Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Pulmonary oedema occurs when increases in pulmonary capillary pressure or the permeability of the alveolar/capillary membrane cause fluid to accumulate in the interstitial space and alveoli.
Pulmonary embolism, with either thrombus or air, partially occludes the pulmonary circulation, causing an increase in alveolar dead space and pulmonary arterial hypertension.
Pulmonary hypertension most commonly results from long-term hypoxia or elevated left atrial pressure and involves reduced nitric oxide production and remodelling of the pulmonary blood vessels.
Pulmonary oedema is defined as an increase in pulmonary extravascular water, which occurs when transudation or exudation exceeds the capacity of the lymphatic drainage. In its more severe forms, there is free fluid in the alveoli.
The pulmonary capillary endothelial cells abut against one another at fairly loose junctions which are of the order of 5 nm wide. These junctions permit the free passage of quite large molecules, including albumin. On their luminal surface endothelial cells are lined by endothelial glycocalyx (EG), which is a complex layer of macromolecules bound to the cell surface that acts as a passive barrier to large molecules and water, controlling permeability of the endothelium (see later). , The endothelial cell and EG barrier allows some macromolecules to pass through, and pulmonary lymph contains albumin at about half the concentration in plasma. Alveolar epithelial cells are connected by tight junctions at their alveolar surface, with a gap of only about 1 nm. Under normal circumstances the tightness of these junctions prevents the escape of large molecules, such as albumin, from the interstitial fluid into the alveoli. However, the proteins that make up the tight junction are not simply passive structural units, and can, for example, in response to nitric oxide, be modified and allow an increase in permeability across the tight junction.
The lung has a well-developed lymphatic system draining the interstitial space through a network of channels around the bronchi and pulmonary vessels towards the hilum. Lymphatic vessels are seen in the juxtaseptal alveolar region (see later discussion) and are commonly found in association with bronchioles. Down to airway generation 11 (see Table 1.1 ), the lymphatics lie in a potential space around the air passages and vessels, separating them from the lung parenchyma. In the hilum of the lung, the lymphatic drainage passes through several groups of tracheobronchial lymph glands, where they receive tributaries from the superficial subpleural plexus. Most of the lymph from the left lung usually enters the thoracic duct, whereas the right side drains into the right lymphatic duct.
| Secondary | |||
|---|---|---|---|
| Primary | respiratory | cardiac | other |
|
|
|
|
The normal lymphatic drainage from human lungs is astonishingly small—only about 10 mL per hour. However, lymphatic flow can increase up to 10 times this value when transudation into the interstitial space is increased. This presumably occurs when pulmonary oedema is threatened, but it cannot be conveniently measured in humans.
For intravascular fluid to enter the alveoli it must traverse three barriers. First, it must move from the microcirculation into the interstitial space (across the EG and endothelium), second through the interstitial space and finally from the interstitial space into the alveoli (across the epithelium; Fig. 29.1 ).
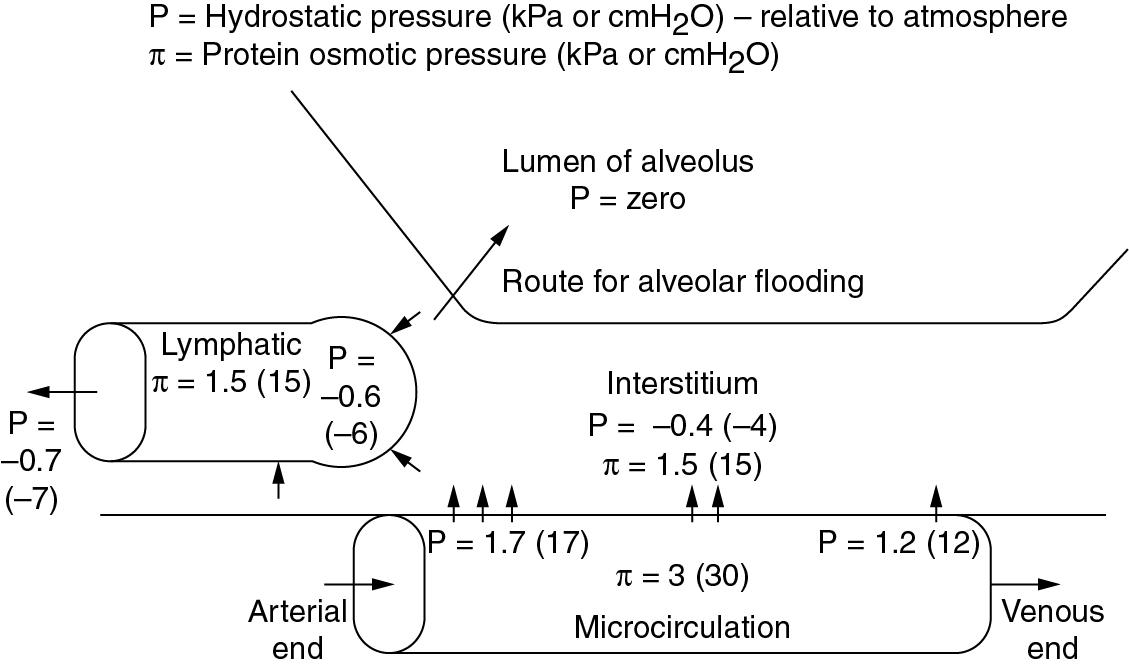
This is promoted by the hydrostatic pressure difference between capillary and interstitial space but counteracted by the osmotic pressure of the plasma proteins. The balance of pressures is normally sufficient to prevent any appreciable transudation, but it may be upset in a wide variety of pathological circumstances.
It is customary to display the relationship between fluid flow and the balance of pressures in the form of the Starling equation. For the endothelial barrier this is as follows:
| |
is the flow rate of transudated fluid which, in equilibrium, will be equal to the lymphatic drainage. |
| K | is the hydraulic conductance (i.e., flow rate of fluid per unit pressure gradient across the endothelium). |
| P C | is the hydrostatic pressure in the pulmonary capillary. |
| P IS | is the hydrostatic pressure in the interstitial space. |
| Σ | is the reflection coefficient, in this case applying to albumin. It is an expression of the permeability of the endothelium to the solute (albumin). A value of unity indicates total reflection corresponding to zero concentration of the solute in the interstitial fluid. A value of zero indicates free passage of the solute across the membrane and, with equal concentrations on both sides of the membrane, the solute could exert no osmotic pressure across the membrane. This normally applies to the crystalloids in plasma. |
| Π C | is the osmotic pressure the solute exerts within the pulmonary capillary. |
| Π IS | is the osmotic pressure the solute exerts in the interstitial space. |
Under normal circumstances in humans, the pulmonary lymph flow ![]() is about 10 mL per hour, with a protein content about half that of plasma. The pulmonary microvascular pressure ( P C ) is in the range of 0 to 2 kPa (0–15 mmHg) relative to atmosphere, depending on the vertical height in the lung field. Furthermore, there is a progressive decrease in capillary pressure from its arterial to its venous end because approximately one-half the pulmonary vascular resistance is across the capillary bed (see Figs 6.2 and 29.1 ). In this context, it is meaningless to think of a single value for the mean pulmonary capillary pressure.
is about 10 mL per hour, with a protein content about half that of plasma. The pulmonary microvascular pressure ( P C ) is in the range of 0 to 2 kPa (0–15 mmHg) relative to atmosphere, depending on the vertical height in the lung field. Furthermore, there is a progressive decrease in capillary pressure from its arterial to its venous end because approximately one-half the pulmonary vascular resistance is across the capillary bed (see Figs 6.2 and 29.1 ). In this context, it is meaningless to think of a single value for the mean pulmonary capillary pressure.
The hydrostatic pressure in the interstitial space ( P IS ) of the lung is not easy to measure, but from animal studies was measured as approximately −0.40 to −1.25 kPa (−4 to −12.5 cmH 2 O). In the excised lung there was no vertical gradient in interstitial pressures such as might have been expected from the effect of gravity, but this was observed when measurements were made with the chest and pleura intact.
The reflection coefficient for albumin (Σ) in the healthy lung is about 0.5. The overall osmotic pressure gradient between blood and interstitial fluid is about 1.5 kPa (11.5 mmHg). Thus there is a fine balance between forces favouring and opposing transudation. There is a considerable safety margin in the upper part of the lung, where the capillary hydrostatic pressure is lowest. However, in the dependent part of the lung, where the hydrostatic pressure is highest, the safety margin is slender.
Like many physiological principles that are several decades old the Starling equation model for capillary fluid movements is an oversimplification. In particular, the EG plays a vital, but incompletely understood, role. , For example, in lung tissue the hydraulic conductance for the endothelium–EG complex is probably not constant, as assumed in the Starling equation, and may vary in different lung regions, with different inflation pressures or at different vascular pressures. Damage to the EG structure by a variety of pathological processes will then result in greater permeability to water and other molecules, leading to oedema.
The interstitial space does not simply act as a passive conduit for fluid transfer to the lymphatics. Proteoglycan and hyaluron molecules are present in the pulmonary interstitial space of animals, and they function like a gel to absorb water to minimize increase in interstitial pressure and prevent hydration of other extracellular structures such as collagen. Regional differences in the properties of these molecules are believed to be responsible for the establishment of a pressure gradient between the septal interstitial space and the juxtaseptal region where lymphatic channels originate. This gradient may promote, and allow some control of, fluid flow from the endothelium to the lymphatics in the normal lung.
With increased fluid transfer across the endothelium, the interstitial space can accommodate large volumes of water with only small increases in pressure, and the interstitial compliance is high. About 500 mL can be accommodated in the interstitial space and lymphatics of the human lungs with a rise of pressure of only about 0.2 kPa (2 cmH 2 O). Eventually, the capacity of the molecules to absorb water is exceeded, and the proteoglycan structure breaks down, possibly leading to disturbances of nearby collagen molecules and therefore basement membrane function, producing alveolar oedema.
The permeability of this barrier to gases is considered in Chapter 8 . It is freely permeable to gases, water and hydrophobic substances, but virtually impermeable to albumin. Fluid is actively cleared from the alveoli in normal human lungs. For methodological reasons, most studies of this system have involved type II alveolar epithelial cells, but the same processes are believed to occur in type I cells and in club cells in the distal airways. On the alveolar side of the cells, the cell membrane contains epithelial sodium channels and cystic fibrosis transmembrane regulator channels (page 334), which actively pump sodium and chloride ions, respectively, into the cell. On the interstitial border of the cells, chloride moves passively out of the cell, and the Na + /K + -ATPase channel actively removes sodium from the cell. Water from the alveolus follows these ion transfers down an osmotic gradient into the interstitial space. Aquaporins are found in human alveolar epithelial cells, suggesting that transcellular water movement may be facilitated by these water channel proteins, but their role in lungs remains unclear, and paracellular water movement probably is more important.
A small amount of active clearance of fluid from the alveoli occurs under normal circumstances, but these systems become vital when pulmonary oedema threatens. Active removal of alveolar fluid by alveolar epithelial cells increases within 1 hour of the onset of oedema. Stimulation of β 2 -adrenoceptors by catecholamines increases the affinity of existing Na + /K + -ATPase channels for sodium and causes new channels to be incorporated into the cell membrane from intracellular endosomal stores. After a few hours, a variety of hormones (e.g., thyroxine, aldosterone, glucocorticoids) and cytokines (e.g., tumour necrosis factor) induce the transcription of new Na + /K + -ATPase channels and increase fluid clearance. These mechanisms are important both for minimizing the severity of pulmonary oedema and clearing oedema fluid once the precipitating cause has resolved.
There is presumably a prodromal stage in which pulmonary lymphatic drainage is increased, but there is no increase in extravascular water. This may progress to the following stages.
In its mildest form there is an increase in interstitial fluid, but without passage of oedema fluid into the alveoli. With the light microscope this is first detected as cuffs of distended lymphatics, typically ‘8’-shaped around the adjacent branches of the bronchi and pulmonary artery ( Fig. 29.2 ). There is fluid accumulation in the alveolar septa. but this is confined to the ‘service’ side of the pulmonary capillary which contains the stroma, leaving the geometry of the ‘active’ side unchanged (see page 8 and Fig. 1.8 ). Thus gas exchange is better preserved than might be expected from the overall increase in lung water.
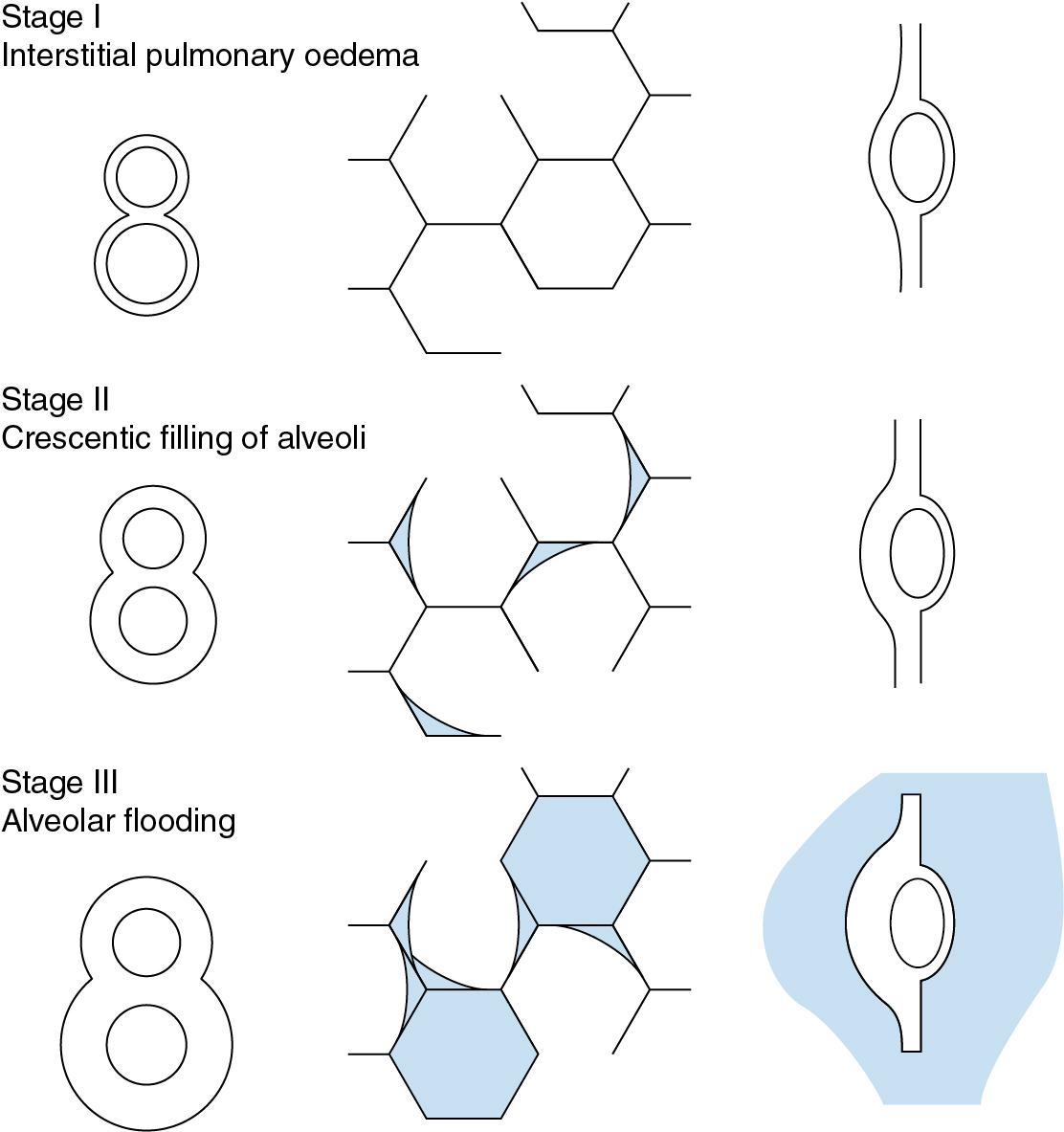
Physical signs are generally minimal in stage I, except perhaps for mild dyspnoea, particularly with exercise. The alveolar/arterial P o 2 gradient is normal or only slightly increased.
With further increase in extravascular lung water, interstitial oedema of the alveolar septa is increased, and fluid begins to pass into some alveolar lumina. It first appears as crescents in the angles between adjacent septa, at least in lungs which have been fixed in inflation ( Fig. 29.2 ). The centre of the alveoli and most of the alveolar walls remain clear, and gas exchange is not grossly abnormal, but dyspnoea at rest is likely, and the characteristic butterfly shadow may be visible on the chest radiograph.
In the third stage, there is quantal alveolar flooding. Some alveoli are totally flooded, whereas others, frequently adjacent, have only the crescentic filling or else no fluid at all in their lumina. It seems that fluid accumulates up to a point at which a critical radius of curvature results in surface tension, sharply increasing the transudation pressure gradient. This produces flooding on an all-or-none basis for each individual alveolus. Because of the effect of gravity on pulmonary vascular pressures (page 77), alveolar flooding tends to occur in the dependent parts of the lungs. Rales can be heard during inspiration, and the lung fields show an overall opacity superimposed on the butterfly shadow.
Clearly there can be no effective gas exchange in the capillaries of an alveolar septum which is flooded on both sides, and blood flow through these alveoli constitutes shunt. This results in an increased alveolar/arterial P o 2 gradient and hypoxaemia, which may be life-threatening. Blood flow to the oedematous lung regions is slightly reduced by hypoxic pulmonary vasoconstriction (page 80), possibly in conjunction with interstitial swelling causing capillary narrowing, but the shunt commonly remains substantial.
Hypercapnia is not generally a problem. In less severe pulmonary oedema, there is usually an increased respiratory drive, due partly to hypoxaemia and partly to stimulation of vagal nociceptors (page 48). As a result the P co 2 is usually normal or somewhat decreased.
When alveolar flooding is extreme, the air passages become blocked with froth, which moves to and fro with breathing. This effectively stops all gas exchange and is rapidly fatal unless treated.
On the basis of the Starling equation, it is possible to make a rational approach to the aetiology of pulmonary oedema. There are three groups of aetiological factors, classified according to their effect on components of the Starling equation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here