Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
© 2018 Elsevier Inc. All rights reserved. Please note that the copyright for the original figures submitted by the contributors is owned by Contributors.
Although they arise from different tissues, chordomas and many chondrosarcomas share similarities in natural history and location such that they can be misdiagnosed for each other; a differential diagnosis is often required to ascertain the pathology of the tumor. Neither tumor is common. Chordomas are found along the spine, mainly at the base of the skull and sacrum, and chondrosarcomas often present in those locations as well. A differential diagnosis is important for the radiation oncologist inasmuch as chordomas tend to be more resistant to radiation than chondrosarcomas and thus require a higher total dose in attempts to effect disease control. Even so, management of both these tumor types at the base of the skull presents similar problems to the radiation oncologist.
The main challenge to effective management of tumors that arise at the base of the skull is the anatomic milieu of the tumor. Standard treatment consists of surgery and irradiation; the combination is employed because the proximity of critical structures such as the brainstem, optic tracts, pituitary gland, and temporal lobes usually renders attempts at complete surgical resection unsuccessful. A recurrence rate of 58% from surgery alone, as reported in one study, is typical. However, the same anatomic factors that constrain the effectiveness of surgery can also hinder the effectiveness of conventional photon irradiation. The radiation oncologist prefers to minimize irradiation of these structures as much as is possible, but three-dimensional control of the distributed radiation dose with X-rays is difficult to achieve because of the inherent characteristics of the photon beam. Consequently, less-than-optimal doses of radiation may need to be delivered to minimize irreversible damage to these structures. Chordoma histology presents an additional problem because these tumors are relatively resistant to standard doses of radiation, which historically has limited the usefulness of conventional photon treatment. In treating chordomas of the base of the skull, adjuvant photon radiation of 60 Gy was used in the past, yielding control rates of 23%–39%. Raising the total dose to 66.6 Gy achieved a local control rate of 50%. Clearly, improvement is needed; a higher dose must be delivered to the target volume while sparing the nearby critical structures.
At our institution and others, protons offer an alternative means of adding radiation therapy to the initial surgical treatment. We use proton radiation therapy to deliver high total doses to these tumors while sparing the nearby critical structures. The ability to do this arises from the physical dose distribution characteristics of the accelerated proton beam.
Protons (hydrogen ions) have mass (1835 times that of an electron) and an electric charge. These characteristics, plus the energy of the accelerated beam, enable the radiation oncologist to control the beam in three dimensions, thus affording the ability to deposit radiation doses more accurately within target volumes while minimizing—or, often, eliminating—the radiation dose to the surrounding nontargeted tissues and critical structures. Mass reduces the extent to which the particle scatters as it passes through tissue; charge enables interaction with orbiting electrons within targeted cells, thus causing the ionization required to begin the process of cell destruction; modulating beam energy enables the radiation oncologist to control the depth of penetration. Thus the radiation oncologist can fully control the dose deposition in three dimensions; this level of control cannot be achieved with X-rays, even those delivered via means such as intensity-modulated radiation therapy.
The fundamental reason for the high controllability of proton beams is illustrated by the Bragg peak, which graphically describes the behavior of protons in tissue. With proton beams, as with other accelerated heavy charged particles, the deposited dose is relatively low upon entering the body and increases slowly until reaching the prescribed depth in tissue; there, the bulk of the dose is deposited within the targeted volume, and virtually no dose is deposited in distal tissues. This is in contrast to beams of X-rays, in which the highest dose is deposited near the entrance to the body and the dose is deposited continually as the beam passes through the targeted volume and exits the body. An unmodulated proton beam deposits its energy in a tiny volume, but the radiation oncologist can spread out the Bragg peak to encompass the target volume while still retaining a relatively low entrance dose and sparing tissues distal to the direction of travel. These properties permit much greater sparing of the nearby critical structures with proton than with photon beams in treating similar target volumes, thus allowing for safe dose escalation ( Fig. 32.1 ).
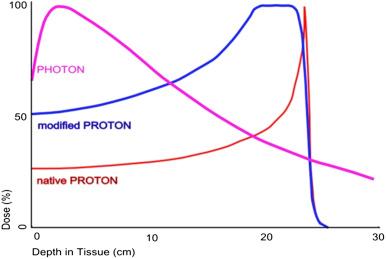
The ability to spare normal tissues is important. The more the physician can reduce or eliminate the dose to normal tissues, the less the likelihood that treatment may need to be compromised or halted because of unacceptable side effects. The ability to reduce or eliminate radiation dose to normal tissues, attributable to the reduced lateral scatter and sharp dose fall-off of the proton beam, allows completion of treatment to the total needed and permits delivery of higher doses without increasing the side effects.
The importance of reducing the volume integral dose to normal tissues has long been noted. In studies spanning more than 40 years, Philip Rubin and several collaborators identified the clinicopathologic courses of radiation injury in organs and tissues throughout the body and measured the tolerance doses for various organs. Tolerances were identified in terms of ranges of total doses after which severe or life-threatening complications were likely to occur within 5 years of therapeutic radiation, i.e., severe sequelae would likely occur in 5% of patients treated at the lower end of the range (TD5/5) and in 50% of patients treated to the dose at the top of the range (TD50/5). Although organs and tissues were separated into categories according to their importance for survival, no “safe” dose (TD0/5) was identified for any organ; rather, in a classic series of graphs, Rubin and Casarett demonstrated that sublethal doses of radiation initiate a course that can eventuate in clinical manifestations of radiation injury, some of which progress to lethality.
Because proton irradiation reduces the volume integral dose to normal tissues and structures, because of the low control rates for chordomas and chondrosarcomas of the base of the skull when treated with conventional photon radiation, and because higher doses improve tumor control for virtually all tumors, investigators have anticipated a role for proton radiation therapy in numerous challenging scenarios. Fowler, using biological modeling, estimated that significant improvements in local tumor control and survival are likely to occur with proton radiotherapy for a variety of tumors. The normal-tissue-sparing properties of proton beams permit effective disease control with similar or lower rates of normal tissue injury and less severe toxicity than would be seen in comparable photon treatment regimens.
Proton radiation treatment thus is an excellent modality for treating chordomas and chondrosarcomas of the base of the skull. Proton beams’ low entrance dose, sharp lateral penumbra, and abrupt cessation of dose at the end of range enable minimizing the volume of normal cells receiving the radiation and, in turn, reduce the likelihood of radiation-related sequelae in normal brain tissue, which is particularly important for tumors near radiosensitive structures such as the brainstem, optic structures, the temporal lobe, and the pituitary hypothalamus. Furthermore, similar to X-ray beams, proton beams have a relatively sparse path of ionization (low linear energy transfer). Especially important to normal tissue traversed by the radiation in its path to the tumor, this property, during fractionated treatments, allows repair of radiation damage to normal cells. For tumors that do not border critical structures, the high controllability and conformability of protons enables delivery of the total prescribed dose over a brief course of stereotactic radiosurgery (SRS) or stereotactic body radiation therapy (SBRT).
Proton therapy is particularly useful for treating small, irregularly shaped postoperative residua of skull base surgery. Proton beams, because of superior dosimetric characteristics, enable precise, conformal delivery of fractionated ionizing energy, conformally and precisely, to skull base tumors. The volume integral dose to nearby normal tissues is reduced when compared with that of photon radiation, and normal cells that are exposed have an opportunity to effect repair.
The seminal article on proton radiation therapy was written by Robert R. Wilson in 1946. Wilson, a physicist who worked with Ernest O. Lawrence and John Lawrence at the University of California at Berkeley, where neutrons were being investigated for cancer treatments, speculated that protons had properties well suited for therapy. Specifically, he noted the properties subsequently established as the foundation of heavy-charged-particle therapy: low entrance dose, a sharp dose fall-off, and controllable range and distribution in tissue.
Protons were not used for treatment, however, until almost a decade later. Tobias and his colleagues at Berkeley employed them beginning in 1954 for identifiable targets, such as the sella, that could be located with the limited imaging capability of X-rays. In 1957, in Sweden, Larsson and colleagues began exploring the therapeutic uses of protons for pituitary tumor or pituitary ablation in diabetes, and in the early 1960s, Kjellberg and colleagues at the Massachusetts General Hospital (MGH), using the cyclotron at Harvard Cyclotron Laboratory (HCL), began studying proton SRS for pituitary ablation and, later, for treating arteriovenous malformations (AVMs).
In the mid-1970s, Suit and colleagues at the MGH/HCL began evaluating fractionated proton therapy for chordomas and chondrosarcomas of the skull base and cervical spine. Proton therapy was used as an adjunct to maximal safe surgical resection and for recurrent tumors. An initial report documented the results of treatment of 10 patients, 9 of whom had chordoma or chondrosarcoma. The investigators reported that local control was achieved for all patients. High doses of radiation, up to 76 cobalt gray equivalents (CGE), were delivered without significant morbidity. In particular, the authors noted that no neurological sequelae supervened. A later report by the same group described the outcomes in 68 patients with chordoma or low-grade chondrosarcoma at the base of the skull who received fractionated high-dose postoperative irradiation through the HCL 160-MeV proton beam. Patients were followed up for at least 17 months and for a median of 34 months; the median tumor dose was 69 CGE, and the daily dose was 1.8–2.1 CGE. The 5-year actuarial local control rate was 82%, and the disease-free survival rate was 76%. The authors reported that the incidence of treatment-related morbidity was acceptable.
In the late 1970s, physicians at Berkeley began treating skull base tumors, first with proton beams and later with qualitatively similar helium ion beams. Patients were treated immediately following surgery or for recurrent disease. Most patients presented with chordomas or chondrosarcomas, but their reported population included other histologies such as meningioma, osteosarcoma, and neurofibrosarcoma. Local control and survival appeared to be improved in skull base tumors following high-dose charged particle treatment (mean dose of 65 GyE), with relative sparing of adjacent normal tissues. The Kaplan–Meier 5-year local control rate was 78% for chondrosarcoma and 63% for chordoma; follow-up ranged from 4 to 191 months with a median duration of 51 months.
Until 1990, all treatments with proton beams were given in high-energy physics laboratories, using accelerators designed for basic research, not patient treatments. In October of that year, however, the world’s first hospital-based proton treatment center was opened at Loma Linda University Medical Center (LLUMC).
Throughout the 1970s, Dr. James M. Slater studied proton and other heavy-charged-particle therapy as a means to deliver effective doses of radiation while sparing normal tissue to the greatest extent possible. As his studies proceeded, he became convinced that charged particles could be used for routine radiotherapy and that the proton was the optimal particle with which to accomplish this objective. He further believed that proton therapy would be best used if the treatment facility were located in a hospital environment, where patients would have ready access to the myriad services used in modern medicine. He therefore initiated a program to develop a hospital-based center at LLUMC with the collaboration of Fermi National Accelerator Laboratory (Fermilab) and the US Department of Energy. The facility, named the James M. Slater, MD, Proton Treatment and Research Center in 2007, has four treatment rooms and five beam lines for treating a variety of tumors. It can accommodate up to 200 patients per day, and, to date, nearly 19,000 patients have been treated there since the facility opened.
The James M. Slater Center has been a model for other hospital-based or hospital-affiliated facilities. Today, there are 17 such centers in the United States, and another 15 are under construction. A greater number of heavy-charged-particle centers, most of them delivering protons, are in operation or development around the world.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here