Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The pulmonary valve is the smallest valve in the body and, in keeping with its size, plays a relatively uncommon role in adult cardiology. Despite the limited involvement of the pulmonary valve in adult cardiac disorders, it is important to review the clinical situations for which pulmonary valve pathology is present. Pulmonic stenosis rarely is identified in adulthood and is a problem much more frequently identified and treated in pediatric patients. Mild to moderate pulmonary regurgitation is, of course, common and normal in adults.
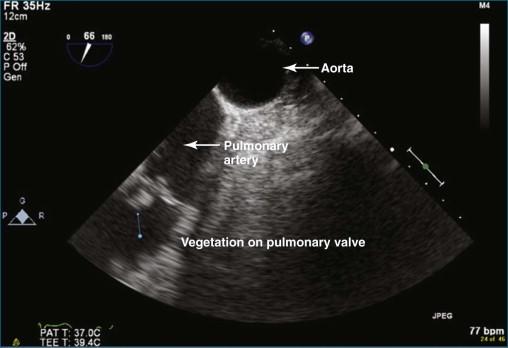
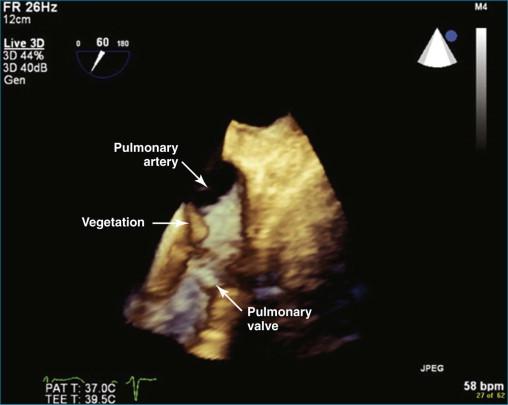
The most common cause of significant pulmonary regurgitation (defined as more than moderate pulmonary regurgitation) in adults is pulmonary hypertension from any cause, with or without dilation of the pulmonary valve ring. Like the tricuspid valve, the pulmonary valve typically becomes incompetent when exposed to high pressure. Less common causes of pulmonary valve regurgitation include infectious endocarditis, carcinoid syndrome, and tumor infiltration of the valve (e.g., papillary fibroelastoma). Pulmonary hypertension can be primary or secondary; the most common secondary cause is left-sided heart failure. Pulmonary artery dilation also can ultimately result in valve ring dilation. Causes of pulmonary artery dilation include connective tissue diseases such as Marfan syndrome, Ehler-Danlos syndrome, systemic lupus erythematosus, and scleroderma. Idiopathic dilation of the pulmonary artery is a clinically described phenomenon. This entity typically is seen in older women and can result in severe pulmonary regurgitation. In the patient population at Harborview Medical Center, infective endocarditis with destruction of the valve is a not-infrequent cause of pulmonary regurgitation. The fact that we have identified several cases of pulmonary valve endocarditis in recent years is notable because the overall incidence of pulmonary valve endocarditis is quite low—estimated to be as low as 2% at autopsy series and clearly less common than tricuspid valve endocarditis. Indeed, between 1960 and 1999, only 36 cases of pulmonic valve endocarditis were reported in structurally normal hearts. Transesophageal echocardiography (TEE) undoubtedly has improved the diagnostic yield of pulmonic valve endocarditis compared with transthoracic echocardiography (TTE), but the true incidence remains uncertain. Congenital absence of the pulmonary valve can be seen in association with tetralogy of Fallot or accompanying a congenital ventricular septal defect (VSD) and less commonly with atrial septal defect (ASD), coarctation of the aorta, or tricuspid atresia. In practice, the valve leaflets are rudimentary and dysplastic rather than completely absent. Isolated dysplastic pulmonary valve is rare and much less common than when seen in association with the previously named defects.
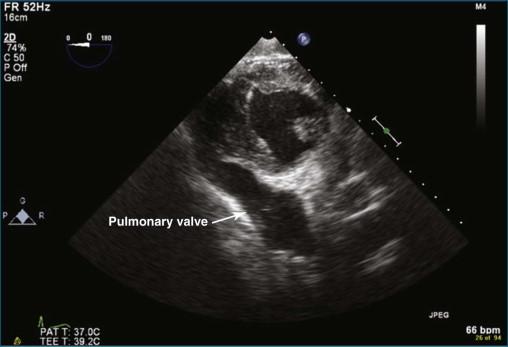
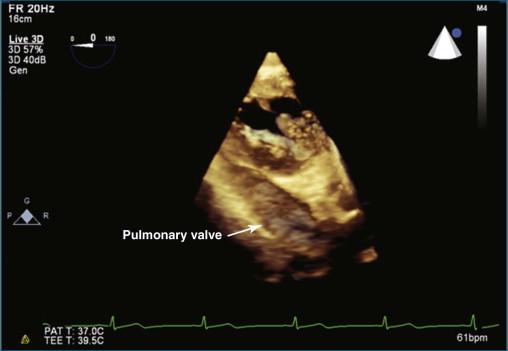
Three-dimensional echocardiography (3DE) of the pulmonary valve is very difficult to perform by TTE in adults, just as imaging the pulmonary valve by two-dimensional echocardiography (2DE) typically is challenging. The limitation typically is ultrasound penetration due to interference from lung tissue. A clear rule of thumb regarding 3DE imaging is that if the 2DE image is poor, the 3DE image is likely to be poor or worse. Figures 10-1 and 10-2 show a TTE image in the parasternal short-axis view with the pulmonic valve adjacent to the aortic valve in 2D ( Video 10-1 ) and 3D ( Video 10-2 ). The pulmonic valve is seen in the longitudinal view, and typically only one, or at best two, of the valve leaflets is identified. Not infrequently, the subcostal view can come in handy for viewing the pulmonary valve with surface imaging. Figure 10-3 and Video 10-3 show subcostal views of the pulmonary valve by 2DE and 3DE.
The first case is an example of detection of a pulmonary embolus using TTE. In this case, there is a saddle embolus. Figure 10-4 and Video 10-4 show 2D views in the parasternal long-axis and apical four-chamber view of a patient who presented with dyspnea and hypotension. The right ventricle is dilated and markedly hypocontractile. McConnell's sign is present, with severe right ventricular dysfunction that spares the apex. Figures 10-5 and 10-6 and Videos 10-5 and 10-6 show examples of an embolus at the bifurcation of the pulmonary artery, hence a “saddle embolus.” The right ventricular dilation and severe systolic dysfunction are emphasized even more with injection of saline contrast into the right ventricle (see Figure 10-6 ; Video 10-7 ).
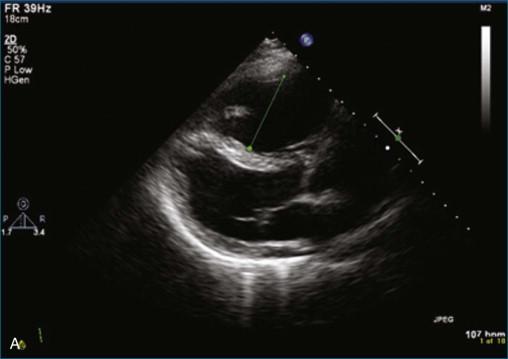
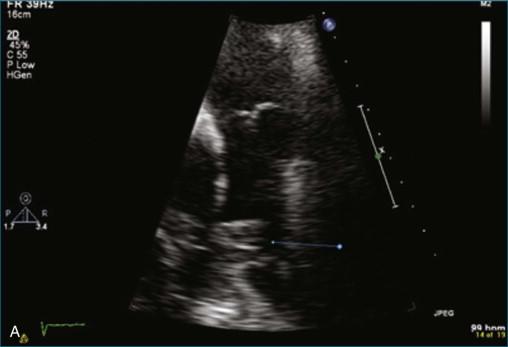
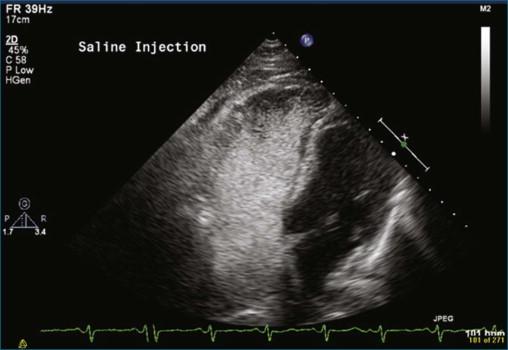
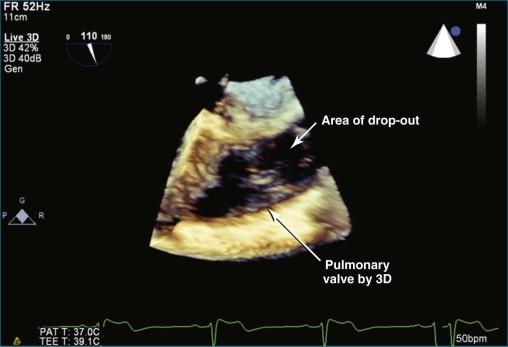
TEE imaging is required to identify more detailed pathology of the valve. However, even standard TEE views do not easily image the pulmonary valve. The most consistent TEE view of the pulmonary valve is attained while simultaneously imaging the aortic valve in the short-axis view, typically at 50 to 90 degrees. The pulmonary valve appears in the longitudinal view and can be interrogated for regurgitation ( Figures 10-7 and 10-8 ; Videos 10-8 to 10-10 ). Inconsistently, the pulmonary valve is seen almost by accident while imaging in the deep gastric view ( Figures 10-9 to 10-11 ; Videos 10-11 and 10-12 ).
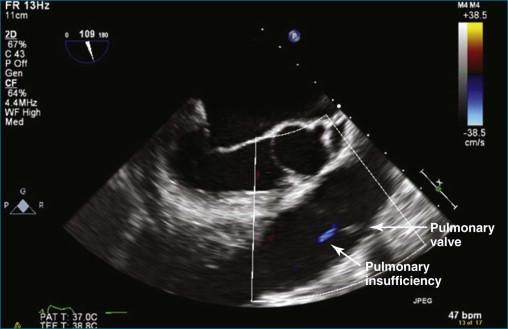
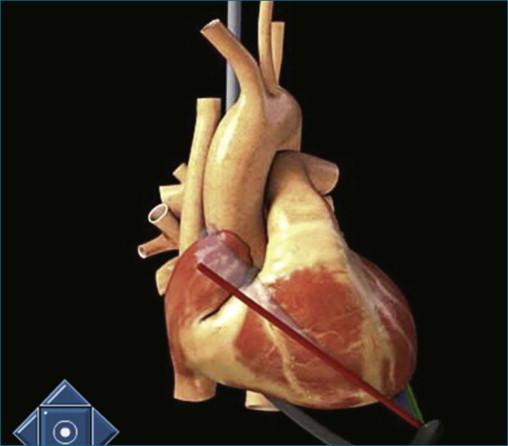
The position of the TEE probe for obtaining the deep gastric view is shown in Figure 10-9 . The steps for obtaining the deep gastric view are as follows:
The TEE probe is advanced past the gastric view (typically 40 cm from the incisors) to an approximate depth of 55 cm. Throughout this manipulation, the probe angle is at zero degrees.
The aortic valve is brought into view by the combination of flexion of the probe using clockwise manipulation of the anterior/posterior control wheel and counterclockwise manipulation of the medial/lateral wheel. This movement has the combined effect of improving contact with the stomach with anteflexion and lateral movement of the probe, aligning with the aortic valve (see Figures 10-10 and 10-11 and Videos 10-11 and 10-12).
By pulling the TEE probe back toward the incisors 3 to 5 cm, the pulmonary valve is brought into view.
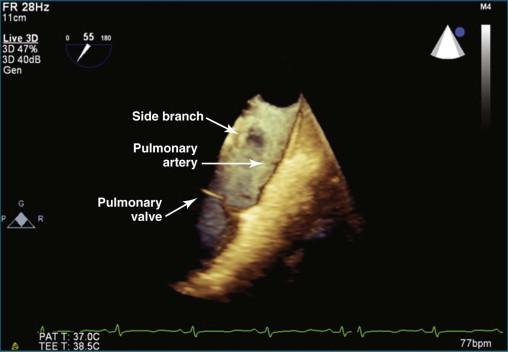
For the most optimal views of the pulmonary valve in either 2DE or 3DE, the high esophageal view is ideal when present because of the proximity of the aorta, specifically the aortic arch. At the level of the aortic arch, the aorta provides a perfect sonographic window to see through and image the right ventricular outflow tract, the pulmonary valve, and the proximal pulmonary artery and its bifurcation ( Figure 10-12 ). The only problem with this view is that it is present in only 50% to 60% of patients. Experience suggests that the view is best seen in patients with right-sided pathology, particularly dilation of the pulmonary artery. Figures 10-13 to 10-16 and Videos 10-13 to 10-25 show several examples of 2D and 3D TEE imaging of the pulmonary artery and valve through the high esophageal window. Figures 10-14 and 10-15 and Videos 10-19 and 10-20 show a case of pulmonary valve endocarditis, again viewed through this window.
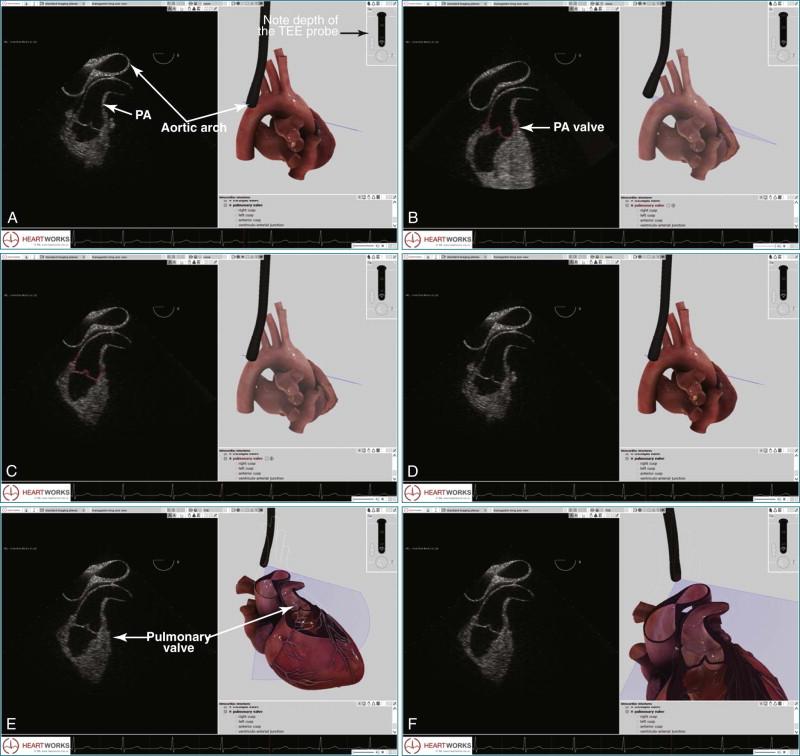
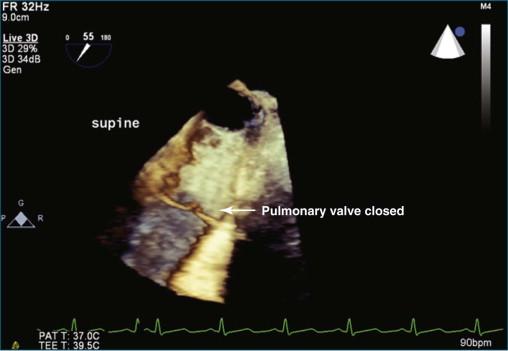
The high esophageal view (see Figure 10-12 ) of the pulmonary valve is obtained by using the following manipulation:
The initial setup requires imaging of the descending aorta with the TEE probe facing posteriorly (see Figure 10-12, B ). The aorta is viewed in a short-axis or transverse plane. The probe angle is at zero degrees.
The TEE probe is then slowly retracted, moving from a depth of 30 to 35 cm to 25 to 27 cm, where the view into the aortic arch becomes prominent and is seen to “lengthen” and have a longitudinal view as opposed to the transverse view (see Figure 10-12, C ). Depth is increased from a typical value of 4 to 6 cm for imaging the aorta to 9 to 11 cm for imaging the pulmonary artery and pulmonary valve.
With the aortic arch seen in its longest transverse length, the probe angle is moved from zero to 50 to 70 degrees, depending on the patient. The entire probe is rotated clockwise, or rightward, to move closer to the pulmonary artery. The medial/lateral control can help this medial movement as well (see Figure 10-12, D ).
For imaging in 3D, the position obtained in Figure 10-12, D , can then be used to switch to live 3D. Alternatively, the 3D zoom mode can be used. The setup for optimal pulmonary artery and valve viewing, including the pulmonary artery bifurcation, is illustrated in Video 10-15.
The operator should not be discouraged by initial failure to obtain this view because it is a high-level manipulation. Also, as mentioned, it is possible only in 50% to 60% of patients. The good news is that it tends to be more successful in patients who have pulmonary artery pathology, as demonstrated by the clinical case that follows.
The pulmonary valve is the only valve that cannot frequently be viewed en face; in fact, it would be very rare to obtain a satisfactory en face view by TTE. 3DTEE, however, occasionally can deliver an en face view of the pulmonary valve (see Video 10-9).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here