Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Pulmonary atresia and intact ventricular septum is a congenital malformation in which the pulmonary valve is atretic and no ventricular septal defect exists. It coexists with variable degrees of right ventricular (RV) and tricuspid valve hypoplasia, and variable degrees of coronary artery abnormalities. This chapter discusses this malformation in the setting of atrioventricular and ventriculoarterial concordant connections.
In 1839, Peacock collected records of seven patients with pulmonary atresia and intact ventricular septum and gave credit to John Hunter for reporting the first case in 1783. Hunter described a premature male who died 13 days after birth. The RV had “scarcely any cavity,” and the tricuspid valve was “especially small.” Coronary sinusoids and RV–coronary artery fistulae were recognized by Grant in 1926 and later by others. RV-dependent coronary circulation began to be recognized at least in 1975 by Essed and colleagues and more recently by others.
In 1955, Greenwold and colleagues at Mayo Clinic described two types of RV in this malformation: (1) small and (2) normal-sized or large. Subsequently, the idea evolved that values for RV cavity size and tricuspid valve dimensions, as well as morphologic details, comprised a spectrum embracing virtually all values between the extremes. Greenwold and colleagues also suggested that pulmonary valvotomy was appropriate treatment when the RV was near normal in size. In 1961, Davignon and colleagues at Mayo Clinic suggested that a systemic–pulmonary artery shunt be performed when the RV was small. Reports of successful surgery from the University of Minnesota, Mayo Clinic, and Henry Ford Hospital appeared in 1961. The combination of a systemic–pulmonary artery shunt with an RV outflow operation was described by Bowman and colleagues in 1971 and by Trusler and colleagues in 1976. In 1993, Hanley and colleagues introduced the concept that optimal outcomes are best achieved when neonatal and subsequent surgical management are specifically tailored to the variable morphology of this malformation.
Hearts with pulmonary atresia and intact ventricular septum include a spectrum extending from mild concomitant abnormalities of the RV and tricuspid valve to the most severe. Whether the entity of pulmonary stenosis and intact ventricular septum, particularly critical pulmonary stenosis in neonates, is part of this spectrum can be debated (see Section I of Chapter 39 ). Evidence supporting the continuum of these two entities is that at least some hearts at adjoining ends of the spectra have similar RV and tricuspid valvar abnormalities. It has been hypothesized that the later the narrowing of the pulmonary valve develops in fetal life, including progression to atresia, the more fully and normally developed are the tricuspid valve, RV cavity, and RV myocardium. Conversely, the earlier these developments occur in fetal life, the more likely that these structures will be hypoplastic and abnormal. This hypothesis is also consistent with the concept that there is a continuity of the spectra of pulmonary atresia with intact ventricular septum and pulmonary stenosis with intact ventricular septum.
The nature of the structure at the junction of the RV and pulmonary trunk is arguable. Van Praagh and colleagues imply that fibrous components are pulmonary valve remnants. Others point out that in many cases, there is only poorly structured imperforate fibrous tissue overlying muscular atresia. In any event, commissural ridges may be prominent and converge to meet in the center of the “valve,” an appearance similar to that in pulmonary valve stenosis. In some patients, commissural ridges are present only in the periphery, the center being a smooth fibrous membrane. In the combined U.K.-Ireland multicenter study of 183 patients, 75% had membranous atresia and 25% muscular atresia. Of greater importance is the nature of the structures immediately below the RV–pulmonary trunk junction (see “ Right Ventricle ” and “ Tricuspid Valve ” in text that follows).
The pulmonary trunk is usually nearly normal in size, but uncommonly is severely hypoplastic. Rarely, the pulmonary trunk is represented only by a fibrous cord.
Right and left pulmonary arteries are usually normal in diameter or slightly hypoplastic. Uncommonly, they are moderately or severely hypoplastic, usually in patients with severely reduced RV cavity size ( Table 40-1 ). Rarely, there are major arborization abnormalities of the native pulmonary arteries in association with large aortopulmonary collaterals. Four such patients have been encountered by F.L. Hanley and colleagues (personal communication, February 2012).
| RV Cavity Size b | n | % of 136 |
|---|---|---|
| Normal (0) | 5 | 4 |
| Mildly reduced (−1, −2) | 28 | 21 |
| Moderately reduced (−3) | 25 | 18 |
| Severely reduced (−4, −5) | 78 c | 57 |
| Subtotal | 136 | 100 |
| Unknown | 27 | |
| T otal | 163 d |
a Based on data from 171 neonates with pulmonary atresia and intact ventricular septum in the Congenital Heart Surgeons Society study. Size of pulmonary arteries was known in 84.
b Cavity size was graded (0, normal to −5, severe hypoplasia) based on echocardiographic and cineangiographic findings.
c Five of six patients with moderate or severe hypoplasia of right and left pulmonary arteries on cineangiography had severely reduced right ventricular cavity size (information available for 84 of 136 patients).
d In addition, eight patients had enlarged right ventricular cavities, and one (12%) of these had moderately hypoplastic pulmonary arteries.
Size of the RV cavity is variable. In about 5% of patients, it is enlarged (see Chapter 38 , Fig. 38-2 and Chapter 39 , Table 39-1 ). Ebstein malformation and severe tricuspid regurgitation may coexist with the latter. Many individuals with this combination die in fetal life. Rarely, the RV wall may be very thin ( Uhl anomaly or parchment right ventricle ) and the cavity nontrabeculated adjacent to the tricuspid valve and heavily trabeculated in its apical half.
Much more frequently, cavity size is reduced, severely so in about 60% of patients. This appears to be the result of massive wall hypertrophy extending into the ventricular cavity. Often, this completely obliterates the infundibular cavity, and the atresia can be termed muscular in such cases. At times, the apical-trabecular cavity is completely obliterated; in the most extreme cases, both portions are obliterated. Although these cavities are obliterated, the respective portions of the RV are not absent. Cavity obliteration can be localized by echocardiography as well as by anatomic studies. Cavity obliteration has been further characterized in the U.K.-Ireland multicenter study. All three components of the RV (inlet, trabecular, and infundibular) were present in all cases, but with different degrees of cavity obliteration from muscular ingrowth. A “unipartite” ventricle due to muscular obliteration of the infundibular and trabecular components was present in 8%. A “bipartite” ventricle due to muscular obliteration of the trabecular component alone was present in 34%. There were no cases of muscular obliteration of the infundibulum alone. A “tripartite” ventricle was present in the remaining 58%.
There is associated diffuse fibrosis of the hypertrophied muscle and, especially when the RV cavity is small, a modest degree of RV endocardial fibroelastosis. Bulkley and colleagues found typical myocardial fiber disarray in 69% of the RV free wall and in 73% of the ventricular septum in this condition. The potential for impaired left ventricular (LV) as well as RV dysfunction is evident.
Coronary sinusoids, or dilated portions of the coronary microcirculation, can be detected by cineangiography in about half of patients ( Table 40-2 ). In some cases, fistulous connections between the RV cavity and these sinusoids form multiple small communications into branches of left or right coronary arteries. Occasionally they converge into a single large vessel that empties into the left anterior descending or right coronary artery. In many cases, the fistulae are minor. In the multicenter U.K.-Ireland study, 58% of patients had completely normal coronary circulation, 15% had minor filling of the coronary arteries with RV injection at catheterization, and about 25% had major fistulae, with about one third of these having no coronary connection to the aorta.
| Category | PS | PA | ||
|---|---|---|---|---|
| No. | % of 86 | No. | % of 145 | |
| Coronary sinusoids—present: | 9 | 10 | 72 | 50 |
| Without RV-cor fistulae | 7 | 8 | 8 | 5 |
| With RV-cor fistulae | 2 | 2 | 64 | 45 |
| Without RV dependence | 2 | 2 | 52 | 36 |
| With RV dependence b | 0 | 0 | 12 | 9 |
| Subtotal | 86 | 145 | ||
| Unknown | 15 | 26 | ||
| T otal | 101 | 171 | ||
a Based on data from 274 patients (two with unknown type of right ventricular outflow obstruction) in the Congenital Heart Surgeons Society study.
b In this study, it was not possible to place each patient in the spectrum of right ventricular dependency (see text). The phrase “with RV dependence” describes patients in whom dependence was judged to be so extreme that decompression of the right ventricle would be fatal.
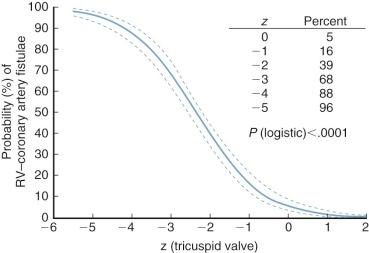
Prevalence of RV–coronary arterial fistulae is inversely related to dimensions of the tricuspid valve (and hence of the RV cavity) ( Fig. 40-1 ) and amount of tricuspid regurgitation. It is also directly related to RV systolic pressure. However, the milieu for development of fistulae may be a consequence of genetically or developmentally induced myocardial abnormalities. Immunohistochemical studies demonstrate abnormal density and orientation of capillaries and myocyte disarray in the presence of fistulae.
Desaturated RV blood, although vital in the presence of proximal coronary obstructions, compromises myocardial oxygen supply in regions to which it is distributed by these fistulae. This may account in part for the myocardial abnormalities described later in this chapter. Complexity of oxygen delivery to the myocardium is evidenced by the fact that the left anterior descending artery (or any coronary artery) may fill through these fistulae from the RV in systole, and from the aorta in the normal manner during diastole. There results a spectrum of percentages of LV myocardium dependent on the RV for coronary perfusion, albeit desaturated; the percentage depends on location and severity of proximal coronary artery stenoses as well as on the fistulae. At some critical point, sufficient myocardium is in jeopardy of developing such severe ischemia when RV hypertension is relieved that RV decompression becomes contraindicated as a surgical option. In an analysis by Giglia and colleagues of 16 patients with coronary angiography and subsequent RV surgical decompression, 7 of 7 (100%; CL 77%-100%) with no coronary stenosis survived, 4 of 6 (67%; CL 39%-88%) with stenosis in one major coronary survived, and 0 of 3 (0%; CL 0%-46%) with stenosis in two major coronaries survived.
When less severe coronary abnormalities are present in association with fistulae, regional LV wall motion abnormalities are commonly identifiable before RV decompression, and they increase after decompression; however, severe global LV dysfunction is unusual.
In about 10% of patients (20% of those with RV–coronary fistulae), coronary circulation or some critical part of it is derived entirely or nearly so from the RV in the manner described, defining RV-dependent coronary circulation . This may occur because of development of arterial obstructions in the left main or right coronary arteries, or both, or in the proximal portion of the left anterior descending artery (R.M. Freedom, personal communication, 1991). No correlates of RV dependence are known beyond those for RV–coronary artery fistulae. Proximal coronary arterial occlusions etiologic to the dependence may develop in fetal life; some develop or progress after birth.
RV-dependent coronary circulation is a major consideration in planning therapy (see Indications for Operation later in this chapter). The difficulty is in deciding how much myocardium at risk is considered too much, triggering the management decision to avoid decompressing the hypertensive RV. To address this difficulty, Calder and colleagues identified coronary abnormalities in 116 patients. They determined that presence of coronary fistulae alone did not correlate with mortality. The presence and extent of coronary interruptions, and the amount of LV myocardium that was RV dependent (determined with a 15-point scoring system), did correlate with mortality.
The problem, however, is even more complex than solely determining the amount of myocardium at risk, because even a small amount of extremely ischemic myocardium may be the source of a life-threatening dysrhythmia. Recently, LV coronary abnormalities unrelated to the presence of fistulae, sinusoids, or coronary interruptions have been discovered. Hwang and colleagues showed that patients with pulmonary atresia with intact ventricular septum, regardless of the presence of fistulae, have decreased density of intramyocardial arterioles relative to normal and hypertrophied hearts.
The tricuspid valve is usually abnormal in this malformation. Occasionally the abnormality is simply small size, but usually the leaflets are thickened and the chordae are abnormal in number and attachment. Local agenesis and incomplete leaflet separation occur. The importance of these abnormalities has been emphasized by Davignon and colleagues and by Paul and Lev.
In about 10% of neonates, z value of the tricuspid valve is less than −5, and in 50% it is −2.2 or less ( Fig. 40-2 ). (See Appendix Fig. 40A-1 for a nomogram for estimating the z value of the tricuspid valve.) Dimensions of the tricuspid valve are well correlated with those of the RV cavity, in contrast to dimensions in neonates with critical pulmonary stenosis, in whom they are not correlated.
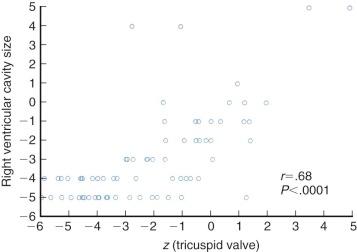
In uncommon cases in which dimensions of the tricuspid valve are large, its leaflets usually show features of Ebstein malformation, with enlargement of the anterior leaflet and downward displacement onto the ventricle of the origin of a dysplastic septal leaflet. The posterior leaflet may or may not be abnormal. These valves are usually severely regurgitant. Another pathologic lesion of the tricuspid valve has been recognized in patients with dilated RV and severe tricuspid regurgitation—that of unguarded tricuspid orifice, in which the inferior (mural) leaflet is absent rather than displaced.
The right atrium is enlarged, and it is more enlarged when there is severe tricuspid regurgitation. An interatrial communication is present in all cases, usually a patent foramen ovale of adequate size. However, shortly after birth the foramen becomes restrictive in some patients. The eustachian valve is frequently prominent.
The left atrium is usually hypertrophied and somewhat enlarged, and the mitral orifice is usually larger than normal. The LV shows some hypertrophy and endocardial fibroelastosis. A convex bulging of the interventricular septum into the LV cavity has been noted, with some speculating that this might produce subaortic obstruction. Clinical and postmortem studies have demonstrated evidence of LV myocardial ischemia in virtually all patients ; perhaps related to this, LV compliance is depressed in many.
The aorta usually has adult morphology without isthmic narrowing and is usually left sided.
The ductus arteriosus is patent at birth but has the usual tendency to close. Orientation of the ductus is typical for pulmonary atresia of all forms, with an obtuse proximal and acute distal angle at its aortic attachment. Usually the bronchial arteries are normal, and important aortopulmonary collateral arteries absent.
Other than Ebstein malformation, coexisting cardiac anomalies are uncommon.
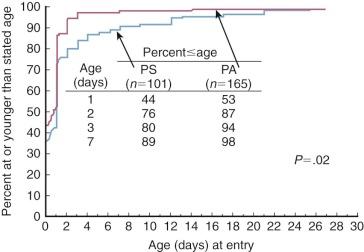
Fetal distress is usually not evident except in those with a large RV. Delivery is typically uncomplicated and at term. The babies are generally well developed ( Fig. 40-3 ) and are likely initially to appear healthy except for cyanosis. Cyanosis is usually obvious on the first day and becomes rapidly more severe as the ductus closes and there is respiratory distress and progressing metabolic acidosis. In the New England series, 81% of babies presented during the first week of life; in a more recent study, 94% presented in the first 3 days ( Fig. 40-4 ).
Absence of an RV impulse in a cyanotic infant with a palpable LV should arouse suspicion of pulmonary atresia and intact ventricular septum, tricuspid atresia, Ebstein malformation, or critical pulmonary stenosis with hypoplastic RV. Typically, no murmur or a systolic murmur of tricuspid regurgitation is heard, sometimes with a thrill. Despite presence of a patent ductus arteriosus, a continuous murmur is seldom heard. The second heart sound is single.
Classic findings on chest radiography include clear lung fields with diminished (or normal) vascular markings and a flat or concave pulmonary trunk segment. Heart size is variable and may be large, even when the RV cavity is small.
P waves can be normal at birth, but evidence of right atrial enlargement develops quickly, and within a few weeks, prominent right atrial P waves are uniformly present. Mean QRS axis in the frontal plane is usually normal or shows a rightward deviation, and the RV hypertrophy pattern usually present in the neonate is absent. However, electrocardiographic evidence of RV hypertrophy may be present even though the RV cavity is small, precluding its use to predict RV cavity size.
Two-dimensional echocardiography is diagnostic ( Fig. 40-5 ). Also, dimensions of the tricuspid valve, RV cavity size, and nature of the outflow obstruction (membranous or muscular) can be determined with confidence. Of importance is the fact that RV–coronary artery fistulae can be identified accurately by two-dimensional echocardiography with pulsed Doppler color flow ultrasound imaging ( Fig. 40-6 ). However, echocardiographic evaluation is limited: fistula size, extent of fistula formation, and presence of proximal coronary arterial stenosis cannot as yet be accurately delineated.


Tricuspid valve z value can be highly predictive of RV-dependent coronary circulation, indicating need for further angiographic evaluation. Fetal echocardiography can identify the anomaly with reasonable accuracy, but this does not appear to affect postnatal outcome favorably. Nevertheless, the morphology determined at fetal echocardiographic evaluation can be used to predict postnatal outcome and thus can be employed as an important prenatal counseling tool.
When pulmonary atresia with intact ventricular septum is diagnosed by echocardiography, cardiac catheterization and angiography are indicated to search for coronary arterial stenoses, coronary sinusoids, and RV-coronary fistulae. If these are present, extent of LV myocardial dependence on the RV must be established.
Right atrial mean pressure is usually higher than left, and a prominent a wave can be seen on the pressure tracing. RV peak pressure is usually higher than or equal to systemic pressure, although it may be less in association with severe tricuspid regurgitation and a thin-walled RV. Systemic arterial saturation is low, but varies according to flow through the patent ductus arteriosus. Size and configuration of the RV cavity can be displayed by RV angiography ( Fig. 40-7 ). In Ebstein anomaly with severe tricuspid regurgitation presenting in the neonatal period in association with high pulmonary vascular resistance, RV systole may fail to open a normal pulmonary valve; high-quality angiography will prevent the mistaken diagnosis of pulmonary atresia.

Quantitative cineangiographic studies indicate reduced RV function in essentially all cases. LV function and end-diastolic volume are also abnormal unless a normal LV mass is present.
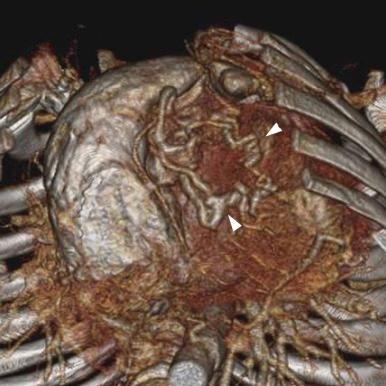
These imaging modalities are not typically used during the neonatal period. They may, however, be useful in evaluating older patients. Evaluation of cavity size and degree of musculature of the RV can be accurately determined using cardiac magnetic resonance (CMR) imaging ( Fig. 40-8 ). Delayed-enhancement CMR can be used to determine the status of the myocardium as well ( Fig. 40-9 ). Volume-rendered computed tomography (CT) angiography imaging can also accurately define abnormal coronary connections ( Fig. 40-10 ).


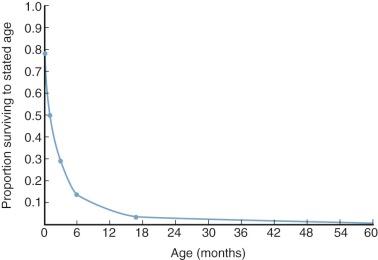
Pulmonary atresia and intact ventricular septum is an uncommon malformation, occurring in 1% to 1.5% of individuals born with congenital heart disease. It was present in 3% of critically ill infants with congenital heart disease in the New England series. It is highly lethal, with about 50% of individuals dying within 2 weeks of birth and about 85% by age 6 months ( Fig. 40-11 ). Death is caused by severe hypoxia and metabolic acidosis and usually coincides with spontaneous closure of the ductus arteriosus. Rarely, patients survive into young adult life. McArthur and colleagues describe a 21-year-old whose pulmonary blood flow came from a right coronary–pulmonary arterial fistula, and Robicsek and colleagues describe another patient of similar age who survived because of a congenital aortopulmonary window.
No single neonatal operation is standard for pulmonary atresia with intact ventricular septum; rather, several surgical procedures, either in isolation or in combination, are indicated based on the details of the morphology in each case (see Indications for Operation later in this chapter for a description of the best initial operation for each morphologic variant). The initial operation most commonly indicated for this entity is concomitant transanular patching, systemic–pulmonary artery shunting, and ductal ligation. The next most common procedure is an isolated systemic-to–pulmonary artery shunt and ductal ligation.
For all of the procedures described, postnatal preoperative management is similar. Following birth, an arterial pressure monitoring catheter is placed, preferably into the left radial artery. (A right radial artery catheter should be avoided because of the possibility that a systemic-to–pulmonary artery shunt originating from the right subclavian artery will be part of the surgical procedure.) A reliable intravenous line is placed into either the femoral or umbilical vein. In general, deep intravenous lines into the upper body, such as into the subclavian or jugular veins, should be avoided to minimize thrombosis in the upper body venous system, because many patients will ultimately require a bidirectional cavopulmonary shunt as part of their overall surgical management. A prostaglandin E 1 (PGE 1 ) infusion is started as soon as possible to maintain ductal patency and is continued until after the surgical procedure. The full dose is 0.1 mg · kg −1 · min −1 ; when the ductus is confirmed to be open, the dose often can be reduced.
Endotracheal intubation and mechanical ventilation are usually necessary, either as part of general resuscitative measures, or as a result of inhibition of intrinsic ventilatory drive due to PGE 1 . A low inspired oxygen fraction and controlled hypercarbia are used to counter the tendency for overcirculation through the ductus arteriosus. Low-dose inotropic support with dopamine or milrinone may be needed to support the systemic circulation.
A median sternotomy is made through an incision that is carried a little farther into the neck than usual to provide better exposure of the brachiocephalic trunk and subclavian arteries. After the pericardium is opened and stay sutures applied, the right pulmonary artery between the aorta and superior vena cava is dissected.
A 3.5-mm expanded polytetrafluoroethylene (PTFE) tube (3.0 mm if the neonate is smaller than about 2.5 kg) is cut to proper length. Without giving heparin at this time, a fine side-biting clamp is placed to exclude the brachiocephalic trunk–subclavian artery junction, and an incision is made in the anterolateral aspect of the arterial wall. An end-to-side anastomosis is made between one end of the expanded PTFE graft and the artery, using 7-0 polypropylene suture. A clamp is placed as far medially on the right pulmonary artery as possible, and another as far laterally as possible but medial to the superior vena cava, and the pulmonary artery is opened longitudinally between them. Alternatively, a single fine side-biting (C) clamp is used. Great care is taken to ensure the clamp does not interfere with ductal flow to the left pulmonary artery or cause coronary artery compression at the aortic origin. The distal end of the expanded PTFE tube is anastomosed to this opening. Clamps are removed.
The ductus arteriosus is dissected and ligated. The pulmonary trunk is dissected minimally. A piece of pericardium is removed, treated with glutaraldehyde, and cut to proper dimensions (see “Decision and Technique for Transanular Patching” under Technique of Operation in Section I of Chapter 38 ). The usual aortic cannula is employed, and a single venous cannula is positioned (see “One Versus Two Venous Cannulae” under Special Situations and Controversies in Section III of Chapter 2 ).
Cardiopulmonary bypass (CPB) is established, with the perfusate warm and normocalcemic. The neonate's temperature is maintained at approximately 34°C during CPB. The surgically created shunt is temporarily occluded by placing a vascular clamp on the PTFE tube. The operation may be done with the heart beating, or cold cardioplegia and controlled reperfusion may be used (see “Cold Cardioplegia, Controlled Aortic Root Reperfusion, and [When Needed] Warm Cardioplegic Induction” in Chapter 3 ).
A longitudinal incision is made in the pulmonary trunk, and the cartilaginous valve plate is incised. The incision is carried proximally onto the RV, taking care to find and stay in the cavity of the infundibulum and to extend it to the sinus portion and distally along the pulmonary trunk, stopping just before the bifurcation. The patch, preferably made from glutaraldehyde-treated autologous pericardium, is sewn into place with 7-0 polypropylene continuous suture. The suture line does not include the full thickness of the incised RV wall, but rather incorporates the epicardium and about 30% of the outer thickness of the RV wall. The foramen ovale must not be closed.
The aortic clamp is released (or controlled reperfusion accomplished), and preparations are made for discontinuing CPB. The vascular clamp is removed from the PTFE tube, and when normothermia is regained, CPB is discontinued and the remainder of the operation completed as usual.
Other initial operations are indicated based on specific morphology. These include isolated placement of a systemic to pulmonary artery shunt and ductal ligation; isolated placement of a transanular patch and ductal ligation using CPB; pulmonary valvotomy, placement of a systemic-to–pulmonary artery shunt, and ductal ligation using CPB; and isolated pulmonary valvotomy and ductal ligation using CPB ( Fig. 40-12 ).

Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here