Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Pulmonary hypertension (PH) in young infants is defined as a resting mean pulmonary artery pressure (mPAP) ≥20 mm Hg.
The mPAP at birth resembles the systemic blood pressure and then drops to infrasystemic levels over the first few days. However, many infants develop pathologic structural/functional changes in the pulmonary circulation due to lung injury and develop PH.
Prematurity-related bronchopulmonary dysplasia (BPD) is a leading cause of PH in infants. With improvements in our ability to salvage ever more premature infants, the histomorphology of BPD has changed from one appearing as altered healing and scarring in the “old BPD” to that of oversimplification of the lung structure in “new BPD,” but the rates of PH continue to be high.
Imaging techniques such as echocardiography and continuous-wave Doppler with various exponents and cardiac catheterization for dye-enhanced radiography can help estimate the severity of PH.
Guidelines are available for clinical management, including for the enhancement of general care, oxygen supplementation, treatment with inhaled nitric oxide, and pharmacotherapy.
Pulmonary hypertension (PH) is a state of “abnormally high pressures” in the pulmonary artery and is defined in neonates and young children as a resting mean pulmonary artery pressure (mPAP) ≥20 mm Hg. These pressure thresholds were originally defined for a postnatal age beyond 3 months after birth but are now being increasingly extrapolated to younger infants. At the neonatal stage, pulmonary arterial pressure is often expressed relative to the systemic blood pressure. As such, during the progressive fall of pulmonary vascular resistance, although the pulmonary pressure may be isosystemic in the immediate postnatal life (first days), it should shortly become infrasystemic after birth. In many of these patients, the pathologic structural/functional changes in the pulmonary circulation are related to the developmental stage at which lung injury occurs. In addition, the timing of injury, as in prior to or after birth, and consequent exposures to fluid, air, and the microbiome are also important determinants of whether these changes are most prominent in lung structure, metabolism, and/or the gas exchange. One of the leading causes of PH in young infants is prematurity-related bronchopulmonary dysplasia (BPD) and related “chronic lung disease” with persistent needs for oxygen. , In the following sections, we describe the pathophysiology, clinical features, evaluation, and management of BPD-related PH.
Infants who develop PH in a setting of BPD do so despite the application of preventive strategies such as antenatal steroid use, surfactant administration, and noninvasive and gentle ventilation. BPD-related PH affects nearly one-third of very low birth weight infants; many survivors need long-term respiratory support and have poor neurodevelopmental outcomes and overall higher morbidity and mortality. , Most of these patients develop extensive changes in pulmonary arteries, veins, and capillaries. , , An important difficulty in understanding the pathophysiology of BPD, and consequently, in the development of effective therapeutic strategies has been the lack of a clear definition of the disease. There are some concerns that the variability of the clinical course and outcomes or the histopathology in tissue specimens obtained from those with lethal disease actually indicate the disease to be a conglomeration of multiple forms of chronic lung injury, and the clinical definitions may be an oversimplification of the complexity of injury to the developing lung. Some of these subgroups may be related different genetic backgrounds, perinatal events, hyperoxia-related cellular injury, infections, altered healing after barotraumatic injury, and multiple other hitherto unknown causes. In the context of BPD, severe PH has been defined as being two-thirds systemic or more, advocated as a threshold of concern in that population frequently evaluated below 3 months. Defining BPD is still shrouded in controversy.
BPD can be thought of as the classic “old BPD” with fibroproliferative changes of the pulmonary parenchyma in infants who require long-term ventilation and the “new BPD” seen in the most premature, extremely low birth weight infants. The old BPD was seen from late 1968 to the 1980s with (1) altered pulmonary healing after severe respiratory distress syndrome (RDS); (2) hyperoxia-induced lung injury superimposed on severe RDS; or (3) a combination of tissue injury secondary to hyperoxia, healing RDS, barotrauma, and poor bronchial drainage and stasis of secretions following endotracheal intubation. ,
The evolution of “new BPD” was rooted in the survival of more premature infants.
As the field of neonatology advanced, ventilatory management was gradually modified with acceptance of the lowest possible oxygen concentrations and gentle, noninvasive ventilation for the shortest durations that were adequate. Furthermore, advances in technology/management strategies in neonatal care, such as the use of exogenous surfactant, and in obstetric care, such as the administration of antenatal steroids, has improved survival in ever more premature infants with increasingly immature lungs.
The pathophysiology of “new BPD” in extremely preterm infants has been associated with multiple, concomitant risk factors related to pulmonary immaturity, ventilation-related lung injury, and altered tissue healing during the subsequent weeks to months. , The alveolar stage of lung development in humans extends from 36 weeks’ gestation to 18 months after birth, but most of the alveolarization occurs within 5 to 6 months after term birth. These infants with “new” BPD show two prominent changes: (1) hyperinflation with fewer, large-sized alveoli, with restricted septation and an overall reduction in alveolar surface area; and (2) paucity and abnormal development of the pulmonary microvasculature. Unlike classic BPD, the airways usually remain free of epithelial metaplasia, smooth-muscle hypertrophy, and fibrosis but show increased elastic tissue. There is focal inflammation. , , Animal models of chorioamnionitis induced by the administration of Escherichia coli or Ureaplasma show altered lung maturation, increased angiogenesis, and inflammation similar to the changes seen in “new” BPD.
The prominent vascular changes in new BPD have been a focus of intense investigation. BPD has been associated with decreased expression of vascular endothelial growth factor (VEGF) and the angiopoietin receptor Tie-2, and consequently, altered angiogenesis and endothelial cell proliferation of endothelial cells. , Decreased endothelial proliferation and development of the pulmonary vascular bed results in a smaller gas exchange surface area, which results in hypoxic vasoconstriction and impaired pulmonary blood flow. VEGF is known to promote angiogenesis and vasculogenesis and acts via nitric oxide (NO) production to promote the normal postnatal reduction in pulmonary vascular resistance. , , Over time, these vascular changes contribute to pulmonary arterial vasoreactivity and cause structural remodeling with intimal hyperplasia and muscularization in the pulmonary vasculature. Preterm infants with severe BPD show abnormal intrapulmonary arteriovenous anastomoses, which may promote the shunting of deoxygenated blood into the pulmonary veins. , , , , , , These vascular abnormalities tend to persist for longer periods in infants with severe BPD and may contribute to hypoxemia with secondary vasoconstriction and vascular remodeling. , , Areas of ventilation-perfusion mismatch, abnormal airway architecture, inflammatory responses, subclinical infections, or oxidative stress may also contribute to BPD lesions. Many of these infants also have pulmonary hypertension and venous disease.
Advanced BPD and associated PH frequently lead to right ventricular (RV) failure. These infants also remain susceptible to secondary events such as viral or bacterial pulmonary infections and associated changes in pulmonary blood flow. RV failure usually manifests with dyspnea, hypoxemia, and poor growth. Physical examination may show tachycardia and/or increased work of breathing. Some infants may have a systolic ejection murmur related to tricuspid regurgitation. RV afterload and RV dilation can cause loss of valvular coaptation.
Echocardiography is useful for screening of PH and evaluation of cardiac anatomy and function. The assessment of secondary changes is reliable at pulmonary pressures ≥40 mm Hg, although echocardiography does not allow direct estimation of pulmonary vascular resistance and may miss pulmonary venous drainage anomalies. In the presence of shunts, echocardiography may still detect but not always decipher the underlying cause of high pulmonary pressures. Current guidelines recommend use of echocardiography for screening of PH at 36 weeks’ postmenstrual age in extreme premature newborns (<29 weeks of gestational age at birth). , Table 40.1 outlines recommendations regarding the evaluation and management of BPD-PH and is largely based on guidelines established by the American Heart Association/American Thoracic Society and the Pediatric Pulmonary Hypertension Network (PPHNet). ,
| Team | A multidisciplinary team (NICU, PICU, Cardiology, Pulmonology, ENT, Nutrition, Occupational Therapy, Developmental Medicine/Neonatal Follow-up, Respiratory Therapy, Nursing) should be involved in the care of infants with BPD-PH. Infants with BPD-PH should have inpatient and outpatient follow-up with the multidisciplinary PH team and at intervals of 3–4 months (or earlier). Echocardiography, biomarkers, hemodynamic studies, and sleep studies should be done at follow-up, when indicated, and depending on the clinical progression and severity of underlying disease. |
| Screening | Echocardiography should be considered for screening of PH in a premature infant if:
|
| Echocardiography | Echocardiography done for PH screening in the context of prematurity should be as complete as possible and include at least:
|
| BNP/NT-pro-BNP | When evaluation is consistent or suspicious for PH, consider baseline and serial measurements of brain natriuretic peptide (BNP) or NT-pro-BNP as a marker of ventricular overload—values do not replace other mean of screening or diagnosis modalities (such as: echocardiography or cardiac catheterization study). |
| Other evaluations | Infants with a diagnosis/suspicion of underlying PH should have exhaustive evaluation for comorbidities that may impact an underlying lung condition, prior to the initiation of pulmonary arterial hypertension (PAH)-targeted medications. Investigations should include:
|
| Cardiac catheterization | Cardiac catheterization should be considered in selected cases, such as:
|
| Oxygen supplementation | Supplemental oxygen therapy should be administered to avoid episodic or sustained hypoxemia to achieve oxygen saturations between 92% and 95% in those with established BPD-PH. |
| iNO | Inhaled nitric oxide (iNO) should be considered in the context of an acute PH crisis and to be weaned after stabilization. The use of sildenafil may be helpful in the weaning of iNO. |
| PAH-targeted therapy | PAH-targeted therapy may be considered in those with BPD-PH after optimal management of their underlying pulmonary/cardiac condition. Pharmacologic treatment should be considered in those with evidence of high pulmonary vascular resistance and RV functional impairment, not related to left-sided heart disease (or pulmonary venous disease). The use of these medications is, for the majority, off-label and should be used with caution. Initiation or adjustment of PAH-targeted pharmacotherapy should be made based on disease severity, tolerance to effects (availability/cost/route of administration), and in conjunction with a specialist with expertise in PH. |
| Infection prevention | When eligible (according to vaccine product), ensure exhaustive and adequate protection with vaccination of the infant with BPD-PH and its entourage (respiratory syncytial virus prophylaxis, pneumococcal vaccination, influenza vaccination, SARS-CoV-2 vaccine [for the family and caregivers]). Crowds should be avoided, and caregivers should be taught appropriate infection prevention strategies (such as hands hygiene) to avoid flare-ups in the context of respiratory infections. |
| Parental/caregiver teaching | Training to recognize signs of respiratory distress or cardiac decompensation: diaphoresis, retraction, work of breathing, cyanosis, abnormal neurologic status. Caregivers should receive training regarding basic maneuvers for cardio-pulmonary resuscitation. |
| Traveling/altitude | Experts should be consulted if the family/caregivers consider traveling by plane (or moving to a higher-altitude area). The infant with BPD-PH may need a fit-to-travel assessment with, possibly, a hypoxic challenge test. |
a Largely based on guidelines by American Heart Association/American Thoracic Society and Pediatric Pulmonary Hypertension Network. BNP, Brain-type natriuretic peptide; BPD, bronchopulmonary dysplasia; ENT , Otorhinolaryngology; iNO, inhaled nitrous oxide; LV, left ventricle; NICU, neonatal intensive care unit; NT-pro-BNP , N-terminal pro-BNP; PICU, pediatric intensive care unit; NT-pro-BNP, N -terminal pro-BNP; PAH, pulmonary arterial hypertension; PH, pulmonary hypertension; PMA, postmenstrual age; RV, right ventricle.
The most reliable modality to assess pulmonary pressure and pulmonary vascular resistance is cardiac catheterization, but it is used less frequently in young infants because it is a relatively risky, invasive procedure. Catheterization can help exclude the possibility of left-sided cardiac anomalies. The procedure can also help assess the pulmonary vascular reactivity to pulmonary vasodilators. Unlike echocardiography, which can detect secondary changes seen at pulmonary pressures ≥40 mm Hg, cardiac catheterization can reliably detect mean pulmonary arterial pressures >20 mm Hg. , ,
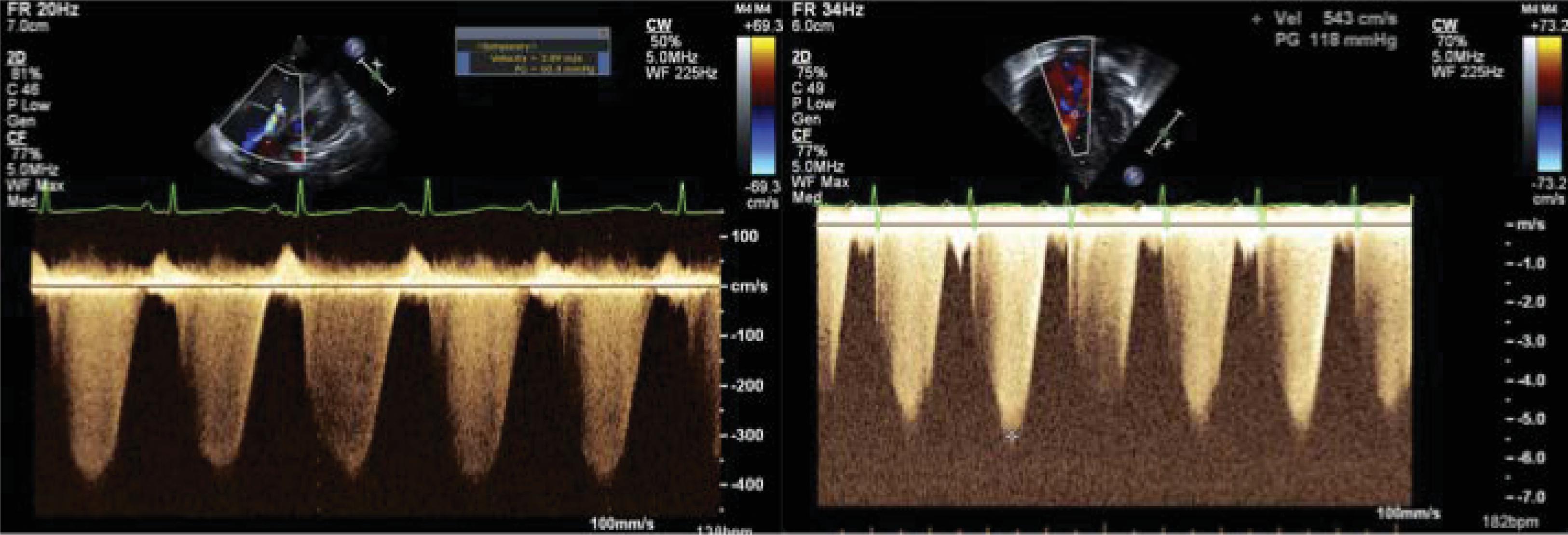
In the absence of structural cardiac anomalies, systolic RV pressure may resemble systolic pulmonary arterial pressure (sPAP). Echocardiography can help estimate RV pressures through measurement of the peak tricuspid regurgitation (TR) jet velocity. Continuous-wave Doppler of the TR jet estimates the peak gradient between the systolic RV pressure and the right atrial (RA) pressure ( Fig. 40.1 ). The modified Bernoulli equation (4 × velocity 2 ) converts the velocity to a pressure gradient. , The sPAP is estimated by adding the presumed RA pressure of 5 to 10 mm Hg. , In the presence of a large ventricular septal defect (VSD) or large patent ductus arteriosus (PDA), the RV compartment is exposed to the systemic pressures during systole. Thus by definition, the TR jet may yield to systolic pressure in the absence of abnormal pulmonary vascular resistance. Similarly, newborns with pulmonary valvular stenosis or pulmonary arterial stenosis may have increased RV pressure (and TR jet) in the absence of increased pulmonary vascular resistances. Notably, TR jet may not be seen in all newborns; a reliable TR jet envelope may be seen only in about 61% of pediatric echocardiographic scans. There may also be erroneous estimations of the RV pressures due to increased angles of insonation (beam nonparallel to the regurgitant jet) and the absence of a complete Doppler envelope.
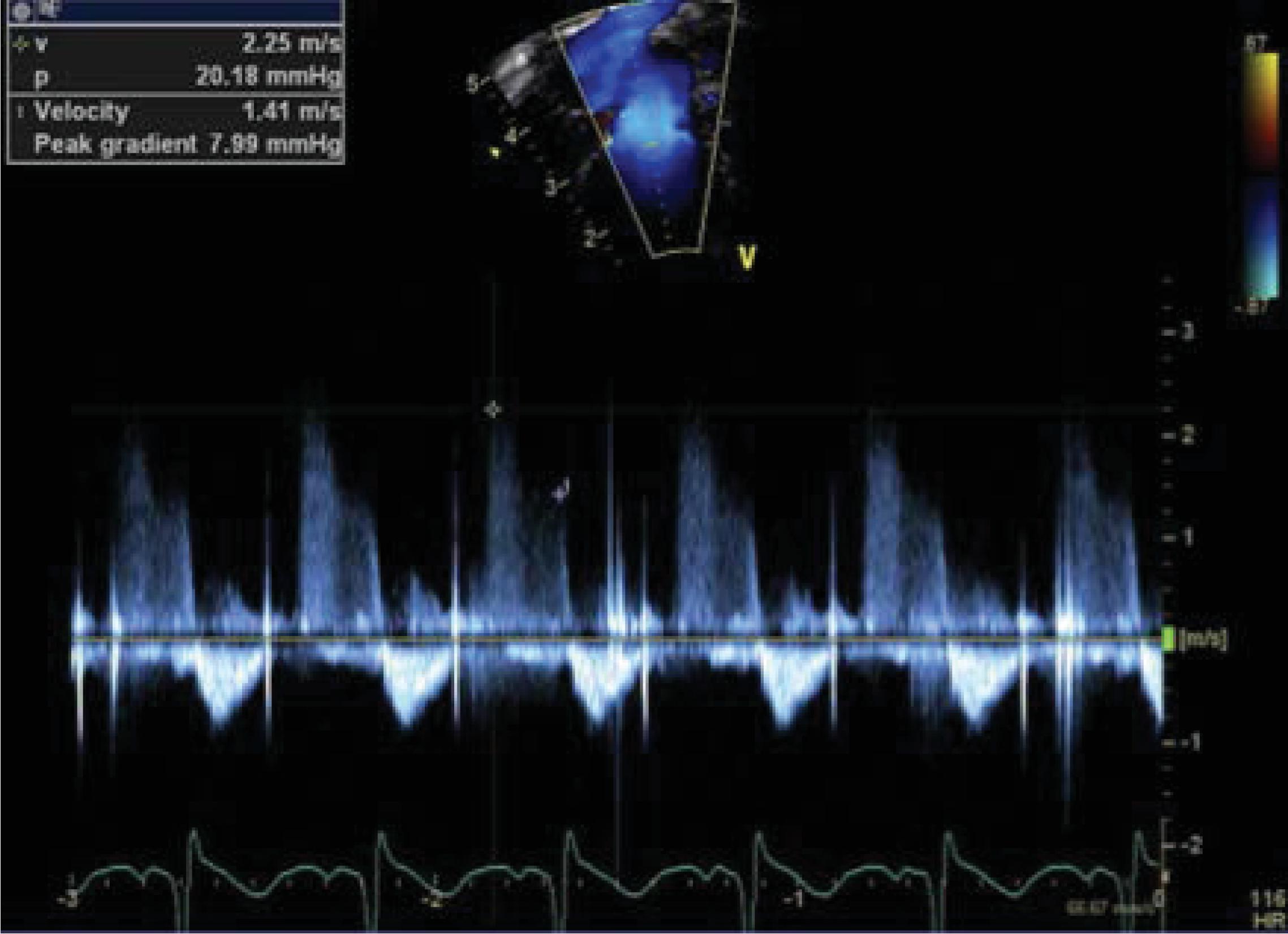
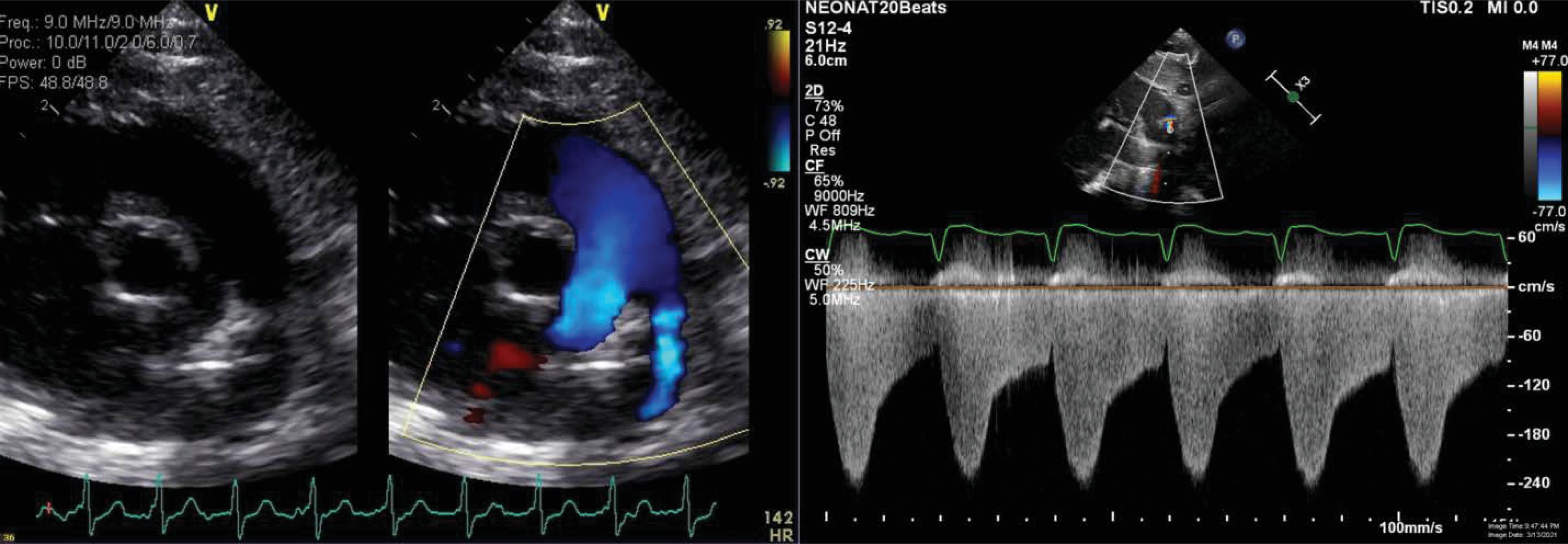
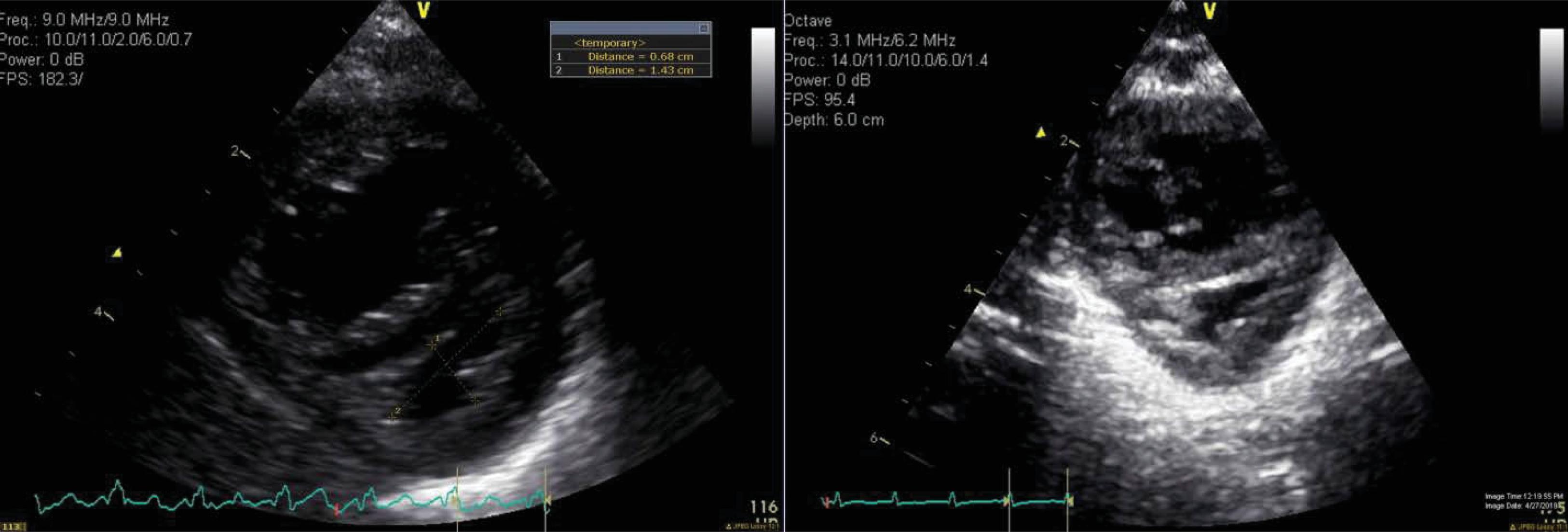
In infants with mild pulmonary valve insufficiency (PI), echocardiography can help estimate the diastolic pulmonary arterial pressure ( Fig. 40.2 ). Indeed, the continuous-wave Doppler of the PI can help estimate the gradient between the peak diastolic pressure in the main pulmonary artery and the RV. A similar approach may be used to estimate sPAP in the context of a restrictive PDA ( Fig. 40.3 ) or a restrictive VSD (in the absence of left-sided inflow or outflow tract congenital anomalies). Indeed, the pressure gradient through the PDA/VSD may inform on the gradient between the systemic and pulmonary compartment. Comparisons of the estimated pulmonary arterial pressures to the corresponding systemic arterial pressures measured using invasive or noninvasive techniques can be helpful; such estimations can allow the classification of PH into infra-, iso-, and suprasystemic pulmonary pressures. The shunting direction through the ductus arteriosus also informs about the relationship between the pulmonary and systemic compartment. One may need to put this into context, because systemic hypotension may be associated with bidirectional or right to left shunting. Similarly, the direction of blood flow assessed by color Doppler at the level of an interatrial communication informs about the end-diastolic pressures of the respective ventricles. A bidirectional or right-to-left atrial shunt may be associated with poor RV compliance in the context of pulmonary hypertension. The right and left ventricle (LV) share muscular fibers and a septum. The septal curvature is assessed in the parasternal short axis view at the peak of systole at the papillary muscle level. , The RV-LV cross-talk may be disturbed in the context of increased RV afterload, leading to septal flattening (isosystemic pulmonary pressures) or bowing into the LV cavity (suprasystemic pulmonary pressures; Fig. 40.4 ). In the context of pulmonary hypertension but infrasystemic pulmonary pressures, a round LV is found and can miss an underlying pathologic process, making it an imprecise indicator. In order to quantify septal distortion, some have used the left ventricular eccentricity index (LVEI). , The LVEI is measured at the end of systole from the parasternal short-axis view at the papillary muscle level. It is the ratio of the LV diameter parallel to the septum to the diameter perpendicular to the septum (with a round LV providing a ratio close to 1.0). Abnormal LVEI in the context of PH has been described when >1.23. ,
An acceleration time to RV ejection time ratio >0.3 measured from the pulsed-wave Doppler envelope of the RV outflow tract, at the tip of the pulmonary valve, has been associated with increased pulmonary artery pressure in infants with BPD. , , Pulmonary artery acceleration time is the time to reach the peak of stroke distance within the main pulmonary artery. It is influenced by underlying RV performance, heart rate, and cardiac output. , , With increasing RV afterload, the stroke distance profile changes toward a more triangular shape from a smooth, curved pattern. Acceleration time is normalized to the heart rate by using the RV ejection time.
Although imprecise, other indirect indicators of pulmonary hypertension should be sought during echocardiography, such as RV hypertrophy or dilation, RA enlargement, pulmonary artery enlargement, hepatic veins dilation, or retrograde flow in the inferior vena cava. A ratio of the RV/LV longitudinal measure (perpendicular to the septum) in the parasternal short axis view at the papillary muscle level can also be used to quantify degree of RV dilation. A ratio greater than 1.0 has been associated with pulmonary hypertension in the pediatric population. , , This measure is influenced by the angle of insonation and is highly operator dependent.
In the context of BPD-PH, RV function should be quantified. RV contraction follows a longitudinal displacement of the inflow toward the outflow tract, a bulging of the septum toward the cavity, and a contraction of the free wall toward the septum. The RV geometry is complex, and assessment of systolic function by 2D echocardiography is usually limited to the use of tricuspid annular plane systolic excursion (TAPSE), fractional area change (FAC), and the use of tissue Doppler imaging (TDI) velocities (or myocardial performance index derived from TDI). Indeed, both markers have been described as predictors of mortality in the BPD-PH population. TAPSE is measured by M-mode in the apical-4-chamber view with the line of interrogation passing through the apex of the RV and the lateral tricuspid valve attachment. It measures the longitudinal displacement of the tricuspid valve during systole. FAC is calculated in the apical-4-chamber view by the formula (RV diastolic area—RV systolic area)/RV diastolic area. Finally, TDI allows for the evaluation of systolic and diastolic myocardial velocities. TDI also allows for the calculation of the RV myocardial perfusion index (or Tei), a combined index of systolic and diastolic performance. The RV myocardial perfusion index has been described has abnormal in the context of pediatric and neonatal PH. , Jain et al. have published normative values for RV function in term newborns. Furthermore, normative data for TAPSE, FAC, and TDI velocities , are available in the newborn population at various gestational ages.
In infants with BPD, the presence of left-sided disease (mitral regurgitation, mitral stenosis, LV systolic or diastolic dysfunction, and LV outflow tract anomalies) and of pulmonary vein stenosis can be informative. Pulmonary vein stenosis is suspected on echocardiography/pulsed-wave Doppler of the pulmonary veins at their ostia, with monophasic Doppler flow profiles with a mean gradient >4 mm Hg. Computed tomography scans can also be helpful for evaluation of pulmonary veins in the context of BPD-PH.
Recent guidelines have also advocated for the evaluation of biomarkers such as the brain-type natriuretic peptide (BNP) or the N -terminal pro-BNP (NT-pro-BNP), which may be increased in the context of RV dysfunction. , BNP is a peptide released by cardiomyocytes secondary to stretch (during dilatation of cardiac structures), , and NT-pro-BNP is its inactive fragment. Infants with PH frequently show elevated levels of these biomarkers. Having said that, these markers may be more helpful for follow-up of disease progression, rather than to establish diagnosis. Vigilance in interpreting these markers is important, because newborns with BPD may have concomitant systemic hypertension and LV hypertrophy, which may also be associated with an increase in BNP/NT-proBNP.
Using a nationwide database in the United States, Stroustrup and Trasande reported the incidence and resource use of infants with BPD. They found that the incidence of BPD decreased by 4.3% per year for 1993 to 2006. There was an increase in the use of noninvasive ventilation, but with it came an increase in the cost and length of hospitalization. , In recent years, BPD has remained stable (or even increased) according to the National Institute of Child Health and Development (NICHD) reports. , Furthermore, advances in neonatology have led to an increase in survival at the extremes of prematurity (22–24 weeks’ gestational age) and birth weight (severe intrauterine growth restriction), possibly explaining the stability in BPD rates. Major predictors of BPD include early gestational age at birth and mechanical ventilation on day seven. Furthermore, fetal growth restriction in infants born in the range of 23 to 27 weeks’ gestation leads to increased risk of developing BPD. , Risk factors for PH in the context of BPD include extreme prematurity, oligohydramnios (associated with pulmonary hypoplasia), intrauterine growth restriction, prolonged mechanical ventilation, maternal preeclampsia and hypertension, and protracted oxygen supplementation. , , , Infants with BPD who have PH have a higher incidence of comorbidities, including retinopathy of prematurity, gastroesophageal reflux, pulmonary aspiration, airway anomalies, and dependence on technology (gastrostomy, gavage, tracheostomy, home oxygen). ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here