Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The respiratory and cardiac systems are integrally related both anatomically and functionally, and as a result there are significant interactions, particularly in the newborn period.
Inappropriately high airway pressure during mechanical ventilation leads to adverse hemodynamic effects including a reduction in left and right ventricular output, a decrease in venous return, and an increase in pulmonary vascular resistance (PVR). Inappropriately low airway pressure can also have detrimental effects by decreasing lung volume and consequently increasing PVR.
PEEP and MAP in the range commonly used in clinical practice in settings of lung diseases with low compliance and in the absence of low lung volume or hyperexpansion have mild effects on hemodynamics.
In addition to the direct effects of ventilator settings on the cardiovascular system, the chosen treatment strategy and aims, such as a particular blood gas goal, can have a significant effect on the cardiovascular system. While the impact of acidosis on cardiovascular system is not well studied, there is accumulating evidence that excessive hypercapnia especially in the first few postnatal days attenuates cerebral blood flow autoregulation and likely contributes to reperfusion injury in preterm infants.
The cardiorespiratory system consists of two organ systems—the heart and cardiovascular system and the lungs and pulmonary vasculature—which are designed to work together to deliver an adequate supply of oxygen to the tissues to meet the demands of oxygen consumption. There are complex anatomical and physiological relationships between these two organ systems. An understanding as to how they interact both normally and in the presence of pathology or interventions, such as the provision of positive pressure, is essential for any clinician working with sick children and infants. Changes in intrathoracic pressure affect both organ systems, and within the cardiovascular system, there are differential effects on the left- and right-sided structures. A delicate balance needs to be maintained between the distending airway pressure needed to optimize lung volume and thus oxygenation, but avoiding excessive pressure that would compromise global cardiac function that is necessary for adequate systemic blood flow, which is essential for normal oxygen delivery. Both optimal oxygenation and normal cardiac output are required to deliver adequate oxygen to the tissues. Reduced tissue oxygenation often has a multifactorial causation; however, many of the issues will be related to what is happening at the level of the cardiorespiratory interaction.
The initial cardiorespiratory interaction is during birth at the time of the circulatory transition and umbilical cord clamping. A normal relationship between the spontaneously breathing infant with normal lungs and cardiovascular system should ensue—in which case there will be balance between the closely associated lungs and heart. If there is pathology, particularly respiratory distress, then the interventions required to support the respiratory system such as positive pressure ventilation may adversely impact the function of the heart. Similarly, abnormalities of cardiac function such as ventricular failure can result in lung congestion and the need for respiratory support. Finally, changes in the pulmonary vasculature, particularly in the neonatal transition where failure of the normal fall in pulmonary vascular resistance (PVR), can impair cardiac function. The predominant influence on the cardiorespiratory interaction is mean airway pressure (MAP) which most directly affects the intrathoracic pressure. The effect of inspiration/expiration in addition to MAP is minimal. Adjunctive respiratory therapies such as inhaled nitric oxide may also rapidly change the balance between cardiac and respiratory systems.
In utero, the fetus has fluid-filled lungs with a very low pulmonary blood flow—about 10% of postnatal flow and minimal tidal volume changes such that any pressure exerted on the heart physically and via venous return is steady and unchanging. The interposition of the placenta and the unique fetal shunts—the ductus venosus, foramen ovale (FO), and patent ductus arteriosus (PDA)—into the fetoplacental circulation results in a fetal circulation quite different to that of the postnatal infant just minutes after birth. Instead of being two parallel circulations, there is admixture of blood at several levels. Oxygenated blood returning from the placenta via the umbilical vein passes through the ductus venosus and into the inferior vena cava (IVC). Blood then streams into the right atrium where due to the anatomy of the right atrium and FO, the oxygenated blood is directed preferentially across the FO into the left atrium (LA), thus improving the oxygenation level of blood passing from LA into left ventricle and subsequently toward the systemic circulation. The presence of the PDA and fetal conditions (hypoxemia, vasoconstricting factors) that cause increased PVR result in preferential blood flow from the right ventricle via the right to left shunting PDA into the systemic circulation. The significantly reduced blood flow to the pulmonary circulation means that the normal main source of preload to the LA (when there is no placental flow), the pulmonary venous return, is significantly limited in utero. Blood passing from the right ventricle via the PDA and from the left ventricle into the aorta travels down the descending aorta and deoxygenated blood is then sent to the placenta via iliac and eventually umbilical arteries to complete the fetoplacental circulation. The main flow to the LA is provided by the placental return and some flow from lower body via the IVC—this is an important consideration when the timing of umbilical cord clamping in the newborn transition is considered. Interruption of the placental blood flow return prior to establishment of the pulmonary blood flow through the lungs puts the transitioning newborn at risk of a loss of preload to the systemic ventricle with a subsequent fall in systemic cardiac output.
The events of the perinatal cardiopulmonary transition constitute the first, and possibly most crucial, cardiorespiratory interaction and there is potential for significant complications ranging from premature interruption of the placental blood flow resulting in an acute drop in cardiac output to failure to properly transition leading to the syndrome of persistent pulmonary hypertension in the newborn (PPHN, Table 9.1 ). For premature infants, the complex transition to neonatal physiology is disrupted by varying degrees of cardiac dysfunction, poorly compliant and underdeveloped lungs, and failure of expected closure of fetal channels (particularly the ductus arteriosus). The combination of these insults can result in atelectasis, persistent elevation in PVR, poor cardiac output, and systemic hypotension. It is therefore of particular importance that ventilation and cord management strategies are optimized to limit hemodynamic instability. Keeping the cardiorespiratory events of the transition in sequence has become an important aspect of management at birth. The key initial event is inflation of the lungs (usually spontaneously by crying, or otherwise augmented with positive pressure devices), resulting in a rapid increase in pulmonary blood flow and reversal of the ductal shunt to become left to right. Additionally, the lung liquid must be rapidly absorbed; animal imaging data suggest that this occurs via transepithelial gradients developed during inspiration. , With the increased pulmonary blood flow, adequate left atrial filling is established and the umbilical cord can now be cut without acutely reducing the left atrial return. In this sequence, oxygenation is provided by the lungs prior to the placental “lung” being removed by cord clamping.
| Event | Intervention | Impact |
|---|---|---|
| Inspiration | Stimulation or PPV | Fluid absorption |
| ↑ Pulmonary blood flow | ||
| Reversal of PDA shunt | ||
| Lung aeration prior to cord clamping | Stimulation, CPAP/PPV a | Placental transfusion |
| Establishing FRC | CPAP/PPV | Improved oxygenation, ↓ risk of PPHN |
| Avoidance of hyperoxia | Judicious use of oxygen | Improved response to iNO |
a Excessively high CPAP and PPV can also negatively impact venous return and cardiovascular function (see text).
Failure to allow the natural sequence of events to unfold may result in significant hemodynamic instability, at least in animal models. Umbilical venous blood accounts for 30% to 50% of venous return to the heart. , Clamping the umbilical cord prior to lung aeration can therefore result in reduced preload, as poor left atrial filling from low pulmonary blood flow is unable to make up for the loss of umbilical venous flow. In combination with raised left ventricular (LV) afterload from a rapid increase in systemic arterial pressure as the low resistance placenta is removed, these acute physiologic changes can result in reduced cardiac output and haemodynamic instability.
These hemodynamic changes are much less marked if the pulmonary blood flow is established by lung aeration prior to cord clamping (termed physiologically based cord clamping). Delaying cord clamping increases the likelihood that the newborn infant will establish breathing/lung inflation before the umbilical cord is clamped. , In addition, the benefits of a delay in cord clamp time have generally been ascribed to the receipt of a placental transfusion. The volume of placental transfusion may be as high as 20 to 30 mL/kg of birth weight in term infants and up to 15 mL/kg in preterm infants. This is dependent on a number of factors, including gravity, time, flow/patency of the umbilical vessels, and spontaneous breathing efforts. Delayed cord clamping is associated with lower rates of early hypotension, inotropic support, blood transfusion, and surfactant use in premature infants compared to early cord clamping. ,
Respiratory efforts result in significant fluctuations in umbilical cord blood flow and can potentially enhance the placental transfusion received by preferentially directing umbilical venous return to the right atrium on inspiration. The provision of positive pressure and initial resuscitative measures while the infant is still attached to the umbilical cord has been hypothesized to provide additional benefit to the transitional circulation, and as a means to facilitate placental transfusion for compromised infants where delayed cord clamping would otherwise be abandoned for neonatal resuscitation. In the BabyDUCC trial, nonvigorous infants born over 32 weeks’ gestation requiring transitional assistance were randomized to either resuscitation during placental transfusion or early cord clamping. The primary outcome of heart rate at 60 to 120 seconds was similar between the two groups, as were oxygen saturations in the delivery room. Observational data show that the vast majority of preterm infants spontaneously breathe by time of arrival to resuscitaire, regardless of whether cord clamping is early or delayed. Two recent randomized controlled trials (RCTs) have compared delayed cord clamping with and without assisted ventilation in premature infants. , Apgar scores, oxygen saturations, delivery room interventions, and hemodynamics in the first 24 hours including rates of inotropic support and blood transfusion appear similar whether respiratory support was initiated prior to cord clamping or not. While the hemodynamic benefits of establishing lung aeration with an intact cord have not yet been proven, multiple additional studies addressing this clinical question are currently underway.
Umbilical cord milking is another alternative for provision of a placental transfusion to the newborn. As transfusion occurs over a shorter time period, there is less opportunity for the effect of the respiratory system on the volume of transfusion and subsequent cardiac flow on effects. Despite this, there still seem to be cardiovascular benefits such as higher mean blood pressure and less inotrope use compared to immediate cord clamping.
Umbilical cord milking may even encourage greater early oxygenation and lower MAP requirement compared to delayed cord clamping. However, multiple recent studies have identified an association with higher incidence of severe intraventricular hemorrhage compared to delayed cord clamping, primarily in the extremely low birth weight (ELBW) group. , , This is likely caused by a rapid surge in cerebral blood flow (CBF), consistent with the effects seen in premature lambs of a large rise in mean carotid artery pressure and blood flow, without any increase in pulmonary blood flow. An RCT reported a greater need for respiratory and hemodynamic support in preterm infants exposed to umbilical cord milking compared to delayed cord clamping—including ventilation, surfactant, inotropic agents, and blood transfusion—though there was discrepancy in gestational ages between the two groups.
For the aforementioned reasons, delayed cord clamping generally remains the preferred cord management approach in premature infants. The 2019 Cochrane Review was largely inconclusive about many of the physiological effects and advantages of the different cord management strategies. Cord clamping to optimize the pulmonary and hemodynamic transition at birth remains an active area of research.
After inflation of the lungs and the commencement of spontaneous negative pressure breathing, there are further cardiorespiratory interactions with venous blood return to the heart being enhanced by the negative pressure generated during normal inspiration. This can be seen on blood flow traces showing a respiratory pattern, more so in adults. If an infant requires positive pressure support, either by continuous positive airway pressure (CPAP) or mechanical ventilation, some degree of impact on the cardiovascular system is inevitable. Mechanical ventilation raises intrathoracic pressure and reduces venous return and preload to the right heart, thus also impairing right ventricular performance, especially if the distending pressure is excessive, relative to the compliance of the lungs. Mechanical ventilation also significantly affects PVR and right ventricular afterload. The left ventricle in contrast receives venous return from within the thorax so is less affected by changes in intrathoracic pressure. Cardiac output from the left ventricle is dependent on blood flow from the right ventricle, so changes in RV output will affect LV output too. Increased pressure in the right ventricle can cause a conformational change in the heart with displacement of the interventricular septum which decreases LV preload and compliance. Although LV contractility is not affected by positive pressure ventilation, the LV output can be significantly affected by changes in the LV myocardial wall tension (difference between LV systolic pressure and mean intrathoracic pressure). , There is also a potentially positive effect of mechanical ventilation and raised intrathoracic pressure on the LV with lower LV afterload due to a decreased transmyocardial pressure gradient in the setting of higher intrathoracic pressure ( Fig. 9.1 ).
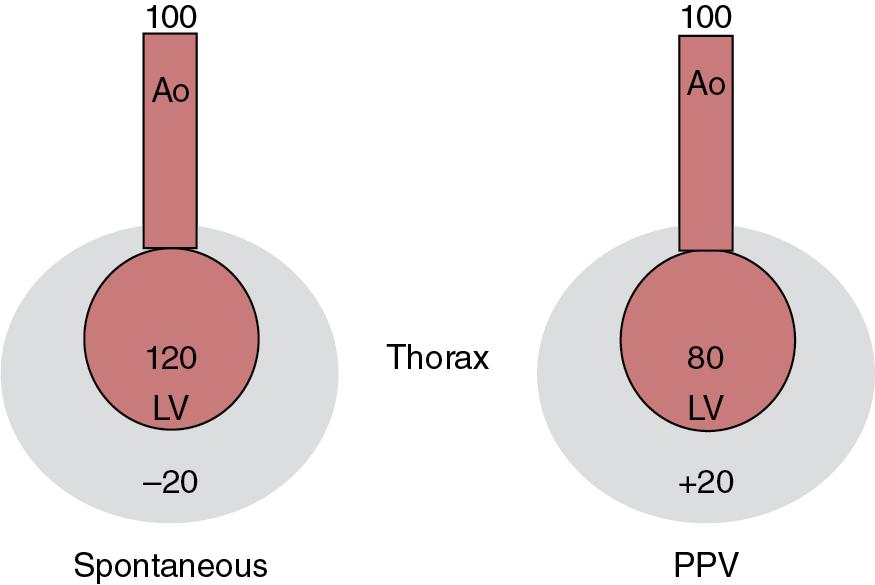
After birth, the neonate is exposed to higher oxygen tension compared to fetal life. Routine use of an oxygen supplement can deleteriously affect the cardiovascular transition. Indeed, animal models have shown a reduction in response to inhaled nitric oxide in sheep with PPHN exposed to high fractional inspiratory oxygen. Each of the elements of cardiovascular function can be affected by the respiratory system—the preload via changes in intrathoracic pressure and pulmonary blood flow, the contractility by direct impingement on ventricles within a confined space, and the afterload by changes in the PVR (both overinflation and underinflation increase PVR) that also translates to inadequate filling of the LA in the setting of reduced pulmonary blood flow. ,
During normal phasic spontaneous breathing with negative intrathoracic pressure, the passive systemic venous return is easily able to fill the right atrium that is at low pressure with the additional augmenting effect of inspiration. The end-diastolic volumes of both ventricles change in different directions—enhanced venous return into the RV from outside the thorax increases RV filling, which makes the LV stiffer and harder to fill. When positive pressure is applied, the opposite happens with impaired RV filling and easier LV filling. The end result of these changes is fluctuations in the systemic arterial pressure. In a ventilated adult, ventilator-induced changes in preload can result in a variable stroke volume and subsequently variation in the pulse pressure. The rise in arterial pressure during a positive pressure breath is counterintuitive as the expectation would be reduction in venous return and subsequently the pulse pressure should decrease. Factors that may account for this include an increase in pulmonary venous return from squeezing of capillaries, decreased LV afterload, mechanical assist of LV contraction by compression, and adrenergic stimulation with increased inotropy. The degree of pulse pressure variation can be used to predict responsiveness to a volume bolus during supportive hemodynamic management. Interestingly, the effect of mechanical ventilation on the right ventricle is opposite—impaired venous return and cardiac output, which in turn reduces the venous return to the LA that will result in a fall in LV output as well. Pulse pressure variation is also seen in neonates in association with cardiopulmonary interactions but is probably not as variable and thus not likely to be as predictive of fluid responsiveness. This may be due to the greater compliance of the newborn chest wall, compared to the adult, which may decrease the transmission of positive pressure to the pleural space and mediastinum.
Preload is an important driver of adequate cardiac contractility, and subsequently cardiac output and the newborn heart are particularly sensitive to changes in preload. Worsening respiratory disease can impact on both right and left sides of the heart ( Table 9.2 ). Higher MAP impairs systemic venous return to the right atrium, necessitating higher central venous pressure (CVP) to counteract the increased intrathoracic pressure, particularly in the setting of positive pressure ventilation. If the reduced systemic venous return is not balanced by increased CVP, the preload to the right ventricle is reduced and thus right ventricular output (RVO) will fall. A reduction in RVO results in reduced pulmonary blood flow which, apart from the effect on oxygenation, will also impact upon the preload and filling to the LA and subsequently the systemic cardiac output. The reduction in pulmonary blood flow can be exacerbated by the state of recruitment of the lungs. Underrecruited lungs will result in collapse in the supporting tissues around blood vessels in the lung, thus increasing PVR. If the lungs are overinflated, the increased pressure from the air-filled structures in the lung will cause compression of pulmonary vasculature and also result in impaired pulmonary blood flow and reduced return to the LA ( Fig. 9.2 ). If there is increased pulmonary vasoconstriction such as in PPHN, this will also impair blood flow through the lungs. An index of severity of raised PVR can be obtained by assessing the pulmonary venous return, in which case the pulmonary venous velocity is reduced. Direct impingement of the heart, either by excessive MAP with overdistended lungs or by dilation of either ventricle, can cause septal bowing and reduction of ventricular cavity, typically of the LV by the RV with PPHN.
| Component | Respiratory alteration | Resultant effect |
|---|---|---|
| Preload | High mean airway pressure | ↓ RV preload |
| High pulmonary vascular resistance | ↓ LV preload | |
| Contractility | High pulmonary vascular resistance | ↓ RV contractility |
| Acidosis secondary to permissive hypercapnia | ↓ Contractility* | |
| Afterload | High pulmonary vascular resistance | ↑ RV afterload |
| Positive intrathoracic pressure | ↓ LV afterload |
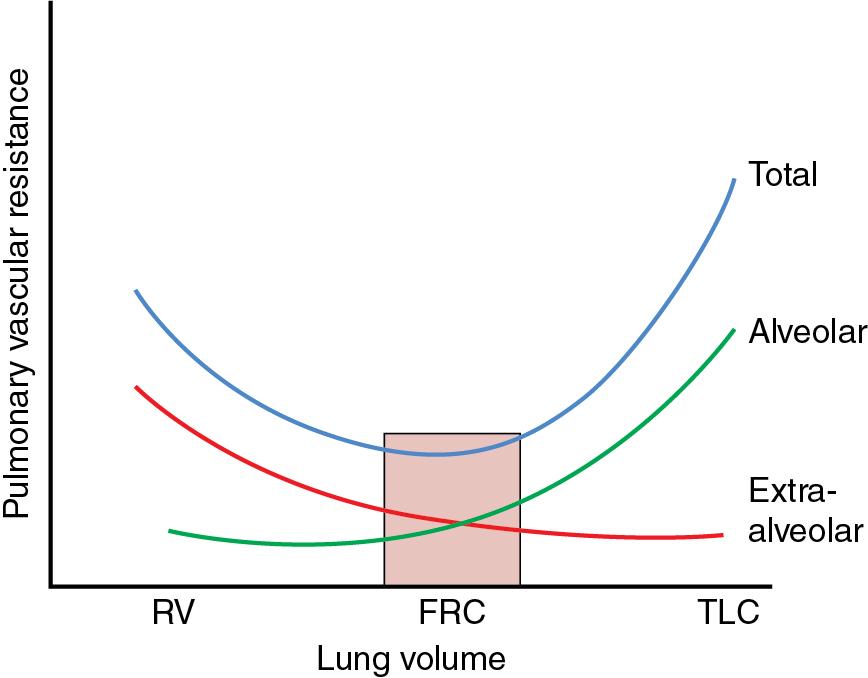
With the positive airway pressures, the negative influence on cardiac output is mediated primarily through reduced systemic venous return, while at higher MAPs, direct effects on PVR and myocardial function become important. In the sickest infants, all of these factors are likely to be important. MAP is often very high, hypoxia and acidosis are common, and pulmonary artery pressure is high as suggested by the commonly observed low ductal blood flow velocity. Both ventricles also show the ability to substantially increase output when the preload is increased by a ductal or atrial shunt, confirming a degree of myocardial reserve. The effect of positive pressure ventilation in reducing systemic venous return and cardiac output may be as important in preterm infants as it has been recognized to be in adults. There are few studies on the effect of ventilation on cardiac output in the preterm infant. Hausdorf and Hellwege demonstrated a 25% to 30% reduction in ventricular stroke and cardiac output, with no effect on blood pressure, by increasing positive end-expiratory pressure (PEEP) from 0 to 8 cm of water (cm H 2 O) in a group of preterm infants. The clinical importance of this is that an aggressive effort to improve arterial oxygenation may reduce perfusion and thus compromise tissue oxygen delivery. Trang suggested that this occurs at PEEP levels above 6 cm H 2 O in preterm infants. However, the absolute level of PEEP or MAP is not as important as the appropriateness of those pressures for the compliance of the lungs; the more compliant the lungs are, the greater the transmission of distending airway pressure to the mediastinum and thus the greater the impairment of cardiac output.
Interventions to counteract the deleterious effects of respiratory diseases and ventilator support on the preload primarily involve judicious use of ventilation settings to avoid excessive MAP and addressing the underlying pathophysiology of the lung disease, for example, reducing PVR. However, there is some evidence from adults and one study in the preterm newborn to suggest that volume expansion can correct some of the preload deficit which results from positive pressure ventilation and improve cardiac output in the short term.
Mechanical ventilation does not appear to directly affect myocardial contractility. However, through a decrease in LV afterload, it may enhance LV contractility. On the other hand, as discussed earlier, inappropriately high or low lung volume can increase PVR and therefore through an increase in RV afterload can impair right ventricular contractility. In addition, ventilator strategies aimed at permissive hypercapnia with resulting acidosis can decrease myocardial contractility at least in adult (see “Effect of Acidosis and Permissive Hypercapnia”). In the term infant, poor cardiac output is most often the result of an asphyxial insult or sepsis. Poor cardiac output in the preterm infant has been seen primarily as a consequence of an immature myocardium, especially in the first few postnatal days of the very premature infant. LV ejection fraction, admittedly a broad measure of myocardial function in the preterm infant, was not significantly associated with either ventricular output. The predominantly left to right pattern of ductal shunting will reduce RV output and atrial shunting will reduce left ventricular output (LVO) by shunting blood from the systemic back to the pulmonary circulations. This effect is apparent even in the first postnatal days.
Afterload also potentially has a large effect on cardiac output in the transitioning newborn infant. The afterload that the right ventricle is exposed to is determined primarily by what is happening in the lungs—both in terms of aeration and in terms of the pulmonary vascular pressure. Optimization of lung recruitment and addressing raised PVR are both important strategies to improve right ventricular function. Judicious management of the shunting through the PDA may also be important with strategies to allow the PDA to remain open and promote a right to left shunt sometimes reducing afterload on the RV and allowing improved forward flow out of the ventricle. Although, as discussed earlier, positive pressure ventilation can enhance LV contractility by reducing the LV afterload (wall stress), systemic vascular resistance plays a more important role in changes in LV afterload than mechanical ventilation.
The newborn myocardium and cardiac function are more vulnerable to both preload and afterload changes than older infants and adults and this is even more so in preterm infants. They have a simpler structure with fewer mitochondria and differences in the myofibrils themselves result in less reserve and ability to compensate for changes in blood volume and peripheral resistance. ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here