Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Pancreatitis often results in sequelae of pseudocyst formation or other complications. The consensus guidelines of the revised Atlanta classification of pancreatitis describe various features of acute pancreatitis, distinguishing between interstitial edematous pancreatitis and the more aggressive necrotizing pancreatitis ( Table 93.1 ). Acute peripancreatic fluid collections are defined specifically as fluid lacking the presence of tissue necrosis. A pancreatic pseudocyst is defined as a fluid collection within or adjacent to the pancreas that becomes completely encapsulated with a mature, nonepithelialized, fibrous, inflammatory wall. This process of acute pseudocyst formation requires at least 4 weeks by definition. Pancreatic pseudocysts are typically homogeneous with minimal or no necrosis present and without a significant solid component on contrast-enhanced computed tomography (CT) imaging ( Fig. 93.1 ). Acute necrotic collections often form in the setting of necrotizing pancreatitis and are defined as collections with variable amounts of fluid and necrosis, without a discrete encapsulating wall. If such a collection becomes completely encapsulated with a mature inflammatory wall, it is defined as walled-off pancreatic necrosis, a more specific term replacing the previous concept of pancreatic abscess ( Fig. 93.2 ). Management of walled-off pancreatic necrosis is discussed in Chapter 94 , though many of the same principles apply regarding stabilization of acute pancreatitis, pancreatic ductal evaluation, and minimally invasive techniques.
| Phases of Acute Pancreatitis | Interstitial Edematous Pancreatitis | Necrotizing Pancreatitis |
|---|---|---|
| Early phase (0–4 weeks) | Acute peripancreatic fluid collection | Acute necrotic collection |
| Late phase (>4 weeks) | Pancreatic pseudocyst | Walled-off pancreatic necrosis |
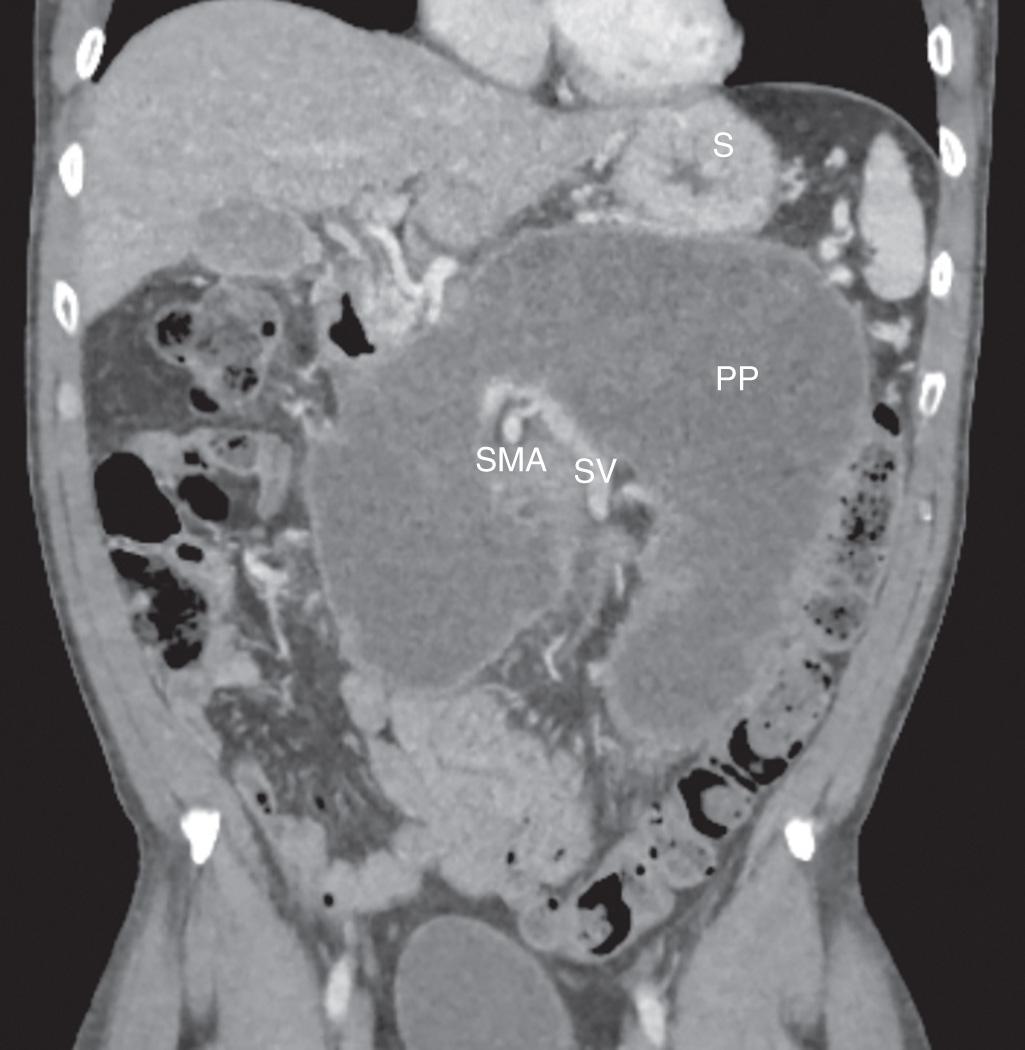
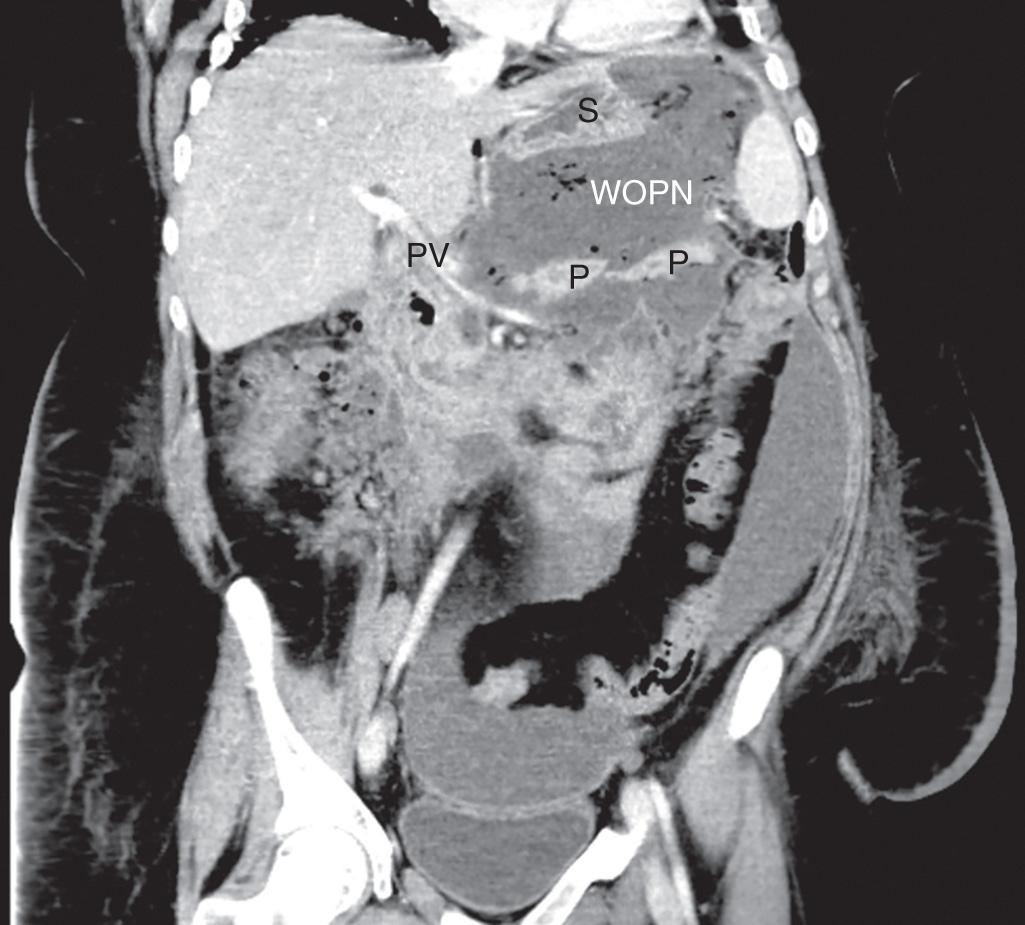
Pancreatic fluid collections and pseudocysts occur in 40% and 5% to 15% of patients, respectively, as a consequence of acute pancreatitis. Etiologies of pancreatitis vary by region, but alcohol abuse is most commonly associated with pseudocyst formation (50% to 70% of cases) in most case series. Any other etiologies of pancreatitis, such as gallstones, trauma, and postprocedural issues, can also result in pseudocysts. Biliary causes of pancreatitis more often lead to pseudocysts after acute pancreatitis, whereas alcohol is typically the cause of pseudocysts in patients with chronic pancreatitis. Release of inflammatory cytokines as part of the immunologic response of pancreatitis can lead to profound reactive fluid accumulation around the pancreatic parenchyma. These fluid collections are typically sterile and disappear with resolution of the pancreatitis. Microcirculatory injury and intraacinar activation of digestive enzymes can damage the parenchyma itself. Disruption or occlusion of the pancreatic ductal system can also lead to fluid collections within and surrounding the pancreas due to extravasation of amylase-rich secretions. Factors affecting the responsiveness of fluid collections to treatment include infection and the presence of significant necrotic debris, as well as ongoing communication with the main pancreatic ductal system.
Pseudocysts are located most commonly in the lesser sac adjacent to the pancreas because the inflammatory response associated with pancreatitis prevents fluid from adequate reabsorption. Less often, pseudocysts will form within the pancreatic parenchyma or extend to other intraperitoneal or retroperitoneal locations. Symptoms of pancreatic pseudocysts commonly include abdominal pain and early satiety. Less frequent symptoms include infection of the pseudocyst, jaundice, or intestinal obstruction from their space-occupying nature, intracystic hemorrhage, and peritonitis from pseudocyst rupture. If the pseudocyst is sizable enough, an abdominal mass corresponding to the pseudocyst can be palpated on physical exam. Most pseudocysts are noticed on abdominal imaging after acute pancreatitis, and pseudocysts account for most pancreatic cystic lesions in patients with a history of pancreatitis. Additionally, chronic pancreatitis can lead to development of a pseudocyst in up to 40% of cases. Neoplastic, congenital, and infectious etiologies of cystic lesions must also be considered in the differential diagnosis and are listed in Table 93.2 . In patients without a history of pancreatitis or pancreatic symptoms, pseudocysts are rarely the cause of an incidentally found pancreatic cyst.
| Inflammatory fluid collections | Acute peripancreatic fluid collection |
| Pancreatic pseudocyst | |
| Acute necrotic collection | |
| Walled-off pancreatic necrosis | |
| Cystic neoplasms of the pancreas | Serous cystadenoma |
| Mucinous cystadenoma/cystadenocarcinoma | |
| Intraductal papillary mucinous neoplasm | |
| Cystic islet cell tumor | |
| Cystic adenocarcinoma | |
| Acinar cell cystadenocarcinoma | |
| Cystic choriocarcinoma | |
| Cystic teratoma | |
| Parasitic cysts | Echinococcal cyst |
| Taenia solium cyst | |
| Dermoid cyst | — |
| Lymphoepithelial cyst | — |
| Congenital simple cyst | — |
| Polycystic disease | Isolated to the pancreas |
| Associated with polycystic kidney disease | |
| Associated with von Hippel-Lindau disease | |
| Associated with cystic fibrosis | |
| Extrapancreatic cysts | Duplication cyst |
| Mesenteric cyst | |
| Splenic cyst | |
| Adrenal cyst |
Contrast-enhanced CT imaging is highly sensitive (close to 100%) for the presence of pancreatic cystic lesions but does not reliably exclude the possibility of a cystic neoplasm. Cystic neoplasms may sometimes show a macrocystic phenotype and mural nodularity is not always present. A known history of pancreatitis strongly suggests the diagnosis of a pseudocyst, but cystic neoplasms can also cause ductal obstruction leading to pancreatitis in 5% to 10% of patients. Radiologic findings of chronic pancreatitis such as glandular calcifications, pancreatolithiasis, and pancreatic atrophy should be noted. The presence of nonenhancing internal dependent debris on T2-weighted magnetic resonance imaging (MRI) sequences was a highly specific finding for pseudocysts in a recent review of pancreatic cystic lesions, and may be helpful in excluding neoplasm. Endoscopic retrograde cholangiopancreatography (ERCP) in the past was considered to be the gold standard for diagnosing pancreatic ductal disruption or stricture, and can usually visualize a pseudocyst if there is communication between it and the pancreatic ductal system. However, ERCP is an invasive procedure carrying its own risk of procedure-related complications, including the risk of contaminating a previously sterile pancreatic fluid collection. Magnetic resonance cholangiopancreatography (MRCP) has demonstrated greater than 90% accuracy in identifying pancreatic duct leakage when compared with ERCP and may be a reliable indicator of the need for early intervention in patients with pancreatitis ( Fig. 93.3 ). Therefore, ERCP is rarely required for diagnosis in the current era.
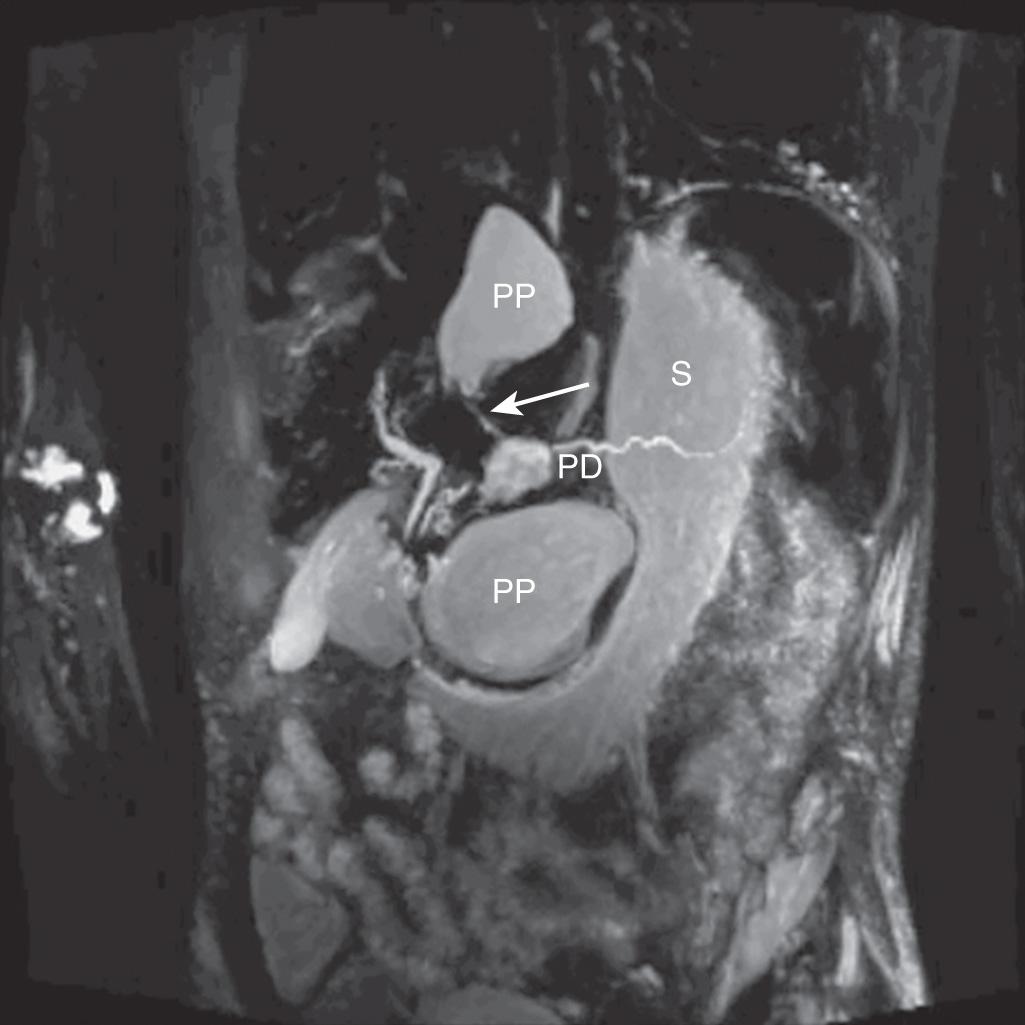
In the absence of diagnostic certainty of a pseudocyst, endoscopic ultrasound is indicated to better characterize the cystic lesion and to aspirate a sample of cyst fluid for analysis. The measurement of cyst fluid carcinoembryonic antigen (CEA) and amylase levels, and the presence or absence of mucin, are together accurate at distinguishing between the possibility of a malignant/premalignant cystic neoplasm and a pseudocyst. The most commonly sampled cystic lesions and their respective cyst fluid values are shown in Table 93.3 . In addition, other cystic tumors such as cystic endocrine neoplasms, cystadenocarcinomas, or solid pseudopapillary neoplasms with a cystic component can occasionally be confused with pseudocysts. Cytology from fine-needle aspiration can often identify epithelial cells that exclude pseudocyst, but such findings lack reliable sensitivity.
| Cyst Fluid Element | Pancreatic Pseudocyst | Serous Cystadenoma | Mucinous Cystic Neoplasm | Intraductal Papillary Mucinous Neoplasm |
|---|---|---|---|---|
| CEA | Low | Low | High | Low to high |
| Amylase | High | Low | Low | High |
| Mucin | Absent | Absent | Present usually | Present usually |
Due to imprecise definitions of pseudocyst and small case series, older data on management of pseudocyst can seem contradictory or difficult to interpret. D'Egidio and Schein introduced a classification scheme that has been widely referenced. This scheme divides pseudocysts into three types: type 1, a postnecrotic pseudocyst after acute pancreatitis, rarely involving ductal disruption; type 2, a postnecrotic pseudocyst after an acute exacerbation of chronic pancreatitis, sometimes showing ductal disruption; and type 3, a retention pseudocyst always related to obstruction and dilation of the pancreatic duct. Despite the heterogeneity of patients in this observational series, important conclusions were shown including the low recurrence rate of pseudocyst after percutaneous drainage in the absence of ductal stricture, a high success rate of internal drainage in patients with chronic pancreatitis, and the necessity of ductal decompression (with endoscopic stent placement or surgery) in patients with ductal stricture because pseudocyst drainage alone in the presence of a stricture was found to be associated with recurrence. Nealon et al. further clarified important anatomic relationships related to the prognosis and management of pancreatic pseudocysts, categorizing pseudocysts into types I to IV by the appearance of the main pancreatic duct and the presence or absence of pseudocyst-duct communication ( Fig. 93.4 ).
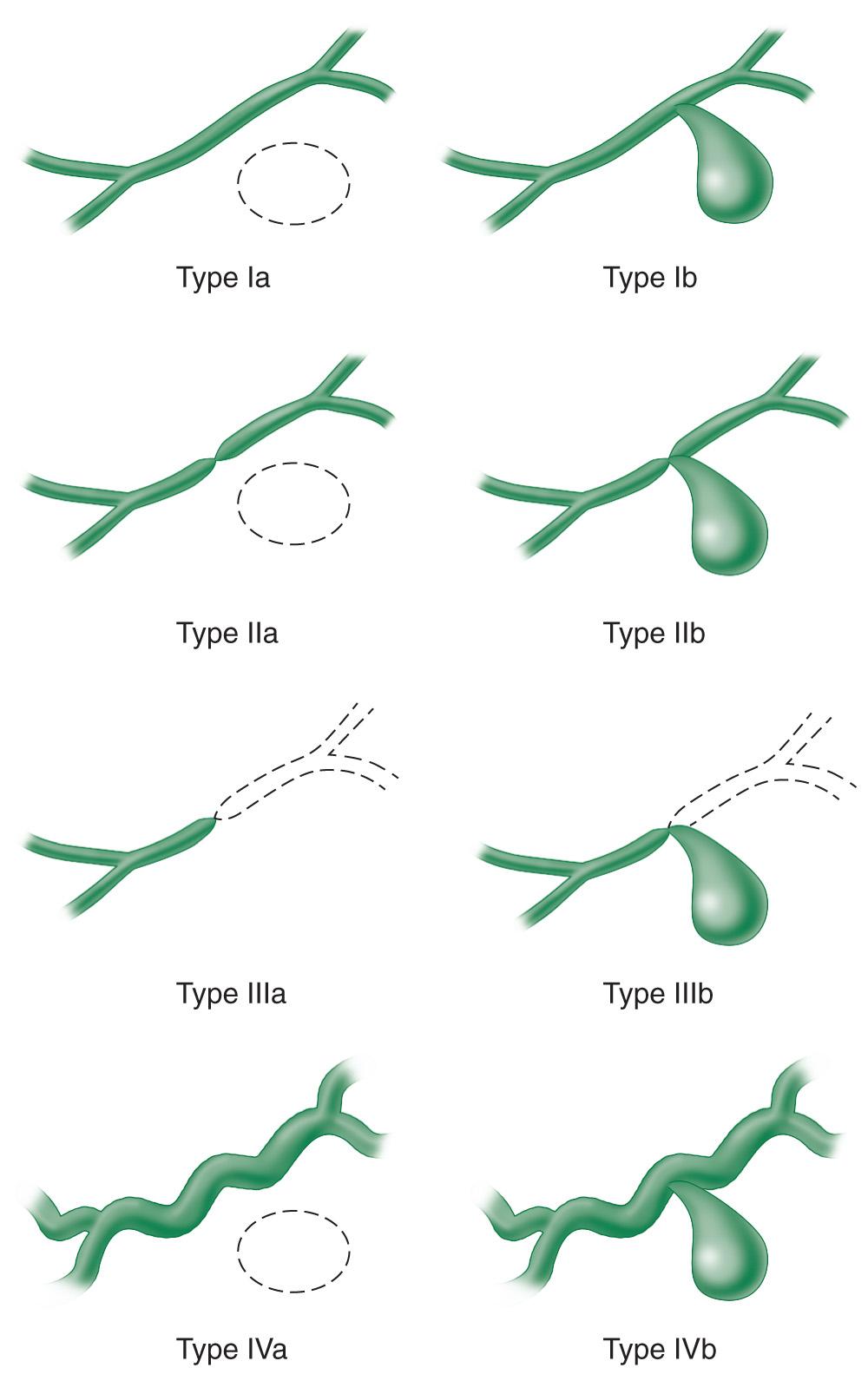
Older data suggested that nonoperative management of pseudocysts would lead to a significant rate of complications. Those reports may have been influenced by a low sensitivity of abdominal imaging at that time for detection of pseudocysts, causing a selection bias for those patients with large pseudocysts more likely not to resolve without intervention and drainage. Two observational studies of pseudocysts published in the early 1990s helped to change this paradigm. Yeo et al. reported on 75 consecutive patients with pseudocysts identified on CT imaging, of whom 48% were asymptomatic and were managed nonoperatively. Of those asymptomatic pseudocyst patients, 60% had complete resolution of their pseudocysts after 1-year follow-up. The lesions of the other 40% of patients remained stable or decreased in size and there was only one patient with a complication (intracystic hemorrhage), which was self-limited and did not require surgery. Although larger pseudocysts were more likely to require surgery, even 27% of those patients with pseudocysts greater than 10 cm in diameter were successfully managed nonoperatively. Vitas and Sarr similarly reported on 68 patients with minimal or no symptoms from a pseudocyst who were initially managed with a nonoperative approach. Almost two-thirds of these patients were successfully managed without intervention after a median follow-up of 51 months, with initial pseudocyst diameter ranging from 2 to 11 cm. Among nonoperative patients with follow-up imaging, 54% of the pseudocysts completely resolved. Only 7% of patients required an emergent operation for a complication related to the pseudocyst, emphasizing nonetheless the need for close initial follow-up when pursuing a nonoperative approach to look for signs of pseudoaneurysm formation or impending vascular erosion. More recently, Cui et al. published a prospective study of pseudocysts after acute pancreatitis (median diameter 9.7 cm) demonstrating resolution or decrease in size of the pseudocyst in 84% of patients. In Nealon's large series of 563 pseudocyst patients, 25% resolved spontaneously and there was equivalent likelihood of resolution between pseudocysts with diameter less than 5 cm or greater than 5 cm. Although underpowered, the series reported by Soliani et al. had similar recurrence rates between pseudocysts with diameter less than 10 cm and greater than 10 cm. Symptoms may be more common in patients developing a pseudocyst after an episode of acute pancreatitis rather than chronic pancreatitis, and in alcoholic-related pancreatitis, which tends to have a more persistent inflammatory process. Normal pancreatic ductal anatomy in the Nealon classification was a significant predictor of spontaneous resolution, with 87% resolution of type I pseudocysts and infrequent resolution of pseudocysts associated with any other ductal anatomy. Nealon type II, III, and IV pseudocysts were typically symptomatic and required intervention.
When a patient's symptoms are significant enough to affect their quality of life or a pseudocyst continues to increase in size on surveillance imaging, elective decompression is indicated ( Box 93.1 ). Open surgical cystoenterostomy has traditionally been the gold standard for internal drainage, with pseudocyst recurrence rates less than 5%. Newer endoscopic and laparoscopic techniques have demonstrated similar efficacy in pseudocyst resolution along with the benefits of a minimally invasive approach, including decreased morbidity and hospital length of stay. These techniques are discussed further in Chapter 94 . In the most favorable large study to date, endoscopic drainage has been shown to have a greater than 90% pseudocyst resolution rate at an experienced center. However, a recent series comparing endoscopic and surgical drainage reported a higher primary success rate for laparoscopic or open surgical procedures, suggesting that the efficacy of endoscopic drainage varies widely and remains significantly operator-dependent. When varices or other large vessels are visualized by endoscopic ultrasound (or CT or MRI) between the stomach and the pseudocyst at a proposed cystogastrostomy site, endoscopic drainage is contraindicated and surgery should be performed.
Observation
Percutaneous catheter drainage
Endoscopic internal drainage
Cystogastrostomy
Cystoduodenostomy
Transpapillary pancreatic duct stenting/pseudocyst drainage
Surgical interventions (open or laparoscopic)
Cystogastrostomy
Roux-en-Y cystojejunostomy
Cystoduodenostomy
Longitudinal pancreaticojejunostomy
Partial pancreatic resection
External catheter drainage
When compared with operative internal drainage, percutaneous drainage of pseudocysts has been shown to result in an increased requirement for repeat interventions and increased mortality, although the latter may result from a selection bias relating to its use in a sicker population of acute pancreatitis patients. When percutaneous drainage fails, there is usually an associated persistent pancreatic fistula and a high rate of sepsis. At hospitals with adequate surgical and endoscopic expertise, percutaneous catheter drainage may be best reserved for patients who are severely malnourished or medically unfit for a more extensive procedure. Percutaneous drainage is helpful for emergent treatment of an infected pseudocyst with sepsis. For stable patients with suspected infection of a pseudocyst, surgical or endoscopic drainage may still be an efficient treatment option.
Operations for internal drainage follow the same basic principles, whether open or laparoscopic. The wall of the pseudocyst must be mature and thick enough to hold suture for anastomosis, which is typically true more than 6 weeks after the initial appearance of the pseudocyst. In chronic pancreatitis, surgery for a pseudocyst may proceed as soon as any acute inflammation has subsided. Given the usual significant inflammation from the antecedent pancreatitis, dissection around the pseudocyst should be minimized whenever possible. If there is any concern about the possibility of a cystic neoplasm, a full-thickness biopsy of the cyst wall can be excised where the anastomosis will be performed and sent for immediate frozen section evaluation. Residual debris or necrotic material within the pseudocyst cavity should also be gently suctioned or debrided prior to anastomosis. Cystogastrostomy, Roux-en-Y cystojejunostomy, or cystoduodenostomy are options for internal drainage depending on the anatomic location of the pseudocyst. Especially in cases of giant pseudocysts, the anastomosis should be located to optimize dependent drainage of the pseudocyst. Longitudinal pancreaticojejunostomy for duct drainage alone may be adequate for treatment of an associated pseudocyst in chronic pancreatitis. In other more complex cases, pancreatic resection may be necessary. External drainage can result in a prolonged pancreaticocutaneous fistula, but may be necessary in emergent septic situations when a more definitive or extensive intervention is not prudent.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here