Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Pulmonary embolus (PE) is a common clinical problem with an annual incidence of 4 to 21 per 10,000 people per year, rising significantly to 1 in 100 patients after 80 years of age. Autopsy studies have shown that PE is the second leading cause of sudden, unexpected, nontraumatic death in outpatients, and it is not diagnosed premortem in most cases. The all-cause, 30-day mortality rate after diagnosed PE is about 8%. The mortality rate of untreated PE has been estimated to be 30%. Although the prompt and accurate diagnosis of PE is important, imaging can lead to undesirable consequences such as increased cost, ionizing radiation exposure, and potential overdiagnosis. Another potential consequence of initial computed tomography (CT) for PE is that additional CT follow-up examinations may be performed. One study that followed 300 patients initially evaluated in the emergency department for PE using CT pulmonary angiography (CTPA) found that over the next 4 years, 900 subsequent CT studies were performed in patients with a mean cumulative dose estimate of 31 mSv (range, 7–297 mSv).
Although imaging has a key role in the diagnosis of PE and in risk stratification, clinical assessment of the patient is the first and most crucial step. The symptoms and signs of acute PE include dyspnea, pleuritic chest pain, tachypnea, tachycardia, and hemoptysis. When PE is suspected, the utilization of clinical decision support improves the outcome for patients and reduces unnecessary imaging. Validated scoring systems, such as the Wells, simplified Wells, and modified Geneva scores, are recommended. Based on these scores, when PE is deemed likely, the clinician should proceed to imaging; in low likelihood patients, a D-dimer test can be performed to help exclude the diagnosis of PE, and imaging should only be obtained if the D-dimer test result is positive.
Although the majority of chest radiographs of patients with acute PE show an abnormality, the radiographic findings are nonspecific. A major role of the chest radiograph is to exclude other diagnoses that might mimic a PE, such as pneumothorax, pneumonia, or rib fractures, and to provide information that helps in interpreting the scintigram.
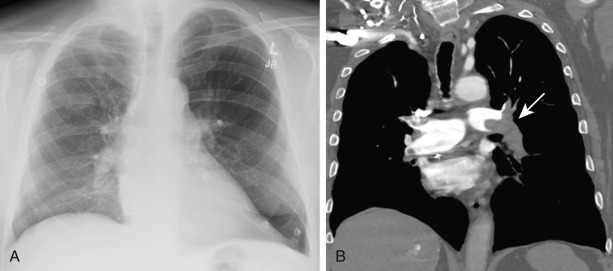
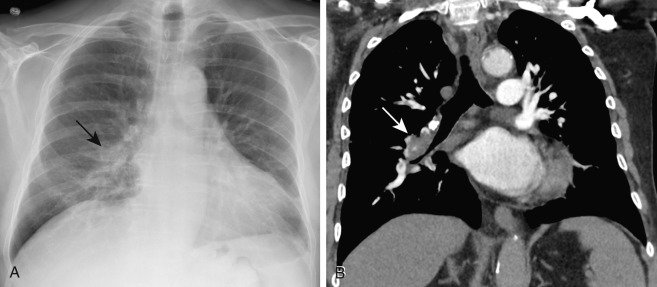
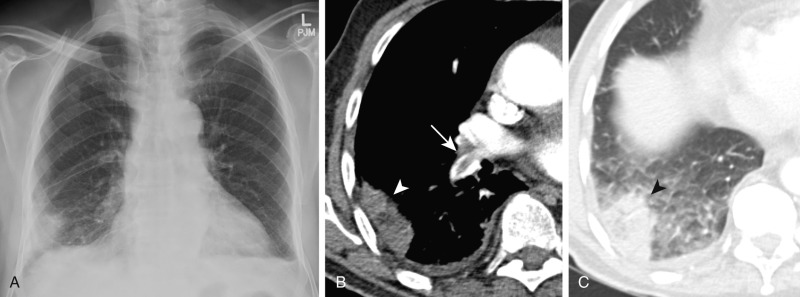
Radiographic signs of a PE without infarction include atelectasis, oligemia of the lung beyond the occluded vessel (Westermark sign) ( Fig. 11.1 ), or a large central pulmonary artery caused by central thrombus (Fleischner sign) ( Fig. 11.2 ) with abrupt tapering of the occluded pulmonary artery distally, creating the “knuckle sign.” Radiographic signs of PE with infarction include peripheral wedge-shaped consolidation abutting the pleura (i.e., Hampton hump) ( Fig. 11.3 ), atelectasis, elevated hemidiaphragm, and pleural effusion. The Prospective Investigation of Pulmonary Embolus Diagnosis (PIOPED) demonstrated that these radiographic signs do not provide sufficient information to accurately establish or exclude the diagnosis of PE.
The scintigram relies on indirect radiologic signs for the diagnosis of PE. Xenon 133 and technetium-99m ( 99m Tc) diethylenetriamine-pentaacetic acid are the usual inhaled ventilation agents, and 99m Tc macroaggregated human albumin is the perfusion agent that is injected intravenously. To make the diagnosis of PE, the perfusion scan must show two or more apex-central, wedge-shaped defects in a segmental or larger vascular distribution, together with evidence of normal ventilation in the same lung segments (ventilation/perfusion [V/Q] mismatch) ( Fig. 11.4 ). When interpreting the scintigram, it is important to evaluate a current chest radiograph.
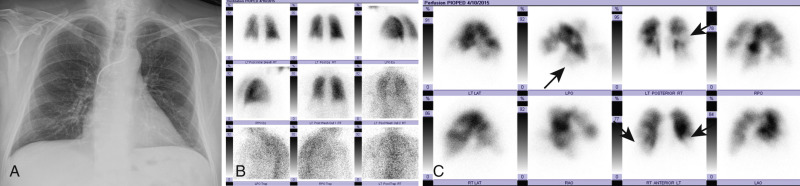
Based on the PIOPED II criteria, V/Q scans are reported according to the likelihood of a PE being present as one of the following: normal, very low probability for PE (<10%), low probability for PE (10%–19%), intermediate probability for PE (20%–79%), or high probability for PE (>80%). Diagnostic accuracy is greatest when the V/Q scan is combined with clinical probability. An in-depth discussion about scintigraphy can be found in Nuclear Medicine: The Requisites .
Lower extremity venous ultrasonography has a role to play in patients with nondiagnostic pulmonary vascular imaging and in patients who cannot or do not want to undergo a CT scan because diagnosing a deep venous thrombus (DVT) is tantamount to diagnosing a PE from the standpoint of anticoagulation. Studies have shown that ultrasonography of the legs is positive for DVT in 10% to 20% of patients with suspected PE but without leg symptoms or signs, and it is positive in approximately 50% of patients with proven embolism. However, all patients with suspected PE in whom the ultrasonography negative for DVT require pulmonary vascular imaging.
Multidetector CT (MDCT) venography of the pelvis and lower extremities is simple and accurate and when combined with CTPA provides a fast and comprehensive analysis of thromboembolic disease. It can be performed with the same contrast bolus used for the chest CT. However, because of radiation dose concerns, this method should not be used routinely in all patients. According to the Fleischner Society's guidelines, CT venography should only be considered when an expeditious complete vascular examination is desired clinically.
On noncontrast chest CT scans, an incidental PE can be identified as a hyperattenuating filling defect ( Fig. 11.5 ). For the evaluation of suspected PE, CTPA has become the modality of choice because of technical advances, its immediate and widespread availability, and its ability to identify alternative diagnoses for the patient's symptoms such as pneumonia, pleural disease (including pneumothorax, pleural effusion, and pleuritis), pericarditis, aortic dissection, and esophagitis. MDCT can acquire contrast-enhanced images of the entire chest with a reconstructed slice thickness of 1 mm in 8 seconds or less. Multidetector scanners allow improved vascular resolution because of narrow slice thickness and less motion artifact because of faster scan times. Diagnostic accuracy for PE detection is constantly increasing, with sensitivity and specificity of recently developed CT scanners reaching up to 94% to 96% and 94% to 100%, respectively.
The fundamental goals of CTPA acquisition is to provide high-contrast opacification of the pulmonary arterial circulation using the lowest possible volume of contrast material, keeping the acquisition time as short as possible to mitigate motion artifacts, and minimizing radiation dose while maintaining acceptable levels of image noise. Contrast bolus timing can be determined either by using a test bolus or by using bolus tracking with the region of interest (ROI) placed in the right ventricle or main pulmonary artery. The appropriate window width and level settings are important to identify small emboli, webs, or flaps. Excessively bright vessel contrast can obscure subtle emboli. Some suggest using PE-specific settings with a window width and level of 700 and 100 HU, respectively. Others have suggested a window width equal to the mean attenuation of the enhanced main pulmonary artery plus two standard deviations and a window level equal to half of this value.
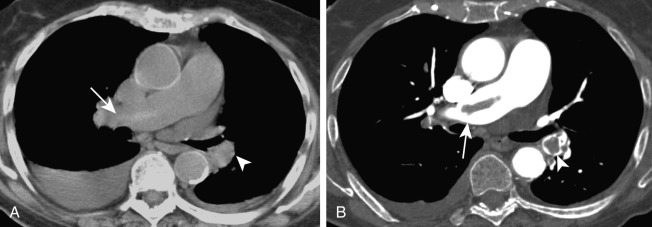
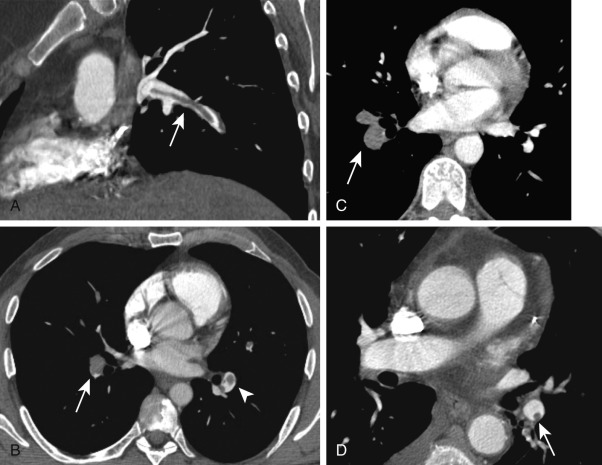
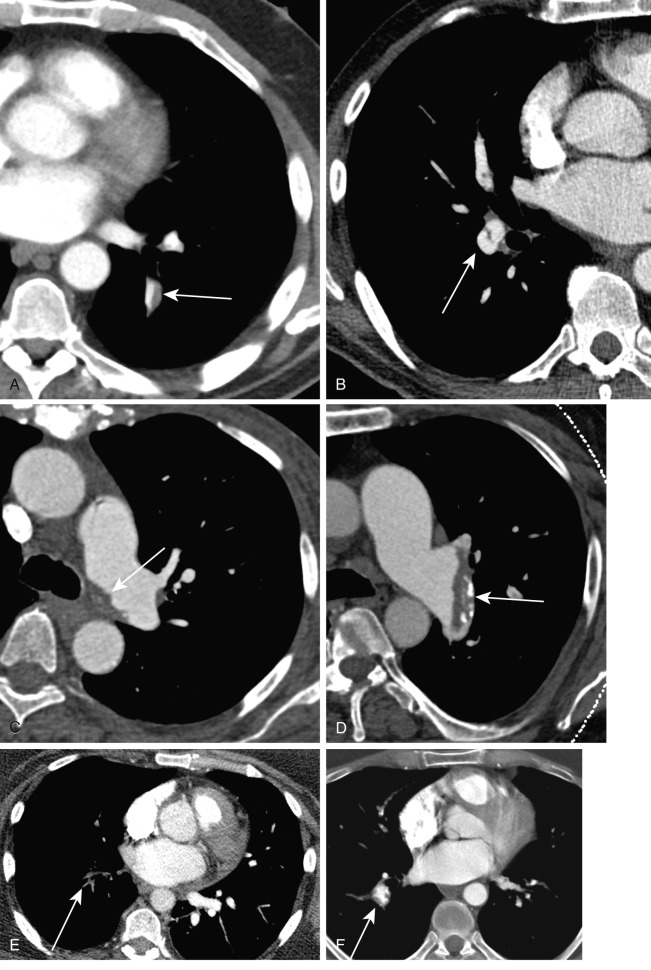
Findings of an acute PE on CTPA include complete arterial occlusion, partial filling defects surrounded by contrast, or a peripheral filling defect that makes an acute angle with the arterial wall ( Fig. 11.6 ). Pulmonary infarcts, when present, are peripheral and extend to the pleural surface ( Fig. 11.7 ). Pulmonary infarcts may appear as consolidation or ground-glass opacities and may contain bubbly lucencies. They generally have a wedge-shaped configuration but may become nodular as they resolve. The diagnostic criteria for acute PE are outlined in Table 11.1 .
| Acute Embolus | Chronic Embolus | |
|---|---|---|
| Filling defect | Complete filling defect Central filling defect surrounded by contrast
Eccentric filling defect with acute angle with pulmonary artery wall |
Complete filling defect Peripheral, crescent-shaped defect with obtuse angle with pulmonary artery wall Web or flap (linear filling defect) Contrast flowing through thickened, often constricted, artery caused by recanalization of chronic thrombus Calcification of the thrombus |
| Vessel size | Normal or dilated | Normal or smaller than adjacent patent vessels (constricted) |
| Ancillary findings | Peripheral, wedge-shaped opacities (infarcts) Perfusion defect (dual energy) Imaging markers of right heart strain |
Extensive bronchial or other systemic collateral vessels Mosaic perfusion pattern in the lungs CT findings of pulmonary hypertension |
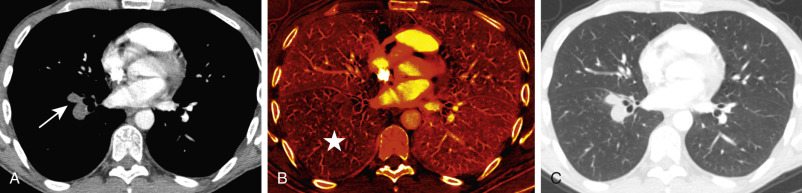
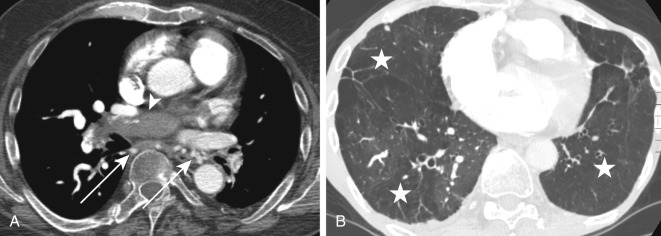
Dual-energy CT (DECT) pulmonary angiography provides several potential advantages over single-energy scans. The ability to reconstruct virtual monoenergetic images at varying energy levels allows the radiologist to retrospectively select the kV that provides the best compromise between vascular attenuation (with increased vascular attenuation at lower kV levels) and acceptable image noise. DECT may help salvage a CTPA study that would be considered nondiagnostic because of suboptimal contrast opacification on a single energy scanner. It can also allow prospective reduction in the required contrast volume. Another advantage of dual-energy CTPA is the ability to superimpose an iodine map overlay on the standard images to create a dual-energy perfusion map of the lungs. These are static images that indirectly display blood volume rather than time-resolved dynamic CT perfusion imaging. These perfusion maps display the iodine-perfused lung tissue, analogous to scintigraphy, and allow visualization of parenchymal perfusion defects distal to vessels affected by PE ( Figs. 11.8 and 11.9 ). This may enhance the detection of smaller, peripheral emboli compared with single-energy CTPA, which does not provide such perfusion maps.
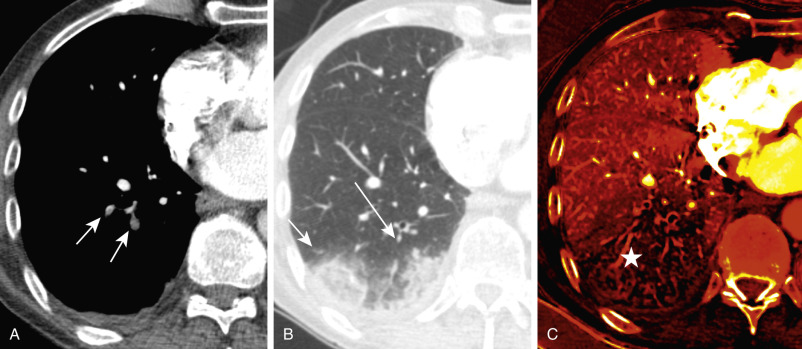
Computed tomography pulmonary angiography also has a role to play in assessing the severity of hemodynamic compromise from acute PE and to identify patients at increased risk for fatal or nonfatal adverse events, thus guiding clinical management toward more aggressive therapy. The main methods used to categorize the hemodynamic relevance and severity of PE are imaging signs of right heart strain, methods for clot burden quantification, and lung perfusion measurements. Imaging markers of right heart strain include a right ventricle–left ventricle ratio of greater than 1 ( Fig. 11.10 ), reflux of contrast into the hepatic veins, dilated main pulmonary artery, and straightening or leftward bowing of the interventricular septum ( Box 11.1 ). To calculate the right ventricle–left ventricle ratio, the ventricular chambers are measured in the axial plane at their widest points between the inner surface of the free wall and the surface of the interventricular septum, which may be at slightly different axial images. The right ventricle–left ventricle ratio has the strongest predictive value and most robust evidence base for adverse clinical outcomes in patients with acute PE. Clot burden assessments, such as the Mastora and Qanadli scores, are cumbersome and not routinely used in clinical practice. Dual-energy perfusion maps may have prognostic value because a correlation between the extent of perfusion defects on the perfusion maps and adverse clinical outcome has been shown.
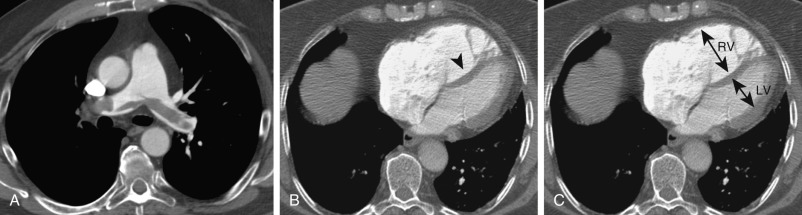
Right ventricle–left ventricular ratio greater than 1
Reflux of contrast into the hepatic veins
Dilated main pulmonary artery
Straightening or leftward bowing of the interventricular septum
Although CTPA has been shown to be highly sensitive and specific when pretest clinical decision support is used, it is surprisingly inaccurate in patients with low pretest probability, with false-positive rates as high as 42%. One study found that PEs diagnosed on CTPA were frequently overdiagnosed (26% of cases) when compared with the consensus opinion of a panel of expert chest radiologists. Discordance occurred more often when the original reported PE was solitary (46% of reported solitary PEs were considered negative on retrospective review) and located in a segmental or subsegmental pulmonary artery (27% of segmental and 59% of subsegmental PE diagnoses were considered negative on retrospective review).
There are pitfalls in the interpretation of CTPA that can result in the misdiagnosis of PE ( Fig. 11.11 ). These include patient-related factors such as respiratory motion, image noise, and flow-related artifact. Motion artifacts are best appreciated on lung window settings and can create the “seagull” sign or can be identified by a rapid change in the position of vessels on contiguous images. Image noise increases as patient size increases and can make the evaluation of segmental and subsegmental vessels difficult, resulting in indeterminate readings or misdiagnosis of PE. Flow-related artifacts, caused by mixing of unopacified blood and contrast material, can result in a transient interruption of contrast enhancement. This transient interruption of contrast enhancement is likely related to deep inspiration, resulting in a wave of unenhanced blood from the inferior vena cava entering the right atrium, right ventricle, and pulmonary arteries just before image acquisition. Flow-related artifacts can usually be identified by ill-defined margins or attenuation level above 78 HU. Another artifact that can limit the interpretation of CTPA is a streak or beam-hardening artifact from dense contrast within the superior vena cava, which can overlie the right pulmonary artery and medial upper lobe pulmonary arteries. In areas of consolidation or atelectasis, an unenhanced or poorly opacified vessel may result because of focally increased vascular resistance with slow flow. This can also result in a misdiagnosis of PE. Recognition of this phenomenon is important because the unenhanced vessel may be normal, containing no PE, or the poor contrast enhancement may obscure a true embolus. A false diagnosis of PE can also be made if a poorly opacified pulmonary vein or a mucus-plugged bronchus is misidentified as a pulmonary artery.
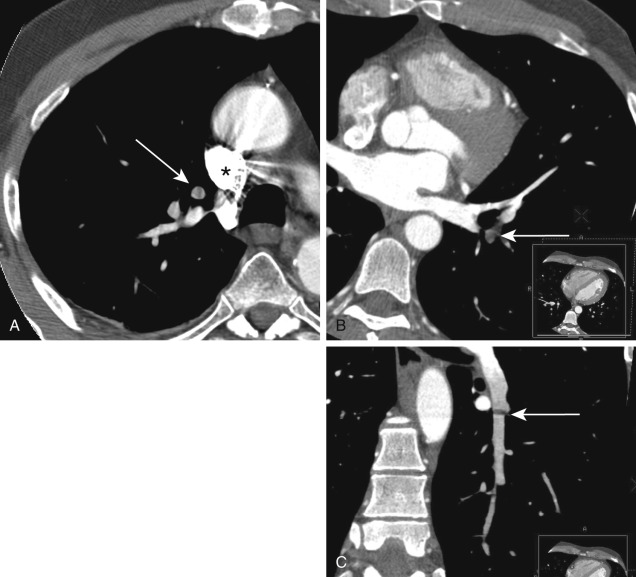
After the diagnosis of PE is made, patients should receive appropriate treatment without delay. The treatment should be tailored to the individual patient and his or her clinical condition. Treatment options include anticoagulation, placement of an inferior vena cava filter, systemic thrombolysis, pulmonary artery catheter–directed pharmacologic thrombolysis ( Fig. 11.12 ), percutaneous mechanical thrombolysis, and surgical embolectomy. Readers are referred to another volume in this book series, Vascular & Interventional Radiology: The Requisites , for a complete description of percutaneous procedures for the management of acute PE.
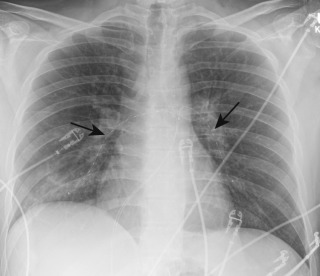
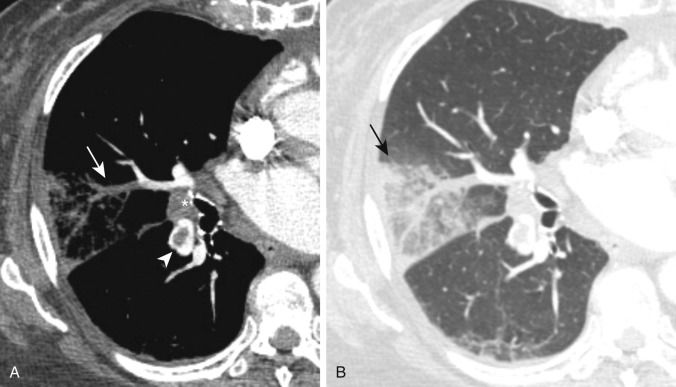
Most pulmonary emboli resolve without sequelae. However, in a small percentage of patients, particularly those with large or recurrent emboli, incomplete resolution of the thrombi may occur. Findings on CTPA of a chronic PE include complete occlusion of a vessel that is smaller than adjacent patent vessels, a web or flap within a contrast-filled artery, a crescent-shaped filling defect that forms obtuse angles with the pulmonary arterial wall, or contrast flowing through thickened pulmonary arteries because of recanalization ( Fig. 11.13 ). There are secondary signs of chronic PE, including extensive bronchial or other systemic collateral vessels, mosaic attenuation of the lungs, or CT changes caused by pulmonary hypertension ( Fig. 11.14 ). The diagnostic criteria for chronic PE are outlined in Table 11.1 .
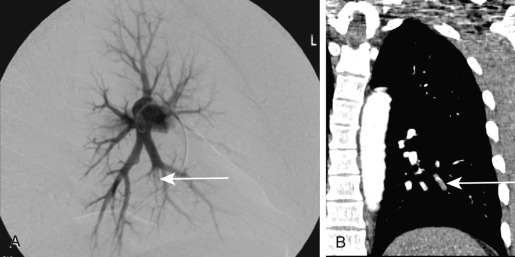
Pulmonary angiography involves percutaneous advancement of a catheter through a peripheral vein through the right atrium and right ventricle to the pulmonary arteries under fluoroscopic guidance. After the injection of intravascular contrast, images are acquired at a rapid rate. The angiographic signs of PE include a partial filling defect within a contrast-filled vessel and complete occlusion of the vessel, producing an abrupt vascular cutoff ( Fig. 11.15 ). Although pulmonary angiography had been considered the gold standard for the diagnosis of PE, it has become almost entirely supplanted by CTPA. Currently, the main clinical utility of conventional pulmonary angiography is for therapeutic intervention and, in selected cases, preoperative evaluation of the pulmonary arteries.
With recent improvements in technology, magnetic resonance angiography (MRA) shows promise for the detection of PE. MRA has the advantage of using neither ionizing radiation nor iodinated contrast. These advantages allow the use of multiphasic acquisitions and the potential for repeated contrast injections, resulting in improved technical success rates when the initial acquisition is compromised by poor bolus timing or patient motion. Disadvantages of MRA compared with CTPA include longer acquisition times, limiting the suitability of long MRI protocols in dyspneic patients, and lower spatial resolution, which likely contributes to the lower sensitivity for the detection of subsegmental emboli. There is also concern about nephrogenic systemic fibrosis, which occurs rarely in patients with poor renal function who receive gadolinium-containing contrast material. Furthermore, the relative lack of familiarity with MRA for evaluation of PE may contribute to technical inconsistency, the primary reason for the underperformance of MRA for the detection of subsegmental emboli in the PIOPED III study.
The pulmonary arteries are evaluated in a segment-by-segment method, similar to the evaluation of CTPA images. Although the criteria for the diagnosis of PE are similar for MRA and CTPA, an important difference is the fact that completely occlusive emboli can appear as abrupt vessel cutoffs on MRA, analogous to cutoff vessels seen in conventional angiography. This may make the identification of occlusive emboli more difficult on MRA than on CTPA because an absent vessel may be more challenging to identify. The PIOPED III study concluded that technically adequate MRA had a sensitivity of 92% and a specificity of 99%, but 25% of patients had technically inadequate images.
Unenhanced MRA techniques can also be used for patients with suspected PE. These include balanced steady-state free precession techniques, three-dimensional (3D) fresh blood imaging using 3D fast spin-echo–based sequences, and arterial spin-labeling techniques.
Nonthrombotic PE is an uncommon condition. It usually involves particles that are so small that they do not present as intraarterial filling defects. Possible causes include droplets of fat, bubbles of air or nitrogen, amniotic fluid, tumors, and foreign bodies such as talc or cement.
Septic emboli can occur when fragments of thrombus include microorganisms, typically bacterial; however, fungi or parasites can also be involved. Please refer to Chapter 14 for more details.
This is a rare complication of a long bone fracture. Other rarer causes include hemoglobinopathy, major burns, pancreatitis, overwhelming infection, tumors, blood transfusion, and liposuction. Please refer to Chapter 10 for more details.
Air embolism can be classified into two types: venous air embolism and arterial air embolism. Venous air embolism occurs when air is introduced into systemic veins and travels to the right ventricle and pulmonary arteries. Causes of venous air embolism to the right ventricle and pulmonary arteries include peripheral intravenous (IV) placement, radiologic IV contrast administration, central line placement or removal, dialysis, pacemaker placement, trauma, and certain surgical procedures (otolaryngologic, orthopedic, obstetrics and gynecology, and especially neurosurgical procedures). Venous air embolism is reported in 12% to 23% of patients undergoing contrast-enhanced CT, but venous air emboli are typically of no clinical significance when small in volume. Moderate amounts of venous air embolism may result in a noncardiogenic pulmonary edema pattern. Large venous air embolism may show hyperlucency in the heart, pulmonary arteries, or hepatic veins and can be fatal.
Arterial air embolism occurs when air enters the pulmonary veins, left ventricle, and systemic arterial circulation. Arterial air embolism may occur by direct entry of air into the pulmonary veins during lung biopsy, radiofrequency or microwave lung ablation, thoracic or cardiac surgery, or penetrating trauma. Paradoxical embolism of a venous air embolism can occur when there is a right-to-left communication through a septal defect, patent foramen ovale, or pulmonary AVMs.
Either venous or arterial air embolism can result from decompression sickness (divers), barotrauma associated with positive pressure ventilation, and penetrating and rarely blunt chest trauma.
The pathologic effects of air embolism occur through vascular obstruction “air block” or vasoconstriction. Air emboli can also cause vascular inflammation and endothelial injury. Arterial air embolism is typically a more serious event and can be morbid even with a small arterial air embolism. The heart and brain are the most vulnerable end organs in arterial air embolism, potentially resulting in stroke, seizure, or myocardial infarction.
This is a rare but highly fatal complication of pregnancy. It occurs when amniotic fluid is forced into the bloodstream through small tears in the uterine veins during normal labor or when the placenta is disrupted by surgery or trauma. Please refer to Chapter 10 for more details.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here