Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Multiple advances in perioperative techniques, especially the maturation of cardiopulmonary bypass, have allowed the practice of cardiac surgery to develop rapidly. Despite these advances that have enabled contemporary cardiac surgery to address a diverse range of cardiac pathologies, neurological injury, ranging from delirium to stroke, has persisted as a relatively common and important complication after cardiac surgery. As a result, extensive research has explored the etiologies of neurologic injury after cardiac surgery to identify interventions that may reduce its incidence. Although the strategies for neuroprotection have some similarities across the types of cardiac surgery, many differences remain as a result of varied etiologies for neurological injury. For the sake of clarity, this chapter will approach neurologic injury and neuroprotective strategies by the type of cardiac procedure in the following four sections: cardiac surgery with cardiopulmonary bypass; off-pump coronary artery bypass grafting; transcatheter aortic valve replacement; and thoracic aortic surgery.
Multiple clinical trials have demonstrated that the incidence of new infarcts on diffusion-weighted magnetic resonance imaging following cardiac surgery with cardiopulmonary bypass (CPB) ranges from 25% to 40%. Although the vast majority of these lesions are not clinically significant, the incidence of clinical stroke after cardiac surgery with CPB has persisted in the 5%–10% range, depending on the diagnostic approach. Stroke in this setting remains an important complication because it significantly increases perioperative mortality and morbidity. Postoperative cognitive dysfunction including delirium is even more common than stroke after cardiac surgery with CPB. This neurological complication has also been associated with significant reductions in independence and quality of life.
Multiple clinical trials have reported patient and procedure-specific risk factors for the development of neurologic injury after cardiac surgery with CPB. These risk factors include advanced age, diabetes, preexisting cerebrovascular disease, severe atherosclerotic vascular disease, previous cardiac surgery, prolonged CPB time, and perioperative hypotension. The stroke risk is also typically significantly increased in the setting of combined cardiac procedures on CPB as follows: coronary artery bypass grafting (CABG) < valve repair/replacement < CABG and valve repair/replacement < multiple valve interventions. The risk factors for neurologic injury in this setting are listed in Table 22.1 .
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cerebral embolization is a common etiology for neurological injury after cardiac surgery with CPB. In a clinical evaluation of an intra-aortic filter device deployed during cardiac surgery with CPB ( N = 2297), detailed histopathological analysis confirmed filter-trapped debris in 98% of study patients. The debris consisted of fibrous atheroma in 79%, platelets and fibrin in 44%, red blood cell thrombus in 8%, adventitial tissue in 3%, and miscellaneous debris in 2% of cases. Patients with neurological injury after cardiac surgery with CPB will often have evidence of multiple cerebral lesions, consistent with cerebral embolization.
Embolic material can be further classified into three categories: macroemboli, microemboli, and gaseous emboli ( Table 22.2 ). Macroemboli are frequently derived from atheromatous plaque, valvular calcium, or thrombus that become dislodged during surgical manipulation to result in a focal stroke when they are clinically significant. Microemboli are typically derived from small lipid particles and platelet-fibrin aggregates and may be responsible in part for delirium after cardiac surgery with CPB. Gaseous emboli can enter the CPB circuit during vascular cannulation and anastomosis, vacuum-assisted venous drainage, as well as during open cardiac chamber procedures. The amount of embolized gaseous microemboli very probably correlates with the degree of subsequent neurological injury, including both delirium and stroke. Larger gaseous emboli may result in discrete stroke.
|
|
|
|
|
Periods of hypotension are relatively common in cardiac surgery compared with other surgical procedures. Most patients with intact cerebral autoregulation can tolerate moderate swings in mean arterial pressure and still maintain adequate cerebral blood flow. Despite this compensation, however, cerebral autoregulation may be impaired in the setting of multiple factors including advanced age, cerebrovascular disease, cardiopulmonary bypass, sepsis, and temperature. As a consequence, patients with impaired cerebral autoregulation may be at greater risk of cerebral hypoperfusion and ischemia during periods of hypotension. Hypoperfusion injuries are relatively common in patients who develop strokes after cardiac surgery with CPB, with a reported incidence of 20%–40% in computed tomographic imaging and 40%–60% in magnetic resonance imaging. Recent trials have demonstrated that the duration and degree of hypotension during cardiac surgery with CPB significantly predicted the risks of stroke ( P < 0.05) as well as major morbidity and mortality ( P < 0.05). A recent trial ( N = 197) randomized adult cardiac surgical patients to one of two mean arterial pressure groups during CPB: higher blood pressure defined as a mean of 70–80 mmHg and lower blood pressure defined as a mean of 40–50 mmHg. The defined primary outcome was the total volume of new cerebral lesions detected on diffusion-weighted magnetic resonance imaging in the first postoperative week. The primary outcome was similar between groups (median difference estimate 0; 95% confidence interval -25 to 0.028; P = 0.99). There were new cerebral lesions evident in over 50% of patients in each group, a finding that is consistent with the existing literature. Taken together, these clinical trials suggest that, in addition to optimal blood pressure management and in consideration of the individual cerebral autoregulation reserve, further multimodal trials are indicated to identify best practice measures for neuroprotection in cardiac surgical procedures with CPB.
The conduct of CPB triggers a profound systemic inflammatory response because blood interacts with the hardware components of the CPB circuit. This inflammatory response includes activation of the coagulation system, fibrinolytic system, complement system, leukocytes, endothelial cells, and platelets. This acute phase reaction may lead to tissue injury, depending on factors such age, genotype, and functional reserve. This systemic inflammatory response may also have neurological effects by causing glial cell injury, cerebral edema, and altering the integrity of the blood–brain barrier. Clinical trials have suggested a role for the systemic inflammatory response in neurologic injury after cardiac surgery with CPB. Future trials will probably investigate the modulation of this systemic inflammatory response in a multimodal approach to neuroprotection in this clinical setting.
Neuronal necrosis can result from cellular injury, including apoptosis. Neuronal apoptosis is mediated by gene activation that results in a protein cascade as the mechanism for cell death. These neuronal pathways depend on individual genomic predisposition for cerebral injury. Multiple genes have been implicated in influencing the risk of neurologic injury after cardiac surgery, including the apolipoproteins, interleukin polymorphisms, and platelet glycoprotein alleles. Future trials will undoubtedly further explore neurologic risk in cardiac surgery with CPB as a function of genotype with the goal of identifying therapeutic targets for neuronal protection in the perioperative period and beyond.
Patients undergoing cardiac surgery with CPB are typically cooled to a level of hypothermia depending on the procedure, surgeon preference, institutional norms, and patient risk factors. As cerebral temperature is lowered, cerebral metabolic rate decreases as does an ischemic tolerance with hypothermia. Hypothermia alters gene regulation of multiple inflammatory pathways to inhibit apoptosis and inflammation, as well as suppress free radical formation.
In contrast, perioperative hyperthermia has been clearly shown to be detrimental to neurological outcomes after adult cardiac surgery with CPB. This variable will be discussed in further detail in the next section.
Contemporary cardiopulmonary circuit designs have been gradually optimized to reduce the risks of the embolic generation and subsequent cerebral vasculature, given that the circuit hardware is a major source of microbubble formation and embolization. Risk factors for microbubble formation include vacuum-assisted venous drainage, roller versus centrifugal pump exposure, bubble versus membrane oxygenator application, hard-shell versus soft-shell venous reservoir, and low venous reservoir levels. Furthermore, in-line arterial filters and venous reservoir filters have also been integrated in contemporary perfusion practice for removal of microbubbles and other embolic particles from the CPB circuit. Although effective, these filters do not remove all emboli, despite reduced pore size. The benefits of reduced pore size must be balanced with the increased risk of hemolysis and damage to other blood components. In addition to air filters, the presence of a dynamic bubble trap in between the arterial filter and aortic cannula has been shown to reduce the incidence of microbubbles passing into the arterial circulation.
In addition to microbubbles and macroemboli, blood suctioned directly from the surgical field via cardiotomy suction is known to contain high levels of lipid particles. These lipid emboli have been associated with capillary occlusion and cerebral ischemia. In an effort to reduce the effects of lipid embolization due to blood returned from the cardiotomy suction, expert consensus has considered processing the blood through a cell-saver device before returning it to the CPB circuit. Two randomized controlled trials have explored this approach, with conflicting results, although both trials demonstrated increased transfusion in the cell-saver group. In a recent randomized trial ( N = 30) testing the benefits of a lipid microemboli filter integrated into the CPB circuit, the intervention group had a significant reduction in lipid microemboli and serum markers for neurological injury. Future trials will target ongoing improvement of clinical outcomes from a multimodal filter strategy to minimize embolic load and protect against neurological injury.
In addition to CPB components, investigators have also investigated the approach to aortic cannulation for CPB to reduce further the risk of cerebral embolization. Multiple clinical trials have examined the effects of aortic cannula design with respect to features such as length and tip design to reduce aortic wall sheer stress and minimize cerebral atheroembolism. The goals in this design process have been to reduce aortic jet velocity, to control direction of the jet, and to decrease turbulence with features such as a funnel and curved tip, including side apertures for dispersal of flow. Furthermore, cannulation of the distal aortic arch has also been established as a cannulation strategy to reduce the burden of cerebral emboli.
Another area of innovation in embolic reduction has been the addition of embolic protection to the design of the arterial cannula itself. There are currently two aortic cannulae that have embolic protection devices incorporated into their design, namely the CardioGard backward suction cannula (CardioGard Medical, Israel) and the Embol-X intra-aortic filtration device (Edwards Lifesciences, Irvine, California). The CardioGard cannula ( Fig. 22.1 ) is a curved-tip, 24-French dual-lumen aortic cannula. The first lumen is a standard forward-flow lumen for CPB arterial outflow and the second lumen is placed to suction, venting back into the venous reservoir of the CPB circuit. This returned blood is then filtered by the venous reservoir filter and the arterial filter. In vitro and animal studies demonstrated a reduction in cerebral embolic load when compared with standard aortic cannulae. A multicenter, randomized controlled trial of 66 patients demonstrated a significant decrease in the new lesion volume in the CardioGard group as measured by diffusion-weighted magnetic resonance imaging.
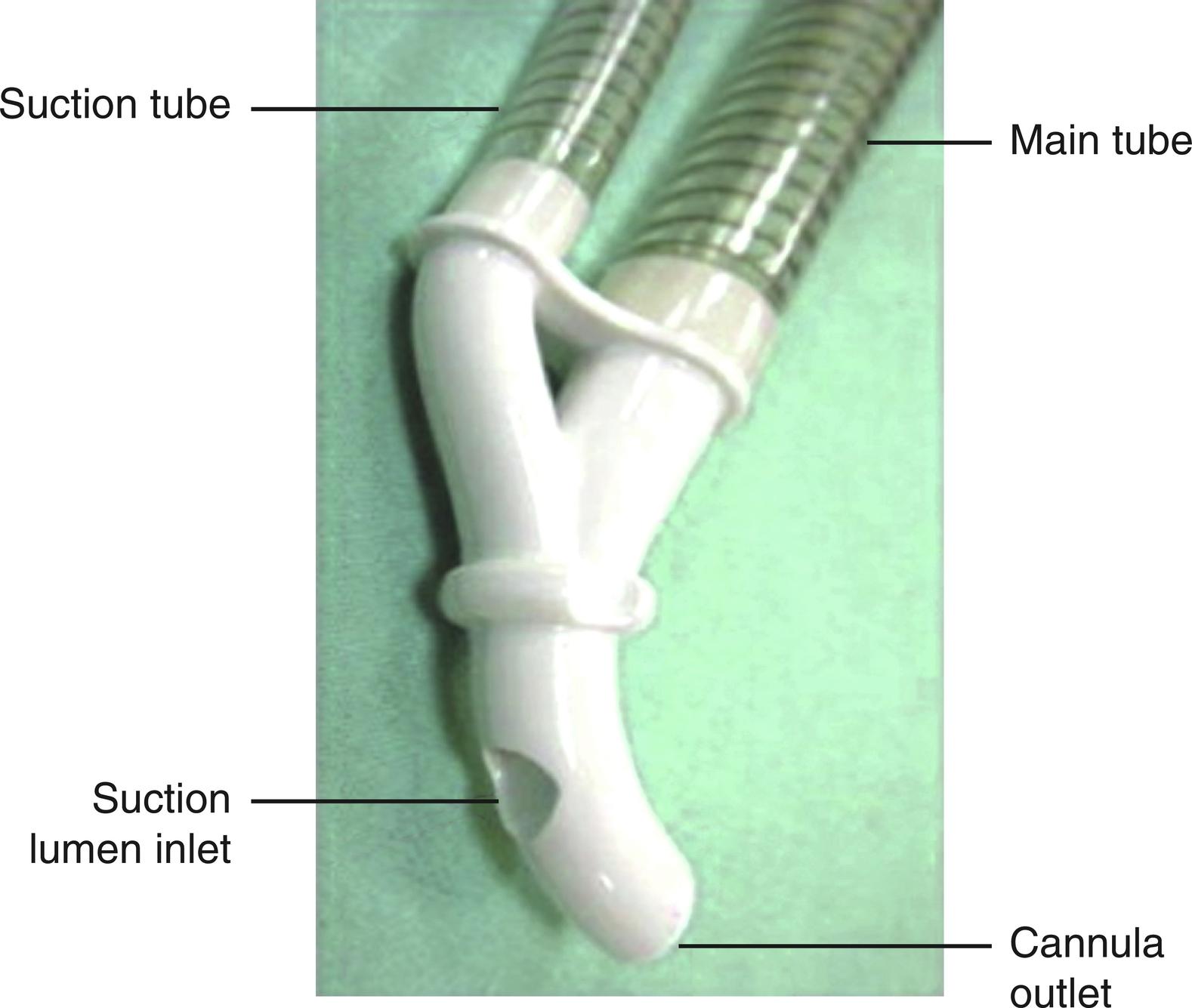
The Embol-X intraaortic filtration device ( Fig. 22.2 ) is a 24-French aortic cannula with a built-in side port through which the filtration device can be deployed and recaptured. In early studies, the device was deployed before the removal of the aortic cross-clamp and left in place until the patient was weaned from CPB. Early results demonstrated safety and facility of the procedure, as well as the effectiveness of the device in capturing embolic debris. Several trials have examined the Embol-X device in standard cannulation in open heart surgery with somewhat conflicting results. One trial has suggested that deployment of this device prior to aortic clamping, as opposed to only after removal, may improve embolic capture.
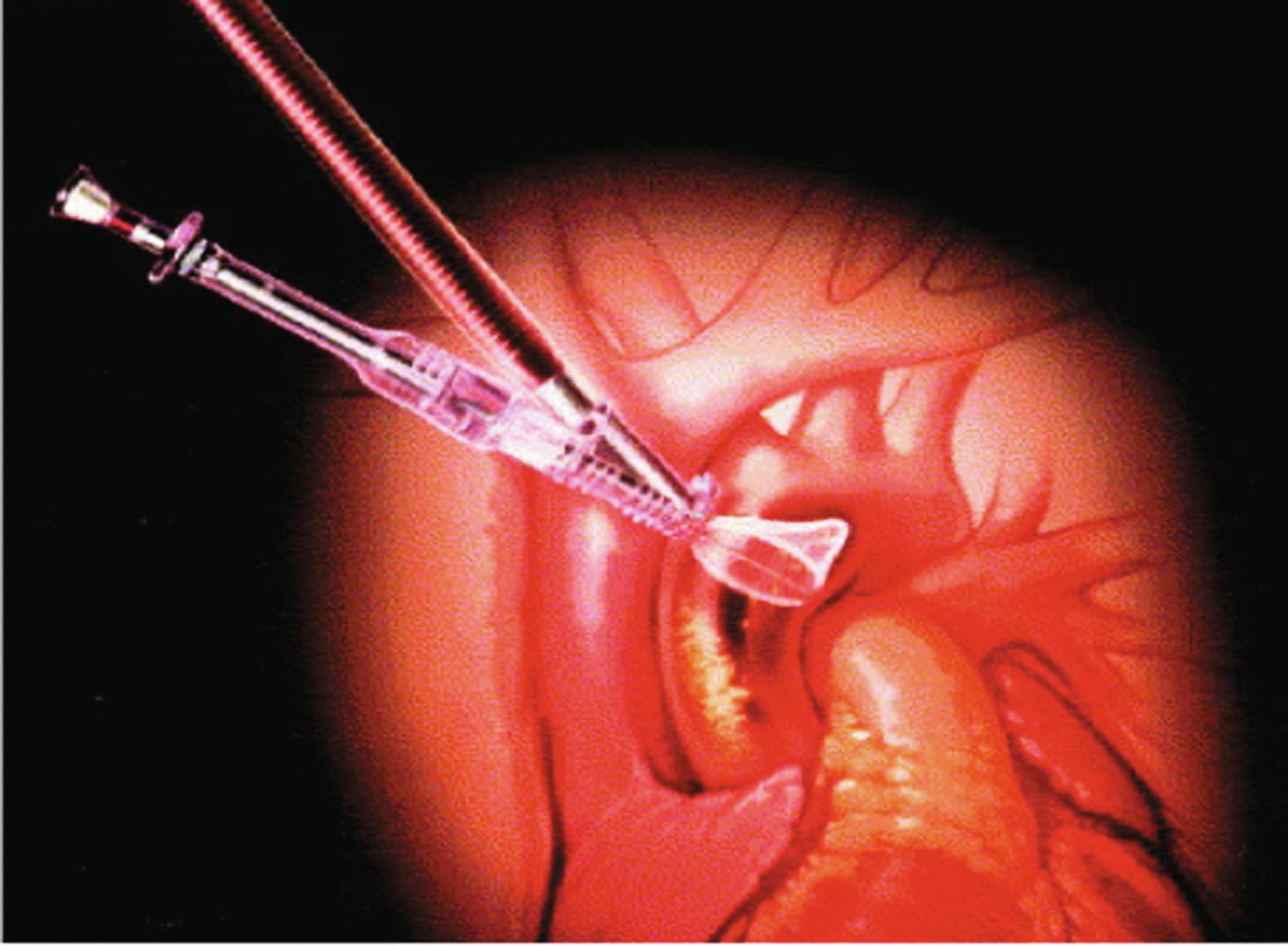
A recent multicenter, randomized controlled trial assigned 383 patients to the Embol-X device (133 patients), the CardioGard backward suction cannula (118 patients), or standard aortic cannula (132 patients) during surgical aortic valve replacement. In this landmark trial, significant embolic debris was found in 99% of the Embol-X filters after retrieval and 75% of the CardioGard suction cannulae. The investigators found no significant difference between groups with regards to clinical endpoints such as clinically apparent stroke and stroke volume as quantified by diffusion-weighted magnetic resonance imaging. There was no difference in mortality, length of hospital stay, and hospital readmission rate. In secondary outcome analysis, embolic protection was significantly associated with reduced risks of perioperative delirium and acute kidney injury. Overall, this important trial did not find major significant neuroprotective benefit from embolic protection. Future adequately powered trials will probably focus on high-risk patients in the search of further neuroprotective benefit from refinements in aortic cannula design.
Cardiac surgical patients during CPB are exposed to a high risk of gaseous emboli because of factors such as open chamber procedures, vascular cannulation, and assisted vacuum venous drainage. Once gas emboli reach the cerebral vasculature, they may occlude small vessels causing ischemia, or activate an inflammatory cascade further exacerbating injury. It has been hypothesized that by flooding the surgical field with highly blood-soluble carbon dioxide, most of the gas introduced into the patient will dissolve, reducing the risk of embolization. Although multiple trials have tested his hypothesis by demonstrating reduced intracardiac gas, they have collectively not proven that there is clinical neuroprotection from this intervention. A recent high-quality meta-analysis demonstrated no significant difference in the incidence of clinical stroke (1.0% versus 1.2%, P = 0.62) or neurocognitive decline (12% versus 21%, P = 0.35) with carbon dioxide exposure, despite a reduction in gaseous microemboli as detected by transesophageal echocardiography. Although there is evidence of reduced gaseous embolic load from flooding the surgical field with carbon dioxide, this has not translated to a reduction in stroke or neurocognitive impairment. A large randomized control trial is currently in progress to examine the effects of carbon dioxide application during coronary artery bypass grafting. The results of this trial may determine the future niche for this intervention in cardiac surgery with and without CPB.
The presence of atheroma in the ascending aorta is a significant risk factor of neurological injury in cardiac surgery as it is a major source of cerebral emboli. Aortic events such as cannulation, clamping, graft anastomosis, and manipulation may dislodge atheromatous debris and precipitate significant cerebral atheroembolism. Furthermore, the “sandblasting” effects of blood flow through the aortic cannula can embolize atheromatous plaques, as outlined earlier.
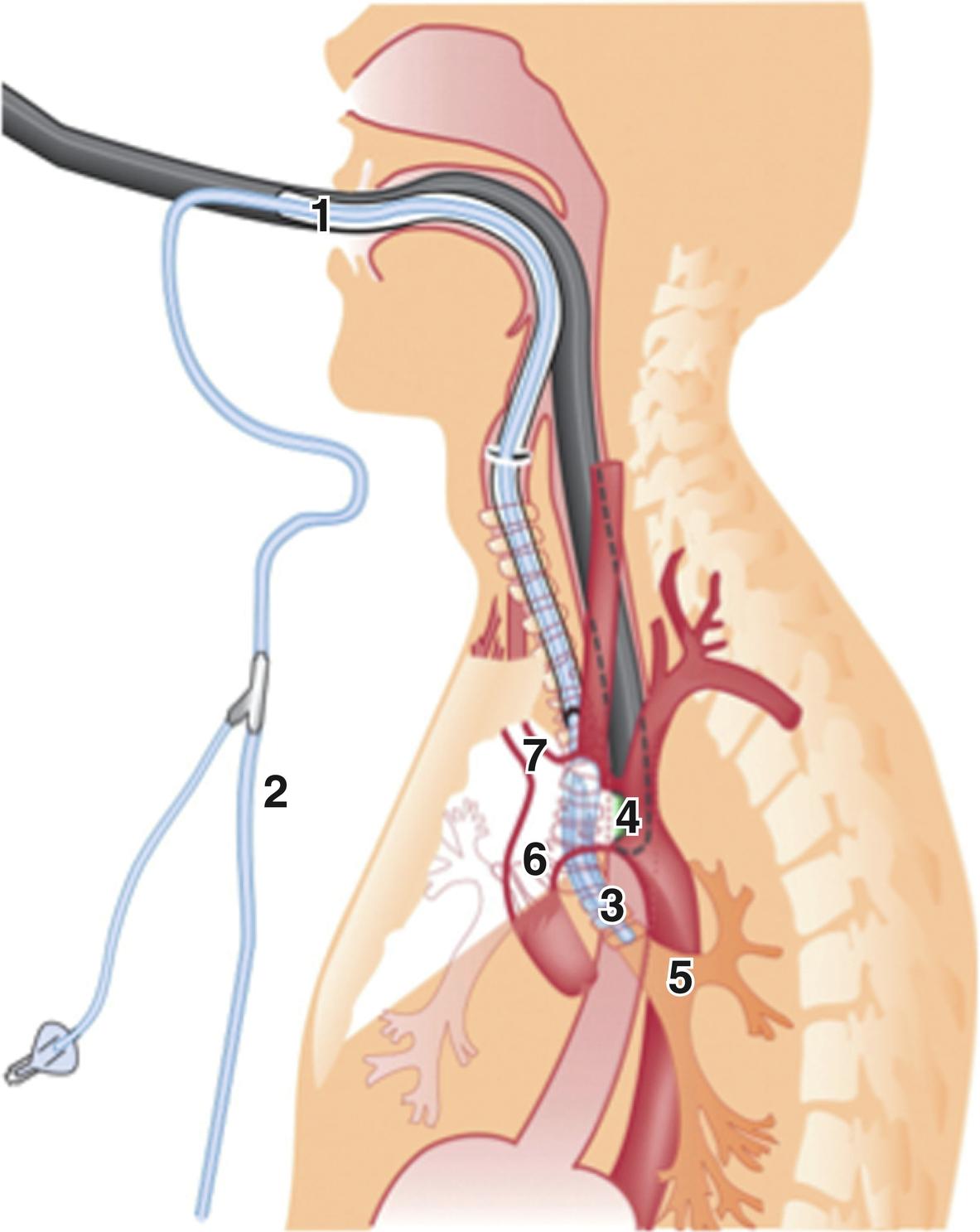
Traditionally, direct digital palpation of the aorta by the surgeon guided final selection of the aortic cannulation site. In contemporary practice, aortic imaging has proven superior to digital palpation for assessment of ascending aortic atheroma. The two imaging techniques to assess aortic atheroma are transesophageal echocardiography and epiaortic ultrasound. The major limitation of transesophageal imaging is that it cannot reliably image the distal ascending aorta and proximal arch because of the interposition of the tracheobronchial tree at this mediastinal level. This “blind spot” of transesophageal imaging has been overcome with the A-view technique. This modified technique involves the introduction of a balloon catheter into the left main bronchus, which is then filled with sterile saline, allowing visualization of the distal ascending aorta from the esophagus ( Fig. 22.3 ).
When epiaortic imaging has been applied to identify atheroma, it is more likely to prompt a change in aortic cannulation site or strategy compared with palpation alone. Large trials have demonstrated that epiaortic imaging was significantly associated with a reduction in stroke risk after adult cardiac surgery with CPB. The cumulative evidence suggests that epiaortic scanning provides the best method overall for grading ascending aortic atheromatous disease to guide aortic cannulation and to reduce consequent stroke risk.
There has been significant debate regarding the optimal pH management strategy during cardiopulmonary bypass. In the adult population, alpha-stat management is most often utilized. In alpha-stat management, the hypocarbia and alkalosis created by hypothermia is not corrected and the pH and carbon dioxide in the blood is measured at a temperature of 37°C. Alpha-stat maintains electrochemical neutrality and preserves the coupling between cerebral metabolic rate and cerebral blood flow (both decrease with cooling). In pH-stat management, carbon dioxide is added to the CPB circuit to account for the increase in solubility as cooling occurs, in an effort to maintain a normal pH and PaCO 2 . The increased levels of CO 2 lead to increased cerebral blood flow and oxygen delivery, but also increase the risk of cerebral embolization. A landmark randomized controlled trial ( N = 316) demonstrated a significant cognitive benefit to alpha-stat management in adult patients undergoing CPB for more than 90 min. The current evidence suggests that in cardiac surgery with deep hypothermic circulatory arrest alpha-stat management may be advantageous in adult cardiac surgery. In pediatric cardiac surgery with CPB, pH-stat management may be preferred for more efficient cooling of the brain and better neuroprotection.
In cardiac surgery with cardiopulmonary bypass, patients are often cooled to a certain hypothermia level, as outlined earlier. The benefits of hypothermic CPB have not been consistently demonstrated across multiple studies. Despite large meta-analyses (cumulative N > 10,000: normothermic versus hypothermic CPB), there was no significant difference in neurological outcomes in both adult and pediatric practice. It remains challenging with the current evidence to rule out hypothermic CPB as being beneficial because of the excessive heterogeneity across clinical studies.
In contrast, perioperative hyperthermia has been clearly shown to be detrimental to neurological outcome in cardiac surgery with CPB. In a clinical trial evaluating the clinical effects of temperature management during CPB and cardioplegia, the investigators found an increased stroke risk in the setting of warm CPB with warm cardioplegia. Perioperative hyperthermia has also been significantly associated with a higher risk of cognitive dysfunction after cardiac surgery with CPB. Multiple clinical trials have shown a reduction in neurological injury after CPB with a slower rewarming strategy during CPB. A comprehensive temperature management strategy during and after cardiac surgery with CPB is an important neuroprotective strategy in perioperative practice.
Cerebral ischemia can result not only from the obstruction of blood flow caused by embolic events, but also from inadequate perfusion pressure despite cerebral autoregulation. Higher blood pressure goals may be required in the settings of chronic hypertension and cerebrovascular disease to ensure adequate cerebral perfusion pressure during CPB. In a single-center randomized trial ( N = 248), a high mean arterial pressure (80–100 mmHg) compared with low mean arterial pressure (50–60 mmHg) during CPB significantly decreased the incidence of combined cardiac and neurological complications (4.8% versus 12.9%: P = 0.026). A second single-center randomized trial ( N = 92) demonstrated that a lower mean arterial pressure (60–70 mmHg) compared with a higher mean arterial pressure (80–90 mmHg) during normothermic CPB was significantly associated with increased risks of delirium ( P = 0.017) and cognitive dysfunction ( P = 0.012). However, a recent large, multicenter trial ( N = 197) found no significant difference in neurological injury with respect to clinical or radiographic stroke whether the mean arterial pressure was higher (70–80 mmHg) or lower (40–50 mmHg) during CPB. These conflicting trials suggest that it may not be appropriate to standardize a target mean arterial pressure during CPB but rather to consider the determination of an individual patient’s cerebral autoregulation thresholds. This determination would then facilitate individualized maintenance of perfusion pressure above the lower limit of cerebral autoregulation during CPB to ensure adequate cerebral perfusion and optimal neuroporotection.
Cerebral oximetry with near-infrared spectroscopy has displayed significant promise for estimating cerebral autoregulation and perfusion. Clinical trials have identified that low cerebral oximetry values significantly predict the risk of delirium after CPB. A recent meta-analysis ( N = 2057: 15 randomized controlled trials) found that cerebral oximetry-guided blood pressure management was associated with a significant reduction in cognitive dysfunction (risk ratio 0.54; 95% confidence interval 0.33–0.90; P = 0.02). Although this meta-analysis has suggested a neuroprotective benefit with cerebral oximetry, this result should be regarded as mostly hypothesis-generating, given the high levels of heterogeneity across the included trials. Future large, randomized trials with standardized monitoring and management protocols will probably evaluate the value of cerebral oximetry in the management of cerebral autoregulation and perfusion during adult cardiac surgery with CPB.
Recent clinical trials have suggested that pulsatile as compared with the typical non-pulsatile perfusion during CPB is more physiologic with enhanced preservation of the microcirculation and attenuation the systemic inflammatory response. Clinical trials thus far have not consistently demonstrated neuroprotection because of pulsatile perfusion during CPB. With such conflicting data, there is no clear neuroprotective benefit of pulsatile flow during CPB, suggesting that future trials should address this gap.
Because of the systemic inflammatory response to CPB and its possible role in cerebral injury, the administration of corticosteroids has been examined for neuroprotective benefit. Multiple clinical trials presented results with conflicting evidence. In high-quality meta-analysis, there was no discernible neuroprotective benefit from steroid exposure. Two recent, large, multicenter randomized controlled, double-blinded trials, the Dexamethasone for Cardiac Surgery (DECS) and Steroids in Cardiac Surgery (SIRS) trials, examined the neuroprotective effects of steroids in cardiac surgery with CPB. The DECS trial randomized 4494 patients to receive 1 mg/kg of dexamethasone or placebo. The investigators found no difference in the composite primary study endpoint that included stroke: the risk of stroke was also equivalent between groups. The SIRS trial randomized 7507 patients to receive 250 mg methylprednisolone at induction and another 250 mg at initiation of CPB. The investigators found no significant difference in stroke rates between study groups. A substudy of the DECS trial also demonstrated no difference in neurocognitive dysfunction in the first year after adult cardiac surgery with CPB both at 1 month (relative risk 1.87; 95% confidence interval 0.90–3.88; P = 0.09) and at 12 months (relative risk 1.98; 95% confidence interval 0.61–6.40; P = 0.24). A recent, large, meta-analysis ( N > 7000: 56 randomized clinical trials) also did not demonstrate any neuroprotective benefits from steroid exposure during cardiac surgery with CPB.
In addition to steroids, lidocaine has also been examined for neuroprotection because of its sodium-channel blocking and potential anti-inflammatory properties. Initial clinical trials demonstrated inconsistent neuroprotective effects of lidocaine. Subsequent meta-analyses have suggested a dose-dependent protection from cognitive dysfunction after cardiac surgery with CPB, although there was significant heterogeneity across the included trials.
Magnesium has also been studied for its neuroprotective effects, including its antiexcitotoxic agent and anti-inflammatory properties. Although it is safe, magnesium does not appear to be neuroprotective in recent clinical trials. Although ketamine may reduce delirium after cardiac surgery, this result has not been consistently evident in clinical trials.
Multiple agents have been tested for neuroprotection, including propofol, calcium channel blockers, beta blockers, antioxidants, erythropoietin, piracetam, the complement inhibitor pexelizumab, the serine protease inhibitor aprotinin, and dexmedetomidine. Despite all this research, significant neuroprotective benefits have yet to be demonstrated.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here