Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The successful transition from single cells to complex multicellular organisms has required the development of mechanisms for cells to communicate with each other, so as to act in concert during processes such as nutrient acquisition, motility, and defense. The most fundamental of these are cell–cell junctions that serve as structural organizers, but also provide information that individual cells can utilize to orient themselves in relation to the remainder of the organism. In larger species that contain multiple organs and cell types, the need to communicate information over long distances has led to the development of diffusible factors that are secreted by one cell and travel to distant cells. These factors can be delivered locally, via the circulation (e.g., hormones and cytokines) or via the nervous system (e.g., neurotransmitters), and are recognized by the appropriate cell surface receptor on the recipient cell. The complex nature of the numerous signals presented to the cell at any given point in time has led to the development of an intricate array of receptor-activated intracellular second messengers that, by undergoing a coordinated series of interactions and enzymatic alterations, can transduce the information presented on the cell surface to effector molecules that mediate the appropriate cellular response.
The successful transition from single cells to complex multicellular organisms has required the development of mechanisms for cells to communicate with each other, so as to act in concert during processes such as nutrient acquisition, motility, and defense. The most fundamental of these are cell–cell junctions that serve as structural organizers, but also provide information that individual cells can utilize to orient themselves in relation to the remainder of the organism. In larger species that contain multiple organs and cell types, the need to communicate information over long distances has led to the development of diffusible factors that are secreted by one cell and travel to distant cells. These factors can be delivered locally, via the circulation (e.g., hormones and cytokines) or via the nervous system (e.g., neurotransmitters), and are recognized by the appropriate cell surface receptor on the recipient cell. The complex nature of the numerous signals presented to the cell at any given point in time has led to the development of an intricate array of receptor-activated intracellular second messengers that, by undergoing a coordinated series of interactions and enzymatic alterations, can transduce the information presented on the cell surface to effector molecules that mediate the appropriate cellular response.
The kidney serves to protect the internal milieu of higher organisms from perturbations due to the accumulation of metabolic products, as well as those resulting from fluctuations in the intake or loss of water and various salts. To regulate this intricate function, the body must continuously monitor the composition and quantity of the extracellular fluid, and then signal the nephron to appropriately regulate glomerular filtration and tubular cell function in response to changes in these parameters. Regulation of these exquisitely precise events requires that the cells of the kidney are able to respond to signals emanating from distant sites, and then efficiently communicate in an intercellular and intracellular manner to coordinate the response. This chapter will provide an overview of several of the most common receptors and intracellular second messenger pathways that are utilized in this process.
In the best studied pathway of cell signaling, a first messenger is secreted by one group of cells and travels either to distant cells (endocrine factors) or to local cells (autocrine or paracrine factors), where it binds to a specific receptor. The first messengers in these classic pathways are generally either proteins (growth factors, cytokines), catecholamines (epinephrine, dopamine) or steroids (mineralocorticoids, sex hormones), although receptors have been identified for multiple circulating factors including lipids (e.g., lysophosphatidic acid), ions (e.g., calcium), eicosanoids (e.g., prostaglandin E 2 ), sugars (e.g., glucose), nucleosides (e.g., ATP), and gases (e.g., nitric oxide). Most of these receptors are located on the cell surface and have an extracellular region (domain) that recognizes and binds to the specific ligand. This ligand-binding domain is connected via one or more transmembrane segments to the intracellular (cytosolic) domain that undergoes a change in conformation or activity in response to ligand binding, and thus initiates the activation and/or modification of intracellular second messengers. In contrast to these cell surface receptors, steroid receptors, which are discussed later in this chapter, are typically located in the cytoplasm. The lipophilic steroid ligands are capable of crossing the cell membrane and binding the receptor, which then initiates signaling events by translocating into the nucleus where the ligand–receptor complex can regulate gene transcription.
Based on their structure, the type of ligand that they bind, and the principle second messengers that are activated, classical cell surface receptors can be grouped into G-protein coupled receptors, receptor tyrosine kinases (RTKs), serine/threonine kinase receptors, and receptor-like phosphatases. However, it has become increasingly clear that other surface proteins serve as signaling initiators to transduce information about the environment surrounding the cell. Thus, cell–cell and cell–matrix adhesion molecules initiate signaling cascades that regulate cell shape, differentiation, proliferation, and survival. The following section will provide a brief overview of these various signaling initiators, focused on those presently considered to be important in regulating normal renal development and maintaining adult kidney homeostasis.
The receptor sub-type that is responsible for mediating the signaling responses of the greatest number of ligands in the kidney is probably the G-protein-coupled receptor (GPCR). GPCRs make up the largest family of cell surface receptors, with over 800 members predicted from the sequence of the human genome (reviewed in ) ( Figure 13.1 ). GPCRs are transmembrane proteins with their amino terminus on the cell exterior, seven transmembrane α helical segments, and the carboxyl terminus in the cell interior. This arrangement results in three extracellular loops and three intracellular loops joining the transmembrane segments. They bind to extracellular ligands such as epinephrine, dopamine, angiotensin II, adenosine, vasopressin, calcium, and parathyroid hormone, and mediate their intracellular actions.
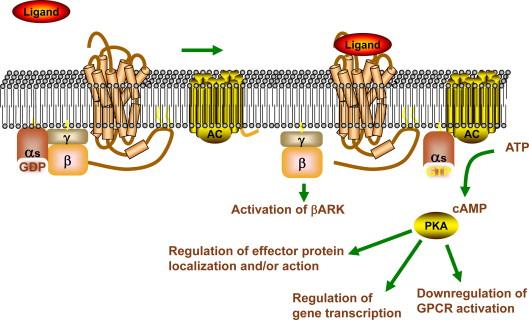
The extracellular loops serve as the primary binding site for the specific GPCR ligand, with the amino terminus also contributing to the binding site for some ligands. The intracellular loops, most critically the 5–6 loop, serve as the binding site for the principal GPCR intracellular effectors, the heterotrimeric G-proteins. These small GDP/GTP-binding protein complexes are made up of α-, β-, and γ-subunits, with the α-subunit serving as the GDP/GTP-binding site, and the βγ-subunits acting both as regulators of α-subunit localization, and independently as intracellular signaling effectors. The existence of multiple different α-, β-, and γ-subunits allows for hundreds of potential combinations of heterotrimeric G-proteins, and thus imparts specificity of response to the individual GPCR and its ligand.
In the absence of receptor activation, the α-subunit is bound to GDP, and associates with the βγ-subunits at the membrane. However, following ligand binding to the extracellular surface of the GPCR, a conformational change of the receptor results in disassociation of GDP, and binding of GTP to the α-subunit. The binding of GTP stimulates disassociation of the α-subunit from the βγ-subunits and the receptor. The GTP-loaded α-subunit can then associate with its intermediary effectors (such as adenylyl cyclase and phospholipase), while the βγ-subunits can associate with and regulate independent effectors, such as ion channels and the β a drenergic r eceptor k inase (βARK) (reviewed in ).
The protein products of the 16 mammalian genes encoding Gα-subunits have been grouped into four classes, the G sα (stimulatory for adenylyl cyclase), G iα (inhibitory for adenylyl cyclase), G q/11α (regulators of phospholipase Cβ (PLCβ)), and G 12α (regulators of RhoGEF). Binding of the appropriate GTP-loaded Gα-subunit to its primary effector results in the activation or inhibition of effector function; for example, adenylyl cyclase catalyzes the cyclation of ATP to form 3′,5′-cyclic AMP (cAMP), an intracellular second messenger that can bind and activate downstream signaling proteins such as p rotein k inase A (PKA). This reaction is activated by GTP-G sα binding to adenylyl cyclase and inhibited by GTP-G iα binding. In addition, βγ binding to adenylyl cyclase can augment its activation by GTP-G sα .
An important concept in all forms of signal transduction is the ability of the cell to carefully control the location, amplitude, and duration of the signal. Signal amplification is the process whereby the cell can regulate the amplitude of the signal. For example, a single GPCR can generate between tens and hundreds of GTP-coupled Gα-subunits, which can subsequently bind to and activate similar numbers of adenylyl cyclase enzymes, which in turn generate multiple copies of cAMP. The number and availability of the intracellular effector enzymes and substrates thus determines the level of signal amplification following the activation of relatively few receptors on the cell surface.
Just as important as signal amplification is the ability of the cell to downregulate the signaling pathway once the desired response has been initiated. For GPCRs, this occurs in several ways. First, the α-subunit is itself a GTPase, meaning that it hydrolyzes GTP to form GDP and inorganic phosphate. This hydrolysis occurs spontaneously following GTP binding to Gα, but can be augmented by the association of specific RGS proteins ( r egulators of G -protein s ignaling) with the GDP/Gα complex, as this interaction stabilizes the inactivated state. Once the Gα-subunit is in the GDP-bound state, it can associate again with the βγ-subunit to regenerate the inactive heterotrimeric G-protein.
In addition, many GPCRs are themselves inactivated by a process called homologous desensitization. As has been noted above, the βγ-subunits can associate with the cytosolic protein βARK. βARK, also known as GRK2, is a member of the G -protein-coupled r eceptor k inases (GRKs) that, following association with G βγ , phosphorylate the intracellular loops and/or C terminus of ligand-associated GPCRs on serine and/or threonine residues. This phosphorylation results in the association of β-arrestin with the receptor, mediating the uncoupling of the ligand–receptor complex from the heterotrimeric G-proteins, and thus diminishing its activity. Binding of β-arrestin has also been shown to target the receptor–ligand complexes to clathrin-coated pits on the cell surface, followed by internalization and either lysosomal degradation or recycling of the inactivated receptor to the cell surface.
The downstream GPCR effector adenylyl cyclase is also subject to phosphorylation-dependent inhibition. As noted above, activated adenylyl cyclase catalyzes the formation of cAMP, which in turn associates with and activates PKA. This enzyme, an intracellular serine/threonine kinase, has multiple phosphorylation substrates within the cell. Phosphorylation of these substrates can regulate their activity, cellular localization, and/or their interaction with other proteins. One phosphorylation substrate is adenylyl cyclase itself, resulting in inhibition of cAMP production. A second substrate is the GPCR. In a process known as heterologous desensitization, activation of PKA by a non-GPCR signal can result in phosphorylation of the GPCR and subsequent inhibition of ligand-mediated GPCR activation.
A prototypic family of GPCRs in the kidney is the dopamine receptors. There are five dopamine receptors presently described (D 1 –D 5 ), and they are further sub-classified into D 1 -like (D 1 and D 5 ) or D 2 -like (D 2 –D 4 ). The D 1 -like receptors are associated with G sα , and therefore activate adenylyl cyclase, whereas the D 2 -like receptors inhibit adenylyl cyclase activity (reviewed in ). In the kidney, D 1 -like and D 2 -like receptors are expressed throughout the tubules. The net effect of activating these receptors is the induction of a salt and water diuresis, although by different mechanisms in the different tubular segments. Dopamine-mediated activation of D 1 receptors in the proximal tubule results in activation of adenylyl cyclase, leading to cAMP-dependent inhibition of the activity of NHE-3, NaPi-2, and the Na,K-ATPase, thus inhibiting proximal sodium reabsorption. In contrast, activation of the D 2 -like receptor D 4 in the cortical collecting duct leads to a water diuresis by preventing vasopressin-stimulated adenylyl cyclase activation. Dopamine receptors also mediate renal vasodilation, and appear to regulate renin secretion. Due to these effects, defects in dopamine receptor function in mice are associated with salt retention, vasoconstriction, and increased blood pressure.
Arginine vasopressin (AVP, also known as anti-diuretic hormone (ADH)) binds to three GPCRs, the G q/11α -linked V1a and V1b receptors, and the G sα -linked V2 receptor. V1 receptors are located on several cell types, including smooth muscle cells of blood vessels, where they mediate the vasoconstrictive (“pressor”) response of vasopressin, while V2 receptors are located on epithelial cells in the collecting duct and mediate water reabsorption. Binding of vasopressin to the V2 receptor stimulates adenylyl cyclase-mediated cAMP production, which in turn causes insertion of vesicles containing the water channel aquaporin-2 into the apical membrane of collecting duct cells. By inhibiting adenylyl cyclase activation in these cells, dopamine can partially counteract this water-reabsorptive effect of vasopressin.
An example of a GPCR that is coupled to PLCβ signaling is the type 1 receptor for angiotensin II (AT 1A R). Angiotensin II is the eight amino acid peptide product of the angiotensin converting enzyme (ACE)-mediated cleavage of angiotensin I. Angiotensin II is capable of binding to and activating two distinct G-protein coupled receptors, the type 1 receptor (AT 1 R) and the type 2 receptor (AT 2 R). The predominant actions of angiotensin in the kidney and adrenal gland are mediated by the AT 1 R, including vasoconstriction, smooth muscle hypertrophy, sodium retention, and aldosterone secretion. Most data presently support the idea that the AT 2 R receptor acts as an antagonist to AT 1 R signaling, although exactly how the AT 2 R signals has been much less apparent. The topology of the AT 2 R is consistent with a seven transmembrane G-protein coupled receptor, yet it has been controversial as to whether AT 2 R in fact signals via traditional G-proteins. There have been several reports that AT 2 R can signal via G iα , although this has not been universally accepted (reviewed in ). Other groups have suggested that AT 2 R signaling is pertussis-toxin insensitive (i.e., not dependent on G iα ), and is instead mediated by production of cyclic GMP. It remains unclear whether the generation of cGMP is a direct result of AT 2 R activation or is mediated in an autocrine/paracrine fashion by AT 2 R-stimulated bradykinin production.
The AT 1 R is in the G q/11α family of GPCRs, meaning that binding of angiotensin II to the receptor stimulates GTP-loading of G qα which in turn associates with and activates phospholipase Cβ (PLCβ) ( Figure 13.2 ). The active form of PLCβ mediates the hydrolysis of phosphotidylinositol 4,5 bisphosphate (PI 4,5 P 2 ) in the membrane to produce diacylglycerol (DAG) and inositol trisphosphate (IP 3 ). IP 3 is hydrophilic and enters the cytoplasm where it activates the IP 3 receptor on the surface of the endoplasmic reticulum, thereby stimulating calcium release from internal stores. The simultaneous production of DAG at the membrane, and local release of stored calcium, leads to the recruitment and activation of the classic, calcium-dependent protein kinases C (PKCs). Activation of PKC appears to be required for angiotensin II-mediated renal efferent arteriole vasoconstriction, Na,K-ATPase recruitment to the membrane in proximal tubule cells (resulting in increased proximal sodium reabsorption), and stimulation of aldosterone secretion by adrenal zona glomerulosa cells.
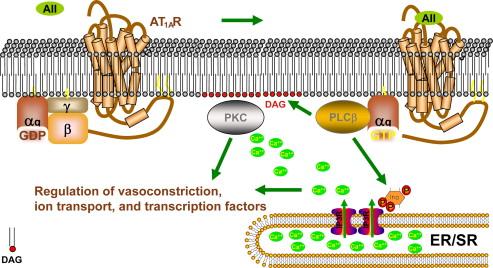
In addition to activation of PLCβ, a second signaling pathway that is activated by the AT 1 R is the m itogen a ctivated p rotein k inase (MAPK) pathway. As mentioned earlier, phosphorylation of GPCRs by βARK results in the recruitment of β-arrestin to the receptor complex, and subsequent receptor internalization. β-arrestin has been found to act as a binding scaffold for the core components of the MAPK pathway, including Raf, MEK, and ERK (see “Intracellular Signaling Pathways,” below; reviewed in ), resulting in the activation of this pathway that mediates cell growth and proliferation. This scaffolding function of β-arrestin appears to be both cell type and receptor specific, and can mediate activation of additional intracellular signaling pathways, including the phosphoinositide 3-kinase (PI 3-K) pathway that will be discussed later in this chapter. Activation of these signaling pathways appears to play an important role during kidney development, since newborn mice null for the type I angiotensin receptor or in which angiotensin signaling has been inhibited, have significant renal developmental abnormalities, including renal arterial hypertrophy and papillary atrophy.
A second means by which GPCRs can activate MAPK signaling was discovered when the levels of β-arrestin were depleted using RNA interference (RNAi). Under these conditions, angiotensin II was still able to activate MAPK, although to a lesser degree. These experiments uncovered a β-arrestin-independent pathway of angiotensin II-dependent MAPK stimulation that occurs via activation of a second cell surface receptor, a process known as receptor transactivation. As noted above, stimulation of G qα by the AT 1A R leads to activation of PKC. PKC, in addition to regulating processes such as ion transport, can activate a cell surface protein called heparin-binding epidermal growth factor (HB-EGF). HB-EGF is one of the ligands for a separate cell surface receptor, the epidermal growth factor receptor (EGFR), and binding of HB-EGF to the EGFR results in the stimulation of multiple signaling events, including MAPK activation (see “Intracellular Signaling Pathways”).
These two independent pathways for activating MAPK signaling provide an example of how scaffolding proteins can compartmentalize signaling within the cell. The β-arrestin-mediated ERK activation is sustained for several hours and occurs in the cytoplasm, whereas the G qα /PKC-dependent ERK activation appears to be more transient and primarily within the nucleus. This ability to localize activated ERK in different cellular compartments allows the cell to differentially regulate specific effector proteins, and thus direct distinct cellular outcomes. In the heart, for example, AT 1 R-mediated G qα /PKC-dependent transactivation of the EGFR, and ultimately MAPK nuclear signaling, is believed to at least partially mediate angiotensin-stimulated cardiac hypertrophy.
Many GPCRs can activate multiple Gα-subunits, depending on cellular location and availability. For example, the parathyroid hormone (PTH) receptor can potentially activate G sα (thus activating adenylate cyclase and PKA), G qα (activating PLCβ and PKC), and G i (inhibiting adenylate cyclase). PTH is an 84 amino acid peptide hormone secreted by the parathyroid gland that acts on bone to increase calcium and phosphate release into the circulation, as well as on the proximal and distal tubules of the kidney to inhibit phosphate reabsorption and stimulate calcium reabsorption, respectively. PTH is proteolytically processed to generate multiple fragments which can bind to and activate the PTH receptor, a class B GPCR (defined by the six conserved cysteine residues that form disulfide bonds in the large extracellular amino terminal domain). Expression of a mutant form of the receptor that selectively fails to activate G qα -dependent PLCβ signaling in mice results in abnormalities in bone ossification without a change in serum calcium. The normal serum calcium in these animals suggests that renal tubular calcium handling in these mice is dependent on G sα - or G i -regulated adenylate cyclase signaling, while bone ossification appears to require G qα -PLCβ signaling. In support of this hypothesis, complete loss of PTH receptor signaling results in hypocalcemia in addition to bone abnormalities. In humans, this is recapitulated by an autosomal recessive mutation in the receptor in patients with Blomstrand chondrodysplasia, a lethal disorder characterized by excessive bone maturation and mineralization.
As noted previously, receptor internalization mediated by β-arrestin is frequently a means by which GPCR are uncoupled from their ligands and signaling is downregulated. For example, PTH related protein (PTHrP) can bind and activate the PTH receptor, leading to GTP-loading of G sα and a transient increase in cAMP followed by receptor desensitization. In contrast, PTH receptor ligands such as PTH 1–34 (the first 34 amino acids of PTH) can induce a more sustained increase in adenylyl cyclase activation and cAMP levels, leading to systemic responses such as increased vitamin D hydroxylation and higher serum calcium levels. Investigation into the mechanism of this difference has demonstrated that binding of PTH 1–34 to the PTH receptor leads to internalization of the active receptor–ligand complex in endosomes that also contain adenylyl cyclase, leading to sustained signaling from this intracellular site. These structures, referred to as signaling endosomes, have been shown to mediate signaling via multiple receptor types in addition to GPCR, and to regulate complex cellular responses such as migration, differentiation, and asymmetric division.
A second class of transmembrane receptors is the kinase receptors. These proteins typically contain an extracellular ligand-binding domain at the amino terminus, a single membrane-spanning domain, and an intracellular carboxy terminus that includes the kinase domain. In most cases, binding of the ligand to the receptor results in homodimerization of two receptor molecules, bringing the intracellular kinase domains into close proximity where they phosphorylate substrate residues on the adjacent receptor. This phosphorylation step generates binding sites for the recruitment of intracellular signaling molecules, as well as further activating the kinase domain so that non-receptor substrates recruited to the complex can also be phosphorylated.
The largest class of kinase receptors is the tyrosine kinase receptors, also known as receptor tyrosine kinases (RTKs) ( Figure 13.3 ). These molecules frequently serve as receptors for extracellular growth factors, circulating proteins that stimulate cell growth and division. Examples of ligand–receptor combinations in this family include epidermal growth factor (EGF) and its receptors ErbB1 (or EGFR) and ErbB2; platelet-derived growth factor (PDGF) and the PDGF receptor; insulin and the insulin receptor; and vascular endothelial growth factor and its major receptors VEGFR1 and VEGFR2 (also called Flt1 and Flk1).
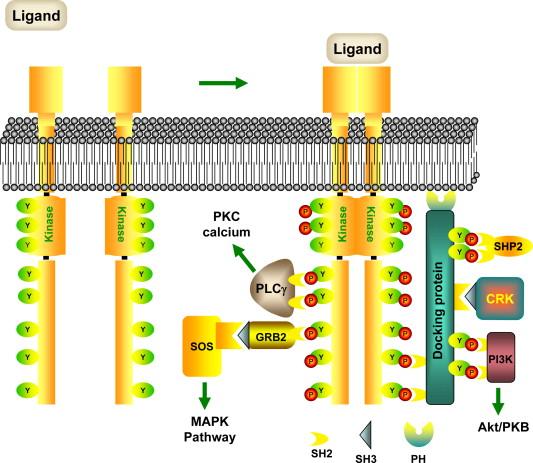
Once the growth factor has bound to and activated the receptor, newly phosphorylated tyrosine residues on the intracellular carboxy terminus of the receptor serve as binding sites for cytosolic or membrane-associated proteins that contain phosphotyrosine-binding domains. The best characterized of these domains are the s rc- h omology 2 (SH2) domains that share characteristic features first described in the phosphotyrosine-binding region of the cytosolic tyrosine kinase Src. SH2 domains are approximately 100 amino acids in length, and provide specificity of interaction in two ways. First, the interaction of the binding pocket of the SH2 domain and the tyrosine residue is only stabilized when the tyrosine residue is phosphorylated. Second, the amino acids immediately flanking the phosphorylated tyrosine residue determine which SH2 domain interaction is preferred. For example, the SH2 domain on the p85 adaptor protein, α-subunit of the lipid enzyme phosphoinositide 3-kinase (PI 3-K, see “Intracellular Signaling Pathways,” below), strongly prefers to bind to phosphotyrosine with a methionine residue at the +3 position. Thus, receptors containing the sequence pTyr-X-X-Met (where X can be almost any amino acid) specifically recruit and activate the PI 3-K.
In the kidney, tyrosine kinase receptors have been implicated in controlling development, mediating hypertrophy, regulating the balance between repair and fibrosis after injury, and promoting the growth of renal carcinomas. During development, glial derived neurotrophic factor (Gdnf) is made by the embryonic metanephric mesenchyme and activates the c-Ret tyrosine kinase receptor that is expressed on the epithelial cells of the adjacent Wolffian duct. The activation of Ret is somewhat unusual, since Gdnf does not directly bind to Ret, but rather binds to a third membrane protein, Gfrα, that mediates dimerization of Ret in response to association with Gdnf (reviewed in ). Activation of Ret in this manner results in the activation of multiple intracellular signaling pathways, including the Erk-MAPK pathway, the PI 3-K pathway, members of the Src family of non-receptor tyrosine kinases, and phospholipase Cγ (PLCγ). Activation of the MAPK and Src pathways (see “Intracellular Signaling Pathways”) have been found to be critical for the outgrowth and branching of the ureteric bud from the Wolffian duct, the first step in the formation of the metanephric kidney.
Signaling by several other tyrosine kinase receptors has been implicated in kidney development, including the fibroblast growth factor (FGF) receptors, hepatocyte growth factor receptor (Met), and the epidermal growth factor receptor. FGF signaling is a complex process that includes 18 known ligands and 4 distinct tyrosine kinase receptors (FGFR1-4) (reviewed in ). Like many receptor ligands, FGFs are secreted glycoproteins that are concentrated in proximity to their cell surface receptor by binding to heparan sulfate proteoglycans on the cell and/or nearby matrix components. The interaction between FGFs and their receptors can be further regulated by cell- or tissue-specific expression of FGF co-receptors such as Klotho. Intracellular signaling by the FGFR is regulated in part by the cytosolic adaptor protein FGFR substrate 2 (FRS2), which is phosphorylated by the FGFR kinase domain, leading to the recruitment and activation of downstream MAPK and PI 3-K signaling. In the developing kidney, FGF7 and FGF10, signaling via the IIIb isoform of FGFR2, have been shown to be critical for normal branching and extension of the collecting system, while FGF8 appears to be required for nephrogenesis by the adjacent metanephric mesenchyme.
Many growth factor receptors are expressed in the mature kidney, and are believed to be critical for maintenance of normal tubule architecture and for regulating the cellular response to injury. Renal tubular epithelial cells express EGF receptors as well as Met, the receptor for hepatocyte growth factor (HGF). These tyrosine kinase receptors directly bind their respective ligands via their extracellular amino terminal domains, followed by homodimerization and activation of intracellular signaling. A major mediator of the intracellular signaling mediated by EGF and HGF is the Gab1 docking protein, which functions in a manner similar to that of FRS2 for the FGFR. Recruitment of Gab1 to Met or the EGFR results in its phosphorylation on multiple tyrosine residues and subsequent association with p85, PLCγ, Grb2, a second adaptor protein known as Crk, and the protein tyrosine phosphatase SHP2. Following acute kidney injury, the level of HGF increases in the kidney, resulting in activation of Met, mediating MAPK, PI 3-K, and PLC signaling. These pathways in turn are believed to be important for inhibition of apoptosis (the PI 3-K pathway), and stimulation of cell migration and proliferation during the repair process (PI 3-K, MAPK, and PLC pathways).
A second group of transmembrane kinase receptors are the serine-threonine kinase receptors. Like the non-receptor kinases PKA and PKC, these receptors catalyze the phosphorylation of serine or threonine residues in their substrate molecules. Perhaps the best studied of these receptors in the kidney is the receptor for transforming growth factor β (TGFβ), a member of the TGFβ superfamily of secreted factors that also includes the bone morphogenic proteins (BMPs) and activin. TGFβ-like proteins signal into the cell via a heterotetrameric complex comprised of two subclasses of serine-threonine kinase receptors, the type I receptor and the type II receptor. Like the tyrosine kinase receptors, these proteins have an extracellular ligand recognition domain, a single transmembrane spanning domain, and an intracellular kinase domain. The different TGFβ-like ligands utilize distinct type I and II receptor combinations. For example, TGFβ1-3 signals via the combination of the type II receptor TβR-II and the type I receptors activin receptor-like kinase 1 (ALK-1) or ALK-5, while BMPs signal through the type II receptors ActR-II or BMPR-II and the type I receptors ALK-2, ALK-3 or ALK-6.
TGFβ receptor signaling begins when the ligand binds to the extracellular domain of its cognate type II receptor ( Figure 13.4 ). The kinase domain of type II receptors is constitutively active, and binding to the extracellular ligand results in the recruitment of the appropriate type I receptor to the complex, where it is phosphorylated and activated by the type II receptor. In this manner, the TGFβ1-dependent association of ALK-5 with TβR-II allows the constitutively active TβR-II to phosphorylate ALK-5 and activate its intracellular serine-threonine kinase domain. The specificity of signaling by TGFβ family members is further regulated by the presence in many cells of the accessory receptors betaglycan and endoglin. These transmembrane proteins lack intracellular kinase domains, and appear to regulate the affinity of TGFβ proteins for the various type II receptors, as well as modifying intracellular signaling by the ligand–receptor complex.
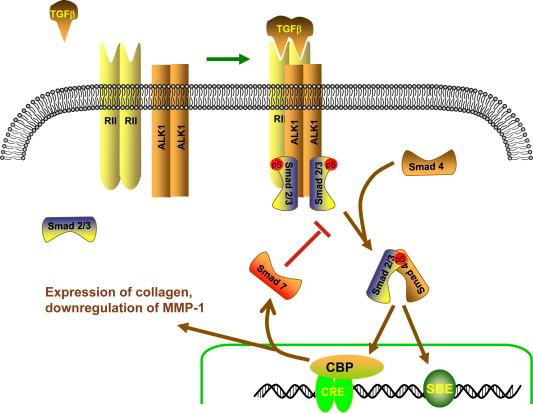
As opposed to tyrosine kinase receptors that signal primarily via recruitment of SH2 domain containing proteins to activate pathways such as MAPK and PI 3-K, TGFβ receptors signal primarily via a distinct signaling pathway, the Smad proteins. Smads are small cytoplasmic proteins that contain a DNA-binding domain and a TβR-I/Smad4-interacting domain. Based on their structure and function, Smads have been divided into three groups, the receptor activated Smads (Smad1, 2, 3, 5, 8), a regulatory Smad (Smad4), and the inhibitory Smads (Smad6, 7). Upon activation of TβR-I, the appropriate receptor activated Smads (e.g., Smad2 and 3 for ALK-5) are phosphorylated on regulatory serine residues in the TβR-I/Smad4 interacting domain, resulting in their disassociation from the receptor and association with Smad4. This Smad2–Smad4 complex then translocates to the nucleus, where the Smad DNA-binding domain can mediate direct association with S mad- b inding e lements (SBE) in the DNA of the promotor region of target genes, as well as association with other transcriptional regulators.
One of the transcriptional targets that is regulated by Smad2–4 signaling is another member of the Smad family, Smad7. Smad7 is an inhibitory Smad that can bind to TβR-I, and prevent Smad2 or Smad3 from associating and being activated. In this manner, TGFβ stimulation of Smad7 transcription provides a negative feedback loop that acts to prevent sustained Smad2 and Smad3 activation by the TGFβ receptor.
Studies in mice that have undergone genetic inactivation of various TGFβ family members demonstrate that Bmp2 and Bmp4 have important roles in normal kidney development (reviewed in ). In the mouse embryo, Bmp4 is expressed in the metanephric mesenchyme surrounding the Wolffian duct and adjacent to the ureteric bud (the epithelial structure that will branch to form the entire collecting system of the kidney), while Bmp2 is expressed in the condensing mesenchyme at the tips of the ureteric bud (the region that will differentiate into the glomerulus and proximal portions of the nephron through the connecting segment). The Bmp receptors Alk3 and Alk6 are expressed on the invading urteric bud itself. While complete loss of Bmp2 or Bmp4 results in embryonic lethality prior to kidney development, mice that are heterozygous for loss of Bmp4 expression exhibit multiple defects in the collecting system of the kidney, including doubling of the collecting system, hydroureter, and dysplastic kidneys, and Bmp2 heterozygotes demonstrate exaggerated uretic bud branching. Thus, it appears that Bmps normally act to inhibit ureteric bud outgrowth and branching during development.
In addition to their role in kidney development, TGFβ proteins have been shown to play a major role in regulating fibrotic responses of the adult kidney by both increasing new matrix deposition and inhibiting matrix degradation. In vitro studies have shown that TGFβ-dependent Smad3–Smad4 nuclear signaling can induce the expression of multiple collagen isoforms, along with their cellular binding partner β1 integrin, and activated Smad3 has been found to mediate decreased transcription of the gene for matrix metalloproteinase-1 ( MMP-1 ) . In support of an important role for the TGFβ-Smad signaling pathway in the development and progression of renal fibrosis in vivo , genetic overexpression of TGFβ in the rat has been shown to induce glomerulosclerosis due to increased extracellular matrix deposition, while mice lacking Smad3 demonstrate less fibrosis following ureteral obstruction.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here