance programs, CQI tries to improve overall performance rather than merely to identify poor performers. CQI has become a central activity for all health care organizations.
As efforts by the medical profession to improve quality were maturing, state and federal agencies were becoming interested in regulating health care, developing standards, and promoting high-quality medical care. By the late 1800s, many states required physician licensure and mandated educational standards for physicians. The National Board of Medical Examiners was founded in 1915 to provide a nationwide examination that licensing authorities could accept and use to judge candidates for licensure.
As the medical field expanded and specialty training became available, it was recognized that some type of certification process for specialists was necessary. To establish a uniform system, the American Board of Medical Specialties was formed in 1933. There are now 24 specialty boards that certify physicians, and this process has evolved to one of continuous professional development and lifelong learning through a Maintenance of Certification program requiring ongoing measurement of six core competencies. Controversy has accompanied the Maintenance of Certification program, and it continues to evolve as several groups attempt to determine the optimal strategy.
One of the first federal quality initiatives was the formation of the U.S. Food and Drug Administration (FDA) in 1906. With the enactment of Social Security in 1935 and Medicare in 1965, the federal government became more involved in setting requirements for the delivery of health care funded by federal dollars.
To monitor the care of Medicare patients, Congress enacted rules called Conditions of Participation , which required hospitals to provide certain services and conduct reviews to determine the appropriateness of hospital admissions. In 1972, amendments to the Social Security Act created the Professional Standards Review Organization program to promote hospital efficiency and eliminate unnecessary hospital use. This program failed to meet expectations and was unpopular because many thought that it emphasized cost containment rather than quality. It was abandoned and replaced by other peer review organizations.
At the same time, substantial changes in hospital reimbursement occurred, with a shift to a cost-per-case system based on assignment to a diagnosis-related group (DRG). The peer review organizations were responsible for validating correct assignment to a DRG and for monitoring hospital admissions, readmissions, surgical procedures, complications, and hospital deaths. Peer review organizations emphasized quality but focused more on outcome metrics than structure and process metrics, and they were not without their critics.
As the amount of Medicare spending increased, the federal government became more involved in monitoring and controlling payments to physicians and hospitals. Before 1989, physician payments for services within the Medicare program were based on usual and customary charges for services by similar physicians in the previous year. Substantial changes in Medicare payments to physicians occurred as a result of the Budget Reconciliation Act of 1989, which redirected payments based on costs rather than charges. Costs were determined for the actual work involved, the overhead required to provide the service, and malpractice costs, with all three elements further adjusted for geographic differences in cost. This caused major changes in practice patterns, which had both positive and negative effects on the quality of care.
Additional federal funding led to the formation of the Agency for Health Care Policy and Research, which had a turbulent history and narrowly escaped being eliminated in 1995, only to be reauthorized in 1999 with a new mandate and new name: the Agency for Healthcare Research and Quality (AHRQ). Many other initiatives and programs were developed by professional organizations and government agencies over the next 10 years, with various degrees of success.
Several initiatives were proposed as part of the Patient Protection and Affordable Care Act (PPAHA public law 111 to 148) of 2010. In addition to sweeping changes in the delivery of health care, several provisions in this legislation specifically targeted the quality of health care, including establishment of a Patient-Centered Outcomes Research Institute, formation of a Medicare Innovation Center with $10 billion to fund payment reform and quality improvement pilots, and development of new systems linking payment to the quality of outcomes. The initiatives in the PPAHA and other progressive efforts promoted a major transformation of the American health care delivery system through the realignment of payment incentives to reward quality rather than quantity of care.
It is important for interventional cardiologists to understand this history as they interact with many parts of the developing quality infrastructure, such as The Joint Commission, board certification, maintenance of certification, increased transparency from public reporting, and payment reforms.
But even though Quality cannot be defined, you know what Quality is. Robert M. Pirsig
A high level of quality is something everyone wants in health care, but it is not easily defined. Patients, physicians, payers, and government have different perspectives on the elements that contribute to high-quality health care. Patients frequently place greater emphasis on the interaction and time they have with their physician than on issues such as adherence to practice guidelines. In contrast, payers and government agencies are likely to place more emphasis on adherence to performance measures and on reductions in unnecessary procedures and costs.
The potential to deliver high-quality care in the cardiac catheterization laboratory centers on the core values promoted by the Institute of Medicine (IOM). In its report Crossing the Quality Chasm, quality is defined as “the degree to which health care systems, services, and supplies for individuals and populations increase the likelihood for desired health outcomes in a manner consistent with current professional knowledge.” The IOM further states that health care should be safe, effective, evidence based, timely, equitable, and patient centered.
Several definitions of quality have been proposed, reflecting the complexity of the health care system and its heterogeneous stakeholders. The RAND Corporation defines quality care as “providing patients with appropriate services in a technically competent manner, with good communications, shared decision making, and cultural sensitivity.”
An increasingly popular operational definition of quality is based on error reduction and the recognition that there are three major types of errors in health care: underuse, overuse, and misuse. Underuse is the failure to provide a medical intervention when it is likely to produce a favorable outcome for a patient. Overuse is the use of a test or therapy even though its benefits do not justify the potential harm or costs. Misuse occurs when a therapy or diagnostic test is used in the wrong way or for the wrong purpose.
However quality is defined for interventional cardiologists, a structure for evaluating and measuring quality is needed to continually improve patient care ( Fig. 69.1 ). The structure suggested provides a framework of quality into which recognized building blocks of quality can be placed and used in everyday workflow. These building blocks include specific elements and activities:
Quality and case reviews are performed on a regular basis by all members of the patient care team in the catheterization laboratory (e.g., cardiologists, nurses, technologists) and by cardiac surgeons, noninvasive cardiologists, and others if necessary.
Clinical practice guidelines, data standards, performance measures, and appropriate use criteria (AUC) are incorporated into daily practice.
The CQI process is used as a tool to evaluate variations in practice and improve performance in the cardiac catheterization laboratory.
Data are submitted to clinical registries such as the CathPCI Registry of the National Cardiovascular Data Registry (NCDR) to benchmark patient outcomes rather than using volume standards or administrative databases.
Data from the NCDR and other local information are used to create dashboards for easy and understandable display of trends.
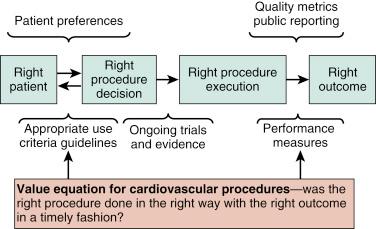
For invasive procedures, the decision to perform the procedure (i.e., right patient and right procedure) may be measured with AUC and clinical practice guidelines. A high-quality health care environment ensures that the patient is receiving the correct treatment for his or her condition. If the patient receives flawless and efficient delivery of the wrong treatment, high-quality care cannot exist. Patient preference is solicited as part of shared decision making along with the clinical recommendation. Execution of the right procedure is influenced by clinical practice guidelines, performance measures, and the best available evidence, but it also includes the operator’s skill and experience as assessed by board certification and maintenance of certification, case reviews, and other peer review activities.
The right clinical result can be assessed by performance and outcome measures, some of which are publicly reported. In this fashion, the interventional practice of a facility and an operator may be evaluated, which provides the basis for determining the value of different practices.
Quality in health care has its origin in the cycle of therapeutic development ( Fig. 69.2 ). The model starts with a hypothesis derived from basic scientific research, animal research, or other observations. It progresses to early clinical research with small, nonrandomized, and unblinded case series and eventually leads to a large, randomized, blinded, and well-designed clinical trial.
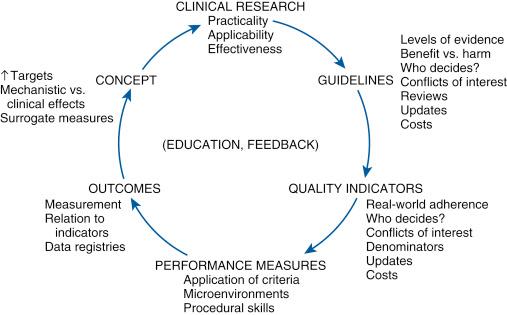
After one or more well-performed clinical trials provide clear information about a clinical question, the substrate is established for a clinical practice guideline. All available evidence and opinions are used to produce the clinical practice guidelines for medical conditions and therapeutic procedures (e.g., PCI guidelines). The AUC and performance measures are then derived from clinical practice guidelines ( Fig. 69.3 ).
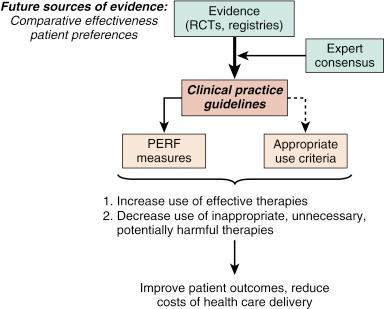
The building blocks of quality fit into Donabedian’s triad, a conceptual framework that has been used extensively. The three domains (i.e., structure, process, and outcome) each identify a major area containing multiple metrics that can be assessed to determine the program’s quality.
Structure refers to the physical components of health care delivery. For the cardiac catheterization laboratory, structural measures include the training, experience, and board certification of the physicians performing procedures; training and competency records for laboratory staff; adequacy of diagnostic, imaging, and therapeutic equipment including radiation safety; appropriate maintenance and calibration logs for equipment; educational opportunities for the staff; and internal methods used for tracking procedural data ( Table 69.2 ).
| Structural Indicators |
| Personnel Indicators |
|
| Equipment Indicators |
|
| Organizational Indicators |
|
| Process Indicators |
|
| Outcome Indicators |
|
To assess the knowledge base of interventional cardiologists, the American Board of Internal Medicine (ABIM) has administered an additional qualification examination for board certification in interventional cardiology since 1999. The maintenance of certification requirements is in evolution. Other requirements include maintenance of a valid certification in internal medicine or cardiovascular disease, documentation of adequate procedure volumes (e.g., 100 cases over 2 years), evidence of participation in a quality improvement project, and completion of self-evaluation modules for medical knowledge and practice performance. Hospitals and payers frequently adopt a requirement for board certification, but there are no data to prove that board-certified physicians provide a higher quality of care than those who are not board-certified. For other laboratory personnel, structural measures can include requirements for all technologists to be registered cardiovascular invasive specialists and to maintain certification in advanced cardiac life support.
Important structural components include education in the form of cardiac catheterization conferences, which provide a forum for discussing difficult management issues, and morbidity and mortality reviews as part of a peer review quality improvement program. To be effective, these programs must emphasize improvement of performance rather than identifying individual physicians as potential one-time outliers. Collection of laboratory data with benchmarking against state or national data is important for assessing clinical outcomes. Examples include the NCDR’s CathPCI Registry and the Acute Coronary Treatment and Intervention Outcomes Network-Get With The Guidelines (ACTION-GWTG) Registry of myocardial infarction. Participation in these programs provides caregivers with standardized tools for data collection, including relevant data elements and definitions, and for the validated risk adjustment of patient outcomes, such as mortality rates. In addition to tracking outcomes, the data reports help to assess adherence to guidelines and performance measures and can serve as a focus for quality improvements within an institution.
Process measures assess how the system works and how health care is delivered (see Table 69.2 ). An example in interventional cardiology is the door-to-balloon (DTB) time for patients with ST-elevation myocardial infarction (STEMI). To achieve an optimal DTB time, many steps spanning several hospital departments and services are necessary. The emergency department triages the patient with a possible STEMI, an electrocardiogram is obtained to confirm the diagnosis, and appropriate treatment is started. The interventional team is alerted, the cardiac catheterization laboratory is prepared, the patient is transported to the laboratory, and the culprit artery is opened. A flaw in any step increases the DTB time and the possibility of not meeting the process measure.
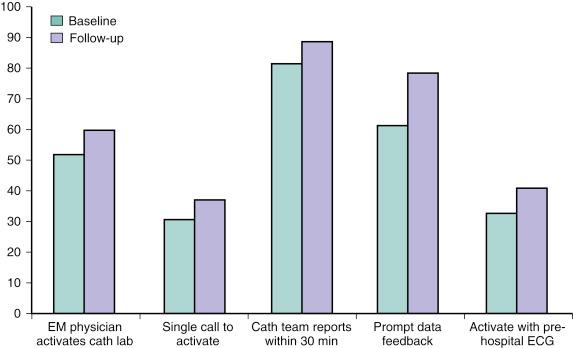
Realizing that DTB times were suboptimal in many facilities, the Door-to-Balloon Alliance was launched in late 2006 with the goal of achieving a DTB time of 90 minutes or less for at least 75% of nontransferred patients. Several process measures that improved DTB times were adopted and tracked, resulting in a significant improvement in national DTB times ( Fig. 69.4 ).
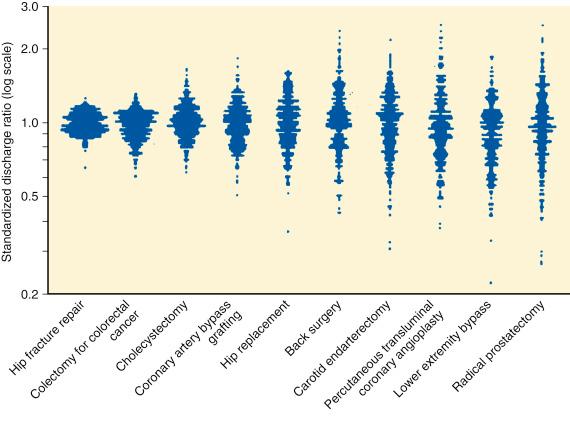
Other examples of process measures include collecting data to determine the appropriateness of performing a PCI or to enable the assessment of performance measures. Proper patient selection for PCI is receiving increasing scrutiny by all health care stakeholders because there is wide variation in its use ( Fig. 69.5 ). Assessing the appropriateness of a PCI involves understanding the patient’s history, clinical presentation, and physical findings; noninvasive testing to determine ischemia severity; assessment of left ventricular function; and interpretation of coronary angiographic findings.
The third component of Donabedian’s triad is outcome , which is the final product or result of previous actions. Outcomes are tangible measures such as procedural risk-adjusted mortality rates and other adverse outcomes such as vascular complications, bleeding, stroke, contrast nephropathy, cardiac tamponade, periprocedural acute myocardial infarction (AMI), and the rate of emergency coronary artery bypass grafting (CABG) (see Table 69.2 ). The measurement of outcomes as quality indicators is attractive because of the implied association between outcome measures and hospital or physician performance. However, these tenuous associations can create challenges for accurate assessment. For example, the definition of a periprocedural AMI (i.e., any troponin level increase versus three or five times the baseline level) and the clinical significance of increased biomarker levels in the periprocedural period are subject to debate. Standardized protocols are needed for biomarker collection during the postprocedural period and for the interpretation of elevated biomarker levels in patients with acute coronary syndrome (ACS).
NCDR data have shown that only 26% of medical centers measure biomarkers after elective PCI, thus limiting use of the peri-PCI outcome measure. Clinical data standards and definitions and standards for statistical models have been developed to help ensure consistency, but important issues remain.
Many PCI-related adverse events—such as death or emergency CABG—are uncommon, especially when assessed at the operator level, because the total number of procedures annually is modest. For example, the 95% confidence interval for a 2% mortality rate for 100 PCI procedures is 0% to 7%. This is a wide range for judging whether the operator has a low or high mortality rate. Quarter-to-quarter or year-to-year outcome measures often vary widely when measured for a single center or operator.
Another important issue is risk adjustment. Factors other than the operator and facility influence outcome comparisons. Patient acuity, demographic features, and comorbid conditions affect the outcome independent of the quality of care provided. Risk adjustment for key clinical outcomes requires accurate and detailed clinical data and a rigorous risk-adjustment methodology to describe provider outcome measurements. Unfortunately, with a focus on risk adjustment, there is the potential for the gaming of outcomes by discharging patients early so that outcomes—such as contrast-induced nephropathy and in-hospital mortality—are not captured. A disproportionate emphasis on outcomes can result in clinical risk-averse behaviors.
The NCDR was started in 1997 to help provider groups and institutions respond to increasing requirements to document the processes and outcomes of care in the cardiac catheterization laboratory. The NCDR is the most comprehensive outcomes-based data repository program in the United States, with seven hospital-based and one practice-based registry. More than 2200 hospitals nationwide participate in one or more of the NCDR registries, which collectively have amassed more than 15 million patient records. Participation in some of the NCDR registries is required by several states and several payers.
Comprehensive data from the CathPCI Registry are provided to member institutions for internal quality assessment and improvement, and confidential data for individual operators are available through a secure portal. Using these data, risk-prediction models have been developed for PCI mortality and bleeding rates ( Table 69.3 ).
| Variable | Scoring Response Categories | Total Points a | Risk of Inpatient Mortality (%) | |||
|---|---|---|---|---|---|---|
| <60 Years | ≥60, <70 Years | ≥70, <80 Years | ≥80 Years | |||
| Age | 0 | 4 | 8 | 14 | 5 | 0.1 |
| Cardiogenic shock | No | Yes | — | — | 10 | 0.1 |
| 0 | 25 | — | — | 15 | 0.2 | |
| Prior CHF | No | Yes | — | — | 20 | 0.3 |
| 0 | 5 | — | — | 25 | 0.6 | |
| Peripheral vascular disease | No | Yes | — | — | 30 | 1.1 |
| 0 | 5 | — | — | 35 | 2.0 | |
| Chronic lung disease | No | Yes | — | — | 40 | 3.6 |
| 0 | 4 | — | — | 45 | 6.3 | |
| GFR | <30 | 30–60 | 60–90 | >90 | 50 | 10.9 |
| 18 | 10 | 6 | 0 | 55 | 18.3 | |
| NYHA functional class IV | No | Yes | — | — | 60 | 29.0 |
| 0 | 4 | — | — | 65 | 42.7 | |
| PCI status (STEMI) | Elective | Urgent | Emergent | Salvage | 70 | 57.6 |
| 12 | 15 | 20 | 38 | 75 | 71.2 | |
| PCI status (no STEMI) | Elective | Urgent | Emergent | Salvage | 80 | 81 |
| 0 | 8 | 20 | 42 | 85 | 89.2 | |
| 90 | 93.8 | |||||
| 95 | 96.5 | |||||
| 100 | 98.0 | |||||
a In the National Cardiovascular Data Registry (NCDR) risk prediction model for percutaneous coronary intervention mortality, points are assigned for the different variables, and the total number of points is used to predict the risk of inpatient mortality.
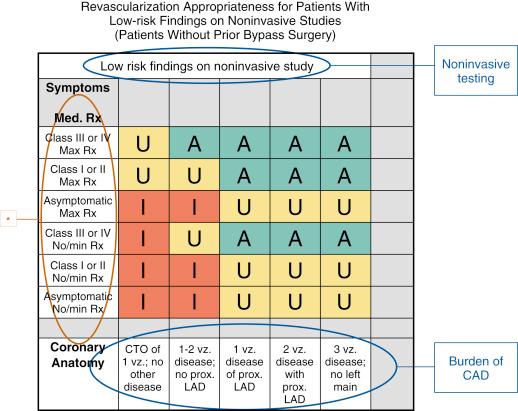
Outcome measures also include AUC. These criteria have been developed to determine whether coronary revascularization is a reasonable approach for a given clinical circumstance ( Fig. 69.6 ). Although acceptable indications in addition to AUC exist, measuring the degree of adherence to the clinical situations covered by the criteria is valuable for assessing the quality of patient selection. Application and assessment of appropriateness of care require collection of the relevant clinical variables. Because of evolving information about fractional flow reserve and revascularization from randomized controlled trials, the revascularization AUCs are updated every 2 to 3 years.
Outcome assessment often overlaps with and represents the aggregate effect of the other two components of the triad, structure and process. Improvement in the end product is therefore the ultimate measure of the overall success of medical care. Ideally, outcome assessment examines an isolated episode of care (i.e., a single PCI procedure) and includes a longitudinal follow-up assessment of the PCI. This may include the impact of care on indirect health-related measures such as patient satisfaction with the overall care process and quantification of the cost of the care delivery, including cost-effectiveness and cost-efficiency calculations.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here