Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Defining and classifying tumors of the hematopoietic and lymphoid tissue accurately is critical for providing optimal treatment to patients with hematologic malignancies. Explicit definitions and terminologies are prerequisites for the precise classification of hematologic malignancies. A reproducible classification that is based on consensus definitions and terminologies is fundamentally essential for proper medical practice and the advancement of medical knowledge. Generally, such classifications should be based upon clinically distinct disease entities that can be grouped together based on shared distinguishable clinical features, as well as phenotypic and molecular markers which allow for reproducible laboratory testing, clinical outcomes, and responses to therapeutic agents. Traditionally, preliminary attempts at disease classification were initiated by the personal experience of experts, and then evolved after many years or even decades of controversy and exhaustive debate. Subsequently, classification schemes change when new scientific discoveries are validated.
Following many years of controversy, a collaborative project of members of the European Association for Haematopathology and the Society for Hematopathology, along with advice from clinical hematologists and oncologists, resulted in the World Health Organization (WHO) Classification of Tumours of Haematopoietic and Lymphoid Tissues. This WHO classification, which is the first true worldwide consensus classification of hematologic malignancies, is based on the principles initially defined in the “Revised European-American Classification of Lymphoid Neoplasms” (REAL) from the International Lymphoma Study Group (ILSG). The most recent WHO classification (4th edition) was formulated in a series of meetings by two clinically advisory committees: one for myeloid neoplasms, and another for acute leukemias and lymphoid neoplasms. The current WHO classification, which addresses new developments related to disease definitions, nomenclature, grading, and clinical relevance, provides the basis for an approach to hematopoietic and lymphoid neoplasm classification that employs clinical features, morphology, immune phenotype, and genetic features to define diseases. The relative importance of each of these components varies among diseases and is dependent on the current state of knowledge. This process of classification is an ever changing process which evolves upon new scientific and clinical advances. These classifications should be used as ever changing tools, not as groupings of disease entities that are fixed in academic concrete. Morphologic assessment remains a key element of the diagnostic evaluation and classification of hematologic malignancies, as many diseases have characteristic diagnostic features. Morphologic evaluation also remains a decisive step for prioritization of ancillary testing choices that may include phenotyping, as well as molecular or genetic testing. Immunophenotypic studies such as flow cytometry and immunohistochemistry (IHC) are used routinely to identify the lineage of the benign or malignant process, and often are necessary to reach a definitive diagnosis. In several lymphoid and myeloid neoplasms, a specific diagnosis can be facilitated by using genetic or molecular abnormalities (e.g., CCND1 in suspected mantle cell lymphoma [MCL] or BCR/ABL for chronic myeloid leukemia [CML]). In addition, some abnormalities can serve as prognostic markers in several diseases (e.g., TP53 deletion or mutation ). However, many entities still lack defining genetic or molecular abnormalities.
In some conditions, rendering an accurate diagnosis requires knowledge of the patient’s clinical features, such as age and prior therapy, as well as the anatomic site and/or extent of disease. Although most of the disease entities described in the WHO classification represent distinct entities, some categories are not as clearly defined and are regarded as provisional entities. In addition, borderline categories have been created for cases that do not clearly fit into one category; thus well-defined categories can remain homogeneous while borderline cases require further study and characterization. Furthermore, the WHO classification stratifies hematologic neoplasms according to lineage into three main groups: myeloid, lymphoid, and histiocytic/dendritic cells. In this chapter we provide a summary of neoplasms in these three groups, emphasizing changes that have had an effect on practice guidelines along with highlights to significant findings that have been identified in these entities since the publication of the most recent WHO classification in 2016.
The myeloid neoplasms are a heterogeneous group of diseases. In general terms, they are clonal hematopoietic malignancies that can arise in or affect a single myeloid lineage (e.g., monocytic). Alternatively, they can be derived from a pluripotent stem cell and can affect multiple or even all myeloid cell types (erythroid, megakaryocytic, monocytic, neutrophilic, basophilic, eosinophilic, and mast cells). The myeloid neoplasms include chronic and acute diseases, along with diseases that evolve from an indolent process to a more aggressive state. As such, they can manifest as proliferative disorders, mostly involving primary mature hematopoietic elements; they can predominately affect immature or blastic cells; or sometimes they can be a combination of these two cell types. The diseases can result in excess production of blood cells, or they can proliferate in the bone marrow (BM) and exhibit ineffective hematopoiesis, resulting in peripheral cytopenias. Because the diseases range from indolent to acute, they exhibit a wide variation in prognosis. Some disorders are fairly indolent and require only supportive care and transfusional support; others slowly progress but have no successful treatment option. Acute and life-threatening disorders are often curable, even within days or weeks after presentation, if appropriately managed. Accurate, timely diagnosis and correct classification has a tremendous impact on patient outcomes. Treatment of a particular hematological malignancy should only proceed once the nature of the blood cancer is known, which allows for the selection of rational choices of treatment options.
The diagnosis of the myeloid neoplasms typically requires a multifaceted team approach that relies on cooperation among the clinician, laboratory personnel, and a skilled pathologist. The precise diagnosis requires compiling accurate historical data, clinical information, general laboratory findings, and carefully interpreted observations from the peripheral blood smear, bone core biopsy, and aspirate, merged with information gained from immunophenotyping, cytogenetic analysis, and molecular analyses ( Table 56.1 ). Although in some cases a diagnosis can be made with a quick examination of the blood smear, with a single molecular test, or with a simple immunophenotype, in general, careful assessment of the total clinical picture provides the most accurate and clinically relevant diagnosis. Future routine diagnostic modalities may include next-generation sequencing, single-nucleotide polymorphism array karyotyping, gene expression arrays, and genome-wide epigenetic studies.
| Current |
|
| Potential Future Clinical Studies |
|
The myeloid neoplasms are grouped as different categories of diseases. These categories seem to be changing constantly, both in name and in the components of the disorders. However, refinements have led to an increased understanding of the clinical, pathologic, and genetic basis of the diseases and ultimately serve, or at least strive to, improve diagnosis, treatment, and outcome. The current classification of myeloid neoplasms includes the categories of myeloproliferative neoplasms (MPNs), acute myeloid leukemias (AMLs), myelodysplastic syndromes (MDS), and MDS/MPNs, which is a category with features that are intermediate between MDS and MPN ( Table 56.2 ). This generally accepted classification system was derived from a number of earlier classification systems and represents the working system of the WHO as of 2008. The recent 2016 update of the WHO classification is based on the additional information gained from molecular testing modalities that further impact the classification and categorization of these neoplasms.
|
The MPNs, formerly referred to as myeloproliferative disorders (see Chapters 69–74 ), are a group of clonal multipotential hematopoietic stem cell disorders that have a proliferative nature, presenting frequently with hypercellular BMs, and an elevation of one or more myeloid cell types in the blood. The MPNs are insidious in onset and chronic in course but have a variable tendency to terminate in BM failure or acute leukemia. The MPNs include CML ( Table 56.3 ), which illustrates how the elucidation of pathways involved in the molecular pathogenesis (i.e., dysregulation of ABL1 tyrosine kinase [TK] signaling) can successfully lead to the rational targeted therapy (i.e., imatinib and other TK inhibitors).
| Disease | Tyrosine Kinase Involvement |
|---|---|
| Chronic myeloid leukemia, BCR-ABL1 + | ABL1 (100%) |
| Myeloid and lymphoid neoplasms with eosinophilia TK abnormalities | PDGFRA, PDGFRB , or FGFRA |
| Polycythemia vera | JAK2 V617F (≈95%), JAK exon 12 (≈4%) |
| Primary myelofibrosis | JAK2 V6174 (≈50%), MPL W515 K/L (5%–9%), CALR (35%) |
| Essential thrombocythemia | JAK2 V617F (≈50%), MPL W515 K/L (≈1%), CALR (35%) |
| Chronic neutrophilic leukemia | CSF3R (83%–90%) |
| Chronic eosinophilic leukemia, not otherwise specified | — |
| Mastocytosis | KIT D816V (≈95%) |
| MPN, unclassified | — |
The MPNs also include BCR-ABL1– negative entities, essential thrombocythemia (ET), polycythemia vera (PV), and primary myelofibrosis (MF) (see Chapter 70, Chapter 71, Chapter 72 ). These MPNs share common features, including multipotential hematopoietic stem cell origin, clonal proliferation, and chronic nature. Although these disorders seem to be distinctive, they can present a diagnostic challenge, and they can have subtle and overlapping presentations. Recent discoveries in the underlying molecular pathology of these entities have demonstrated that, similar to CML and eosinophilic disorders, they too share TK signaling dysregulation, at least to some degree. This is attributable to an associated mutation in the TK, JAK2 ( JAK2 V617F), which is present in about 50% to 95% of cases of Ph-negative MPNs. Variants of JAK2 mutations involving exon 12 are also observed in 2% to 3% of PV patients. This association has led to significant revisions in the 2008 WHO criteria for diagnosis, but it also has raised questions as to how a single mutation can be associated with such heterogeneous phenotypic characteristics. Proposed revisions in 2016 WHO classifications for the diagnosis of the Ph-negative MPNs include suggested modifications of lower hemoglobin thresholds for PV. BM biopsy results and the presence of JAK2 mutations now represents a major criterion. A subnormal erythropoietin level became a minor criterion, and the endogenous erythroid colony assay is no longer included in the current WHO diagnostic criteria for MPNs. These changes are intended to capture patients with subtle presentations, such as those with masked PV. Experts also have recommended inclusion of mutations in calreticulin (CLAR) and the thrombopoietin receptor, MPL, which is identified in 20% to 30% of ET and MF cases, as well as the colony-stimulating factor 3 receptor mutations in the next revision.
All three (JAK2, CLAR, and MPL) driver mutations are negative in approximately 10% of cases (so-called “triple negative” [TN] cases). In these TN cases, the non-clonal differential diagnostic considerations pose significant diagnostic challenges. As such large-scale next generation sequencing is increasingly being used to establish not only the clonal nature of the disease, but also to guide molecular risk stratification and management decisions, including clinical trial selection for these patients when evaluated for MF.
JAK2 V617F inhibitors are among several agents in use to treat patients with MPNs. In 2011 the US Food and Drug Administration (FDA) approved ruxolitinib for the treatment of patients with intermediate or high-risk MF. Subsequently, the FDA also designated ruxolitinib as an orphan product, as it demonstrated potentially significant improvement in safety and efficacy over other available therapies for PV. A number of ruxolitinib-based combination therapies, as well as novel agents that target various pathways, are currently being investigated and will be further discussed in detail later (see Chapter 70, Chapter 71, Chapter 72 ).
Chronic neutrophilic leukemia (CNL) (see Chapter 72 ) and mast cell disease (see Chapter 75 ) are currently included in the category of MPNs. CNL is now better defined with the integration of CSF3R mutation as a diagnostic tool, and is distinct from atypical CML.
Mast cell disease is yet another disease related to abnormal TK signaling—in this case, the KIT gene. Its classification system generally considers systemic mastocytosis and cutaneous mastocytosis as main entities but also includes a mast cell proliferation associated with clonal non-mast cell hematopoietic malignancies; this can be another myeloid malignancy or, less commonly, a lymphoid malignancy. With the advances of comprehensive mutational profiling, it is proposed that additional mutations in SRSF2 , ASXL1 , and/or RUNX1 identify a high-risk group of patients with KIT D816V + advanced systemic mastocytosis. A more recently improved understanding of the cellular and molecular basis of eosinophilic disorders has translated into more biologically oriented classification schemes that carry therapeutic implications. Thus, in 2008, the WHO established a semimolecular classification scheme of separately listed disease subgroups including “myeloid and lymphoid neoplasms with eosinophilia and abnormalities of platelet-derived growth factor receptors (PDGFRA, PDGFRB), or fibroblast growth factor receptor 1(FGFR1),” chronic eosinophilic leukemia (CEL), not otherwise specified (NOS), lymphocyte-variant hypereosinophilia, and idiopathic hypereosinophilic syndrome (see Chapter 74 ). Although quite uncommon, these are noteworthy because, similar to CML, they have also been found to be caused by dysregulation of TK signaling (caused by mutations in PDGFRA , PDGFRB , or FGFRA ) and at least partially successfully treated with TK inhibitors.
It is important to emphasize that the modern diagnosis of the MPNs does not rest solely with the routine microscopic examination. The diagnostic work-up is more far-reaching and must include reviewing the clinical history and pertinent physical findings, as well as obtaining and assessing laboratory values, including recent complete blood cell counts. Examination of a well-prepared peripheral blood smear and both BM aspirate and biopsy specimens are still crucial. However, ancillary studies, such as cytogenetic and molecular analysis, as well as other more specific laboratory evaluations, are just as important in formulating the correct diagnosis, and in particular, in distinguishing MPNs from reactive myeloid proliferations. This is particularly true, for example, regarding the MPN driver mutations, and the impact not only on deciding the diagnosis but also on the prognosis and treatment of MPN patients.
The historical and emerging classifications systems for AML dramatically illustrate the marked heterogeneity of these diseases (see Chapters 59 and 60 ). Despite this heterogeneity, however, to date a major breakthrough has not been made in the development of specific initial treatment for AML as an entity apart from acute promyelocytic leukemia. However, to a large extent this concept is changing as newer agents targeting molecularly or genetically defined forms of AML have become available (e.g., FMS-like tyrosine kinase-3 [FLT3] inhibitors for FLT3-mutated cases). It is anticipated that the gap between the growing number of unique or genetically defined acute leukemia types and the somewhat limited treatment options may begin to close.
The French–American–British (FAB) classification of AML, introduced in 1976 with its subsequent revision in 1985, provided the first real framework for classifying the AMLs. It also provided fairly reproducible definitions of diseases. The classification was mainly based on morphologic and cytochemical features of the leukemic blasts in the BM. In general, the FAB scheme required that 30% of the BM-nucleated elements be blasts; then, it further defined AML subtypes based on the presence of maturation in the granulocytic series, the presence of a monocytic component, and the presence of an erythroid component. Later, as immunophenotyping allowed for improved identification of myeloid precursors, acute megakaryoblastic leukemia and AML with minimal differentiation were added. The types of AML noted in the revised FAB scheme included one with minimal differentiation (M0), AML without maturation (M1), AML with maturation (M2), and acute promyelocytic leukemia (M3). Also included was a type with combined monocytic and myeloid (neutrophilic) components (M4), a pure monocytic or monoblastic type (M5A, M5B), a type with a prominent erythroid component (M6), and a megakaryoblastic type (M7) ( Table 56.4 ).
| French–American–British (FAB, 1985) | Cytogenetic | WHO (2001) | WHO (2008) | WHO (2016) |
|---|---|---|---|---|
|
|
|
|
|
The FAB classification provided a framework and defined criteria for different types of acute leukemia. However, leukemia-associated genetic changes, first recognized in the 1980s, seemed to provide more important prognostic information and did not always correlate well with the FAB-defined entities. As the cytogenetic abnormalities became more widely appreciated and their prognostic implications better understood, classification schemes based solely on the “favorable,” “intermediate,” and “adverse” prognostic cytogenetic findings were introduced and used along with, or as an alternative to, the FAB scheme (see Table 56.4 ).
The AML WHO classification published in 2001 provided a different strategy from that used by the FAB in two important ways: it redefined AML as requiring only 20% blasts in the BM or blood, and it emphasized the importance of the associated cytogenetic abnormalities. The change in blast percentage came from the recognition that patients with 20% to 30% blasts, who previously were classified as having MDS (refractory anemia [RA] with excess of blasts in transformation [RAEB-T] in the FAB MDS classification), often had outcomes similar to those with AML and frequently required treatment as if they had been diagnosed with AML. Furthermore, the inclusion of karyotypic abnormalities recognized the important prognostic information associated with these abnormalities, as well as the fact that a karyotypic abnormality could point to the underlying molecular pathogenesis. As we learned from our experience with CML, this was critical in developing new drugs and treatment strategies. However, the WHO classification recognized that not all acute leukemias could be defined by cytogenetic abnormalities and that some needed to be defined either clinically or still based on morphologic findings. In this regard, the 2001 classification recognized four major subclasses of AML: AML with recurring cytogenetic abnormalities; therapy-related AML (subclassified further as those following etoposide treatment and those with a history of cytotoxic drug therapy or radiation); AML with multilineage dysplasia; and cases that did not fit into the other subclasses were referred to as AML not otherwise categorized (see Table 56.4 ).
A 2008 revision of the 2001 classification scheme emphasized risk-based stratification and made a number of changes (see Table 56.4 ). In particular, it expanded the cytogenetic abnormality-associated cases to those with some less common translocations. It added provisional entities defined by mutations often occurring in cytogenetically normal cases, namely those with NPM1 and CEBPA mutations. It combined the different types of therapy-related MDS/AML and renamed this category therapy-related myeloid neoplasm (t-MN). It also redefined the dysplasia-associated cases by allowing the cases to be identified by history (of previous MDS), morphology (with multilineage dysplasia), or by cytogenetics (with defined chromosomal changes associated with dysplasia). Additional categories were also added, including AML associated with Down syndrome, acute panmyelosis with MF, granulocytic sarcoma, and blastic plasmacytoid dendritic cell tumor (see Chapter 64 ). The 2008 classification eventually defined more than 25 different types of AML. The advent of newer technologies, such as single-nucleotide polymorphism array karyotyping and next-generation sequencing, facilitated the recognition of several relevant driver mutations. Molecular analysis for leukemic driver mutations, particularly in the cytogenetically normal AML subgroup (40% to 50% of AML patients), has been incorporated into routine clinical practice to assess disease progression and prognosis. Important mutations involve FLT3 , NPM1 , KIT , CEBPα , TET2 , DNMT3A , and IDH1 . Although the relevance of many of these mutations to prognosis is defined, some are still debated and their coexisting consequences are yet to be determined.
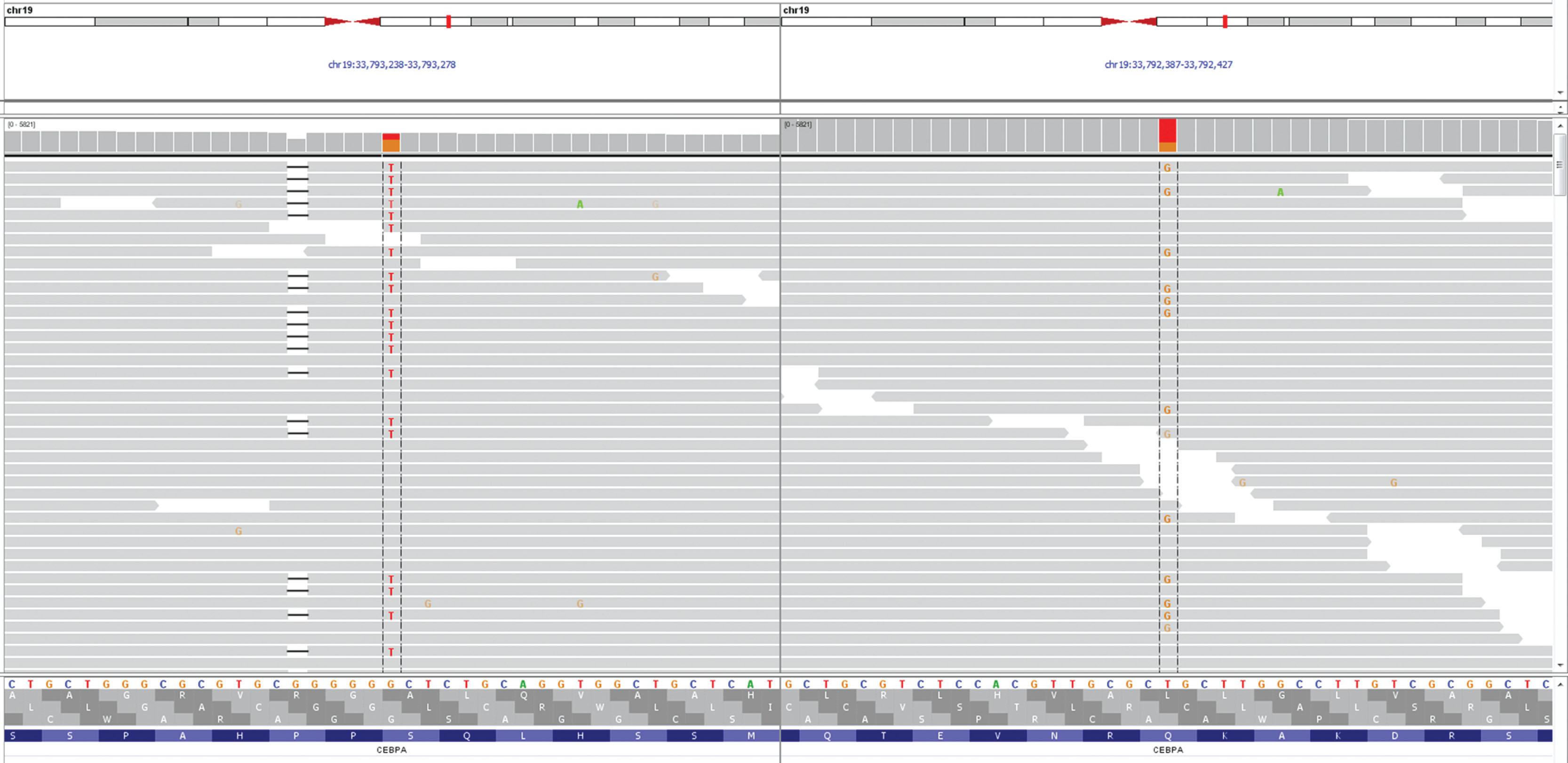
With the development of more sensitive analysis, the subclassification of AML patients has become more detailed. The revisions to AML classification in 2016 remain largely unchanged for the entities of AML and NOS, with the exception of including the erythroid/myeloid subtype of acute erythroid leukemia with MDS. The 2016 revisions include recognition of new cytogenetic subgroups, such as a provisional entity of AML with BCR-ABL and accompanied cryptic deletions of antigen receptors, particularly immunoglobulin heavy chain (IGH) gene rearrangement; this was recently shown to be specific for de novo disease. This update also included new and revised subgroups based on the presence of myeloid gene mutations. For example, AML with RUNX1 mutations include only de novo cases. AML with CEBPα now are heterozygous/double mutations ( Fig. 56.1 ). In addition, NPM1 and double mutations in CEBPα trump multilineage dysplasia in de novo disease without MDS-related cytogenetic abnormalities other than del(9q). There is also a greater emphasis on the recognition of familial myeloid neoplasms, with an added classifications section.20 Overall, the 2016 WHO classification of AML has limited changes but recognizes the importance of myeloid gene mutation studies without making the classification overly complex. Cytogenetic studies, screening for gene rearrangements, and assessment of gene panels, including NPM1, CEBPA, RUNX1 , are currently required to assign AML to the recurrent genetic abnormalities category.
The earliest recognition of myelodysplastic disorders as a clinical entity came with the identification of long-standing and treatment-refractory anemia as occasionally representing a preleukemic disorder (see Chapters 61 and 65 ). MDS patients classically present with peripheral cytopenias, a hypercellular BM with ineffective hematopoiesis and uni- or multi-lineage dysplasia, with or without an increase in blast cell numbers. Although this is fairly typical, it belies the wide pathologic spectrum of MDS, which includes cases that are diagnostically challenging, and which can be difficult to distinguish on one hand from benign causes of cytopenias in older adult patients, and on the other hand from AML and other more aggressive clonal myeloid neoplasms.
The FAB group proposed the first formal classification of MDS in 1982. In this classification scheme, the myelodysplastic disorders were divided into four subtypes based on increasing blast cell numbers or as chronic myelomonocytic leukemia (CMML). The four entities included RA, refractory anemia with ring sideroblasts (RARS), refractory anemia with excess of blasts (RAEB), and refractory anemia with excess of blasts in transformation (RAEB-T). These entities were differentiated mainly by the percentage of blasts seen in the BM ( Table 56.5 ). As noted earlier, in the FAB scheme AML was defined by the presence of 30% or more blasts in the blood or BM.
| FAB 1982 | WHO 2001 | WHO 2008 | WHO 2016 |
|---|---|---|---|
| RA | RA |
|
MDS with single lineage dysplasia (MDS-SLD) |
| RARS | RARS | RARS | MDS-RS with single lineage dysplasia (MDS-RS-SLD) |
|
RCMD (-RS) |
|
|
| RAEB | RAEB-1 | RAEB-1 | MDS with excess blasts (MDS-EB-1) |
| RAEB-2 | RAEB-2 | MDS with excess blasts (MDS-EB2) | |
| MDS-U | MDS, unclassifiable (MDS-U) | ||
| MDS with 5q- | MDS with isolated del(5q) | ||
| RCC (provisional) | Refractory cytopenia of childhood | ||
| RAEB-T | |||
| CMML |
The FAB included CMML in the MDS category, although it was recognized that CMML differed in that it had a proliferative component with increased numbers of circulating monocytes. At times, leukocytosis was a predominant feature of the so-called myeloproliferative type of CMML, but this was considered to be a dysplastic form and was classified as a type of MDS when the white blood cell count was less than 13,000/μL.
The 2001 WHO classification of MDS included significant changes to the classification of both MDS (see Table 56.5 ) and AML (as discussed earlier). As mentioned previously, the most notable change was the reduction in the blast percentage required for a diagnosis of AML from 30% to 20%, leading to the elimination of the RAEB-T category. The 2001 classification also included a new subtype of MDS that, despite the lack of increased blasts (less than 5%), had a more aggressive course, probably owing to the presence of more pronounced multilineage dysplasia. This category was called refractory cytopenia with multilineage dysplasia (RCMD), and it included a substantial proportion of cases previously grouped in the low-grade RA and RARS categories. Although the recognition of RCMD served to deemphasize the importance of the blast percentage when determining prognosis, these classifications subdivided the RAEB category into two types, with 5% to 9% blasts (RAEB-1) and 10% to 19% blasts (RAEB-2), paradoxically emphasizing the prognostic significance of blast percentage in this category.
An additional significant change in the WHO 2001 MDS classification scheme included the exclusion of CMML from the MDS category, and the development of a separate nosologic group for CMML and other diseases in which there were features of both myelodysplasia and myeloproliferation at the time of diagnosis. These “overlap” disorders are mentioned briefly later.
Further refinements in the WHO classification scheme for MDS were made subsequently in 2008 (see Table 56.5 ). These included expanding low-grade MDS from RA to refractory cytopenia with unilineage dysplasia (RCUD), thereby recognizing that the megakaryocyte or granulocyte lineage could be equally affected. The 2008 WHO classification scheme also emphasized the key role of cytogenetic analysis in the diagnosis of MDS, particularly in cases with otherwise insufficient morphologic evidence to substantiate a diagnosis of MDS. This is reflected in the inclusion of the subtype MDS unclassified (MDS-U), defined by the presence of cytopenias, less than 1% peripheral blasts, less than 10% dysplastic cells in any lineage, and less than 5% BM blasts with the presence of specific cytogenetic abnormalities commonly associated with MDS. In addition, the WHO 2008 classification now includes “MDS with an isolated del(5q)” including the “5q minus syndrome.” This syndrome had been recognized for some time and is characterized typically by its presentation in middle-aged women with macrocytic anemia, splenomegaly, normal-to-elevated platelet counts, hypolobated megakaryocytes in the BM, and an isolated del(5q). The specific types of MDS have been increased and there are more than 10 different entities.
The 2016 WHO revision introduced nomenclature changes including replacement of the terminology “refractory anemia” and “refractory cytopenia” with “myelodysplastic syndrome with single lineage dysplasia.” Other changes in the 2016 revision include the considerations of the prognostic significance of myeloid gene mutations in MDS, revising the diagnostic criteria for MDS entities with ring sideroblasts based on the detection of SF3B1 mutations, slightly modifying the cytogenetic criteria for MDS with isolated del(5q), reclassifying most cases of the erythroid/myeloid type of acute erythroleukemia, and recognizing the familial link in some cases of MDS. Flow cytometric immunophenotyping is recognized as a useful ancillary technique in the evaluation of MDS.
In 2001 WHO introduced the overlap syndromes—that is, the MDS/MPNs ( Table 56.6 )—because at the time there was disagreement among committee members as to whether CMML was an MDS, as the FAB suggested, or an MPN, as a number of investigators suggested. This group of diseases was defined to include disorders that share features of the MPNs and of MDS at the time of initial presentation but do not fit well into either group. Some of the entities in the MPD/MPN category still are not well understood and may represent a disease in transition from MDS or MPN, although a patient should not be placed in this category if initially diagnosed as MDS or MPN. However, without complete knowledge of the historical pathology of each individual patient, it is useful to have this category in order to construct a more reasonable classification. The overlap syndromes include CMML, including the juvenile type and juvenile myelomonocytic leukemia (JMML; see Chapter 65 ), in addition to “atypical” CML (atypical CML, BCR-ABL1 negative), and an “unclassifiable” category that includes refractory anemia with ring sideroblasts and thrombocytosis (RARS-T) (see Chapter 73 ). The MDS/MPNs share proliferative features in some cell lineages but also have dysplastic features, including ineffective hematopoiesis, in others. Similar to the MPNs, the overlap syndromes require a full evaluation of clinical and morphologic findings and evaluation of ancillary studies before a firm diagnosis can be rendered.
|
In the 2008 WHO classification, RARS-T was proposed as a provisional entity; however, the strong association with SF3B1 mutation and MPN-driver mutations (JAK2, MPL and CALR) supported its inclusion as a full entity in the 2016 revision.
The most common mutations reported with CMML include TET2, SRSF2 , ASXL1 , and genes of the RAS pathway. In fact, the co-existence of SRSF2 and TET2 mutations is particularly helpful in the appropriate clinical setting and can provide support to the diagnosis of CMML. Despite the progress in the molecular characterization of MPN/MDS neoplasm, significant molecular overlap with abnormalities have been noted in the spectrum of other myeloid neoplasms. As such, no disease-defining molecular abnormalities have been identified in this group, with the notable exception of JMML, which is caused by either somatic or germline mutations in one of the RAS pathway genes (PTPN11, NF-1, NRAS, KRAS, CBL) . The diagnosis of MPN/MDS neoplasms requires an integrated approach to incorporate clinicopathologic findings along with cytogenetic and molecular findings.
Hodgkin lymphoma was first described by Thomas Hodgkin in 1932 and is a distinct entity ( Table 56.7 ) that can be distinguished from the majority of lymphomas that are designated non-Hodgkin lymphomas (NHL) (see Chapters 78 and 79 ). Historically, different classification systems have been proposed for NHL ( Table 56.8 ). Henry Rappaport utilized the histologic features and the architectural arrangement of the neoplastic cells and their cytology when developing a classification system in 1956, which became widely accepted. This system was created prior to the advent of modern immunology; as such, the Lukes–Collins classification in 1975 attempted to relate cell morphology to immunologic function. Subsequently in 1982, the Working Formulation for classifying NHL replaced the Rappaport and Lukes–Collins classification. This system had three groups based on patient prognosis: low, intermediate, and high grade. In 1994, the REAL classification implemented a new approach for classifying NHL, taking into account immunologic, genetic, and clinical features, and not solely relying on histopathologic characteristics of the tumor cells. In 2001, the WHO classification successfully provided a common language and was adopted as the standard for clinicians and investigators worldwide. The modifications made in the 2008 classification are the result of a successful coordination between pathologists, clinicians, and biologists.
|
| 1832 | Hodgkin | A report of seven lymphoma cases |
| 1966 | Rappaport | Rappaport Classification |
| 1974 | Lukes–Collins | Lukes–Collins Classification |
| 1978 | Lennert | Keil Classification |
| 1982 | National Cancer Institute | Working Formulation of Non-Hodgkin Lymphoma |
| 1988 | Stansfeld et al. | Updated Keil Classification |
| 1994 | Harris et al. | REAL Classification |
| 2001 | Jaffe et al. | 2001 WHO Classification |
| 2008 | Swerdlow et al. | 2008 WHO Classification |
B-cell, T-cell, and natural killer (NK)-cell neoplasms often represent clonal expansion of these cells at certain developmental stages. Although B-cell neoplasms tend to mimic stages of normal B-cell development, some common B-cell neoplasms, such as hairy cell leukemia, do not conform to a normal B-cell differentiation stage. Additionally, some lymphomas show overt heterogeneity or lineage plasticity; consequently, the normal counterpart of neoplastic cells cannot be used as the sole basis for developing a classification system. The 2008 WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues schema routinely employs a multiple-parameter approach that is based on clinical, morphologic, and biologic features, keeping in mind that a precise separation between entities is not possible in certain cases. Thus, the WHO recognized “gray zones” in which tumor cells may cross boundaries between currently used categories, such as the boundaries between classic Hodgkin lymphoma (CHL) and primary mediastinal large B-cell lymphoma.
In 2008 WHO expanded the classification of lymphoid neoplasms, with more consideration being given to disease definitions, nomenclature, grading, and clinical relevance. Since then, disease definitions have continued to evolve and expand, with new entities and variants being recognized. The most recent 2016 WHO revisions developed a focus on early or in situ lesions, as well as definition of the earlier steps of neoplastic transformation, age as a disease-defining feature (e.g., diffuse large-cell lymphoma of the older adult; Table 56.9 ), and site-specific impact on disease definition. In addition, there was an emphasis on overlapping or borderline entities, with fuzzy demarcation of morphologic, molecular, and genetic characteristics as areas of diagnostic challenge.
|
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here