Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Echocardiographic evaluation of prosthetic valves is similar, in many respects, to the evaluation of native valve disease. However, some important differences exist. First, several types of prosthetic valves are available, with differing fluid dynamics for each basic design and differing flow velocities for each valve size. Second, the mechanisms of valve dysfunction are somewhat different from those for native valve disease. Third, the technical aspects of imaging artificial devices—specifically, the problem of acoustic shadowing—significantly affect the diagnostic approach when prosthetic valve dysfunction is suspected ( Table 13.1 ).
| Mechanical AVR | Surgical Bioprosthetic AVR | Transcatheter Bioprosthetic AVR | Mechanical MVR | Bioprosthetic MVR | |
|---|---|---|---|---|---|
| Fluid dynamics | Complex fluid dynamics depending on valve type | Central orifice, laminar flow, blunt flow profile | Central orifice, laminar flow, blunt flow profile | Complex fluid dynamics depending on valve type | Central orifice, laminar flow, blunt flow profile |
| Echo imaging | Shadowing and reverberations limit valve imaging. | Echogenic sewing ring and 3 struts Trileaflet porcine or pericardial tissue similar to that of a native aortic valve |
Increased echogenicity of aortic sinuses and annulus due to supporting stent Biologic valve leaflets appear similar to a native aortic valve |
Shadowing and reverberations limit valve imaging on TTE. Valve occluder motion well seen on TEE |
Stented valve, flow directed toward septum Trileaflet porcine or pericardial tissue similar to that of a native aortic valve |
| Normal Doppler findings | Antegrade velocity <3 m/s with triangular flow curve Mild eccentric AR due to occluder closure |
Antegrade velocity <3 m/s with triangular flow curve No to trace central AR |
Antegrade velocity <3 m/s with triangular flow curve Mild valvular or paravalvular AR |
Antegrade velocity <1.9 m/s with short  Mild eccentric MR due to occluder closure |
Antegrade velocity <1.9 m/s with short  No to trace central MR |
| Advantages/disadvantages | Excellent long-term durability Requires long-term anticoagulation |
Variable durability, longer in older patients Does not require anticoagulation |
Unknown long-term durability Currently recommended in higher-risk patients Does not require anticoagulation |
Excellent long-term durability Requires long-term anticoagulation |
Variable durability, longer in older patients Does not require anticoagulation (unless needed for AF) |
| Complications | Valve thrombosis Pannus Paravalvular AR Endocarditis |
Leaflet degeneration Stenosis Regurgitation Pannus Paravalvular AR Endocarditis |
Leaflet degeneration Stenosis Regurgitation Pannus Paravalvular AR Endocarditis |
Valve thrombosis Pannus Paravalvular MR Endocarditis |
Leaflet degeneration Stenosis Regurgitation Pannus Paravalvular MR Endocarditis |
| Echo follow-up (in addition to annual clinical evaluation) | Baseline postop Changing signs or symptoms |
Baseline postop Changing signs or symptoms Annual exams starting 5 years after implantation |
Baseline postop Changing signs or symptoms Annual exams recommended at this time |
Baseline postop Changing signs or symptoms |
Baseline postop Changing signs or symptoms Annual exams starting 5 years after implantation |
Echocardiographers frequently are asked to evaluate prosthetic valve function because of the increasing number of prosthetic valves implanted annually and the greater longevity of patients with prosthetic valves. Both an understanding of the basic approach to echocardiographic evaluation (as outlined in this chapter) and detailed knowledge of the specific flow dynamic for the size and type of prosthesis in an individual patient are needed for appropriate patient management.
The three basic types of prosthetic valves ( Figs. 13.1 and 13.2 ) are:
Tissue valves, or bioprostheses
Homograft valves
Mechanical valves
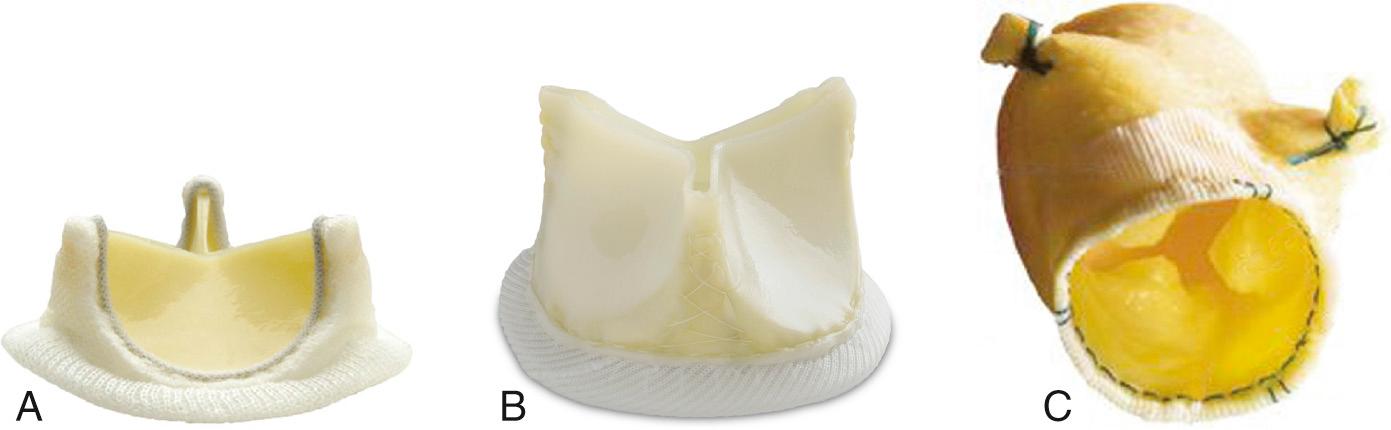
Bioprosthetic heart valves are implanted either surgically or by a transcatheter approach.
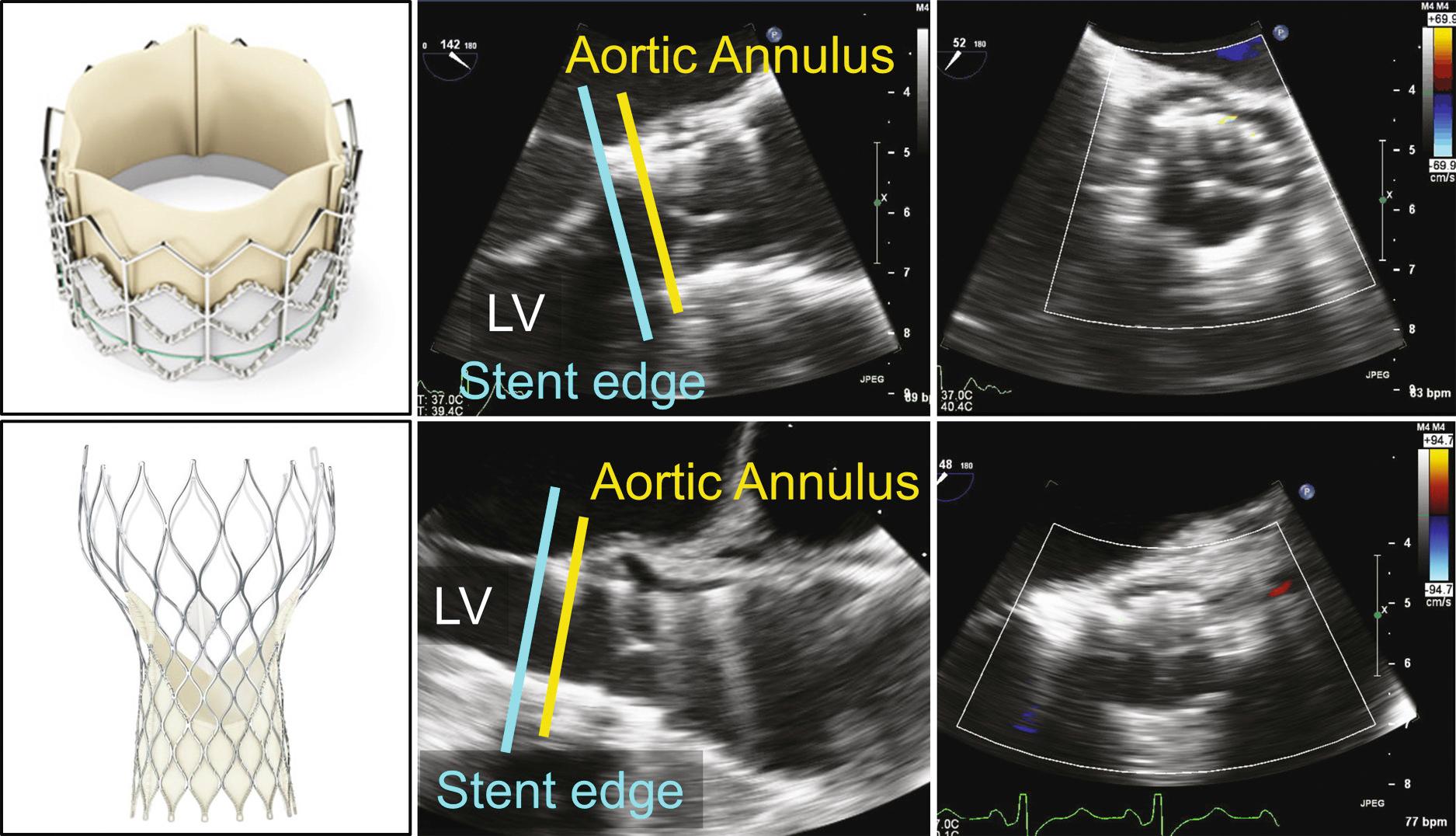
Tissue valves are composed of three biologic leaflets with an anatomic structure similar to that of the native aortic valve. With stented prosthetic valves, the leaflets (typically porcine), or pericardium (usually bovine or equine) shaped to mimic normal leaflets, are mounted on a cloth-covered rigid support that functions as the crown-shaped aortic annulus with a raised “stent” at each of the three commissures ( Fig. 13.3 ). Variations in the support structure and leaflet types abound in commercially available valves; some include anticalcification treatments. “Stentless” tissue valves also have been developed that use a flexible cuff of fabric or tissue, instead of rigid stents, to support the valve leaflets. Stentless valves often are implanted as part of a composite tissue valve and aortic root. In the past, bioprosthetic valves were implanted only surgically using cardiopulmonary bypass to support the circulation while the valve was implanted. Now, specially designed bioprosthetic valves can be implanted by a transcatheter approach with the tissue leaflets mounted on a compressible stent ( Fig. 13.4 ).
Homograft valves are cryopreserved human aortic or pulmonic valves harvested at autopsy. Typically, the valve and great vessel are preserved as a block, to be trimmed appropriately at the time of implantation in the aortic or pulmonic position. Whereas the fluid dynamics of a homograft are similar to those of a native valve, flow velocities are slightly higher and valve areas are slightly smaller than for a normal native valve because of the space occupied by the homograft annulus in the patient's outflow tract. Because of late severe tissue calcification with homograft valves, the approach usually is reserved for adults with complex aortic root abscesses.
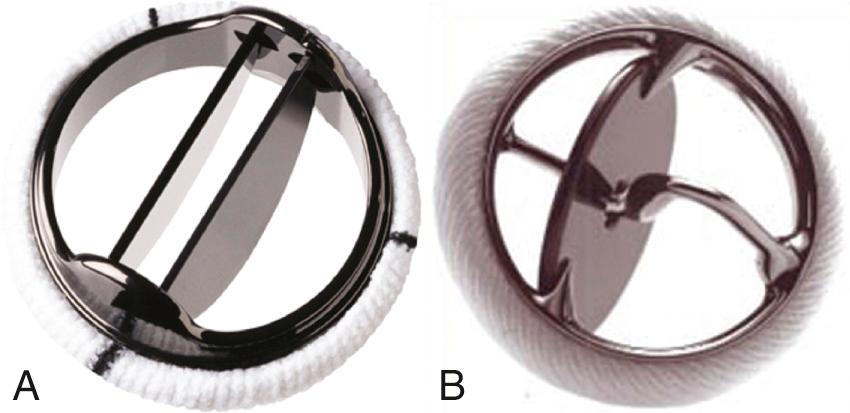
Various mechanical valves currently are available. In addition, several other types of valves, which were implanted in the past, are still in situ in some patients. The two basic types of currently implanted mechanical valves are:
A bileaflet valve in which two semicircular disks hinge open to form two large lateral orifices and a smaller central orifice ( Figs. 13.5 and 13.6 )
A tilting-disk valve in which a single circular disk opens at an angle to the annulus plane, being constrained in its motion by a smaller “cage,” a central strut, or a slanted slot in the valve ring
In the past, ball-cage mechanical valves also were used and are still occasionally encountered. With a ball-cage valve, a spherical occluder is contained by a metal “cage” when the valve is open and fills the orifice in the closed position.
Valved conduits are used in congenital heart surgery and in ascending aortic repairs when both a new passageway for blood flow and a valve are needed. Conduits are constructed from biologic (e.g., a homograft) or artificial (e.g., various types of fabric) material, either with a stented tissue or a mechanical valve. Fluid dynamics similar to those for a valve implanted in the native annulus. A stentless valve in root prosthesis also can be used in this situation.
The types of disease processes that affect prosthetic valves are distinctly different from those seen with native valvular heart disease and can be classified into three groups:
Structural failure
Thromboembolic complications
Endocarditis
Failure of a bioprosthetic valve to open or close properly (mechanical failure) usually is the result of slowly progressive tissue degeneration with fibrocalcific changes of the leaflets, a process that results in increased resistance to opening (stenosis) or failure to coapt during valve closure (regurgitation). Typically, failure of tissue valves occurs 10 or more years after valve implantation. Acute bioprosthetic valve stenosis is rare. Acute bioprosthetic regurgitation can occur with a leaflet tear, usually adjacent to a region of calcification.
Failure of a mechanical valve can occur because of faulty design or wear and tear of the prosthetic material resulting in disk escape or incomplete valve closure. However, these complications were seen only with older-generation valves. Current-generation mechanical valves are reliable and very durable. More often, mechanical valve stenosis or regurgitation is due to thrombus formation or pannus ingrowth around the valve, thus impairing disk excursion or closure.
With both bioprosthetic and mechanical valves, paravalvular regurgitation can occur around the sewing ring because of loss of suture material postoperatively; this is most often related to fibrocalcific disease in the valve annulus. The new onset of paravalvular regurgitation late after surgery raises the possibility of an infectious process (endocarditis) resulting in valve dehiscence.
Prosthetic valves, particularly mechanical valves, are prone to thrombus formation with consequent systemic embolic events or valve dysfunction. Echocardiographic evaluation for prosthetic valve thrombus is limited, except with very large masses, because of shadowing and reverberations. In addition, clinical events may be associated with clots smaller than the limits of clinical ultrasound resolution. Thus, echocardiography cannot exclude the possibility of thrombus on a prosthetic valve; in patients with embolic events, the prosthetic valve itself is a potential source of embolus.
Infection of a valve prosthesis is a serious clinical problem, so suspected endocarditis is a frequent indication for echocardiography in patients with prosthetic valves. Endocarditis on a bioprosthesis may result in vegetations similar to those seen on a native valve. However, with a mechanical valve, the infection often is paravalvular, and no discrete vegetation may be present.
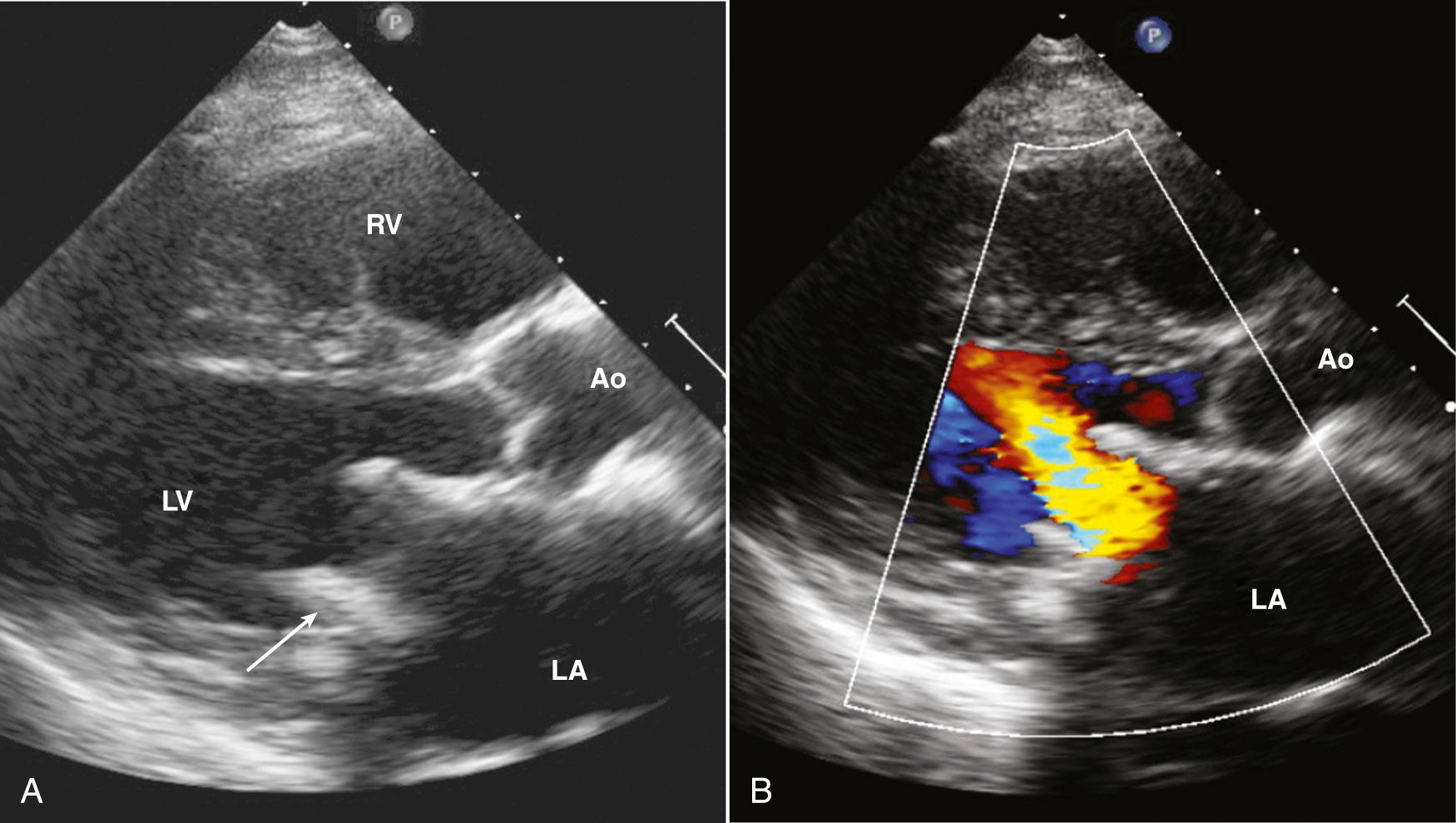
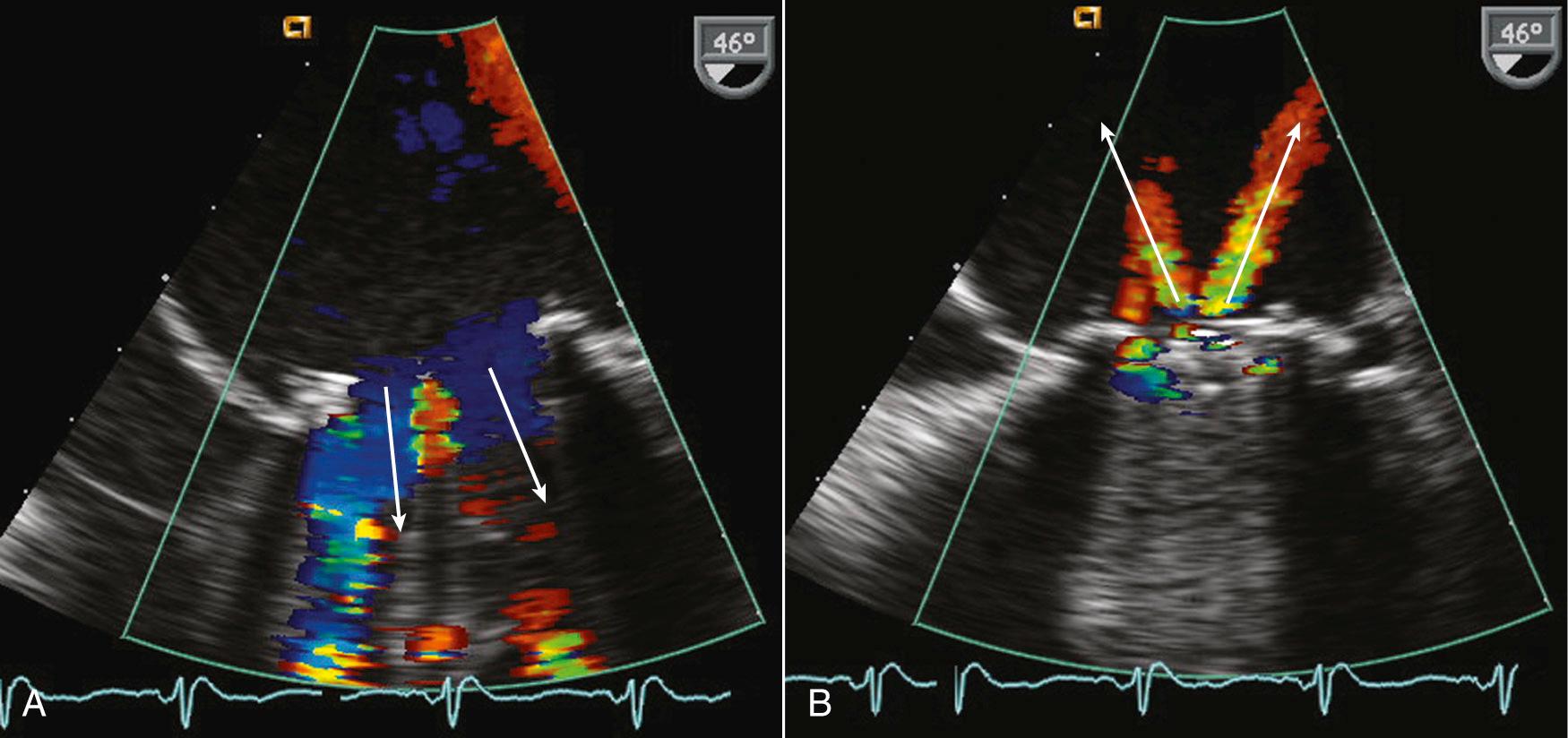
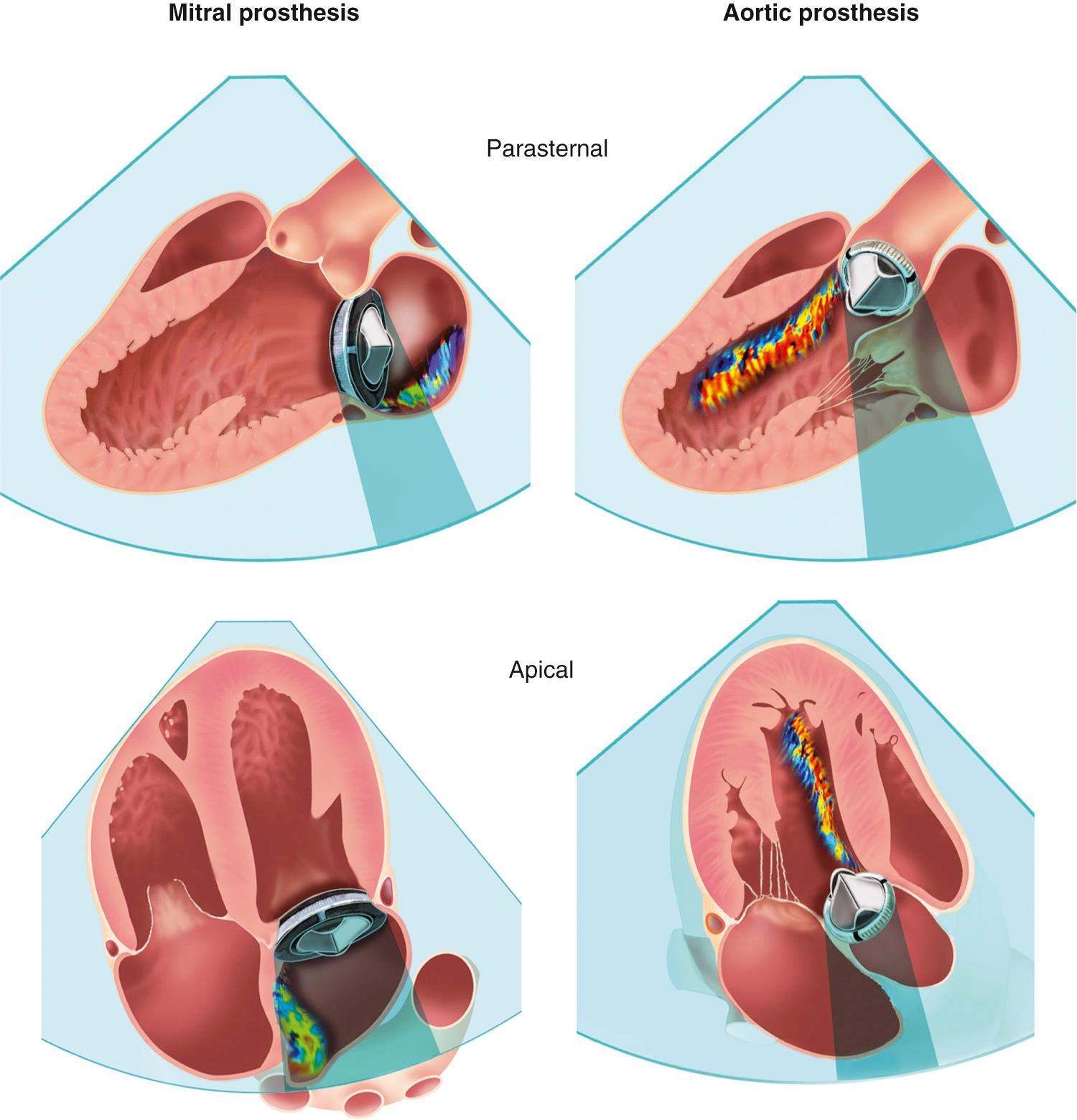
Evaluating prosthetic valves by echocardiography has two major challenges. The normal fluid dynamics of the prosthetic valve must be distinguished from prosthetic valve dysfunction. However, the most technically limiting aspect of the echocardiographic evaluation of prosthetic valves is the problem of acoustic shadowing. The sewing rings of both surgical bioprosthetic and mechanical valves, the supporting stent of transcatheter valves, and the occluders of mechanical valves all are strong echo reflectors, resulting in acoustic shadows and reverberations ( Fig. 13.7 ). These reverberations and shadows obscure the motion of the valve structures and block detection of imaging and Doppler abnormalities in the acoustic shadow region. During the examination, considerable effort is directed toward using windows and views that avoid these imaging artifacts. Transesophageal echocardiography (TEE) is particularly useful in the evaluation of prosthetic mitral valves because it provides acoustic access from the left atrial (LA) side of the valve. Three-dimensional (3D) imaging often is helpful, although acoustic shadowing and reverberations still limit optimal valve visualization.
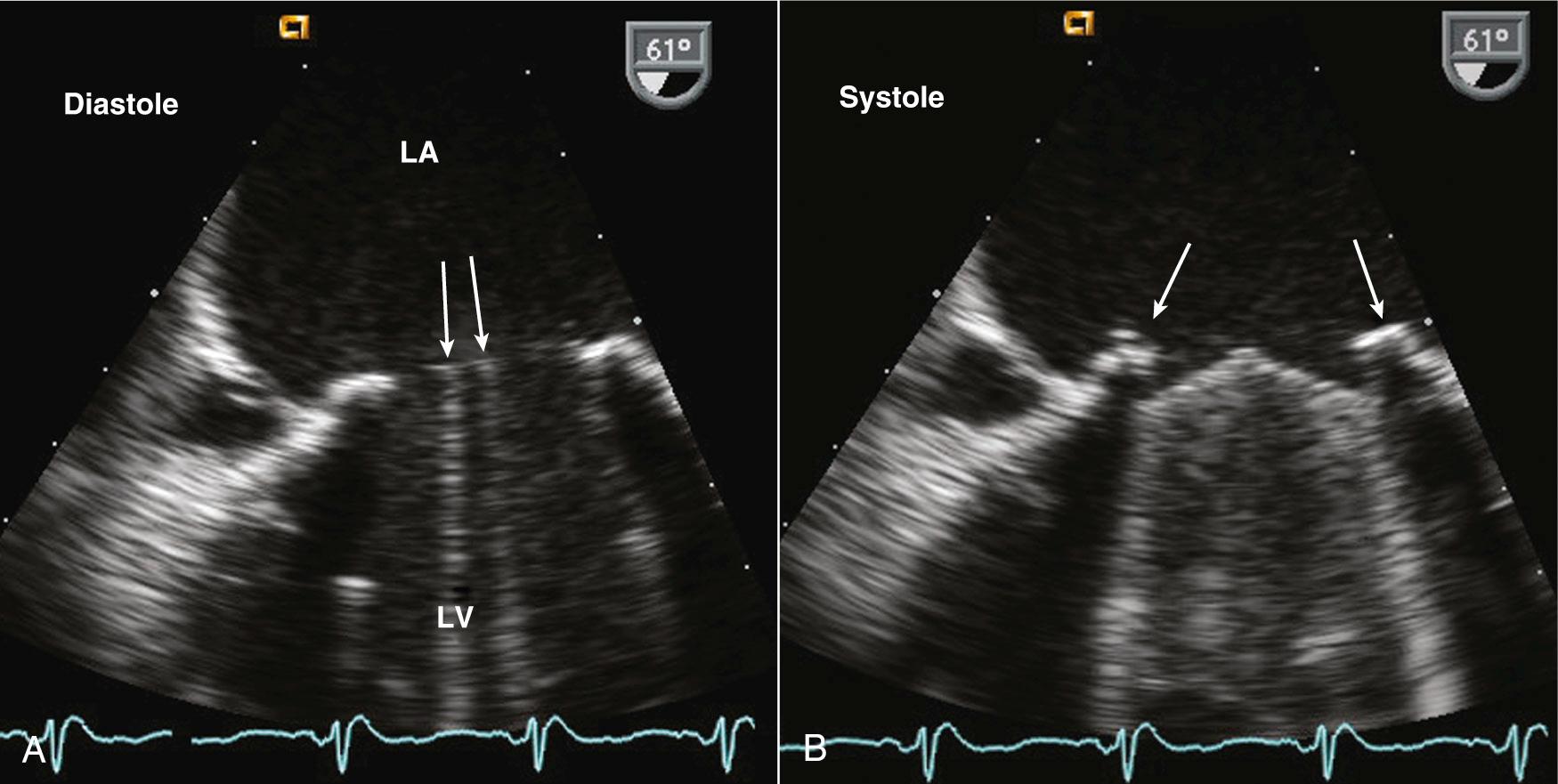
Stented tissue prosthetic valves have a trileaflet structure similar to that of a native aortic valve. An M-mode recording through the leaflets shows the typical “boxlike” opening in systole (for the aortic position) or diastole (for the mitral position), as is seen with a normal native aortic valve. However, with conventional valve designs, the echogenic sewing ring and struts limit visualization of the leaflets, with the specific ultrasound appearance of the supporting structures depending on the specific model ( Fig. 13.8 ). When examining a patient with an unfamiliar valve type, a quick look online at valve photographs can be helpful. Stentless bioprosthetic valves have an echocardiographic appearance very similar to that of a native aortic valve, other than increased echogenicity in the aortic root in the early postoperative period. This valve is best identified by reviewing the chart or asking the patient about any cardiac surgical procedures before beginning the study.
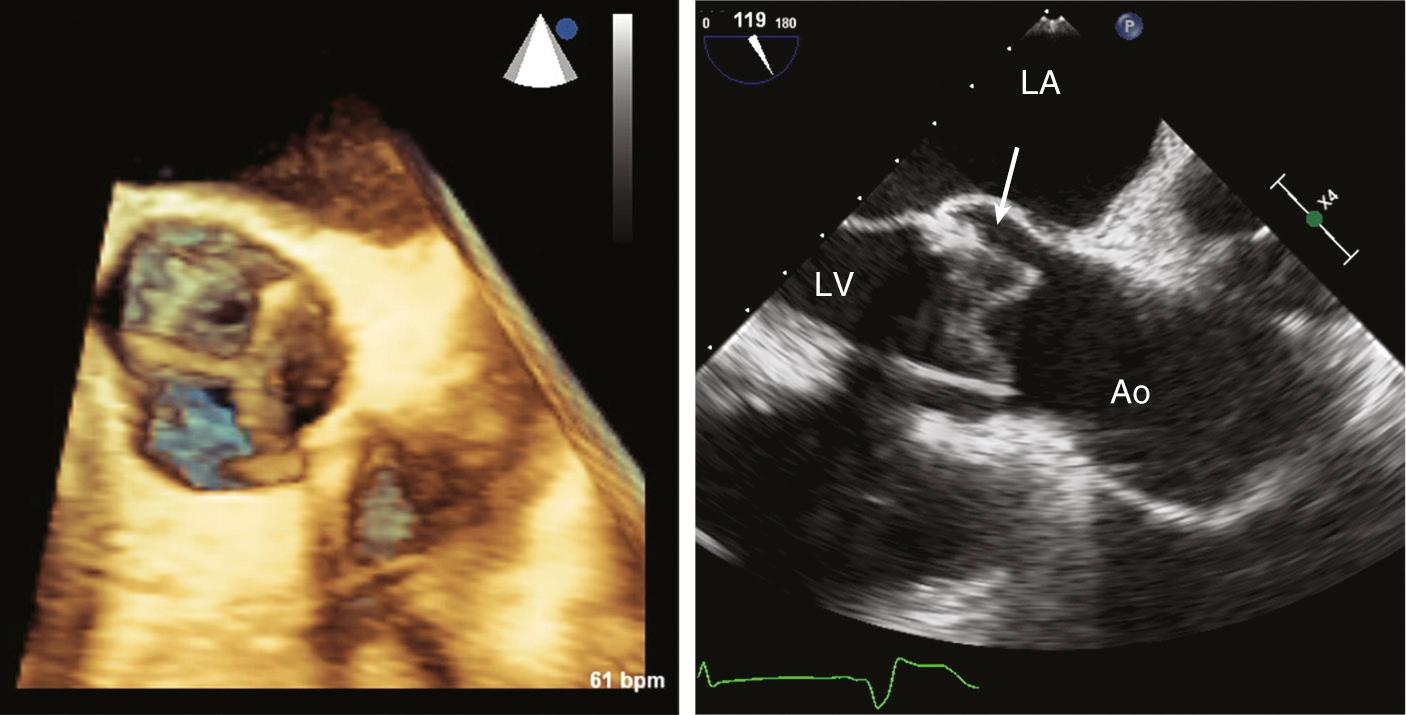
Transcatheter valves (see Fig. 13.4 ) in the aortic or pulmonic position appear similar to normal native valves, with three thin valve leaflets. However, increased echogenicity or thickness of the para-annular region is evidence. Some transcatheter aortic valves have a longer supporting cage that extends into the left ventricular (LV) outflow tract or ascending aorta. Transcatheter mitral valves have a supporting cylindrical cage that extends into the LV chamber, with a variable appearance depending on specific valve type.
Aortic homografts appear similar to native aortic valves except for some increased thickness in the LV outflow tract and the ascending aorta at the proximal and distal suture sites. Typically, the homograft is implanted using the mini-root technique with the homograft replacing a segment of the native aorta. This approach necessitates reimplantation of the coronary arteries. In the past, the aortic homograft sometimes was positioned inside the patient's native aorta with appropriate trimming to maintain patency of the coronary ostia. In patients with endocarditis, the attached anterior mitral leaflet of the homograft is an option for patching a ventricular septal defect or abscess cavity. The echocardiographic appearance of a homograft is very similar to that of a native aortic valve, except for the associated surgical changes. Standard parasternal long- and short-axis image planes provide optimal visualization of valve leaflet anatomy and motion.
Improved images of prosthetic tissue valves can be obtained from a TEE approach, particularly for valves in the mitral position, because the ultrasound beam has a perpendicular orientation to the leaflets with no intervening structures from this approach. With aortic valve prostheses, TEE imaging is less rewarding because the posterior part of the sewing ring shadows the valve leaflets. When images of the leaflets themselves are suboptimal, Doppler data can provide valuable information.
The longevity of bioprosthesis valves typically is limited by slowly progressive tissue failure with fibrocalcific changes resulting in leaflet deformity (leading to regurgitation), increased stiffness (leading to stenosis), or both. Echocardiographically, increased echogenicity and irregularity of the leaflets are typical, although images of the leaflets often are suboptimal because of shadowing and reverberation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here